Adenosine A3 Receptor (A3AR) Agonist for the Treatment of Bleomycin-Induced Lung Fibrosis in Mice
Abstract
1. Introduction
2. Results
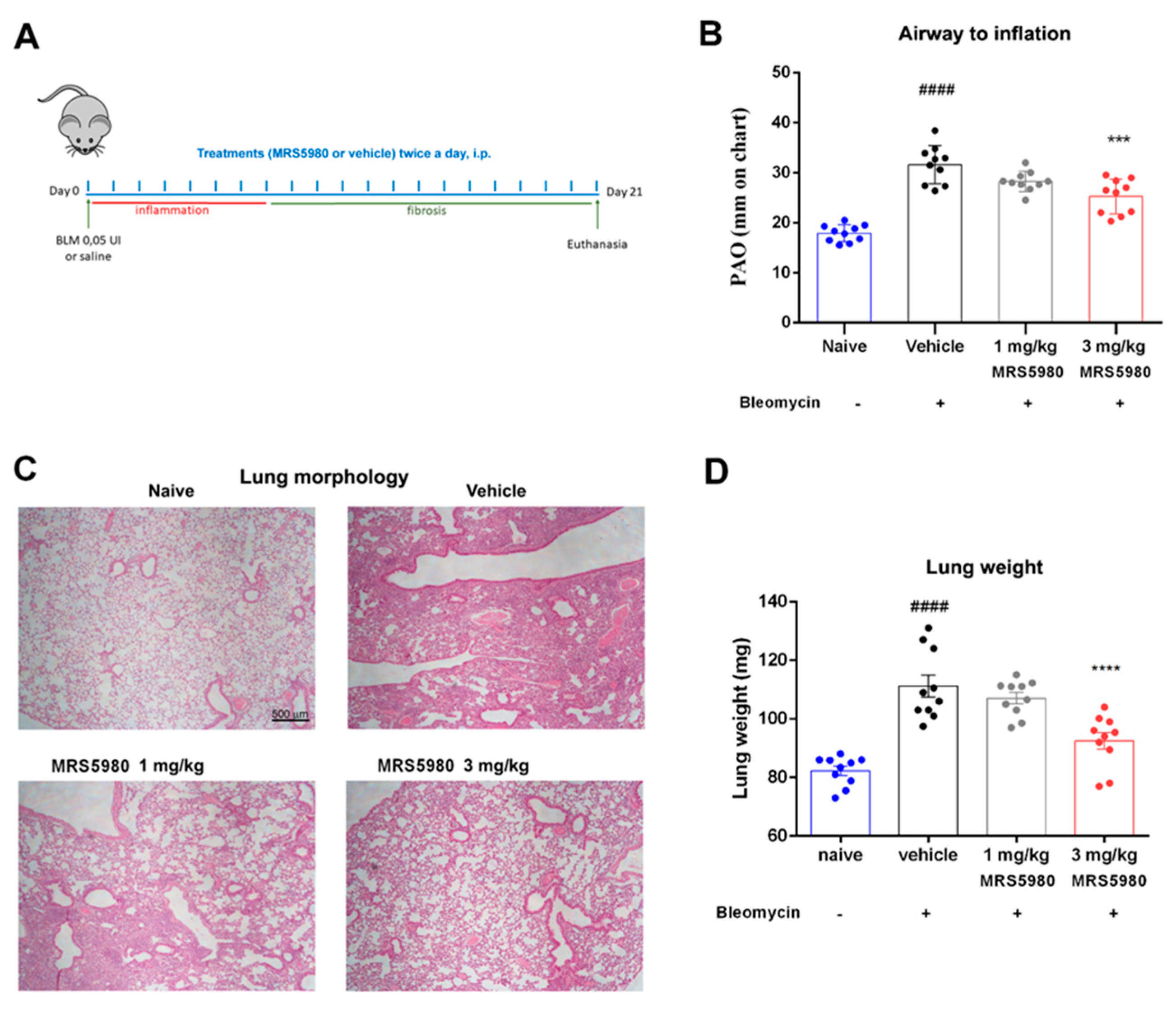
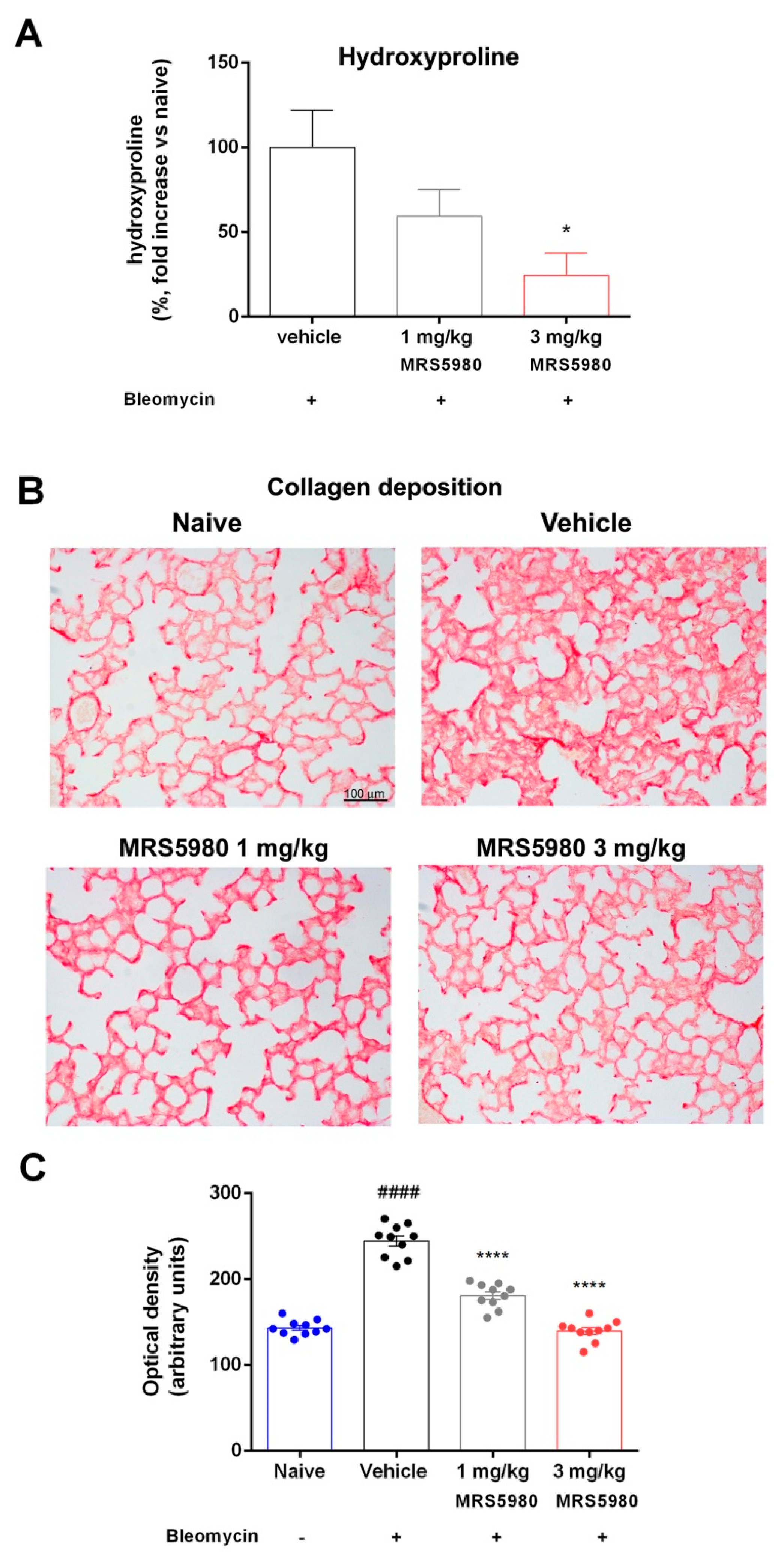
2.1. A3AR Agonist Effects in Ameliorating Lung Function and Pulmonary Architecture
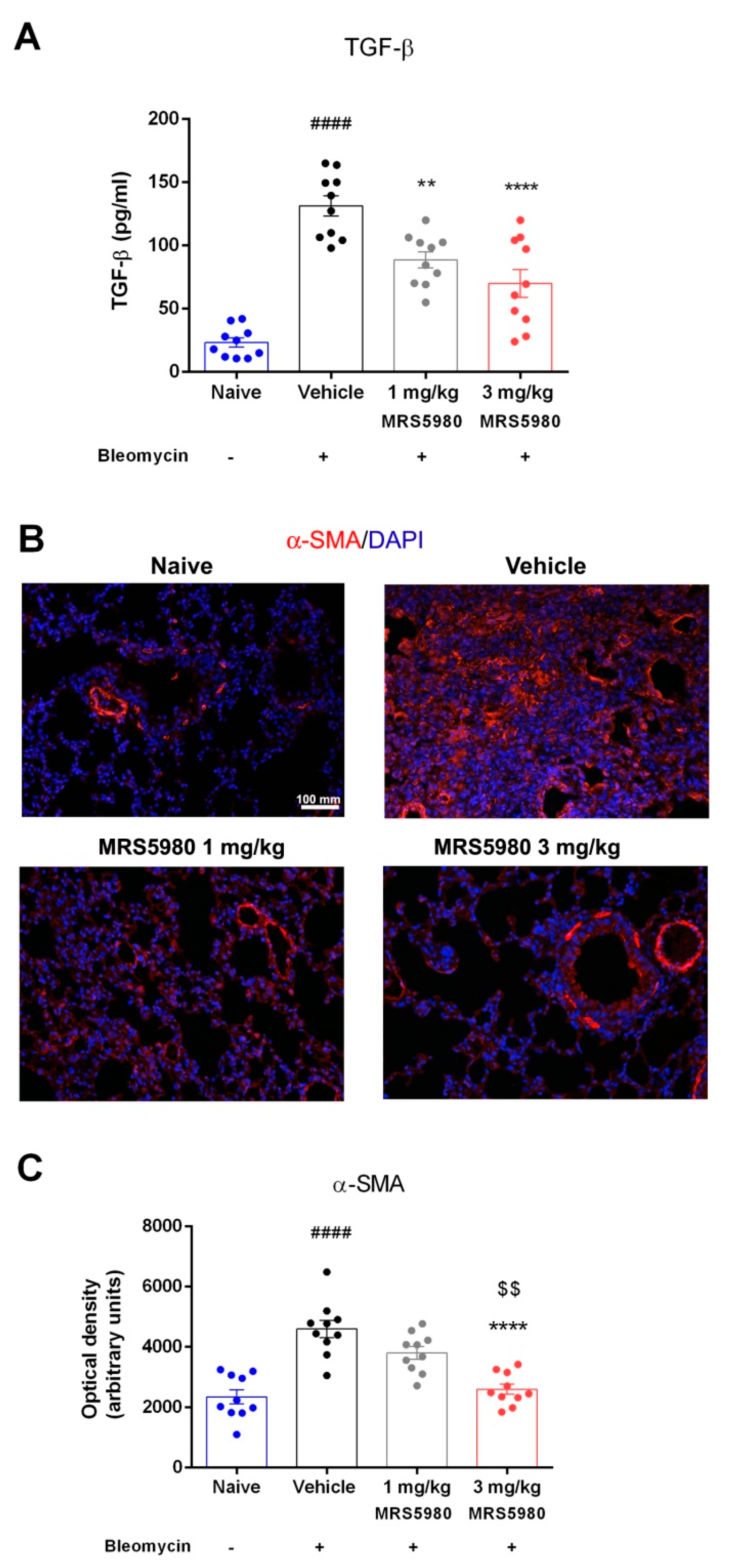
2.2. TGF-β Signaling Pathway Assessment and Evaluation of Fibroblasts Activation
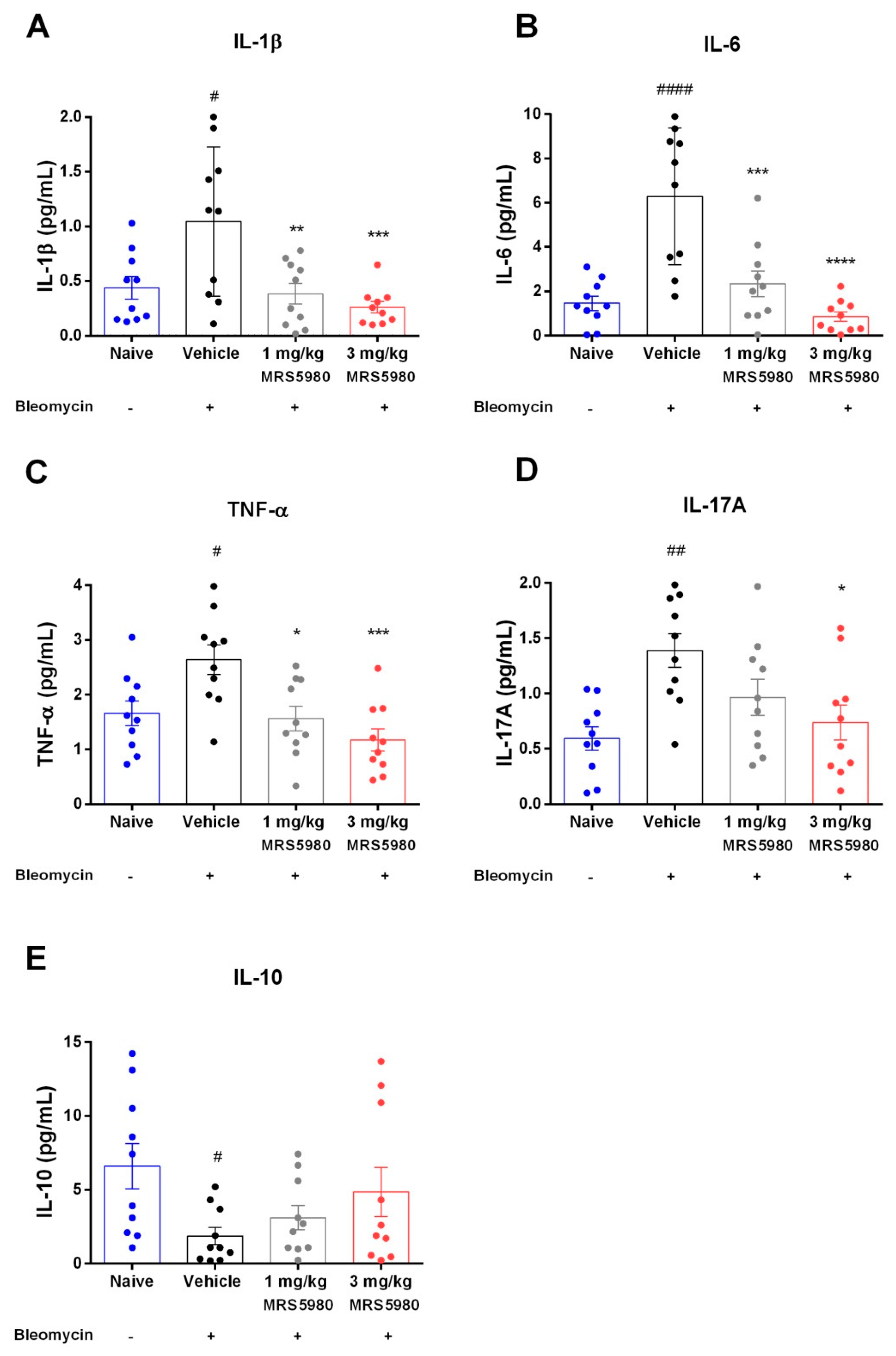
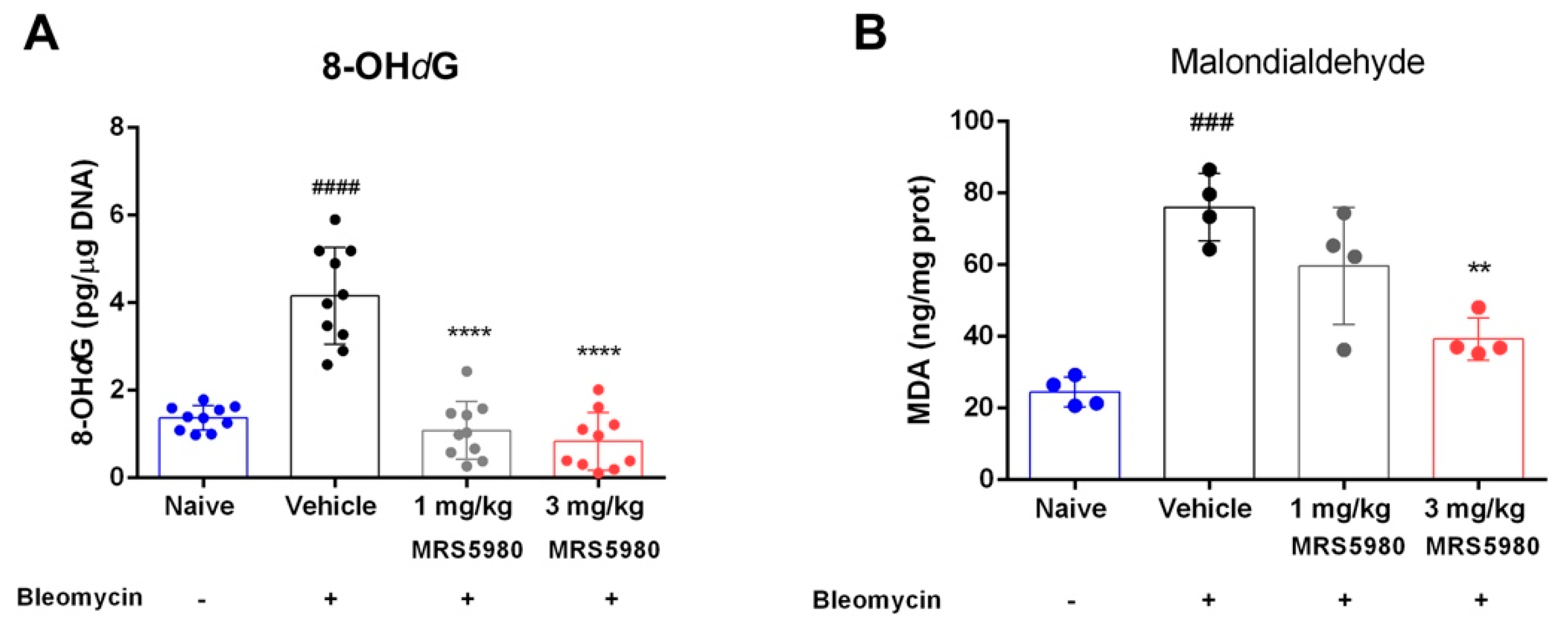
2.3. Evaluation of Inflammation and Oxidative Stress Parameters
3. Discussion
Study limitations
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Animals
4.3. Surgery and Treatments
4.4. Functional Assay of Fibrosis
4.5. Blood Withdrawal and Lung Tissue Sampling
4.6. Histology and Assessment of Collagen Deposition, Goblet Cell Hyperplasia, Smooth Muscle Layer Thickness and Mast Cell Degranulation
4.7. Hydroxyproline Assay
4.8. Determination of α-SMA Deposition
4.9. Cytokine Measurements
4.10. Determination of 8-Hydroxy-2-Deoxyguanosine
4.11. Malondialdehyde (MDA) Determination
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frankel, S.K.; Schwarz, M.I. Update in Idiopathic Pulmonary Fibrosis. Curr. Opin. Pulm. Med. 2009, 15, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, L.; Pini, A.; Rosa, A.C.; Lanzi, C.; Durante, M.; Chazot, P.L.; Krief, S.; Schreeb, A.; Stark, H.; Masini, E. Role of Histamine H4 Receptor Ligands in Bleomycin-Induced Pulmonary Fibrosis. Pharmacol. Res. 2016, 111, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Kolodsick, J.E.; Peters-Golden, M.; Larios, J.; Toews, G.B.; Thannickal, V.J.; Moore, B.B. Prostaglandin E2 Inhibits Fibroblast to Myofibroblast Transition via E. Prostanoid Receptor 2 Signaling and Cyclic Adenosine Monophosphate Elevation. Am. J. Respir. Cell. Mol. Biol. 2003, 29, 537–544. [Google Scholar] [CrossRef]
- Sauleda, J.; Núñez, B.; Sala, E.; Soriano, J. Idiopathic Pulmonary Fibrosis: Epidemiology, Natural History, Phenotypes. Med. Sci. 2018, 6, 110. [Google Scholar] [CrossRef]
- Raghu, G.; Rochwerg, B.; Zhang, Y.; Garcia, C.A.C.; Azuma, A.; Behr, J.; Brozek, J.L.; Collard, H.R.; Cunningham, W.; Homma, S.; et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2015, 192, e3–e19. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Gili, E.; Fagone, E.; Fruciano, M.; Iemmolo, M.; Vancheri, C. Effect of Pirfenidone on Proliferation, TGF-β-Induced Myofibroblast Differentiation and Fibrogenic Activity of Primary Human Lung Fibroblasts. Eur. J. Pharm. Sci. 2014, 58, 13–19. [Google Scholar] [CrossRef]
- Maher, T.M.; Strek, M.E. Antifibrotic Therapy for Idiopathic Pulmonary Fibrosis: Time to Treat. Respir. Res. 2019, 20, 205. [Google Scholar] [CrossRef]
- Morschl, E.; Molina, J.G.; Volmer, J.B.; Mohsenin, A.; Pero, R.S.; Hong, J.S.; Kheradmand, F.; Lee, J.J.; Blackburn, M.R. A3 Adenosine Receptor Signaling Influences Pulmonary Inflammation and Fibrosis. Am. J. Respir. Cell Mol. Biol. 2008, 39, 697–705. [Google Scholar] [CrossRef]
