Abstract
Understanding of the gut microbiome’s role in human physiology developed rapidly in recent years. Moreover, any alteration of this microenvironment could lead to a pathophysiological reaction of numerous organs. It results from the bidirectional communication of the gastrointestinal tract with the central nervous system, called the gut–brain axis. The signals in the gut–brain axis are mediated by immunological, hormonal, and neural pathways. However, it is also influenced by microorganisms in the gut. The disturbances in the gut–brain axis are associated with gastrointestinal syndromes, but recently their role in the development of different types of pain was reported. The gut microbiome could be the factor in the central sensitization of chronic pain by regulating microglia, astrocytes, and immune cells. Dysbiosis could lead to incorrect immune responses, resulting in the development of inflammatory pain such as endometriosis. Furthermore, chronic visceral pain, associated with functional gastrointestinal disorders, could result from a disruption in the gut microenvironment. Any alteration in the gut–brain axis could also trigger migraine attacks by affecting cytokine expression. Understanding the gut microbiome’s role in pain pathophysiology leads to the development of analgetic therapies targeting microorganisms. Probiotics, FODMAP diet, and fecal microbiota transplantation are reported to be beneficial in treating visceral pain.
1. Introduction
The gut has the most populous and diverse system of anaerobic and aerobic microorganisms in the human body [1,2,3]. It is composed mainly of bacteria. However, yeasts, archaea, or parasites living in the large area of the gastrointestinal tract often play a substantial role in this microenvironment [1,2,4,5]. The first years of life, including delivery, are crucial for the development of this complex system [6,7]. Especially at this time, selective pressure is induced by essential host and environmental factors such as breastfeeding or formula feeding, weaning age, diet, infections, and antibiotics [6,7].
The gut microbiota lives in homeostasis with its host. These interactions are regulated by an integral gut barrier and immune system [8,9]. The gastrointestinal tract communicates bidirectionally with the central nervous system via direct and indirect mechanisms [10]. This intricate interplay is called the gut–brain axis (GBA) [11] (Figure 1). Immunological, hormonal, and neural signals play vital roles in this interaction [10,12]. At the same time, the gastrointestinal response to central stimulation is influenced by microorganisms [11]. The microbiota participates in supplying the gut with necessary nutrients and maintaining its barrier integrity. Both terminals of the GBA use serotonin as a vital transmitter [13] Some behavioral changes regulated by serotoninergic transmission seem to depend on the microbiome [13]. Moreover, the GBA affects other systems [10,14,15,16].
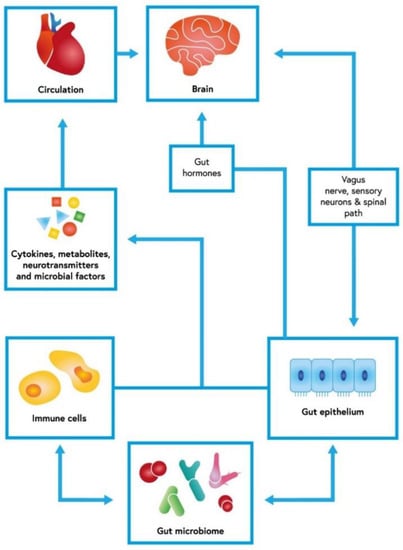
Figure 1.
Gut–brain axis with an interconnected net of dependencies. The cerebral function could be modified by gut microbiome and its influence on gut epithelium and immune response. This bidirectional axis uses cytokines and other soluble factors, but also neuronal communication. The short-chain fatty acids (SCFAs) produced by fiber-fermenting bacteria probably have immunomodulatory functions. By binding to G-protein coupled receptors (GPR41, GPR43, and GPR109A), SCFAs exert an anti-inflammatory response in the gut mucosa [17,18].
Disrupted homeostasis in the GBA was first associated with gastrointestinal symptoms and disorders such as inflammatory bowel disease (IBD) or irritable bowel syndrome (IBS) [19]. Moreover, alterations in the composition of the commensal bacterial species populating the gastrointestinal tract are risk factors for a variety of diseases, including cancer [10,14,20,21,22,23]. A plant diet has an opposite effect promoting colonization of the gut by protective bacteria and inducing the production of short-chain fatty acids (SCFAs) by species such as Faecalibacterium prausnitzii or Roseburia intestinalis [24,25].
Subsequently, studies connecting the microbiota with elements of pain pathogenesis were performed. SCFAs are microbial metabolites that affect T-regulatory cells controlling inflammation [26]. Microorganisms produce neurotransmitters that, together with ingested nutrients, stimulate enteroendocrine cells to produce multiple hormones [27,28]. There is growing evidence relating the microbiome to stress, anxiety, neurological diseases, and depression [29,30,31]. Brain functions affected by microorganisms might augment nociceptive transmission [32,33,34,35].
Initiation of pain transmission is induced by nociceptors, which convert noxious stimuli into nerve impulses [36,37]. Then, the signal is modulated by multiple neurons of different types and functions or non-neuronal cells such as glia [36,37,38,39]. Nevertheless, sustained pain depends on emotional or cognitive experience [36,40]. It is regulated peripherally and centrally by substances whose production is affected by the microbiome. Pain should serve as protection from tissue damage [37]. Nonetheless, chronic pain leads to a lower quality of life [32,41]. Thus, a better understanding of its mechanism is crucial to improving the lives of millions of people worldwide. Moreover, targeting the gut microbiota seems to be a promising novel therapeutic approach for pain management.
As the aforementioned processes continue to receive increasing attention, we addressed the role of the gut microbiota in pain regulation and discussed the possibility of pain therapy by targeting the gut microbiota. In this narrative review, we collected results from in vitro and in vivo studies on the association between the GBA, pain, and its management.
2. Neuropathic Pain and Central Mechanisms of Pain Regulation
Neuropathic pain occurs as a result of nerve-damaging trauma or somatosensory nervous system disease, including its central and peripheral components [42]. Various conditions, such as diabetes, alcoholism, hypothyroidism, or spinal stenosis, contribute to the development of neuropathic symptoms [42]. This type of pain manifests as abnormal sensations usually felt by patients for the first time. They perceive areas of skin with a sensory deficit, paraesthesia, either spontaneous or evoked pain and thermal or mechanical hypersensitivity [42]. Some drugs used in chemotherapy treatment, such as platinum, vincristine, or toxoids, may cause chemotherapy-induced peripheral neuropathy (CIPN) [43]. Over 30% of patients fighting cancer suffer from such severe CIPN-related pain that they are not receiving sufficient treatment dosages [44].
The gastrointestinal tract consists of various microorganisms, which are reported to play a significant role in neuroinflammatory responses. Neuroimmune activation is considered one of the primary mechanisms determining the central sensitization of chronic pain. It was shown in recent studies that the periphery, including gastrointestinal cells, might arouse brain cells [45]. The gut microbiota particularly regulates microglial function [46]. By affecting the activity of different cells, such as astrocytes, endothelial cells, microglia, monocytes, macrophages, pericytes and T-cells, the gut microbiota may regulate neuroinflammation (Figure 2). When those cells are activated, they start to produce multiple pro-inflammatory mediators such as C–C motif chemokine ligand 2 (CCL2 or MCP-1), CXCL-1, interleukin-1β (IL-1β), interferon-γ (IFN-γ), MMP-2/9, and tumor necrosis factor-α (TNF-α) [12]. Cytokines and chemokines secreted by microglia or astrocytes influence synaptic neurotransmission by increasing glutamate and decreasing gamma-amino-butyric acid (GABA) levels, resulting in pain hypersensitivity [47,48]. Taking all the data under consideration, the gut microbiota can play a major role in central sensitization underlying chronic pain associated with neuroinflammation; hence, it may contribute to the development of diverse neurological diseases [49]. Ding et al., in their article, examined the influence of the gut microbiota on neuropathic pain in chronic-constriction injury of the sciatic nerve (CCI) and whether it is associated with T-cell immune responses. CCI is an animal model widely used to represent neuropathic pain. The study showed that the gut microbiota, via modulation of both pro- and anti-inflammatory T-cell responses, induces the development of neuropathic pain. Moreover, the gut microbiota also has an impact on nociceptive behavior in sciatic nerve CCI. The study found that changes in the gut microbiota caused by the administration of oral antibiotics reduced CCI neuropathic pain. It manifested as weakened mechanical allodynia and thermal hyperalgesia [50]. Another study reported that the gut microbiota might lead to peripheral nerve trauma-induced neuropathic pain. Yang et al. showed that rats with spared nerve injury (SNI) and gut microbial dysbiosis might be prone to neuropathic pain and depression-like phenotypes, including anhedonia [46]. By contrast, in the study by Huang et al. in rat models, no significant association between oral probiotics such as L. reuteri LR06 or Bifidobacterium BL5b and anti-nociceptive effects on CCI-induced neuropathic pain was demonstrated [51]. Recent studies showed that the gut microbiota is involved in the pathogenesis of CIPN pain and modifies the effects of chemotherapeutics on tumor growth [52,53]. Shen et al. found that the gut microbiota takes part in the evolution of mechanical hyperalgesia induced by chemotherapy. In their study, mice after antibiotic treatment and germ-free mice both experienced reduced mechanical hyperalgesia after oxaliplatin administration. Moreover, restoration of the germ-free mouse microbiota revoked the protective effect [54]. Another study reported that neuropathic pain induced by paclitaxel therapy might be relieved with a DSF probiotic (high concentration of L. plantarum, S. thermophilus, B. breve, L. paracasei, L. delbrueckii, L. acidophilus, B. longum, B. infantis). Castelli et al. implied that the use of a probiotic as an adjuvant during chemotherapy might be beneficial in counteracting pain associated with CINP [55].
