Ouabain-Induced Changes in the Expression of Voltage-Gated Potassium Channels in Epithelial Cells Depend on Cell–Cell Contacts
Abstract
1. Introduction
2. Results
2.1. Biophysical Properties of the Endogenous Potassium Currents of MDCK Cells in Mature Epithelial Monolayers
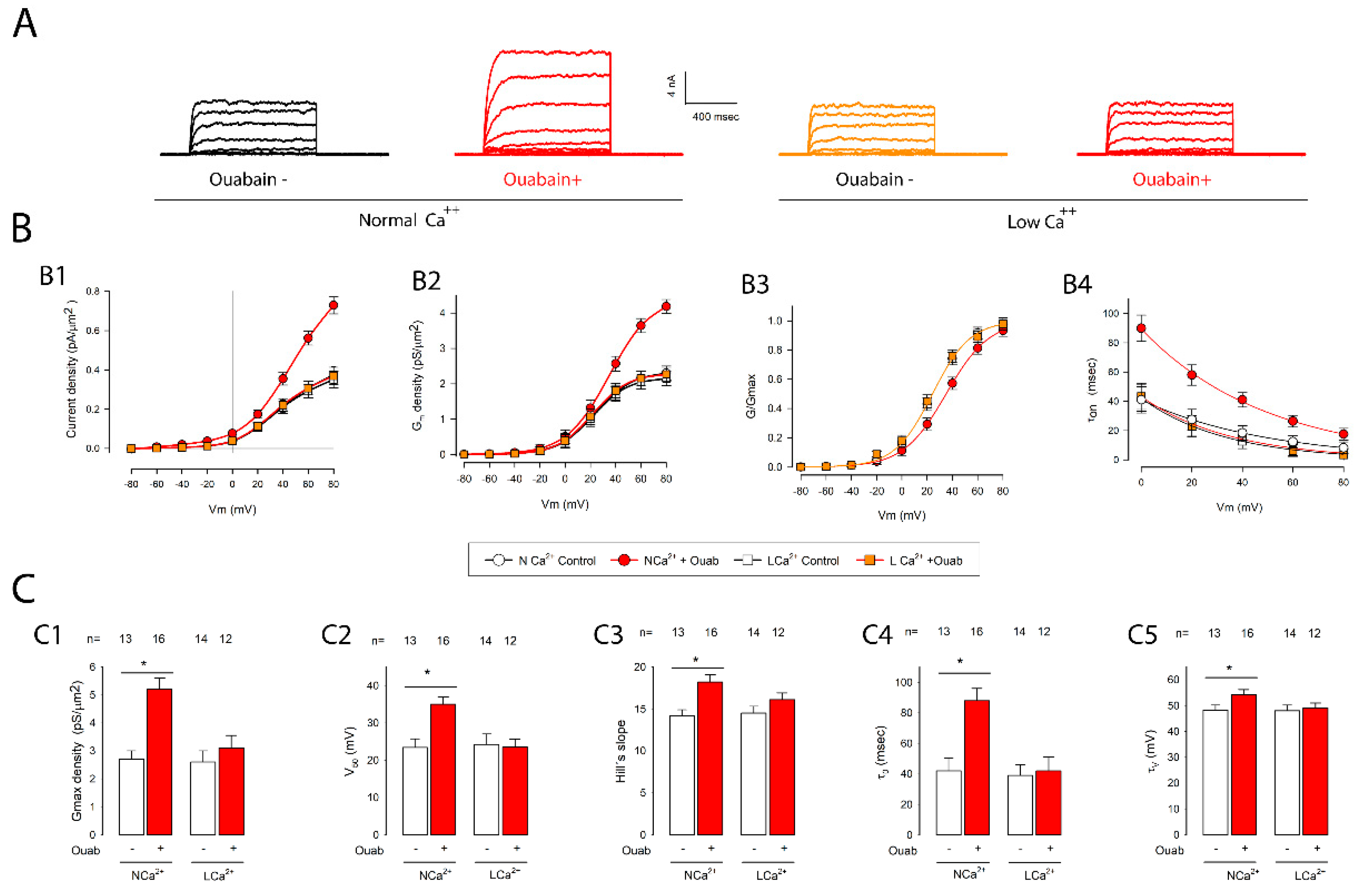
2.2. Ouabain Induced Statistically Significant Changes in the Biophysical Properties of Potassium Currents in Cells Assembled in Mature Epithelial Monolayers (MEMs)
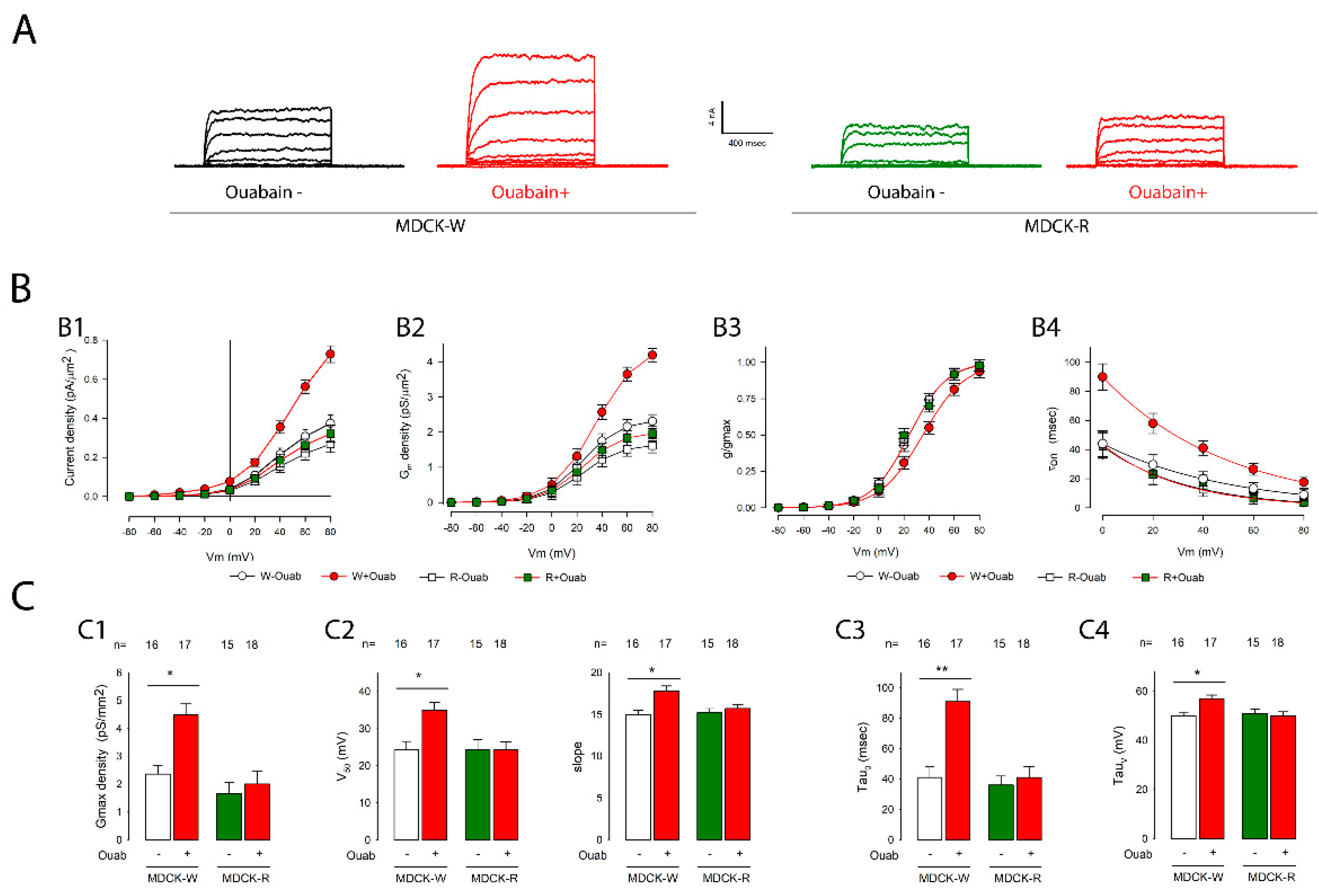
2.3. Ouabain Accelerated the Recovery of Voltage-Gated K+ Channels Lost during Trypsinization When Cells Were Seeded at Confluency
2.4. Ouabain Did Not Produce Changes to Potassium Currents in Subconfluent Cells
2.5. Removal of Extracellular Ca2+ Abolished the Changes Induced by Ouabain on Potassium Currents in Cells in MEMs
2.6. Na+/K+-ATPase Is the Receptor Mediating the Action of Ouabain on Potassium Currents
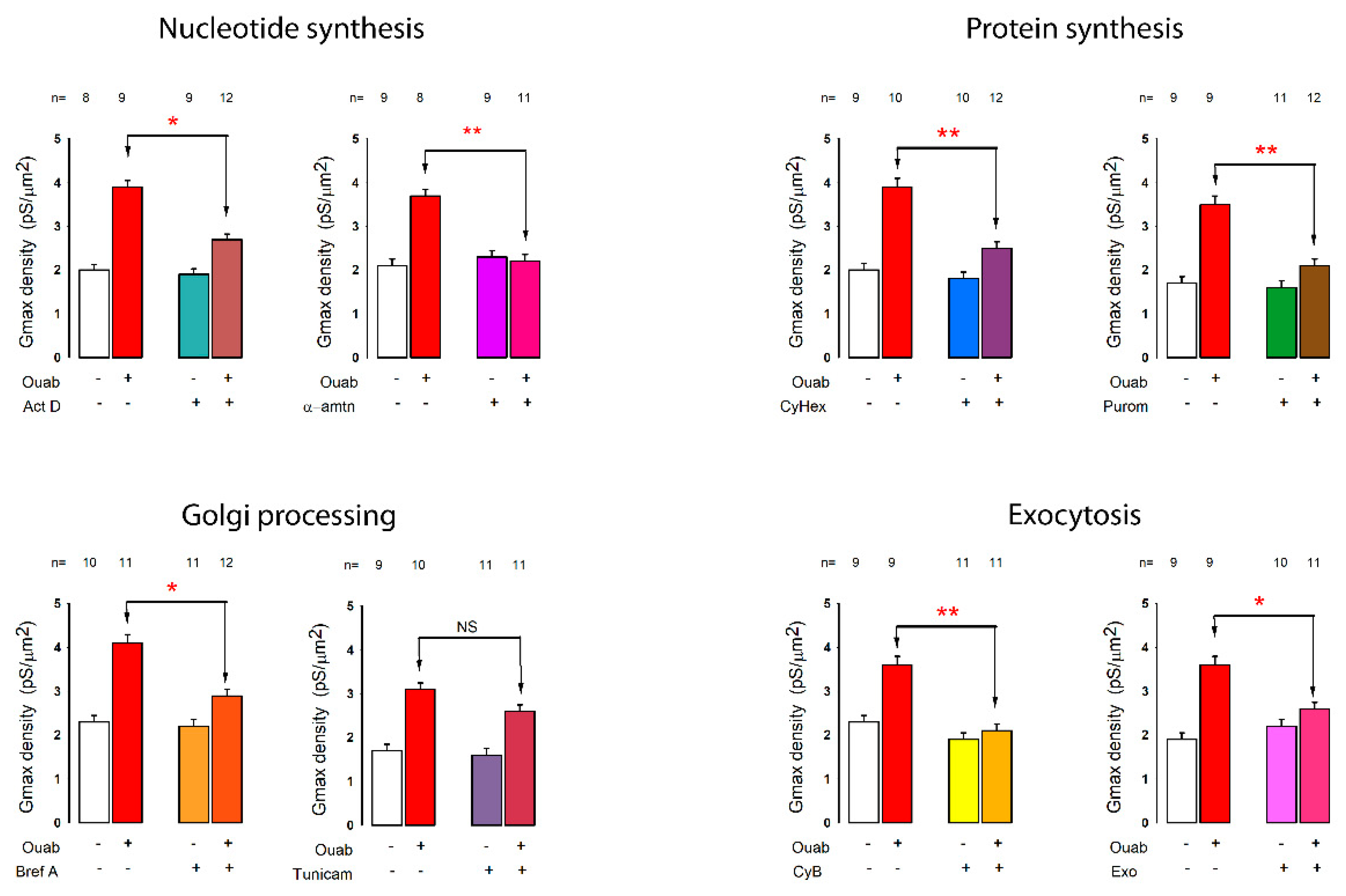
2.7. Ouabain Stimulated the Synthesis, Assembly and Exportation of New Ion Channel Units
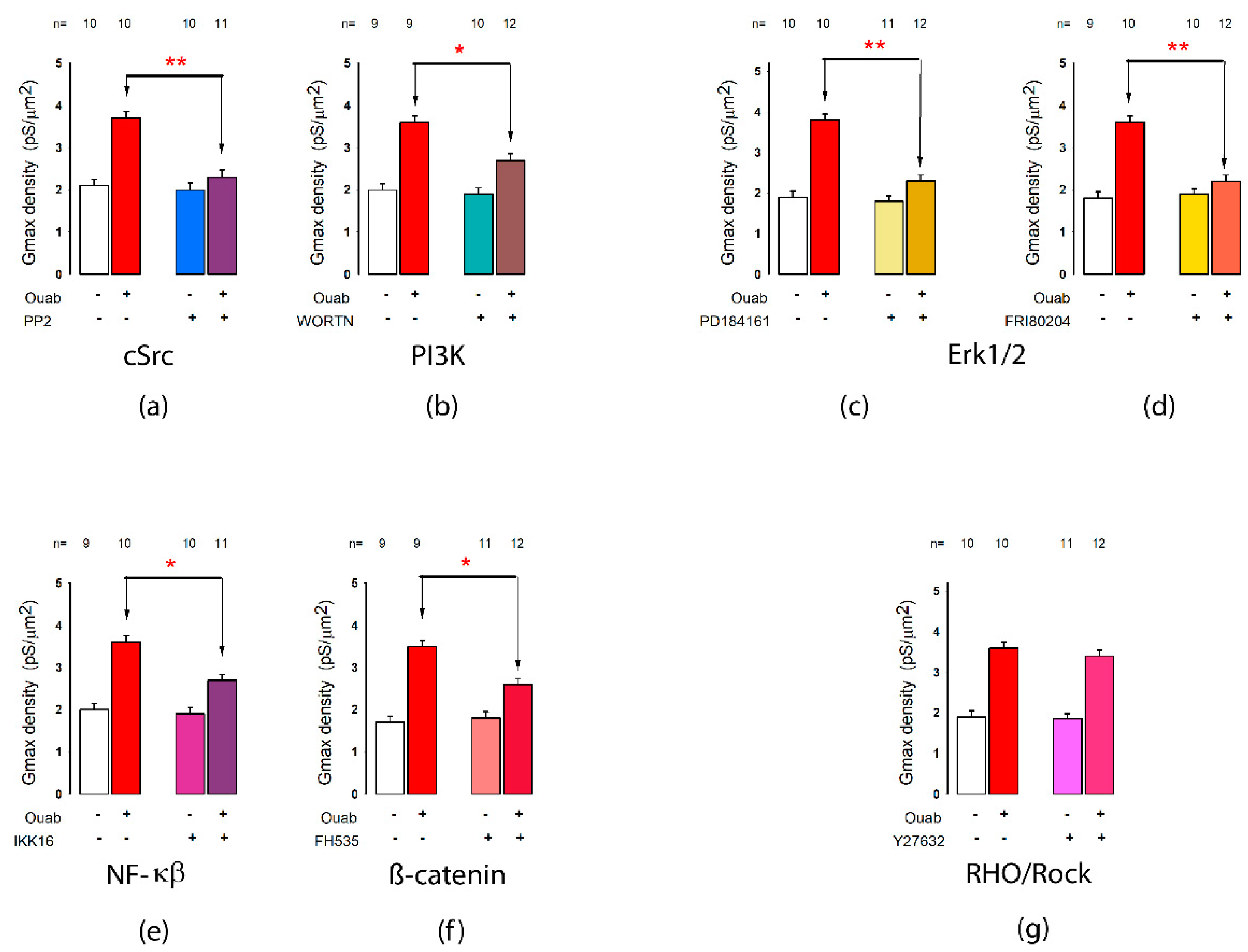
2.8. Signal Transduction Pathways Involved in the Action of Ouabain on Potassium Currents
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Electrophysiological Recording of Cells
4.3. Measurement of Membrane Capacitance
4.4. Solutions
4.5. Chemicals and Drugs