- della Latta, V.; Cabiati, M.; Rocchiccioli, S.; del Ry, S.; Morales, M.A. The Role of the Adenosinergic System in Lung Fibrosis. Pharmacol. Res. 2013, 76, 182–189. [Google Scholar] [CrossRef]
- Tilley, S.L.; Tsai, M.; Williams, C.M.; Wang, Z.-S.; Erikson, C.J.; Galli, S.J.; Koller, B.H. Identification of A3 Receptor- and Mast Cell-Dependent and -Independent Components of Adenosine-Mediated Airway Responsiveness in Mice. J. Immunol. 2003, 171, 331–337. [Google Scholar] [CrossRef]
- Burnstock, G.; Brouns, I.; Adriaensen, D.; Timmermans, J.P. Purinergic Signaling in the Airways. Pharmacol. Rev. 2012, 64, 834–868. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.; Castonguay, A.; Cottet, M.; Little, J.W.; Chen, Z.; Symons-Liguori, A.M.; Doyle, T.; Egan, T.M.; Vanderah, T.W.; De Konnick, Y.; et al. Engagement of the GABA to KCC2 Signaling Pathway Contributes to the Analgesic Effects of A3AR Agonists in Neuropathic Pain. J. Neurosci. 2015, 35, 6057. [Google Scholar] [CrossRef] [PubMed]
- Janes, K.; Esposito, E.; Doyle, T.; Cuzzocrea, S.; Tosh, D.K.; Jacobson, K.A.; Salvemini, D. A3 Adenosine Receptor Agonist Prevents the Development of Paclitaxel-Induced Neuropathic Pain by Modulating Spinal Glial-Restricted Redox-Dependent Signaling Pathways. Pain 2014, 155, 2560–2567. [Google Scholar] [CrossRef] [PubMed]
- Little, J.W.; Ford, A.; Symons-Liguori, A.M.; Chen, Z.; Janes, K.; Doyle, T.; Xie, J.; Luongo, L.; Tosh, D.K.; Maione, S.; et al. Endogenous Adenosine A3 Receptor Activation Selectively Alleviates Persistent Pain States. Brain 2015, 138, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Coppi, E.; Cherchi, F.; Fusco, I.; Failli, P.; Vona, A.; Dettori, I.; Gaviano, L.; Lucarini, E.; Jacobson, K.A.; Tosh, D.K.; et al. Adenosine A3 Receptor Activation Inhibits Pronociceptive N-Type Ca2+ Currents and Cell Excitability in Dorsal Root Ganglion Neurons. Pain 2019, 160, 1103. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.Z.; Tosh, D.K.; Tanaka, N.; Wang, H.; Krausz, K.W.; O’Connor, R.; Jacobson, K.A.; Gonzalez, F.J. Metabolic Mapping of A3 Adenosine Receptor Agonist MRS5980. Biochem. Pharmacol. 2015, 97, 215. [Google Scholar] [CrossRef] [PubMed]
- Cowley, P.M.; Roberts, C.R.; Baker, A.J. Monitoring the Health Status of Mice with Bleomycin-Induced Lung Injury by Using Body Condition Scoring. Comp. Med. 2019, 69, 95–102. [Google Scholar] [CrossRef]
- Chitra, P.; Saiprasad, G.; Manikandan, R.; Sudhandiran, G. Berberine Inhibits Smad and Non-Smad Signaling Cascades and Enhances Autophagy against Pulmonary Fibrosis. J. Mol. Med. 2015, 93, 1015–1031. [Google Scholar] [CrossRef]
- She, Y.X.; Yu, Q.Y.; Tang, X.X. Role of Interleukins in the Pathogenesis of Pulmonary Fibrosis. Cell Death Discov. 2021, 7, 52. [Google Scholar] [CrossRef]
- Veerappan, A.; O’Connor, N.J.; Brazin, J.; Reid, A.C.; Jung, A.; McGee, D.; Summers, B.; Branch-Elliman, D.; Stiles, B.; Worgall, S.; et al. Mast Cells: A Pivotal Role in Pulmonary Fibrosis. DNA Cell Biol. 2013, 32, 206. [Google Scholar] [CrossRef]