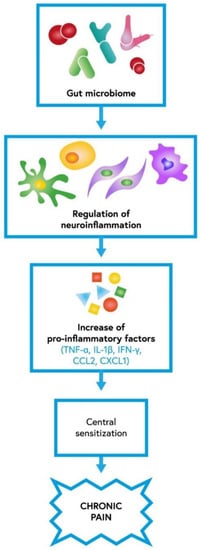
Figure 2.
The role of gut microbiota in neuroinflammation which contributes to central sensitization underlying chronic pain; IL-1β—interleukin-1β, IFN-γ—interferon-γ, TNF-α—tumor necrosis factor-α, CCL2—C–C motif chemokine ligand 2, CXCL1—C-X-C motif chemokine 1 [12].
3. Inflammation and Inflammatory Pain
3.1. Endometriosis
Dysbiosis in the GI tract disrupts immune function, which leads to the elevation of inflammatory cytokines and alteration of immune cell profiles. Those factors may play a role in the connection between the GI tract and endometriosis, as both have a high prevalence in patients [56]. As the GI tract possesses an organized lymphoid structure with many immune cells, the gut microbiota stimulates its growth and function, as shown in a study by Hooper et al. [57]. They further showed that dysbiosis alters the composition of immune cells, triggering inflammation [56]. In the case of the vaginal microbiota, it has been shown that a non-Lactobacillus-dominant (NLD) microbiota is associated with overgrowth of pathogenic bacteria, causing bacterial vaginosis. This may decrease reproductive potency, and a vaginal microbiota rich in Gardnerella, Prevotella, and Bacteroides sp. may increase the risk of endometriosis or pelvic inflammatory disease (PID) [58,59,60,61,62]. In recent years, it was also discovered that the uterus, previously thought to be a sterile environment, has its own microbiota. A healthy woman’s microbiota consists primarily of Firmicutes, Bacteroides, Proteobacteria, and Actinobacteria, according to a study by Baker et al., and a review by Moreno et al. identified the five most represented genera in the endometrial microbiota [62,63,64].
Ata et al. studied women with stage III/IV endometriosis and compared their microbiota from the gut, cervix, and vagina to that of a control group of healthy women. The cervical microbiota of women with endometriosis had an increased number of pathogenic species, and stool samples had higher Shigella and Escherichia concentrations [65]. Other studies also found a correlation between the increase in bacteria associated with bacterial vaginosis or opportunistic pathogens in the reproductive tract with endometriosis in women [61,65,66,67,68,69,70,71,72,73,74].
3.2. Chronic Pelvic Pain
Chronic pelvic pain (CPP) is a long-lasting pain that lowers quality of life, with many possible causes, such as endometriosis or chronic bacterial prostatitis [75,76]. Recent discoveries regarding the gut microbiome and visceral pain led to hypotheses about the correlation between CPP and the human microbiota. Shoskes et al. determined that patients with CPP had lower gut microbiota diversity than the control group, especially amongst Prevotella [77]. A study by Du et al. created a mouse model with experimental autoimmune prostatitis (EAP). EAP mice developed changes in the gut microflora, resulting in a distorted balance in Th17/Treg cells and decreased levels of short-chain fatty acids (SCFAs) in both serum and feces. Microbiota of healthy mice had notably fewer Firmicutes, Nitrospirae, or Fusobacteria than those with EAP. Additionally, the EAP mice had bacteria producing SCFAs, including Bacteroides, Butyricicoccus, and Ruminococcaceae. Changes in Th17/Treg balance were later reversed by supplementation of the SCFA propionate [78]. Their findings were consistent with other studies regarding chronic non-bacterial prostatitis [79,80]. Pelvic allodynia may also be caused by deficient lipase acyloxyacyl hydrolase (AOAH), an enzyme present in microglia. A study by Rahman-Enyart et al. suggests that AOAH plays a role in the modulation of pelvic pain, and its production is dependent on changes in the gut microbiome [81]. As new studies show, the microbiota is a crucial part of overall health, and its changes are correlated with many illnesses; however, further research is needed to make a comprehensive understanding of this topic possible.
4. Visceral Pain, Peripheral Mechanisms of Pain Regulation, and IBS
Visceral pain is a medical term for pain originating from the internal organs within the thorax or abdomen and is divided into acute and chronic pain. Acute visceral pain, caused by typically identifiable causes, is treated with appropriate therapeutic agents, including over the counter (OTC) medications such as non-steroidal anti-inflammatory drugs (NSAIDs) or acetaminophen, and is relatively easy to cure. On the other hand, chronic visceral pain can be difficult to treat even with opioids, and its unknown pathology led to the creation of the term functional gastrointestinal disorders (FGIDs), a collection of many disorders in pediatric and adult patients. FGID includes terms such as irritable bowel syndrome (IBS), infant colic and abdominal migraine, or functional dyspepsia. In the gastrointestinal tract (GI tract), nociceptor nerve endings are found throughout the layers of the GI tract. They respond to many stimuli from the tract and transfer them to their cell bodies in the dorsal horn of the spinal cord [82]. After being transferred to the contralateral side of the spinal cord, the signal is then transmitted to the limbic part of the brain via the spinothalamic tract. A response is then created, and a descending inhibitory circuitry is activated, causing a release of inhibitory neurotransmitters.
In recent years, scientists studied how the microbiome of the GI tract may influence the visceral pain response. The microbial population of a person stabilizes after the first 3 years of life and from then on is relatively stable [82]. Its greatest changes are noticed during disease states; however, while disorders affecting the GI tract are the more obvious causes, GI tract dysbiosis has been observed in many other illnesses. Non-intestinal disorders, such as obesity, allergy, asthma, or autoimmune diseases can also be a factor [82,83,84,85]. Additionally, the use of broad-spectrum antibiotic treatment changes the gut microbiota, and using such antibiotics without strong clinical purpose may become a factor in IBS. In a study by Vicentini et al., mice treated with broad-spectrum antibiotics showed effects on the structure and function of the GI tract, resulting in the loss of enteric neurons in enteric plexuses. Post-treatment supplementation of short-chain fatty acids (SCFAs), naturally produced by a healthy gut microbiome, restored neuronal loss in both submucosal and myenteric plexuses [86]. Similarly, a study by De Palma et al. focused on replicating IBS dysbiosis in rats. With fecal microbiota transplant in rats, visceral hypersensitivity increased when compared to gnotobiotic rats receiving a healthy microbiota, suggesting a link between IBS-associated hypersensitivity and the intestinal microbiota [87,88].
4.1. IBS
The influence of the gut microbiota and its dysbiosis on the pathophysiology of IBS was investigated in many studies that compared differences in the GI tract microbiome between IBS patients and controls [89,90,91,92]. Those studies showed that the intestinal microbiome of IBS patients had reduced amounts of Bacteroides, Prevotella, and Parabacteroides sp. Noor et al. and Maccaferi et al. showed that IBS patients had an increased population of Bacillus, Bifidobacteria, Lactobacillus, Clostridium, and Eubacterium rectale [93,94,95]. Those studies led to research about probiotic intervention and its benefits for IBS patients. In a study by Sisson et al., Symprove, a probiotic containing three Lactobacillus types and one Enterococcus, was shown to improve symptom severity in IBS [96]. In another study by Guglielmetti et al., Bifidobacterium bifidum MIMBb75 alleviated IBS symptomology by decreasing pain, discomfort, digestive upset, or bloating [97]. Further studies on probiotics for IBS presented another bacterial species with a positive influence on the relief of IBS symptoms [13,98]. Butyrate producers such as Faecalibacterium sp. have an anti-inflammatory impact on the GI tract, and F. prausnitzii is a source of serine protease that was shown to have anti-nociceptive activity by decreasing the excitability of dorsal root ganglia neurons [99,100,101,102]. Unknown IBS pathophysiology led to the creation of the term ‘psychobiotics’, referring to probiotics and bacterial metabolites that signal directly to the brain. In a randomized controlled trial (RCT) of 44 adults with IBS, patients were treated with Bifidobacterium longum NCC3001. Patients showed a significant reduction in depression and an increase in quality of life with no change in IBS symptom severity or the fecal microbiota profile. This suggested that there is some direct signaling of B. longum metabolites to the central nervous system (CNS) [103].
4.2. Peripheral Mechanism of Pain Regulation
The enteric nervous system (ENS) is formed by about 200–600 million neurons and is often referred to as the ‘second brain’. This network plays a part in maintaining GI tract function and reaches the lamina propria of the mucosa. ENS neurons form the subserous, myenteric, and submucosal plexuses and carry impulses to and from the brain. Intrinsic primary afferent neurons (IPANs) initiate secretory, motor, and vasomotor reactions from stimuli within the mucosa and from the central nervous system (CNS) [104]. Enteric sensory neurons receive the information through neurotransmitters and hormones released by enteroendocrine (EEC) and enterochromaffin (EC) enteric cells.
Enteric hormones such as serotonin (5-HT), glucagon-like peptide 1 (GLP-1), or peptide YY (PYY) are thought to have an impact on visceral pain and its management [104]. 5-HT excreted by EC cells activates receptors on EC cells and extrinsic primary afferent nerve (EPAN) terminals. This triggers enteric reflexes such as secretion, peristalsis, and perception of pain and inflammation [105,106,107].
Microbes in the GI tract microbiome can synthesize various neurotransmitters and metabolites involved in gut–brain communication, as shown in recent studies [108,109,110,111]. This includes SCFAs, tryptophan metabolites, GABA, dopamine, and noradrenaline [104]. One of the SCFAs, butyrate, was proposed as an agent with an indirect effect on regulating inflammatory visceral pain. Its injection in rat and mouse brains stimulated the production of brain-derived neurotrophic factor (BDNF), which favors neurogenesis, memory formation, and mood stabilization [112,113,114].