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hollman, A. Plants and cardiac glycosides. Br. Heart J. 1985, 54, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Bejček, J.; Jurášek, M.; Spiwok, V.; Rimpelová, S. Quo vadis Cardiac Glycoside Research? Toxins 2021, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Botelho, A.F.M.; Pierezan, F.; Soto-Blanco, B.; Melo, M.M. A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon 2018, 158, 63–68. [Google Scholar] [CrossRef] [PubMed]
- García-Ramos, J.; Peralta, B. Las acciones de la digital y de la ouabaína sobre algunas propiedades del músculo cardíaco [The actions of digitalis and ouabain on some properties of the cardiac muscle]. Arch. Inst. Cardiol. Mex. 1946, 16, 93–108. (In Spanish) [Google Scholar] [PubMed]
- Patel, S. Plant-derived cardiac glycosides: Role in heart ailments and cancer management. Biomed. Pharmacother. 2016, 84, 1036–1041. [Google Scholar] [CrossRef]
- Brody, T.M. Ouabain-induced inhibition of cardiac (Na+ + K+)-Atpase and the positive inotropic response. Ann. N. Y. Acad. Sci. 1974, 242, 684–687. [Google Scholar] [CrossRef]
- Blaustein, M.P. The pump, the exchanger, and the holy spirit: Origins and 40-year evolution of ideas about the ouabain-Na+ pump endocrine system. Am. J. Physiol. Physiol. 2018, 314, C3–C26. [Google Scholar] [CrossRef]
- Haustein, K.O. Therapeutic range of cardiac glycosides. In Cardiac Glycoside Receptors and Positive Inotropy; Erdmann, E., Ed.; Steinkopff: Heidelberg, Germany, 1984. [Google Scholar]
- Fishman, M.C. Endogenous digitalis-like activity in mammalian brain. Proc. Natl. Acad. Sci. USA 1979, 76, 4661–4663. [Google Scholar] [CrossRef]
