Preventive Effect of Probiotics on Oral Mucositis Induced by Cancer Treatment: A Systematic Review and Meta-Analysis
Abstract
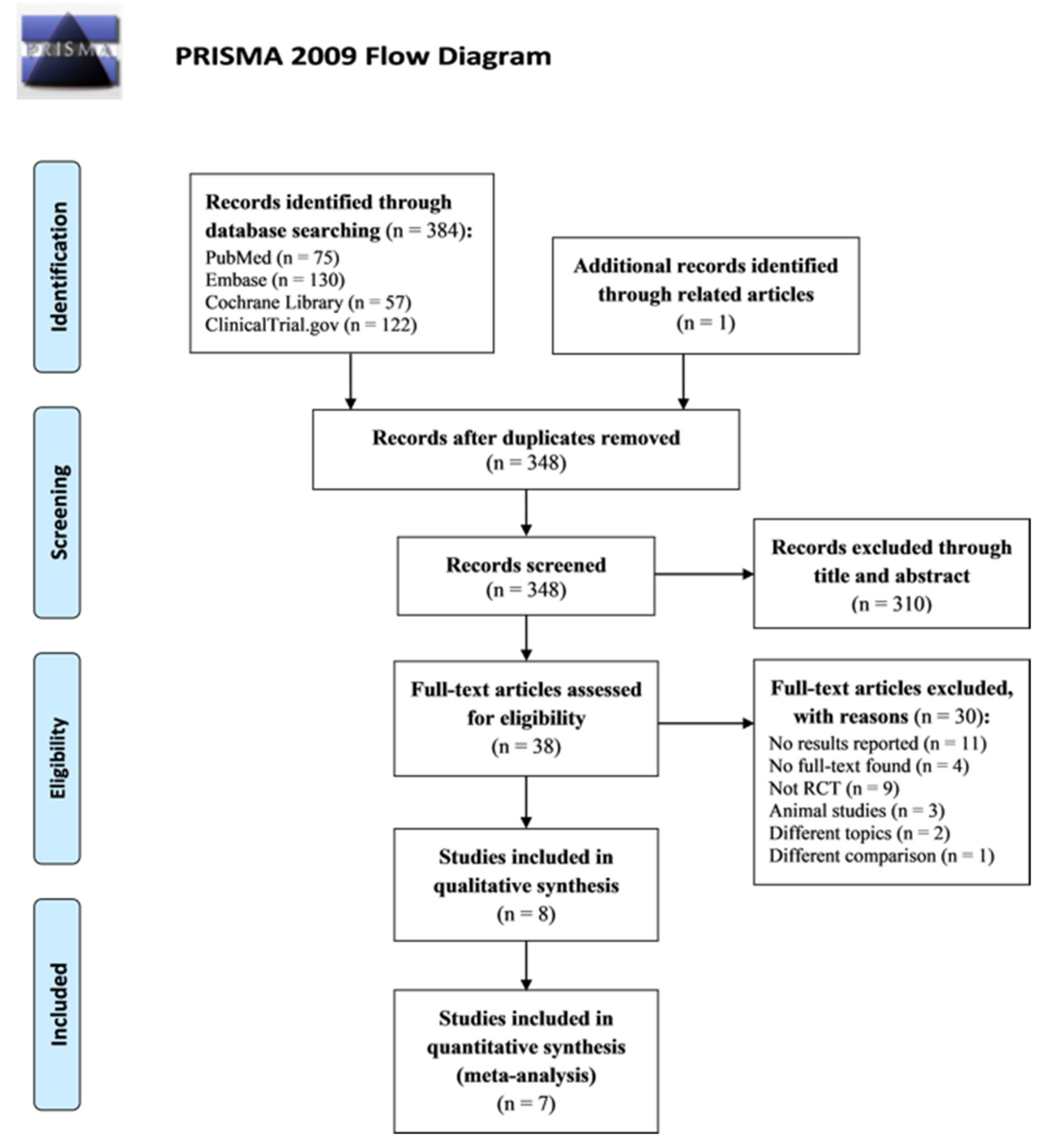
:1. Introduction
2. Results
2.1. Characteristics of Trials
2.2. Primary Outcome
2.2.1. Incidence of Oral Mucositis
2.2.2. Incidence of Severe Oral Mucositis
2.3. Secondary Outcomes
2.3.1. Requirement of Enteral Nutrition
2.3.2. Body Weight Loss
2.3.3. Quality of Life
3. Discussion
4. Materials and Methods
4.1. Selection Criteria
4.2. Search strategy and Study Selection
4.3. Data Extraction
4.4. Methodological Quality Appraisal
4.5. Outcome Assessments
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Al-Dasooqi, N.; Sonis, S.T.; Bowen, J.M.; Bateman, E.; Blijlevens, N.; Gibson, R.J.; Logan, R.M.; Nair, R.G.; Stringer, A.M.; Yazbeck, R.; et al. Emerging evidence on the pathobiology of mucositis. Support Care Cancer 2013, 21, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Sonis, S.T. Pharmacotherapy for the management of cancer regimen-related oral mucositis. Expert. Opin. Pharmacother. 2016, 17, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.M.; Thomas, S.M.; Sartoski, S.; Scott, J.G.; Sobilo, K.; Bewley, S.; Salvador, L.K.; Salazar-abshire, M. Strategies to Mitigate Chemotherapy and Radiation Toxicities That Affect Eating. Nutrients 2021, 13, 4397. [Google Scholar] [CrossRef] [PubMed]
- Pulito, C.; Cristaudo, A.; la Porta, C.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral mucositis: The hidden side of cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 210. [Google Scholar] [CrossRef]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of oral mucositis in patients who have cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef] [Green Version]
- Yarom, N.; Hovan, A.; Bossi, P.; Ariyawardana, A.; Jensen, S.B.; Gobbo, M.; Saca-Hazboun, H.; Kandwal, A.; Majorana, A.; Ottaviani, G.; et al. Systematic review of natural and miscellaneous agents, for the management of oral mucositis in cancer patients and clinical practice guidelines—Part 2: Honey, herbal compounds, saliva stimulants, probiotics, and miscellaneous agents. Support Care Cancer 2020, 28, 2457–2472. [Google Scholar] [CrossRef]