Bacteria such as Escherichia, Fusobacterium, Prevotella, Enterococcus casseliflavus, or Bacteroides can produce tryptophan, which later passes the blood–brain barrier (BBB), influencing serotoninergic neurotransmission in the CNS. In a study by Agus et al., it was shown that during gut inflammation, an increase in tryptophan conversion to kynurenine may be responsible for the development of anxiety and mood shifts [115]. During inflammation, there is an enhanced plasma level of kynurenine, which may favor its passage through the BBB and later metabolism into kynurenic acid (KynA) and quinolinic acid (QuiA), the latter of which is described as a neurotoxic agent [111].
Another microbial product, glutamate, is produced by certain microbial strains in the healthy GI tract [116,117,118,119]. It is a major neurotransmitter in the CNS and acts as a neuroactive molecule. A recent study suggested that glutamate may also regulate gut sensory and motor functions via receptors in the ENS [120,121]. During stress-induced dysbiosis, glutamate receptor expression is altered. In antibiotic-treated mice with dysbiosis, there were decreased levels of hippocampal NMDA and BDNF, which were later restored by prebiotic treatment [31,122,123,124].
GABA is an important neurotransmitter in the brain. Bravo et al. studied its role in pain management and suggested that GABA can inhibit visceral hypersensitivity, altering abdominal pain [125]. Oral administration of Lactobacillus rhamnosus in mice increased GABA levels in the CNS. Additionally, in a study by Perez-Berezo et al., administration of the E. coli Nissle 1917 (EcN) strain showed an increase in analgesic lipopeptide production, activation of GABA receptors on IPANs, and inhibition of visceral hypersensitivity [126].
5. Headache and Its Association with Drugs
Headache is one of the most frequently reported symptoms [127], and various types have been described. Primary headaches can be divided into four groups: migraine, tension headache, trigeminal autonomic cephalgia, and other primary headache disorders [127]. Migraine, a neurological disorder characterized by headache, nausea, vomiting, and photophobia or phonophobia [128,129], is one of the most common types of headaches [17]. The hemicrania occurs due to hypothalamus activation and further pituitary adenylate cyclase-activating polypeptide (PACAP) secretion, which is responsible for vasodilatation [17]. Moreover, migraine is related to GI illnesses, which include celiac syndrome, irritable bowel syndrome, or infection by Helicobacter pylori [12,30]. There is also an association between the gut microbiome and the pathogenesis of migraine. The gut–brain axis triggers the migraine attack through pro-inflammatory factors, gut microbiome composition, neuropeptides, serotonin pathways, stress hormones, and nutritional substances. The physical or psychological stress factors may lead to gut microbiome changes such as dysbiosis [30]. This, in turn, causes an increase in calcitonin gene-related peptide (CGRP) secretion [17], which is correlated with migraine symptoms and has antibacterial effect on strains such as E. coli, E. faecalis, and L. acidophilus [17,30]. This particular type of headache is associated with pro-inflammatory factors. During migraine attacks, increased secretion of serum cytokines such as IL-1b, IL-6, IL-8, and TNF-a was observed. Moreover, Arzani et al. reported that in germ-free mice, the hypernociception induced by inflammatory mediators is reduced [30]. These could result from increased expression of IL-10 in germ-free mouse models [130]. This cytokine is an important regulator of inflammatory responsiveness [130]. These lines of evidence emphasize the importance of the gut microbiome in migraine and have prompted research on whether probiotic supplementation is a beneficial therapy for the condition [12]. The data on the efficacy of probiotic supplementation in migraine are incoherent. Sensenig et al. showed that most patients who were given probiotics, such as L. bulgaricus, L. acidophilus, E. faecium, and B. bifidum, reported an improvement in quality of life [131]. By contrast, another study showed no significant differences between a group of patients who suffered from migraine and were supplemented with probiotics and the one that was not supplemented with probiotics [12,132].
To summarize, the association between the gut microbiome and migraine is clear. Studies show not only a correlation in pathogenesis but also a possible way of treating migraine with probiotics. However, there is still a lot to be discovered [12].
Opioid Tolerance
Opioids are known for their anti-nociceptive, anti-tussive and anti-diarrheal properties. They are the major drugs used in cancer and post-surgical pain treatment [133], although their severe side effects, such as tolerance, dependence, emesis, or constipation, lead to significant restrictions in their use [12]. GI symptoms associated with these drugs are known as opioid bowel dysfunction (OBD) and are the result of the stimulation of opioid receptors in the GI tract [134]. The research shows that chronic use of opioids may result in dysbiosis [12,135], damage to the gut barrier, bacterial translocation, and secretion of pro-inflammatory factors. Opioid tolerance was associated with a lack of Bifidobacteria and Lactobacillaceae in mice [12,25]. The enteric glia are responsible for the proper functioning of the GI tract [12]. Furthermore, they are also relevant to the development of the ectypal inflammatory reaction to long-term use of opioid drugs [136]. The bacterial product bacterial lipopolysaccharide (LPS) was reported to be associated with the production of pro-inflammatory cytokines during long-term opioid treatment [136]. Due to the chronic use of morphine, we can observe increased activity in enteric glia of the P2X receptor [12,136], a calcium-permeable ion channel activated by ATP and associated with cytokine secretion by enteric glia [25]. This leads to an enhanced inflammatory reaction [25]. LPS is also related to the intensified expression of connexin 43 (Cx43), a gap junction protein that mediates the secretion of ATP [136]. Cx43 can be blocked by non-specific connexin inhibitor (CBX), which results in a decreased inflammatory response [136].
Another study showed that administration of broad-spectrum antibiotics prevents GI side effects and tolerance to opioid-related drugs with long-term use of morphine [137]. Analgesic tolerance can be avoided by oral vancomycin due to its active properties against Gram-positive bacteria, the translocation of which is significant in the tolerance process [12,25,137]. Furthermore, germ-free mice have reduced morphine tolerance, which can be reclaimed by gut microbiome reconstitution [138]. In addition, opioid tolerance can be a result of the inactivation of tetrodotoxin-resistant (TTX-R) Na+ channels in dorsal root ganglia (DGR) neurons, which can be reversed by oral vancomycin administration [139]. In conclusion, the above-described studies prove the importance of the gut microbiota in opioid tolerance occurrence. They show the role of the gut flora in the genesis of morphine tolerance and indicate how the side effects of opioid drug use may enhance the entire process.
6. The Gut Microbiota as a Therapeutic Target in Chronic Pain
6.1. Probiotics and Prebiotics
Probiotics are living microorganisms that can provide health benefits to the host [140]. A growing body of research supports the thesis that probiotics are effective in modifying the balance of the gut microbiota [141,142]. Some of their proven beneficial effects include improved digestion, boosted immunity, and decreased cholesterol levels [143]. Some of the more recent studies suggest that probiotics might be effective in alleviating the symptoms of chronic intestinal disorders, such as Crohn’s disease [144].
Several preclinical animal studies have demonstrated the beneficial effects of probiotics on visceral pain [145,146,147]. In multiple studies, probiotics exerted beneficial effects on visceral hypersensitivity. In rats, probiotic VSL#3 decreased visceral hypersensitivity potentially through the mast cell-PAR2-TRPV1 pathway, which then affects the release of potent mediators that affect the enteric nerves and smooth muscles [145]. Moreover, supplementation with Clostridium butyricum, a commensal bacterium, may inhibit colonic inflammation in a mouse model of IBS through its action on nod-like receptor pyrin domain-containing protein 6 [146]. In a similar model, Roseburia hominis alleviated visceral hypersensitivity and prevented the expression of occludin from decreasing [147]. Moreover, in rats, B. infantis 35624 significantly reduced visceral pain, suggesting that it may be effective in treating symptoms of IBS [148].
Several human studies have also revealed the benefits of using probiotics for chronic pain. A randomized, double-blind study on 101 pediatric patients suffering from IBS (NCT01180556) revealed that a 4-week supplementation of L. reuteri DSM 17938 reduced both the frequency and the intensity of abdominal pain in children [149]. Moreover, a probiotic mixture of Bifidobacterium infantis M-63, breve M-16V, and longum BB536 (NCT02566876) was successful in attenuating the symptoms of abdominal pain in IBS but not in functional dyspepsia. Likewise, the intervention group noted the markedly higher quality of life improvement in comparison with a placebo (48% vs. 17%, p = 0.001) [150]. A 2009 review by Newlove-Delgado et al. retrospectively investigating the use of probiotics in children with recurrent abdominal pain suggested that those preparations are likely to improve pain symptoms in the short term, that is, up to 3 months (OR = 1.63; 95% CI = 1.07–2.47) [151]. By contrast, a randomized, placebo-controlled trial by Spiller et al. failed to identify any clinical benefit, including intestinal pain and discomfort, of S. cerevisiae I-3856 supplementation at a dose of 1000 mg per day, in comparison to a placebo [152].
Prebiotics are fibers and other non-digestible ingredients that benefit the host by selectively boosting the growth and activity of select microorganisms in the colon, mainly lactobacilli and bifidobacteria. They are considered either as an addition to probiotics or an alternative to them. Several pre-clinical dissertations have emerged underlining the beneficial role of prebiotics in terms of attenuating chronic pain, such as PDX/GOS reducing chronic visceral pain induced by intracolonic zymosan injection in rats [153]. In human studies, a prebiotic galacto-oligosaccharide mixture supplemented for 2 weeks reduced abdominal pain associated with GI disorders in adults. The treatment arm reported significantly lower scores for bloating, flatulence, and pain. However, there was no improvement in quality of life throughout the study [154]. Lastly, a study on the symbiotic containing Bacillus coagulans on 88 pediatric patients showed a reduction of abdominal pain that was present after treatment (60% vs. 39.5%, p = 0.044) but not after 12 weeks of follow-up [155].