- Canestaro, W.J.; Forrester, S.H.; Raghu, G.; Ho, L.; Devine, B.E. Drug Treatment of Idiopathic Pulmonary Fibrosis Systematic Review and Network Meta-Analysis. Chest 2016, 149, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.A.; Cuzzocrea, S.; Esposito, E.; Campolo, M.; Niehoff, M.L.; Doyle, T.M.; Salvemini, D. Adenosine A 3 Receptor as a Novel Therapeutic Target to Reduce Secondary Events and Improve Neurocognitive Functions Following Traumatic Brain Injury. J. Neuroinflammation 2020, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, E.; Coppi, E.; Micheli, L.; Parisio, C.; Vona, A.; Cherchi, F.; Pugliese, A.M.; Pedata, F.; Failli, P.; Palomino, S.; et al. Acute Visceral Pain Relief Mediated by A3AR Agonists in Rats: Involvement of N-Type Voltage-Gated Calcium Channels. Pain 2020, 161, 2179–2190. [Google Scholar] [CrossRef]
- Singh, A.K.; Mahalingam, R.; Squillace, S.; Jacobson, K.A.; Tosh, D.K.; Dharmaraj, S.; Farr, S.A.; Kavelaars, A.; Salvemini, D.; Heijnen, C.J. Targeting the A 3 Adenosine Receptor to Prevent and Reverse Chemotherapy-Induced Neurotoxicities in Mice. Acta Neuropathol. Commun. 2022, 10, 1–17. [Google Scholar] [CrossRef]
- Durante, M.; Squillace, S.; Lauro, F.; Giancotti, L.A.; Coppi, E.; Cherchi, F.; di Cesare Mannelli, L.; Ghelardini, C.; Kolar, G.; Wahlman, C.; et al. Adenosine A3 Agonists Reverse Neuropathic Pain via T Cell-Mediated Production of IL-10. J. Clin. Investig. 2021, 131, e139299. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.M.; Kleeberger, S.R. Mouse Models of Bleomycin-Induced Pulmonary Fibrosis. Curr. Protoc. Pharmacol. 2008, chapter5, 46. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.H.; Lazo, J.S. Plasma and Pulmonary Pharmacokinetics of Bleomycin in Murine Strains That Are Sensitive and Resistant to Bleomycin-Induced Pulmonary Fibrosis. J. Pharmacol. Exp. Ther. 1988, 247, 1052–1058. [Google Scholar] [PubMed]