- Bowen, W.H.; Lawrence, R.A. Comparison of the cariogenicity of cola, honey, cow milk, human milk, and sucrose. Pediatrics 2005, 116, 921–926. [Google Scholar] [CrossRef]
- Fidler Mis, N.; Braegger, C.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Hulst, J.; Indrio, F.; Lapillonne, A.; et al. Sugar in Infants, Children and Adolescents: A Position Paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 681–696. [Google Scholar] [CrossRef] [Green Version]
- Santos-Silva, A.R.; Rosa, G.B.; Eduardo, C.P.; Dias, R.B.; Brandao, T.B. Increased risk for radiation-related caries in cancer patients using topical honey for the prevention of oral mucositis. Int. J. Oral. Maxillofac. Surg. 2011, 40, 1335–1336. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Sarao, L.K.; Arora, M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. Biomed. Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef] [Green Version]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Thomsen, M.; Vitetta, L. Adjunctive Treatments for the Prevention of Chemotherapy- and Radiotherapy-Induced Mucositis. Integr. Cancer Ther. 2018, 17, 1027–1047. [Google Scholar] [CrossRef] [Green Version]
- Delia, P.; Sansotta, G.; Donato, V.; Frosina, P.; Messina, G.; de Renzis, C.; Famularo, G. Use of probiotics for prevention of radiation-induced diarrhea. World J. Gastroenterol. 2007, 13, 912–915. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, H.; Xia, C.; Dong, Q.; Chen, E.; Qiu, Y.; Su, Y.; Xie, H.; Zeng, L.; Kuang, J.; et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer 2019, 125, 1081–1090. [Google Scholar] [CrossRef] [Green Version]
- Hassan, H.; Rompola, M.; Glaser, A.W.; Kinsey, S.E.; Phillips, R.S. Systematic review and meta-analysis investigating the efficacy and safety of probiotics in people with cancer. Support Care Cancer 2018, 26, 2503–2509. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Feng, J.; Gao, M.; Zhao, C.; Yang, J.; Gao, H.; Lu, X.; Ju, R.; Zhang, X.; Zhang, Y. Oral Administration of Probiotics Reduces Chemotherapy-Induced Diarrhea and Oral Mucositis: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 823288. [Google Scholar] [CrossRef]