6.2. FODMAP Diet
Recent studies have demonstrated that functional GI symptoms can be induced by colonic gas production in patients with visceral hypersensitivity. In those patients, short-chain fermentable carbohydrates increase small intestinal water volume, resulting in increased colonic gas production. Therefore, dietary restriction of short-chain fermentable carbohydrates (low-FODMAP diet) should theoretically ameliorate the symptoms of IBS. In pre-clinical studies, the low-FODMAP diet (LFD) altered the gut microbial composition, resulting in reduced fecal lipopolysaccharide of Gram-negative bacteria. In contrast to a high-FODMAP diet, there is a significant reduction of Akkermancia muciniphila and Actinobacteria [156]. Therefore, it could be beneficial in reducing gut mucosal inflammation and restoring the barrier function of the gut, ultimately leading to the alleviation of visceral pain [156].
In a clinical setting, the FODMAP diet has led to a reduction in IBS severity, with decreased frequency of pain episodes (p < 0.01) and increased quality of life [157]. In another study by Pedersen et al., LFD resulted in a greater reduction of disease severity but no improvement in quality of life [158]. In a double-blind, placebo-controlled trial on 40 patients with IBS by Hustoft et al., LFD with fructans lowered the severity of nausea, vomiting, and flatulence [159]. Overall, up to 86% of IBS patients improve clinically in terms of GI symptoms, as well as abdominal pain, bloating, and constipation, while following the diet [160].
6.3. Fecal Microbiota Transplantation
Fecal microbiota transplantation (FMT) involves the transfer of microbial flora from a healthy donor stool to the recipient’s intestinal tract to normalize the target intestinal microbiota composition and function. One of the most notable examples of the use of FMT in clinical practice is Clostridium difficile infection (CDI), which often occurs in patients whose microbiota has been suppressed by prolonged antibiotic therapy. Recent evidence suggests that the gut microbiota composition is linked to the occurrence of abdominal pain and its frequency, duration, and intensity in the general population [161]. Moreover, in an animal model of colitis, FMT administration to control rats resulted in long-lasting visceral hypersensitivity [162]. Several mechanisms have been proposed through which FMT might affect chronic pain, including competition with pathogenic bacteria, protection of the intestinal barrier, or stimulation of the intestinal immune system [163]. An open-label study on FMT in humans with IBS showed marked improvement in abdominal pain that was associated with the abundance of Akkermansia muciniphila [164].
Moreover, allogenic FMT resulted in a significant decrease in symptoms of IBS (p = 0.02), which was not present in the autologous FMT group (p = 0.16) [165]. Furthermore, a metagenomic sequencing study revealed that following FMT the taxonomic profile of the recipient shifts towards a donor-like profile, inducing long-term changes in the gut microbiota, which mirror the clinical effect of the treatment [166].
In order to perform successful FMT, several criteria must be met. Firstly, donor selection should be strict, excluding those at risk of harboring an infectious agent. Moreover, recipients must not receive major immunosuppressive therapy, or suffer from serious comorbidities that would put them at risk [167]. While FMT is relatively safe, some of the studies suggest its potential drawbacks. One study suggested that FMT might be associated with diarrhea, abdominal cramping, belching, and nausea within 3 h post-FMT [168]. Moreover, there exists a possibility of development of long-term adverse effects due to alteration of the gut microbiota. More long-term, follow-up studies are required to address this issue [169].
7. Conclusions
In recent years, numerous studies have provided data on the role of the gut microbiome and its influence on other tissues. It is known that alteration in the microbiome could be one of the factors contributing to the development of cancer and neurological, gastrointestinal, cardiovascular, and metabolic diseases. Lately, many studies have also investigated the role of the human microbiome in the pathogenesis of different types of pain (Table 1). Proper assessment and control of pain are essential for improving quality of life in many patients. Despite the availability of various pain management methods, there is still a great need for research on factors contributing to pain pathogenesis and novel therapies. Recent studies suggest that the human microbiome may be an essential component of the pathogenesis of multiple types of pain. Neuropathic pain could result from the gut microbiome’s influence on T-cell immune response, disrupting the regulation of pro- and anti-inflammatory cytokine production. Furthermore, alteration of the immune cell response and cytokine production by the gut microbiome could contribute to the development of inflammatory diseases, such as endometriosis. Chronic visceral pain remains a challenge to efficient treatment. The human microbiome contributes to the still unknown pathogenesis of FGIDs, providing a promising direction for further studies. Additionally, common symptoms such as headaches are influenced by the gut microbiome. An altered gut–brain axis could trigger a migraine. Moreover, the regulation of inflammatory mediators that contributes to migraine is disrupted by dysbiosis. The gut microbiome could also impact the efficacy of pain management, leading to opioid tolerance. The contribution of the human microbiome to the pathogenesis of multiple types of pain leads to its use as a possible target for analgesic therapies. Pro- and prebiotics are already widely used in clinical practice. They are reported to be effective in reducing chronic visceral pain and migraine. However, there is still a great need for further evaluation of their efficacy and influence on patients’ quality of life. Another approach assumes the modification of the gut microbiome with a specific diet, such as a low-FODMAP diet, which could be beneficial for patients with IBS, reducing symptoms and pain episodes. The usage of FMT is recommended in the treatment of Clostridium difficile infection.

Table 1.
Summary of novel studies that investigated the role of the human microbiome in the pathogenesis of different types of pain.
Moreover, FMT is reported to efficiently reduce visceral pain among IBS patients. However, these studies have some limitations. There is a strong need for further evaluation of concepts and previous results. Additional long-term studies are required to assess the potential side effects of gut microbiota alteration. Moreover, the differences in the methodology of the studies impede the precise comparison of the results. Pittayanon et al., in their systematic review, reported concerns about deficiencies in studies’ methodology and statistical analysis [95]. The shortcomings, such as lack of data on administrated antibiotics, and differences in the microbiome evaluation methods, are reasons for inconsistency in reviewed papers.
Despite the significant development in the understanding of the human microbiome in the pathogenesis of pain, there are still many areas to be investigated. A detailed evaluation of the influence of the altered microbiome on the gut–brain axis could be a critical factor in understanding the impact of dysbiosis on several tissues and pain development [18]. The detailed characterization of the gut microbiome in chronic, visceral, or headache states and their interaction with the gut–brain axis could deliver novel insight into the pathogenesis of a different type of pain. Further molecular studies could develop novel targets for analgetic treatment that could significantly improve numerous patients’ quality of life.
Author Contributions
Conceptualization, K.U. and A.P.; methodology, Ł.U.; formal analysis, J.R.; investigation, B.S.; writing—original draft preparation, A.G. and J.S.; writing—review and editing, F.M. and A.P.; visualization, B.S.; supervision, J.R.; project administration, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Piotr Michalski from the Academy of Art in Szczecin for his assistance in composing the figures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sommer, F.; Bäckhed, F. The Gut Microbiota—Masters of Host Development and Physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.J.M.; Wells, J.M. Regulation of Human Epithelial Tight Junction Proteins by Lactobacillus plantarum In Vivo and Protective Effects on the Epithelial Barrier. Am. J. Physiol. Gastrointest. Liver. Physiol. 2010, 298, G851–G859. [Google Scholar] [CrossRef] [PubMed]