- Durante, M.; Sgambellone, S.; Lanzi, C.; Nardini, P.; Pini, A.; Moroni, F.; Masini, E.; Lucarini, L. Effects of PARP-1 Deficiency and Histamine H4 Receptor Inhibition in an Inflammatory Model of Lung Fibrosis in Mice. Front. Pharmacol. 2019, 10, 525. [Google Scholar] [CrossRef]
- Lucarini, L.; Durante, M.; Sgambellone, S.; Lanzi, C.; Bigagli, E.; Akgul, O.; Masini, E.; Supuran, C.T.; Carta, F. Effects of New NSAID-CAI Hybrid Compounds in Inflammation and Lung Fibrosis. Biomolecules 2020, 10, 1307. [Google Scholar] [CrossRef]
- Gao, Z.G.; Jacobson, K.A. Purinergic Signaling in Mast Cell Degranulation and Asthma. Front. Pharmacol. 2017, 8, 947. [Google Scholar] [CrossRef]
- Rudich, N.; Dekel, O.; Sagi-Eisenberg, R. Down-Regulation of the A3 Adenosine Receptor in Human Mast Cells Upregulates Mediators of Angiogenesis and Remodeling. Mol. Immunol. 2015, 65, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Tatler, A.L.; Jenkins, G. TGF-β Activation and Lung Fibrosis. Proc. Am. Thorac. Soc. 2012, 9, 130–136. [Google Scholar] [CrossRef]
- Bonniaud, P.; Margetts, P.J.; Ask, K.; Flanders, K.; Gauldie, J.; Kolb, M. TGF-Beta and Smad3 Signaling Link Inflammation to Chronic Fibrogenesis. J. Immunol. 2005, 175, 5390–5395. [Google Scholar] [CrossRef] [PubMed]