- Lichtstein, D.; Samuelov, S. Endogenous ‘ouabain like’ activity in rat brain. Biochem. Biophys. Res. Commun. 1980, 96, 1518–1523. [Google Scholar] [CrossRef]
- Bagrov, A.Y.; Shapiro, J.I.; Fedorova, O.V. Endogenous Cardiotonic Steroids: Physiology, Pharmacology, and Novel Therapeutic Targets. Pharmacol. Rev. 2009, 61, 9–38. [Google Scholar] [CrossRef]
- Schoner, W.; Bauer, N.; Müller-Ehmsen, J.; Krämer, U.; Hambarchian, N.; Schwinger, R.; Moeller, H.; Kost, H.; Weitkamp, C.; Schweitzer, T.; et al. Ouabain as a Mammalian Hormone. Ann. N. Y. Acad. Sci. 2003, 986, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Hamlyn, J.M. Natriuretic Hormones, Endogenous Ouabain, and Related Sodium Transport Inhibitors. Front. Endocrinol. 2014, 5, 199. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.C.; Schwartz, A. Na+, K+-ATPase, the transport enzyme: Evidence for its proposed role as pharmacologic receptor for cardiac glycosides. Ann. N. Y. Acad. Sci. 1974, 242, 646–656. [Google Scholar] [CrossRef]
- Blaustein, M.P.; Hamlyn, J.M. Ouabain, endogenous ouabain and ouabain-like factors: The Na+ pump/ouabain receptor, its linkage to NCX, and its myriad functions. Cell Calcium 2020, 86, 102159. [Google Scholar] [CrossRef] [PubMed]
- Askari, A. The other functions of the sodium pump. Cell Calcium 2019, 84, 102105. [Google Scholar] [CrossRef] [PubMed]
- Cereijido, M.; Robbins, E.S.; Dolan, W.J.; A Rotunno, C.; Sabatini, D.D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J. Cell Biol. 1978, 77, 853–880. [Google Scholar] [CrossRef] [PubMed]