- Hugon, P.; Dufour, J.C.; Colson, P.; Fournier, P.E.; Sallah, K.; Raoult, D. A Comprehensive Repertoire of Prokaryotic Species Identified in Human Beings. Lancet Infect. Dis. 2015, 15, 1211–1219. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14. [Google Scholar] [CrossRef]
- Bengmark, S. Ecological Control of the Gastrointestinal Tract. The Role of Probiotic Flora. Gut 1998, 42, 2–7. [Google Scholar] [CrossRef]
- Vuong, H.E.; Yano, J.M.; Fung, T.C.; Hsiao, E.Y. The microbiome and host behavior. Annu. Rev. Neurosci. 2017, 40, 21–49. [Google Scholar] [CrossRef]
- Schmidt, T.S.B.; Raes, J.; Bork, P. The human gut microbiome: From association to modulation. Cell 2018, 172, 1198–1215. [Google Scholar] [CrossRef]
- Dardmeh, F.; Nielsen, H.I.; Alipour, H.; Kjaergaard, B.; Brandsborg, E.; Gazerani, P. Potential nociceptive regulatory effect of probiotic Lactobacillus rhamnosus PB01 (DSM 14870) on mechanical sensitivity in diet-induced obesity model. Pain Res. Manag. 2016, 2016, 5080438. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Russo, R.; Cristiano, C.; Avagliano, C.; De Caro, C.; La Rana, G.; Raso, G.M.; Canani, R.B.; Meli, R.; Calignano, A. Gut–brain axis: Role of lipids in the regulation of inflammation, pain and CNS diseases. Curr. Med. Chem. 2018, 25, 3930–3952. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef]
- Guo, R.; Chen, L.H.; Xing, C.; Liu, T. Pain Regulation by Gut Microbiota: Molecular Mechanisms and Therapeutic potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, Tryptophan Metabolism and the Brain-Gut-Microbiome Axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Li, X.; Watanabe, K.; Kimura, I. Gut microbiota dysbiosis drives and implies novel therapeutic strategies for diabetes mellitus and related metabolic diseases. Front. Immunol. 2017, 8, 1882. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host–gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Heiss, C.N.; Olofsson, L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocrinol. 2019, 31, e12684. [Google Scholar] [CrossRef]
- Léa, L.T.; Caula, C.; Moulding, T.; Lyles, A.; Wohrer, D.; Titomanlio, L. Brain to Belly: Abdominal variants of migraine and functional abdominal pain disorders associated with migraine. J. Neurogastroenterol. Motil. 2021, 27, 482–494. [Google Scholar]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Rea, K.; O’Mahony, S.M.; Dinan, T.G.; Cryan, J.F. The role of the gastrointestinal microbiota in visceral pain. Handb. Exp. Pharmacol. 2017, 239, 269–287. [Google Scholar]
- Kolodziejczyk, A.A.; Zheng, D.; Shibolet, O.; Elinav, E. The role of the microbiome in NAFLD and NASH. EMBO Mol. Med. 2019, 11, e9302. [Google Scholar] [CrossRef]
- Wahlstrom, A. Outside the liver box: The gut microbiota as pivotal modulator of liver diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 912–919. [Google Scholar] [CrossRef]
- Massari, F.; Mollica, V.; di Nunno, V.; Gatto, L.; Santoni, M.; Scarpelli, M.; Cimadamore, A.; Lopez-Beltran, A.; Cheng, L.; Battelli, N.; et al. The Human Microbiota and Prostate Cancer: Friend or Foe? Cancers 2019, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Cimadamore, A.; Santoni, M.; Massari, F.; Gasparrini, S.; Cheng, L.; López-Beltrán, A.; Montironi, R.; Scarpelli, M. Microbiome and Cancers, with Focus on Genitourinary Tumors. Front. Oncol. 2019, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Neish, A.S. Microbes in Gastrointestinal Health and Disease. Gastroenterology 2009, 136, 65–80. [Google Scholar] [CrossRef]
- Santoni, M.; Miccini, F.; Battelli, N. Gut Microbiota, Immunity and Pain. Immunol. Lett. 2021, 229, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of Immune Cell Function by Short-Chain Fatty Acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Chey, W.Y.; Jin, H.O.; Lee, M.H.; Sun, S.W.; Lee, K.Y. Colonic Motility Abnormality in Patients with Irritable Bowel Syndrome Exhibiting Abdominal Pain and Diarrhea. Am. J. Gastroenterol. 2001, 96, 1499–1506. [Google Scholar] [CrossRef]
- Psichas, A.; Reimann, F.; Gribble, F.M. Gut Chemosensing Mechanisms. J. Clin. Invest. 2015, 125, 908–917. [Google Scholar] [CrossRef]
- Amirkhanzadeh Barandouzi, Z.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered Composition of Gut Microbiota in Depression: A Systematic Review. Front. Psychiatry 2020, 11, 1–10. [Google Scholar]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M.; EHF-SAS. Gut-Brain Axis and Migraine Headache: A Comprehensive review. J. Headache Pain 2020, 21, 15. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal Microbial Colonization Programs the Hypothalamic-Pituitary-Adrenal System for Stress Response in Mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Drożdżal, S.; Rosik, J.; Lechowicz, K.; Machaj, F.; Szostak, B.; Majewski, P.; Rotter, I.; Kotfis, K. COVID-19: Pain Management in Patients with SARS-CoV-2 Infection-Molecular Mechanisms, Challenges, and Perspectives. Brain Sci. 2020, 10, 465. [Google Scholar] [CrossRef]
- Neufeld, K.A.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Effects of Intestinal Microbiota on Anxiety-Like Behavior. Commun. Integr. Biol. 2011, 4, 492–494. [Google Scholar] [CrossRef]
- Bercik, P.; Verdu, E.F.; Foster, J.A.; Macri, J.; Potter, M.; Huang, X.; Malinowski, P.; Jackson, W.; Blennerhassett, P.; Neufeld, K.A.; et al. Chronic Gastrointestinal Inflammation Induces Anxiety-Like Behavior and Alters Central Nervous System Biochemistry in Mice. Gastroenterology 2010, 139, 2102–2112.e1. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Braz, J.; Solorzano, C.; Wang, X.; Basbaum, A.I. Transmitting pain and itch messages: A contemporary view of the spinal cord circuits that generate gate control. Neuron 2014, 82, 522–536. [Google Scholar] [CrossRef]
- Ji, R.R.; Chamessian, A.; Zhang, Y.Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef]
- Scholz, J.; Woolf, C.J. Can we conquer pain? Nat. Neurosci. 2002, 5, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Binder, A.; Wasner, G. Neuropathic Pain: Diagnosis, Pathophysiological Mechanisms, and Treatment. Lancet Neurol. 2010, 9, 807–819. [Google Scholar] [CrossRef]
- Cavaletti, G.; Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity. Nat. Rev. Neurol. 2010, 6, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Lavoie Smith, E.M.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2014, 32, 1941–1967. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Zhang, X.-D.; Miao, W.-Y.; Sun, Y.-J.; Xiong, G.; Wu, Q.; Li, G.; Yang, P.; Yu, H.; Li, H.; et al. PDGFRβ Cells Rapidly Relay Inflammatory Signal from the Circulatory System to Neurons via Chemokine CCL2. Neuron 2018, 100, 183–200.e8. [Google Scholar] [CrossRef]
- Yang, C.; Fang, X.; Zhan, G.; Huang, N.; Li, S.; Bi, J.; Jiang, R.; Yang, L.; Miao, L.; Zhu, B.; et al. Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl. Psychiatry 2019, 9, 57. [Google Scholar] [CrossRef]
- Gao, Y.J.; Ji, R.R. Chemokines, Neuronal-Glial Interactions, and Central Processing of Neuropathic Pain. Pharmacol. Ther. 2010, 126, 56–68. [Google Scholar] [CrossRef]
- Matsuda, M.; Huh, Y.; Ji, R.R. Roles of Inflammation, Neurogenic Inflammation, and Neuroinflammation in Pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Ding, W.; You, Z.; Chen, Q.; Yang, L.; Doheny, J.; Zhou, X.; Li, N.; Wang, S.; Hu, K.; Chen, L.; et al. Gut Microbiota Influences Neuropathic Pain through Modulating Proinflammatory and Anti-inflammatory T Cells. Anesth. Analg. 2021, 132, 1146–1155. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, C.; Wang, J.; Guo, Q.; Zou, W. Oral Lactobacillus reuteri LR06 or Bifidobacterium BL5b supplement do not produce analgesic effects on neuropathic and inflammatory pain in rats. Brain Behav. 2019, 9, e01260. [Google Scholar] [CrossRef]
- Mukaida, N. Intestinal microbiota: Unexpected alliance with tumor therapy. Immunotherapy 2014, 6, 231–233. [Google Scholar] [CrossRef]
- Vázquez-Baeza, Y.; Callewaert, C.; Debelius, J.; Hyde, E.; Marotz, C.; Morton, J.T.; Swafford, A.; Vrbanac, A.; Dorrestein, P.C.; Knight, R. Impacts of the Human Gut Microbiome on Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 253–270. [Google Scholar] [CrossRef]
- Shen, S.; Lim, G.; You, Z.; Ding, W.; Huang, P.; Ran, C.; Doheny, J.; Caravan, P.; Tate, S.; Hu, K.; et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat. Neurosci. 2017, 20, 1213–1216. [Google Scholar] [CrossRef]
- Castelli, V.; Palumbo, P.; D’Angelo, M.; Moorthy, N.K.; Antonosante, A.; Catanesi, M.; Lombardi, F.; Iannotta, D.; Cinque, B.; Benedetti, E.; et al. Probiotic DSF counteracts chemotherapy induced neuropathic pain. Oncotarget 2018, 9, 27998–28008. [Google Scholar] [CrossRef]