- Tosh, D.K.; Deflorian, F.; Phan, K.; Gao, Z.G.; Wan, T.C.; Gizewski, E.; Auchampach, J.A.; Jacobson, K.A. Structure-Guided Design of A(3) Adenosine Receptor-Selective Nucleosides: Combination of 2-Arylethynyl and Bicyclo [3.1.0]Hexane Substitutions. J. Med. Chem. 2012, 55, 4847–4860. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.C.; Pini, A.; Lucarini, L.; Lanzi, C.; Veglia, E.; Thurmond, R.L.; Stark, H.; Masini, E. Prevention of Bleomycin-Induced Lung Inflammation and Fibrosis in Mice by Naproxen and JNJ7777120 Treatment. J. Pharmacol. Exp. Ther. 2014, 351, 308–316. [Google Scholar] [CrossRef]
- McGrath, J.C.; Drummond, G.B.; McLachlan, E.M.; Kilkenny, C.; Wainwright, C.L. Guidelines for Reporting Experiments Involving Animals: The ARRIVE Guidelines. Br. J. Pharmacol. 2010, 160, 1573–1576. [Google Scholar] [CrossRef]
- Pini, A.; Viappiani, S.; Bolla, M.; Masini, E.; Bani, D. Prevention of Bleomycin-Induced Lung Fibrosis in Mice by a Novel Approach of Parallel Inhibition of Cyclooxygenase and Nitric-Oxide Donation Using NCX 466, a Prototype Cyclooxygenase Inhibitor and Nitric-Oxide Donor. J. Pharmacol. Exp. Ther. 2012, 341, 493–499. [Google Scholar] [CrossRef]
- Lucarini, L.; Pini, A.; Gerace, E.; Pellicciari, R.; Masini, E.; Moroni, F. Poly(ADP-Ribose) Polymerase Inhibition with HYDAMTIQ Reduces Allergen-Induced Asthma-like Reaction, Bronchial Hyper-Reactivity and Airway Remodelling. J. Cell. Mol. Med. 2014, 18, 468–479. [Google Scholar] [CrossRef]
- ImageJ 1.33 image analysis program. Available online: https://imagej.nih.gov/ij/ (accessed on 9 January 2017).