- De Sanctis, V.; Belgioia, L.; Cante, D.; la Porta, M.R.; Caspiani, O.; Guarnaccia, R.; Argenone, A.; Muto, P.; Musio, D.; de Felice, F.; et al. Lactobacillus brevis CD2 for Prevention of Oral Mucositis in Patients with Head and Neck Tumors: A Multicentric Randomized Study. Anticancer. Res. 2019, 39, 1935–1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limaye, S.A.; Haddad, R.I.; Cilli, F.; Sonis, S.T.; Colevas, A.D.; Brennan, M.T.; Hu, K.S.; Murphy, B.A. Phase 1b, multicenter, single blinded, placebo-controlled, sequential dose escalation study to assess the safety and tolerability of topically applied AG013 in subjects with locally advanced head and neck cancer receiving induction chemotherapy. Cancer 2013, 119, 4268–4276. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.A.; Aruna, D.; Irukulla, M. Efficacy of Bacillus clausii UBBC—07 spores in the amelioration of oral mucositis in head and neck cancer patients undergoing radiation therapy. Cancer Treat. Res. Commun. 2022, 31, 100523. [Google Scholar] [CrossRef] [PubMed]
- Österlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br. J. Cancer 2007, 97, 1028–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Rath, G.K.; Chaudhary, S.P.; Thakar, A.; Mohanti, B.K.; Bahadur, S. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: A randomized double-blind placebo-controlled study. Eur. J. Cancer 2012, 48, 875–881. [Google Scholar] [CrossRef]
- Topuz, E.; Derin, D.; Can, G.; Kürklü, E.; Cinar, S.; Aykan, F.; Cevikbaş, A.; Dişçi, R.; Durna, Z.; Sakar, B.; et al. Effect of oral administration of kefir on serum proinflammatory cytokines on 5-FU induced oral mucositis in patients with colorectal cancer. Investig. New Drugs 2008, 26, 567–572. [Google Scholar] [CrossRef]
- Xia, C.; Jiang, C.; Li, W.; Wei, J.; Hong, H.; Li, J.; Feng, L.; Wei, H.; Xin, H.; Chen, T. A Phase II Randomized Clinical Trial and Mechanistic Studies Using Improved Probiotics to Prevent Oral Mucositis Induced by Concurrent Radiotherapy and Chemotherapy in Nasopharyngeal Carcinoma. Front. Immunol. 2021, 12, 618150. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009, Cited 2022. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (accessed on 4 August 2022).