- Jiang, I.; Yong, P.J.; Allaire, C.; Bedaiwy, M.A. Intricate Connections between the Microbiota and Endometriosis. Int. J. Mol. Sci. 2021, 22, 5644. [Google Scholar] [CrossRef]
- Hooper, L.v.; Littman, D.R.; Macpherson, A.J. Interactions between the Microbiota and the Immune System. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Martin, D.H.; Marrazzo, J.M. The Vaginal Microbiome: Current Understanding and Future Directions. J. Infect. Dis. 2016, 214 (Suppl. 1), S36–S41. [Google Scholar] [CrossRef]
- Muzny, C.A.; Łaniewski, P.; Schwebke, J.R.; Herbst-Kralovetz, M.M. Host-Vaginal Microbiota Interactions in the Pathogenesis of Bacterial Vaginosis. Curr. Opin. Infect. Dis. 2020, 33, 59–65. [Google Scholar] [CrossRef]
- Blander, J.M.; Longman, R.S.; Iliev, I.D.; Sonnenberg, G.F.; Artis, D. Regulation of Inflammation by Microbiota Interactions with the Host. Nat. Immunol. 2017, 18, 851–860. [Google Scholar] [CrossRef]
- Dols, J.A.M.; Molenaar, D.; van der Helm, J.J.; Caspers, M.P.M.; Angelino-Bart, A.d.K.; Schuren, F.H.J.; Speksnijder, A.G.C.L.; Westerhoff, H.V.; Richardus, J.H.; Boon, M.E.; et al. Molecular Assessment of Bacterial Vaginosis by Lactobacillus Abundance and Species Diversity. BMC Infect. Dis. 2016, 16, 180. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine Microbiota: Residents, Tourists, or Invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Møller, B.R.; Kristiansen, F.v.; Thorsen, P.; Frost, L.; Mogensen, S.C. Sterility of the Uterine Cavity. Acta Obstet. Gynecol. Scand. 1995, 74, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the Endometrial Microbiota Has an Effect on Implantation Success or Failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Ata, B.; Yildiz, S.; Turkgeldi, E.; Brocal, V.P.; Dinleyici, E.C.; Moya, A.; Urman, B. The Endobiota Study: Comparison of Vaginal, Cervical and Gut Microbiota Between Women with Stage 3/4 Endometriosis and Healthy Controls. Sci. Rep. 2019, 9, 2204. [Google Scholar] [CrossRef]
- Bourlev, V.; Volkov, N.; Pavlovitch, S.; Lets, N.; Larsson, A.; Olovsson, M. The relationship between microvessel density, proliferative activity and expression of vascular endothelial growth factor-A and its receptors in eutopic endometrium and endometriotic lesions. Reproduction 2006, 132, 501–509. [Google Scholar] [CrossRef]
- Hernandes, C.; Silveira, P.; Rodrigues Sereia, A.F.; Christoff, A.P.; Mendes, H.; Valter de Oliveira, L.F.; Podgaec, S. Microbiome Profile of Deep Endometriosis Patients: Comparison of Vaginal Fluid, Endometrium and Lesion. Diagnostics 2020, 10, 163. [Google Scholar] [CrossRef]
- Akiyama, K.; Nishioka, K.; Khan, K.N.; Tanaka, Y.; Mori, T.; Nakaya, T.; Kitawaki, J. Molecular detection of microbial colonization in cervical mucus of women with and without endometriosis. Am. J. Reprod. Immunol. 2019, 82, e13147. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X.; Tang, H.; Zeng, L.; Wu, R. Microbiota Composition and Distribution along the Female Reproductive Tract of Women with Endometriosis. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 15. [Google Scholar] [CrossRef]
- Perrotta, A.R.; Borrelli, G.M.; Martins, C.O.; Kallas, E.G.; Sanabani, S.S.; Griffith, L.G.; Alm, E.J.; Abrao, M.S. The Vaginal Microbiome as a Tool to Predict rASRM Stage of Disease in Endometriosis: A Pilot Study. Reprod. Sci. 2020, 27, 1064–1073. [Google Scholar] [CrossRef]
- Deng, T.; Shang, A.; Zheng, Y.; Zhang, L.; Sun, H.; Wang, W. Log (Lactobacillus crispatus/Gardnerella vaginalis): A new indicator of diagnosing bacterial vaginosis. Bioengineered 2022, 13, 2981–2991. [Google Scholar] [CrossRef]
- Tohill, B.C.; Heilig, C.M.; Klein, R.S.; Rompalo, A.; Cu-Uvin, S.; Brown, W.; Duerr, A. Vaginal flora morphotypic profiles and assessment of bacterial vaginosis in women at risk for HIV infection. Infect. Dis. Obstet. Gynecol. 2004, 12, 121–126. [Google Scholar] [CrossRef]
- Dols, J.A.; Smit, P.W.; Kort, R.; Reid, G.; Schuren, F.H.; Tempelman, H.; Bontekoe, T.R.; Korporaal, H.; Boon, M.E. Microarray-Based Identification of Clinically Relevant Vaginal Bacteria in Relation to Bacterial Vaginosis. Am. J. Obstet. Gynecol. 2011, 204, 305.e1–305.e7. [Google Scholar] [CrossRef]
- Verstraelen, H.; Verhelst, R. Bacterial vaginosis: An update on Diagnosis and Treatment. Expert Rev. Anti Infect. Ther. 2009, 7, 1109–1124. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Gaskin, D.J.; Richard, P. The Economic Costs of Pain in the United States. J. Pain 2012, 13, 715–724. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Wang, H.; Polackwich, A.S.; Tucky, B.; Altemus, J.; Eng, C. Analysis of Gut Microbiome Reveals Significant Differences between Men with Chronic Prostatitis/Chronic Pelvic Pain Syndrome and Controls. J. Urol. 2016, 196, 435–441. [Google Scholar] [CrossRef]
- Du, H.X.; Yue, S.Y.; Niu, D.; Liu, C.; Zhang, L.G.; Chen, J.; Chen, Y.; Guan, Y.; Xiao-Liang, H.; Chun, L.; et al. Gut Microflora Modulates Th17/Treg Cell Differentiation in Experimental Autoimmune Prostatitis via the Short-Chain Fatty Acid Propionate. Front. Immunol. 2022, 13, 1. [Google Scholar] [CrossRef]
- Ohadian Moghadam, S.; Momeni, S.A. Human Microbiome and Prostate Cancer Development: Current Insights into the Prevention and Treatment. Front. Med. 2021, 15, 11–32. [Google Scholar] [CrossRef]
- Crocetto, F.; Boccellino, M.; Barone, B.; di Zazzo, E.; Sciarra, A.; Galasso, G.; Settembre, G.; Quagliuolo, L.; Imbimbo, C.; Boffo, S.; et al. The Crosstalk between Prostate Cancer and Microbiota Inflammation: Nutraceutical Products Are Useful to Balance This Interplay? Nutrients 2020, 12, 2648. [Google Scholar] [CrossRef]
- Rahman-Enyart, A.; Yaggie, R.E.; Bollinger, J.L.; Arvanitis, C.; Winter, D.R.; Schaeffer, A.J.; Klumpp, D.J. Acyloxyacyl Hydrolase Regulates Microglia-Mediated Pelvic Pain. Streicher JM, Editor. PLoS ONE 2022, 17, e0269140. [Google Scholar] [CrossRef] [PubMed]
- Moloney, R.D.; Johnson, A.C.; O’Mahony, S.M.; Dinan, T.G.; Greenwood-Van Meerveld, B.; Cryan, J.F. Stress and the Microbiota–Gut–Brain Axis in Visceral Pain: Relevance to Irritable Bowel Syndrome. CNS Neurosci. Ther. 2016, 22, 102. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.H.; Dieguez, D.; Miller, L.M.; Young, H.A. Does the Microbiota Play a Role in the Pathogenesis of Autoimmune Diseases? Gut 2015, 64, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.; Moran, C.; Shanahan, F. The Microbiota in Inflammatory Bowel Disease. J. Gastroenterol. 2015, 50, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Valitutti, F.; Cucchiara, S.; Fasano, A. Celiac disease and the microbiome. Nutrients 2019, 11, 2403. [Google Scholar] [CrossRef]
- Vicentini, F.A.; Keenan, C.M.; Wallace, L.E.; Woods, C.; Cavin, J.-B.; Flockton, A.R.; Macklin, W.B.; Belkind-Gerson, J.; Hirota, S.A.; Sharkey, K.A. Intestinal Microbiota Shapes Gut Physiology and Regulates Enteric Neurons and Glia. Microbiome 2021, 9, 210. [Google Scholar] [CrossRef]
- De Palma, G.; Lynch, M.D.J.; Lu, J.; Dang, V.T.; Deng, Y.; Jury, J.; Umeh, G.; Miranda, P.M.; Pastor, M.P.; Sidani, S.; et al. Transplantation of Fecal Microbiota from Patients with Irritable Bowel Syndrome Alters Gut Function and Behavior in Recipient Mice. Sci. Transl. Med. 2017, 9, eaaf6397. [Google Scholar] [CrossRef]
- Crouzet, L.; Gaultier, E.; Del’Homme, C.; Cartier, C.; Delmas, E.; Dapoigny, M.; Fioramonti, J.; Bernalier-Donadille, A. The Hypersensitivity to Colonic Distension of IBS Patients Can Be Transferred to Rats through Their Fecal Microbiota. Neurogastroenterol. Motil. 2013, 25, e272–e282. [Google Scholar] [CrossRef]
- Malinen, E.; Rinttilä, T.; Kajander, K.; Mättö, J.; Kassinen, A.; Krogius, L.; Saarela, M.; Korpela, R.; Palva, A. Analysis of the Fecal Microbiota of Irritable Bowel Syndrome Patients and Healthy Controls with Real-Time PCR. Am. J. Gastroenterol. 2005, 100, 373–382. [Google Scholar] [CrossRef]
- Lyra, A.; Rinttilä, T.; Nikkilä, J.; Krogius-Kurikka, L.; Kajander, K.; Malinen, E.; Mättö, J.; Mäkelä, L.; Palva, A. ‘Diarrhoea-Predominant Irritable Bowel Syndrome Distinguishable by 16S rRNA Gene Phylotype Quantification. World J Gastroenterol. 2009, 15, 5936–5945. [Google Scholar] [CrossRef]
- Tana, C.; Umesaki, Y.; Imaoka, A.; Handa, T.; Kanazawa, M.; Fukudo, S. Altered Profiles of Intestinal Microbiota and Organic Acids May be the Origin of Symptoms in Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2010, 22, 512-e115. [Google Scholar] [CrossRef]
- Mayer, E.A.; Savidge, T.; Shulman, R.J. Brain-Gut Microbiome Interactions and Functional Bowel Disorders. Gastroenterology 2014, 146, 1500–1512. [Google Scholar] [CrossRef]