- Cereijido, M.; Stefani, E.; Palomo, A.M. Occluding junctions in a cultured transporting epithelium: Structural and functional heterogeneity. J. Membr. Biol. 1980, 53, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Simmons, N. Cultured monolayers of MDCK cells: A novel model system for the study of epithelial development and function. Gen. Pharmacol. Vasc. Syst. 1982, 13, 287–291. [Google Scholar] [CrossRef]
- Simmons, N.L. Ion transport in ‘tight’ epithelial monolayers of MDCK cells. J. Membr. Biol. 1981, 59, 105–114. [Google Scholar] [CrossRef]
- Larre, I.; Lazaro, A.; Contreras, R.G.; Balda, M.S.; Matter, K.; Flores-Maldonado, C.; Ponce, A.; Flores-Benitez, D.; Rincon-Heredia, R.; Padilla-Benavides, T.; et al. Ouabain modulates epithelial cell tight junction. Proc. Natl. Acad. Sci. USA 2010, 107, 11387–11392. [Google Scholar] [CrossRef]
- Castillo, A.; Ortuño-Pineda, C.; Flores-Maldonado, C.; Larre, I.; Rendón, J.M.; Hinojosa, L.; Ponce, A.; Ogazón, A.; Serrano, M.; Valdes, J.; et al. Ouabain Modulates the Adherens Junction in Renal Epithelial Cells. Cell. Physiol. Biochem. 2019, 52, 1381–1397. [Google Scholar] [PubMed]
- Larre, I.; Ponce, A.; Fiorentino, R.; Shoshani, L.; Contreras, R.G.; Cereijido, M. Contacts and cooperation between cells depend on the hormone ouabain. Proc. Natl. Acad. Sci. USA 2006, 103, 10911–10916. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.; Larre, I.; Castillo, A.; Garcia-Villegas, R.; Romero, A.; Flores-Maldonado, C.; Martinez-Rendón, J.; Contreras, R.G.; Cereijido, M. Ouabain Increases Gap Junctional Communication in Epithelial Cells. Cell. Physiol. Biochem. 2014, 34, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.; Larre, I.; Castillo, A.; Flores-Maldonado, C.; Verdejo-Torres, O.; Contreras, R.G.; Cereijido, M. Ouabain Modulates the Distribution of Connexin 43 in Epithelial Cells. Cell. Physiol. Biochem. 2016, 39, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Larre, I.; Castillo, A.; Flores-Maldonado, C.; Contreras, R.G.; Galvan, I.; Muñoz-Estrada, J.; Cereijido, M. Ouabain modulates ciliogenesis in epithelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 20591–20596. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L. Ion Channels in Biophysics and Physiology, 1st ed.; Springer Nature: Singapore, 2021; pp. 154–196. [Google Scholar]
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2001; pp. 154–196. [Google Scholar]
- Cereijido, M. Electrical properties of Madin-Darby canine kidney cells. Fed. Proc. 1984, 43, 2230–2235. [Google Scholar]
- Stefani, E.; Cereijido, M. Electrical properties of cultured epithelioid cells (MDCK). J. Membr. Biol. 1983, 73, 177–184. [Google Scholar] [CrossRef]
- Lang, F.; Paulmichl, M. Properties and regulation of ion channels in MDCK cells. Kidney Int. 1995, 48, 1200–1205. [Google Scholar] [CrossRef][Green Version]
- Aiton, J.F.; Brown, C.D.A.; Ogden, P.; Simmons, N.L. K+ transport in ‘tight’epithelial monolayers of MDCK cells. J. Membr. Biol. 1982, 65, 99–109. [Google Scholar] [CrossRef]