- Trotti, A.; Byhardt, R.; Stetz, J.; Gwede, C.; Corn, B.; Fu, K.; Gunderson, L.; McCormick, B.; Morris, M.; Rich, T.; et al. Common toxicity criteria: Version 2.0. an improved reference for grading the acute effects of cancer treatment: Impact on radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 13–47. [Google Scholar] [CrossRef]
- WHO. WHO Handbook for Reporting Results of Cancer Treatment; WHO: Geneva, Switzerland, 1979. [Google Scholar]
- D’Antonio, L.L.; Zimmerman, G.J.; Cella, D.F.; Long, S.A. Quality of life and functional status measures in patients with head and neck cancer. Arch Otolaryngol. Head Neck Surg. 1996, 122, 482–487. [Google Scholar] [CrossRef]
- Shu, Z.; Li, P.; Yu, B.; Huang, S.; Chen, Y. The effectiveness of probiotics in prevention and treatment of cancer therapy-induced oral mucositis: A systematic review and meta-analysis. Oral. Oncol. 2020, 102, 104559. [Google Scholar] [CrossRef]
- Villa, A.; Sonis, S.T. Mucositis: Pathobiology and management. Curr. Opin. Oncol. 2015, 27, 159–164. [Google Scholar] [CrossRef]
- Sonis, S.T. The biologic role for nuclear factor-kappaB in disease and its potential involvement in mucosal injury associated with anti-neoplastic therapy. Crit. Rev. Oral. Biol. Med. 2002, 13, 380–389. [Google Scholar] [CrossRef]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Yeoh, A.S.-J.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M.K. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: Pathobiology, animal models and cytotoxic drugs. Cancer Treat Rev. 2007, 33, 448–460. [Google Scholar] [CrossRef]
- Sonis, S.T. The Chicken or the Egg? Changes in Oral Microbiota as Cause or Consequence of Mucositis During Radiation Therapy. EBioMedicine 2017, 18, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Ferreira, J.; Hong, C.H.L.; Tan, K.S. Lactobacillus reuteri DSM 17938 and ATCC PTA 5289 ameliorates chemotherapy-induced oral mucositis. Sci. Rep. 2020, 10, 16189. [Google Scholar] [CrossRef]
- Jones, R.M.; Desai, C.; Darby, T.M.; Luo, L.; Wolfarth, A.A.; Scharer, C.D.; Ardita, C.S.; Reedy, A.R.; Keebaugh, E.S.; Neish, A.S. Lactobacilli Modulate Epithelial Cytoprotection through the Nrf2 Pathway. Cell Rep. 2015, 12, 1217–1225. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, J.; Zhang, H.; Zheng, X.; Wang, J.; Jia, X.; Peng, X.; Xie, Q.; Zou, J.; Zheng, L.; et al. Probiotic Streptococcus salivarius K12 Alleviates Radiation-Induced Oral Mucositis in Mice. Front. Immunol. 2021, 12, 684824. [Google Scholar] [CrossRef]
- Riehl, T.E.; Alvarado, D.; Ee, X.; Zuckerman, A.; Foster, L.; Kapoor, V.; Thotala, D.; Ciorba, M.A.; Stenson, W.F. Lactobacillus rhamnosus GG protects the intestinal epithelium from radiation injury through release of lipoteichoic acid, macrophage activation and the migration of mesenchymal stem cells. Gut 2019, 68, 1003–1013. [Google Scholar] [CrossRef]
- Makarova, K.; Slesarev, A.; Wolf, Y.; Sorokin, A.; Mirkin, B.; Koonin, E.; Pavlov, A.; Pavlova, N.; Karamychev, V.; Polouchine, N.; et al. Comparative genomics of the lactic acid bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 15611–15616. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Harris, H.M.B.; McCann, A.; Guo, C.; Argimón, S.; Zhang, W.; Yang, X.; Jeffery, I.B.; Cooney, J.C.; Kagawa, T.F.; et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 2015, 6, 8322. [Google Scholar] [CrossRef] [Green Version]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostok, M.; Hütt, P.; Rööp, T.; Smidt, I.; Štšepetova, J.; Salumets, A.; Mändar, R. Potential vaginal probiotics: Safety, tolerability and preliminary effectiveness. Benef. Microbes 2019, 10, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Liu, X.R. Role of probiotics containing Lactobacillus reuteri in adjunct to scaling and root planing for management of patients with chronic periodontitis: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4495–4505. [Google Scholar] [PubMed]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedin-Do, A.; Taherian-Esfahani, Z.; Ghafouri-Fard, S.; Ghafouri-Fard, S.; Motevaseli, E. Immunomodulatory effects of Lactobacillus strains: Emphasis on their effects on cancer cells. Immunotherapy 2015, 7, 1307–1329. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. Dis. 2015, 60 (Suppl. S2), S129–S134. [Google Scholar] [CrossRef] [Green Version]
- Sotoudegan, F.; Daniali, M.; Hassani, S.; Nikfar, S.; Abdollahi, M. Reappraisal of probiotics’ safety in human. Food Chem. Toxicol. 2019, 129, 22–29. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Palmieri, B.; Aponte, M.; Morales-Medina, J.C.; Iannitti, T. Mechanisms and therapeutic effectiveness of lactobacilli. J. Clin. Pathol. 2016, 69, 187–203. [Google Scholar] [CrossRef] [Green Version]
- Robin, F.; Paillard, C.; Marchandin, H.; Demeocq, F.; Bonnet, R.; Hennequin, C. Lactobacillus rhamnosus meningitis following recurrent episodes of bacteremia in a child undergoing allogeneic hematopoietic stem cell transplantation. J. Clin. Microbiol. 2010, 48, 4317–4319. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Tilak, T.; Bakhshi, S.; Raina, V.; Kumar, L.; Chaudhary, S.; Sahoo, R.; Gupta, R.; Thulkar, S. Lactobacillus brevis CD2 lozenges prevent oral mucositis in patients undergoing high dose chemotherapy followed by haematopoietic stem cell transplantation. ESMO Open 2016, 1, e000138. [Google Scholar] [CrossRef]
- Ouwehand, A.C. A review of dose-responses of probiotics in human studies. Benef. Microbes 2017, 8, 143–151. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]



| Author [Year] | Inclusion Criteria | No. of Patients | Age, Year, Mean ± SD | Male (n, %) | Intervention |
|---|---|---|---|---|---|
| CT + RT | |||||
| Jiang,2019 [17] | Locally advanced NPC, without distant metastasis; aged 18–70 y; KPS ≥ 80; RT (70 Gy) and CT (cisplatin-based) | C: 35 I: 64 | C: 50.40 ± 10.25 I: 51.69 ± 9.79 | C: 21, 60% I: 37, 63.8% | C: placebo (starch), 3 capsules × 2 times/day × 7 weeks I: probiotic combination (B. longum, L. lactis, and E. faecium), 3 capsules × 2 times/day × 7 weeks |
| Mirza, 2022 [23] | HNC; aged between 30–60 y; RT (60–70 Gy) with or without CT (cisplatin-based) | C: 23 I: 23 | 55 (35–60) † | C: 21, 91.3% I: 22, 95.7% | C: placebo (distilled water 5 mL) × 2 times/day, until the completion of RT course I: B. clausii oral suspension, 5 mL × 2 times/day, until the completion of RT course |
| Sanctis, 2019 [21] | HNC (except larynx, parotid and other salivary glands tumors); aged ≥ 18 y; KPS > 70; RT (68–70 Gy) and CT (cisplatin-based; neo-adjuvant allowed for NPC) | C: 32 I: 36 | C: 60 (39–77) † I: 58.4 (34–74) † | C: 27, 75% I: 26, 81.2% | C: sodium bicarbonate, at least 3 times/day, until the end of cancer treatment. I: L. brevis CD2 lozenges, 1 lozenge/2–3 h × 6 times/day to be dissolved in the mouth and then swallowed, up to 1 week after the end of the cancer treatment |
| Sharma, 2012 [25] | HNSCC stage II–IV; RT (70 Gy) and CT (cisplatin-based, 40 mg/m2) | C: 99 I: 101 | C: 50.09 ± 10.04 I: 52.35 ± 9.43 | C: 91, 91.9% I: 94, 93.1% | C: placebo (mixture of the sugars and salts), 1 lozenge/2–3 h × 6 times/day to be dissolved in the mouth and then swallowed × 8 weeks I: L. brevis CD2 lozenges, 1 lozenge/2–3 h × 6 times/day to be dissolved in the mouth and then swallowed × 8 weeks |