- Maccaferri, S.; Candela, M.; Turroni, S.; Centanni, M.; Severgnini, M.; Consolandi, C.; Cavina, P.; Brigidi, P. IBS-Associated Phylogenetic Unbalances of the Intestinal Microbiota Are Not Reverted by Probiotic Supplementation. Gut Microbes 2012, 3, 406–413. [Google Scholar] [CrossRef]
- Noor, S.O.; Ridgway, K.; Scovell, L.; Kemsley, E.K.; Lund, E.K.; Jamieson, C.; Johnson, I.T.; Narbad, A. Ulcerative Colitis and Irritable Bowel Patients Exhibit Distinct Abnormalities of the Gut Microbiota. BMC Gastroenterol. 2010, 10, 134. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut Microbiota in Patients with Irritable Bowel Syndrome-A Systematic Review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef]
- Sisson, G.; Ayis, S.; Sherwood, R.A.; Bjarnason, I. Randomised Clinical Trial: A Liquid Multi-Strain Probiotic vs. Placebo in the Irritable Bowel Syndrome—A 12 Week Double-Blind Study. Aliment. Pharmacol. Ther. 2014, 40, 51–62. [Google Scholar] [CrossRef]
- Guglielmetti, S.; Mora, D.; Gschwender, M.; Popp, K. Randomised Clinical Trial: Bifidobacterium bifidum MIMBb75 Significantly Alleviates Irritable Bowel Syndrome and Improves Quality of Life—A Double-Blind, Placebo-Controlled Study. Aliment. Pharmacol. Ther. 2011, 33, 1123–1132. [Google Scholar] [CrossRef]
- Yoon, J.S.; Sohn, W.; Lee, O.Y.; Lee, S.P.; Lee, K.N.; Jun, D.W.; Lee, H.L.; Yoon, B.C.; Choi, H.S.; Chung, W.S.; et al. Effect of Multispecies Probiotics on Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Gastroenterol. Hepatol. 2014, 29, 52–59. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From Microbiology to Diagnostics and Prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef]
- Sessenwein, J.L.; Baker, C.C.; Pradhananga, S.; Maitland, M.E.; Petrof, E.O.; Allen-Vercoe, E.; Noordhof, C.; Reed, D.E.; Vanner, S.J.; Lomax, A.E. Protease-Mediated Suppression of DRG Neuron Excitability by Commensal Bacteria. J. Neurosci. 2017, 37, 11758–11768. [Google Scholar] [CrossRef]
- Lomax, A.E.; Pradhananga, S.; Sessenwein, J.L.; O’Malley, D. Bacterial modulation of visceral sensation: Mediators and mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G363–G372. [Google Scholar] [CrossRef] [PubMed]
- Pradhananga, S.; Tashtush, A.A.; Allen-Vercoe, E.; Petrof, E.O.; Lomax, A.E. Protease-dependent excitation of nodose ganglion neurons by commensal gut bacteria. J. Physiol. 2020, 598, 2137–2151. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients with Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef] [PubMed]
- Morreale, C.; Bresesti, I.; Bosi, A.; Baj, A.; Giaroni, C.; Agosti, M.; Salvatore, S. Microbiota and Pain: Save Your Gut Feeling. Cells 2022, 11, 971. [Google Scholar] [CrossRef] [PubMed]
- Mawe, G.M.; Hoffman, J.M. Serotonin Signalling in the Gut-Functions, Dysfunctions and Therapeutic Targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D. Review Article: Serotonin Receptors and Transporters—Roles in Normal and Abnormal Gastrointestinal Motility. Aliment. Pharmacol. Ther. Supplement. 2004, 20, 3–14. [Google Scholar] [CrossRef]
- Costedio, M.M.; Hyman, N.; Mawe, G.M. Serotonin and its role in colonic function and in gastrointestinal disorders. Dis. Colon. Rectum. 2007, 50, 376–388. [Google Scholar] [CrossRef]
- Bistoletti, M.; Bosi, A.; Banfi, D.; Giaroni, C.; Baj, A. The Microbiota-Gut-Brain Axis: Focus on the Fundamental Communication Pathways. Prog. Mol. Biol. Transl. Sci. 2020, 176, 43–110. [Google Scholar]
- Kuwahara, A.; Matsuda, K.; Kuwahara, Y.; Asano, S.; Inui, T.; Marunaka, Y. Microbiota-gut-brain axis: Enteroendocrine cells and the enteric nervous system form an interface between the microbiota and the central nervous system. Biomed. Res. 2020, 41, 199–216. [Google Scholar] [CrossRef]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along the Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20, 1482. [Google Scholar] [CrossRef]
- Bosi, A.; Banfi, D.; Bistoletti, M.; Giaroni, C.; Baj, A. Tryptophan Metabolites Along the Microbiota-Gut-Brain Axis: An Interkingdom Communication System Influencing the Gut in Health and Disease. Int. J. Tryptophan. Res. 2020, 13, 1178646920928984. [Google Scholar] [CrossRef]
- Varela, R.B.; Valvassori, S.S.; Lopes-Borges, J.; Mariot, E.; Dal-Pont, G.C.; Amboni, R.T.; Bianchini, G.; Quevedo, J. Sodium butyrate and Mood Stabilizers Block Ouabain-Induced Hyperlocomotion and Increase BDNF, NGF and GDNF Levels in Brain of Wistar Rats. J. Psychiatr. Res. 2015, 61, 114–121. [Google Scholar] [CrossRef]
- Jornada, L.K.; Moretti, M.; Valvassori, S.S.; Ferreira, C.L.; Padilha, P.T.; Arent, C.O.; Fries, G.R.; Kapczinski, F.; Quevedo, J. Effects of mood stabilizers on hippocampus and amygdala BDNF levels in an animal model of mania induced by ouabain. J. Psychiatr. Res. 2010, 44, 506–510. [Google Scholar] [CrossRef]
- Guo, C.; Huo, Y.J.; Li, Y.; Han, Y.; Zhou, D. Gut-Brain Axis: Focus on Gut Metabolites Short-Chain Fatty Acids. World J. Clin. Cases 2022, 10, 1754–1763. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Sanchez, S.; Rodríguez-Sanoja, R.; Ramos, A.; Demain, A.L. Our Microbes Not Only Produce Antibiotics, They Also Overproduce Amino Acids. J. Antibiot. 2017, 71, 26–36. [Google Scholar] [CrossRef]
- Wendisch, V.F.; Jorge, J.M.P.; Pérez-García, F.; Sgobba, E. Updates on industrial production of amino acids using Corynebacterium glutamicum. World J. Microbiol. Biotechnol. 2016, 32, 105. [Google Scholar] [CrossRef]
- Nakayama, Y.; Hashimoto, K.-i.; Sawada, Y.; Sokabe, M.; Kawasaki, H.; Martinac, B. Corynebacterium glutamicum Mechanosensitive Channels: Towards Unpuzzling “Glutamate Efflux” for Amino Acid Production. Biophys. Rev. 2018, 10, 1359–1369. [Google Scholar] [CrossRef]
- Zareian, M.; Ebrahimpour, A.; Bakar, F.A.; Mohamed, A.K.S.; Forghani, B.; Ab-Kadir, M.S.B.; Saari, M. A Glutamic Acid-Producing Lactic Acid Bacteria Isolated from Malaysian Fermented Foods. Int. J. Mol. Sci. 2012, 13, 5482–5497. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, R.; Bai, T.; Xiang, X.; Qian, W.; Song, J.; Hou, X. EphrinB2/ephB2-Mediated Myenteric Synaptic Plasticity: Mechanisms Underlying the Persistent Muscle Hypercontractility and Pain in Postinfectious IBS. FASEB J. 2019, 33, 13644–13659. [Google Scholar] [CrossRef]
- Neunlist, M.; Michel, K.; Reiche, D.; Dobreva, G.; Huber, K.; Schemann, M. Glycine Activates Myenteric Neurones in Adult Guinea-Pigs. J. Physiol. 2001, 536, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Caputi, V.; Marsilio, I.; Filpa, V.; Cerantola, S.; Orso, G.; Bistoletti, M.; Paccagnella, N.; De Martin, S.; Montopoli, M.; Dall’Acqua, S.; et al. Antibiotic-Induced Dysbiosis of the Microbiota Impairs Gut Neuromuscular Function in Juvenile Mice. Br. J. Pharmacol. 2017, 174, 3623–3639. [Google Scholar] [CrossRef] [PubMed]
- Gronier, B.; Savignac, H.M.; Di Miceli, M.; Idriss, S.M.; Tzortzis, G.; Anthony, D.; Burnet, P.W. Increased Cortical Neuronal Responses to NMDA and Improved Attentional Set-Shifting Performance in Rats following Prebiotic (B-GOS ®) Ingestion. Eur. Neuropsychopharmacol. 2018, 28, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Lin, W.J.; Salton, S.R. Role of a VGF/BDNF/TrkB Autoregulatory Feedback Loop in Rapid-Acting Antidepressant Efficacy. J. Mol. Neurosci. 2019, 68, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse Via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Perez-Berezo, T.; Pujo, J.; Martin, P.; Le Faouder, P.; Galano, J.-M.; Guy, A.; Knauf, C.; Tabet, J.C.; Tronnet, S.; Barreau, F.; et al. Identification of an Analgesic Lipopeptide Produced by the Probiotic Escherichia coli Strain Nissle 1917. Nat. Commun. 2017, 8, 1314. [Google Scholar] [CrossRef]
- May, A. Hints on Diagnosing and Treating Headache. Dtsch. Arztebl. Int. 2018, 115, 299–308. [Google Scholar] [CrossRef]
- Aurora, S.K.; Shrewsbury, S.B.; Ray, S.; Hindiyeh, N.; Nguyen, L. A Link between Gastrointestinal Disorders and Migraine: Insights into the Gut–Brain Connection. Headache J. Head Face Pain 2021, 61, 576–589. [Google Scholar] [CrossRef]
- Dodick, D.W. Migraine. Lancet 2018, 391, 1315–1330. [Google Scholar] [CrossRef]
- Amaral, F.A.; Sachs, D.; Costa, V.V.; Fagundes, C.T.; Cisalpino, D.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Silva, T.A.; Nicoli, J.R.; et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc. Natl. Acad. Sci. USA 2008, 105, 2193–2197. [Google Scholar] [CrossRef]
- Sensenig, J.; Marrongelle, J.; Johnson, M.; Staverosky, T. Permission Treatment of Migraine with Targeted Nutrition Focused on Improved Assimilation and Elimination. Altern. Med. Rev. 2001, 6, 488–494. [Google Scholar]
- De Roos, N.M.; van Hemert, S.; Rovers, J.M.P.; Smits, M.G.; Witteman, B.J.M. The effects of a multispecies probiotic on migraine and markers of intestinal permeability–results of a randomized placebo-controlled study. Eur. J. Clin. Nutr. 2017, 71, 1455–1462. [Google Scholar] [CrossRef]