- Marcos-GarcÉs, V.; Harvat, M.; Molina Aguilar, P.; FerrÁndez Izquierdo, A.; Ruiz-SaurÍ, A. Comparative Measurement of Collagen Bundle Orientation by Fourier Analysis and Semiquantitative Evaluation: Reliability and Agreement in Masson’s Trichrome, Picrosirius Red and Confocal Microscopy Techniques. J. Microsc. 2017, 267, 130–142. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius Red Staining: A Useful Tool to Appraise Collagen Networks in Normal and Pathological Tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef]
- Lucarini, L.; Durante, M.; Lanzi, C.; Pini, A.; Boccalini, G.; Calosi, L.; Moroni, F.; Masini, E.; Mannaioni, G. HYDAMTIQ, a Selective PARP-1 Inhibitor, Improves Bleomycin-Induced Lung Fibrosis by Dampening the TGF-β/SMAD Signalling Pathway. J. Cell. Mol. Med. 2017, 21, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Micheli, L.; Durante, M.; Lucarini, E.; Sgambellone, S.; Lucarini, L.; Mannelli, L.D.C.; Ghelardini, C.; Masini, E. The Histamine H 4 Receptor Participates in the Anti-Neuropathic Effect of the Adenosine A 3 Receptor Agonist IB-MECA: Role of CD4 + T Cells. Biomolecules 2021, 11, 1447. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgambellone, S.; Marri, S.; Catarinicchia, S.; Pini, A.; Tosh, D.K.; Jacobson, K.A.; Masini, E.; Salvemini, D.; Lucarini, L. Adenosine A3 Receptor (A3AR) Agonist for the Treatment of Bleomycin-Induced Lung Fibrosis in Mice. Int. J. Mol. Sci. 2022, 23, 13300. https://doi.org/10.3390/ijms232113300
Sgambellone S, Marri S, Catarinicchia S, Pini A, Tosh DK, Jacobson KA, Masini E, Salvemini D, Lucarini L. Adenosine A3 Receptor (A3AR) Agonist for the Treatment of Bleomycin-Induced Lung Fibrosis in Mice. International Journal of Molecular Sciences. 2022; 23(21):13300. https://doi.org/10.3390/ijms232113300
Chicago/Turabian StyleSgambellone, Silvia, Silvia Marri, Stefano Catarinicchia, Alessandro Pini, Dilip K. Tosh, Kenneth A. Jacobson, Emanuela Masini, Daniela Salvemini, and Laura Lucarini. 2022. "Adenosine A3 Receptor (A3AR) Agonist for the Treatment of Bleomycin-Induced Lung Fibrosis in Mice" International Journal of Molecular Sciences 23, no. 21: 13300. https://doi.org/10.3390/ijms232113300
APA StyleSgambellone, S., Marri, S., Catarinicchia, S., Pini, A., Tosh, D. K., Jacobson, K. A., Masini, E., Salvemini, D., & Lucarini, L. (2022). Adenosine A3 Receptor (A3AR) Agonist for the Treatment of Bleomycin-Induced Lung Fibrosis in Mice. International Journal of Molecular Sciences, 23(21), 13300. https://doi.org/10.3390/ijms232113300