| Xia, 2021 [27] | Locally advanced NPC, without distant metastasis; aged 18–70 y; RT (70 Gy) and CT (cisplatin-based) | C: 38 I: 39 | C: 51.70 ± 11.21 I: 52.61 ± 10.56 | C: 11, 32% I: 11, 31% | C: placebo (no information), 1 capsule 2 times/day × 7 weeks I: probiotic combination (L. plantarum MH-301, B. animalis subsp. Lactis LPL-RH, L. rhamnosus LGG-18, and L. acidophilus), 1 capsule × 2 times/day × 7 weeks |
| CT only | |||||
| Limaye, 2013 [22] | Newly diagnosed HNSCC; scheduled to receive ≥ 2 cycles of induction CT (cisplatin-based) | C: 8 I1: 5 I2: 6 I3: 6 | C: 54 (18–63) † I1: 61 (42–66) † I2: 54 (26–64) † I3: 52 (42–56) † | C: 7, 88% I1: 5, 100% I2: 2, 33% I3: 5, 83% | C: placebo rinse 15 mL × 6 times/day I1: AG013 oral rinse (containing L. lactis) 15 mL × 1 time/day I2: AG013 oral rinse (containing L. lactis) 15 mL × 3 times/day I3: AG013 oral rinse (containing L. lactis) 15 mL × 6 times/day |
| Österlund, 2007 [24] | Colorectal cancer, stage II-IV, s/p surgical resection; aged 18–75 y; ECOG ≤ 2; CT (leucovorin and 5-FU-based) | C: 52 I: 98 | C: 57 (31–75) † I: 61 (35–74) † | C: 25, 48.1% I: 51, 52% | C: no information I: L. rhamnosus GG, 1 capsule × 2 times/day during the whole CT course |
| Topuz, 2008 [26] | Newly diagnosed colorectal cancer, stages II-IV; ECOG ≤ 2; CT (5-FU based, median: 6 cycle) | C: 20 I: 17 | C: 58 (34–72) † I: 51 (19–75) † | C: 12, 60% I: 12, 70.6% | C: sodium chloride × 2 times/day, first 5 days of each CT cycle I: oral lavage with kefir (containing Lactobacillus spp., Bifidobacterium spp., etc.) and swallow 250 mL × 2 times/day after meal, first 5 days of each CT cycle |
| Author [Year] | Bias Caused by Adequacy of Randomization | Bias Caused by Deviations from Intended Interventions | Bias Caused by Missing Data of Dropouts | Bias in Measurement of the Outcomes | Bias in Selection of the Reported Results | Overall Risk of Bias |
|---|---|---|---|---|---|---|
| Jiang 2019 [17] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Limaye 2013 [22] | Some concerns 1 | Low risk | High risk 2 | Some concerns 3 | Low risk | High risk |
| Mirza 2022 [23] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Österlund 2007 [24] | High risk 4 | Low risk | Low risk | Low risk | Low risk | High risk |
| Sanctis 2019 [21] | High risk 5 | High risk 6 | Low risk | Low risk | Low risk | High risk |
| Sharma 2012 [25] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Topuz 2008 [26] | Some concerns 7 | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Xia 2021 [27] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-C.; Wu, C.-R.; Huang, T.-W. Preventive Effect of Probiotics on Oral Mucositis Induced by Cancer Treatment: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 13268. https://doi.org/10.3390/ijms232113268
Liu Y-C, Wu C-R, Huang T-W. Preventive Effect of Probiotics on Oral Mucositis Induced by Cancer Treatment: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2022; 23(21):13268. https://doi.org/10.3390/ijms232113268
Chicago/Turabian StyleLiu, Yu-Cheng, Chia-Rong Wu, and Tsai-Wei Huang. 2022. "Preventive Effect of Probiotics on Oral Mucositis Induced by Cancer Treatment: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 23, no. 21: 13268. https://doi.org/10.3390/ijms232113268
APA StyleLiu, Y.-C., Wu, C.-R., & Huang, T.-W. (2022). Preventive Effect of Probiotics on Oral Mucositis Induced by Cancer Treatment: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 23(21), 13268. https://doi.org/10.3390/ijms232113268