- Smith, H.S. Opioid Metabolism. Mayo Clin. Proc. 2009, 84, 613–624. [Google Scholar] [CrossRef]
- Pappagallo, M. Incidence, Prevalence, and Management of Opioid Bowel Dysfunction. Am. J. Surg. 2001, 182, S11–S18. [Google Scholar] [CrossRef]
- Acharya, C.; Betrapally, N.S.; Gillevet, P.M.; Sterling, R.K.; Akbarali, H.; White, M.B.; Ganapathy, D.; Fagan, A.; Sikaroodi, M.; Bajaj, J.S. Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment. Pharmacol. Ther. 2017, 45, 319–331. [Google Scholar] [CrossRef]
- Bhave, S.; Gade, A.; Kang, M.; Hauser, K.F.; Dewey, W.L.; Akbarali, H.I. Connexin-Purinergic Signaling in Enteric Glia Mediates the Prolonged Effect of Morphine on Constipation. FASEB J. 2017, 31, 2649–2660. [Google Scholar] [CrossRef]
- Kang, M.; Mischel, R.A.; Bhave, S.; Komla, E.; Cho, A.; Huang, C.; Dewey, W.L.; Akbarali, H.I. The Effect of Gut Microbiome on Tolerance to Morphine Mediated Antinociception in Mice. Sci. Rep. 2017, 7, 42658. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, J.; Ban, Y.; Jalodia, R.; Chupikova, I.; Fernandez, I.; Brito, N.; Sharma, U.; Abreu, M.T.; Ramakrishnan, S.; et al. Morphine Tolerance is Attenuated in Germfree Mice and Reversed by Probiotics, Implicating the Role of Gut Microbiome. Proc. Natl. Acad. Sci. USA 2019, 116, 13523–13532. [Google Scholar] [CrossRef]
- Mischel, R.A.; Dewey, W.L.; Akbarali, H.I. Tolerance to Morphine-Induced Inhibition of TTX-R Sodium Channels in Dorsal Root Ganglia Neurons Is Modulated by Gut-Derived Mediators. iScience 2018, 2, 193–209. [Google Scholar] [CrossRef]
- Williams, N.T. Probiotics. Am. J. Health Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef]
- Arora, T.; Singh, S.; Sharma, R.K. Probiotics: Interaction with gut microbiome and antiobesity potential. Nutrition 2013, 29, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Hart, A.L.; Kamm, M.A.; Stagg, A.J.; Knight, S.C. Mechanisms of action of probiotics: Recent advances. Inflamm. Bowel Dis. 2009, 15, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Gawad, I.A.; El-Sayed, E.M.; Hafez, S.A.; El-Zeini, H.M.; Saleh, F.A. The hypocholesterolaemic effect of milk yoghurt and soy-yoghurt containing bifidobacteria in rats fed on a cholesterol-enriched diet. Int. Dairy J. 2005, 15, 37–44. [Google Scholar] [CrossRef]
- Ohland, C.L.; MacNaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Dai, C.; Jiang, M. Mechanisms of Probiotic VSL#3 in a Rat Model of Visceral Hypersensitivity Involves the Mast Cell-PAR2-TRPV1 Pathway. Dig. Dis. Sci. 2019, 64, 1182–1192. [Google Scholar] [CrossRef]
- Zhao, K.; Yu, L.; Wang, X.; He, Y.; Lu, B. Clostridium butyricum regulates visceral hypersensitivity of irritable bowel syndrome by inhibiting colonic mucous low grade inflammation through its action on NLRP6. Acta Biochim. Biophys. Sin. 2018, 50, 216–223. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Wang, Y.; Liu, C.; Zhang, L.; Zhu, S.; Liu, S.; Duan, L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J. Gastroenterol. Hepatol. 2019, 34, 1368–1376. [Google Scholar] [CrossRef]
- Mckernan, D.P.; Fitzgerald, P.; Dinan, T.G.; Cryan, J.F. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol. Motil. 2010, 22, 1029-e268. [Google Scholar] [CrossRef]
- Weizman, Z.; Abu-Abed, J.; Binsztok, M. Lactobacillus reuteri DSM 17938 for the Management of Functional Abdominal Pain in Childhood: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Pediatr. 2016, 174, 160–164.e1. [Google Scholar] [CrossRef]
- Giannetti, E.; Maglione, M.; Alessandrella, A.; Strisciuglio, C.; De Giovanni, D.; Campanozzi, A.; Miele, E.; Staiano, A. A mixture of 3 bifidobacteria decreases abdominal pain and improves the quality of life in children with irritable bowel syndrome. J. Clin. Gastroenterol. 2017, 51, e5–e10. [Google Scholar] [CrossRef]
- Newlove-Delgado, T.; Abbott, R.A.; Martin, A.E. Probiotics for Children with Recurrent Abdominal Pain. JAMA Pediatr. 2019, 173, 183–184. [Google Scholar] [CrossRef]
- Spiller, R.; Pélerin, F.; Decherf, A.C.; Maudet, C.; Housez, B.; Cazaubiel, M.; Jüsten, P. Randomized double blind placebo-controlled trial of Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: Improvement in abdominal pain and bloating in those with predominant constipation. United Eur. Gastroenterol. J. 2015, 4, 353–362. [Google Scholar] [CrossRef]
- Kannampalli, P.; Pochiraju, S.; Chichlowski, M.; Berg, B.M.; Rudolph, C.; Bruckert, M.; Miranda, A.; Sengupta, J.N. Probiotic Lactobacillus rhamnosus GG (LGG) and prebiotic prevent neonatal inflammation-induced visceral hypersensitivity in adult rats. Neurogastroenterol. Motil. 2014, 26, 1694–1704. [Google Scholar] [CrossRef]
- Vulevic, J.; Tzortzis, G.; Juric, A.; Gibson, G.R. Effect of a prebiotic galactooligosaccharide mixture (B-GOS®) on gastrointestinal symptoms in adults selected from a general population who suffer with bloating, abdominal pain, or flatulence. Neurogastroenterol. Motil. 2018, 30, e13440. [Google Scholar] [CrossRef]
- Saneian, H.; Pourmoghaddas, Z.; Roohafza, H.; Gholamrezaei, A. Synbiotic containing Bacillus coagulans and fructo-oligosaccharides for functional abdominal pain in children. Gastroenterol. Hepatol. Bed Bench 2015, 8, 56–65. [Google Scholar]
- Zhou, S.-Y.; Gillilland, M.; Wu, X.; Leelasinjaroen, P.; Zhang, G.; Zhou, H.; Ye, B.; Lu, Y.; Owyang, C. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J. Clin. Investig. 2018, 128, 267–280. [Google Scholar] [CrossRef]
- Harvie, R. A Reduction in FODMAP Intake Correlates Strongly with a Reduction in IBS Symptoms—The FIBS Study. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2014. [Google Scholar]
- Pedersen, N.; Andersen, N.N.; Végh, Z.; Jensen, L.; Ankersen, D.V.; Felding, M.; Simonsen, M.H.; Burisch, J.; Munkholm, P. Ehealth: Low FODMAP diet vs Lactobacillus rhamnosus GG in irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 16215. [Google Scholar] [CrossRef]
- Hustoft, T.N.; Hausken, T.; Ystad, S.O.; Valeur, J.; Brokstad, K.; Hatlebakk, J.G.; Lied, G.A. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2017, 29, e12969. [Google Scholar] [CrossRef]
- Nanayakkara, W.S.; Skidmore, P.M.; O’Brien, L.; Wilkinson, T.J.; Gearry, R.B. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: The evidence to date. Clin. Exp. Gastroenterol. 2016, 9, 131–142. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Bonfiglio, F.; Belheouane, M.; Vallier, M.; Sauer, S.; Bang, C.; Bujanda, L.; Andreasson, A.; Agreus, L.; Engstrand, L.; et al. Faecal microbiota composition associates with abdominal pain in the general population. Gut 2018, 67, 778–779. [Google Scholar] [CrossRef]
- Lucarini, E.; Di Pilato, V.; Parisio, C.; Micheli, L.; Toti, A.; Pacini, A.; Bartolucci, G.; Baldi, S.; Niccolai, E.; Amedei, A.; et al. Visceral sensitivity modulation by faecal microbiota transplantation: The active role of gut bacteria in pain persistence. Pain 2022, 163, 861–877. [Google Scholar] [CrossRef] [PubMed]
- Khoruts, A.; Sadowsky, M.J. Understanding the mechanisms of faecal microbiota transplantation. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Aguliar, R.M.; Wantia, N.; Clavel, T.; Vehreschild, M.J.G.T.; Buch, T.; Bajbouj, M.; Haller, D.; Busch, D.; Schmid, R.M.; Stein-Thoeringer, C.K. An Open-Labeled Study on Fecal Microbiota Transfer in Irritable Bowel Syndrome Patients Reveals Improvement in Abdominal Pain Associated with the Relative Abundance of Akkermansia Muciniphila. Digestion 2019, 100, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Holster, S.; Lindqvist, C.M.; Repsilber, D.; Salonen, A.; de Vos, W.M.; König, J.; Brummer, R.J. The Effect of Allogenic Versus Autologous Fecal Microbiota Transfer on Symptoms, Visceral Perception and Fecal and Mucosal Microbiota in Irritable Bowel Syndrome: A Randomized Controlled Study. Clin. Transl. Gastroenterol. 2019, 10, e00034. [Google Scholar] [CrossRef]
- Goll, R.; Johnsen, P.H.; Hjerde, E.; Diab, J.; Valle, P.C.; Hilpusch, F.; Cavanagh, J.P. Effects of fecal microbiota transplantation in subjects with irritable bowel syndrome are mirrored by changes in gut microbiome. Gut Microbes 2020, 12, 1794263. [Google Scholar] [CrossRef]
- Borody, T.J.; Khoruts, A. Fecal microbiota transplantation and emerging applications. Nat. Rev. Gastroenterol. Hepatol. 2011, 20, 88–96. [Google Scholar] [CrossRef]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; De Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.W.M.; Tijssen, J.G.P.; et al. Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef]
- Gupta, S.; Allen-Vercoe, E.; Petrof, E.O. Fecal microbiota transplantation: In perspective. Therap. Adv. Gastroenterol. 2016, 9, 229–239. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).