- Brown, C.D.A.; Simmons, N.L. K+ transport in ‘tight’epithelial monolayers of MDCK cells: Evidence for a calcium-activated K+ channel. Biochim. Biophys. Acta (BBA)-Biomembr. 1982, 690, 95–105. [Google Scholar] [CrossRef]
- Friedrich, F.; Weiss, H.; Paulmichl, M.; Lang, F. Activation of potassium channels in renal epithelioid cells (MDCK) by extracellular ATP. Am. J. Physiol.-Cell Physiol. 1989, 256, C1016–C1021. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.; Lang, F. Ion channels activated by swelling of Madin Darby Canine Kidney (MDCK) cells. J. Membr. Biol. 1992, 126, 109–114. [Google Scholar] [CrossRef]
- Ponce, A.; Cereijido, M. Polarized Distribution of Cation Channels in Epithelial Cells. Cell. Physiol. Biochem. 1991, 1, 13–23. [Google Scholar] [CrossRef]
- Ponce, A.; Contreras, R.; Cereijido, M. Polarized Distribution of Chloride Channels in Epithelial Cells. Cell. Physiol. Biochem. 1991, 1, 160–169. [Google Scholar] [CrossRef]
- Ponce, A.; Bolívar, J.; Vega, J.; Cereijido, M. Synthesis of Plasma Membrane and Potassium Channels in Epithelial (MDCK) Cells. Cell. Physiol. Biochem. 1991, 1, 195–204. [Google Scholar] [CrossRef]
- Talavera, D.; Ponce, A.; Fiorentino, R.; Contreras, R.G.; Cereijido, M. Expression of potassium channels in epithelial cells depends on calcium-activated cell-cell contacts. J. Membr. Biol. 1995, 143, 219–226. [Google Scholar] [CrossRef]
- Cereijido, M.; Talavera, D.; Ponce, A.; Vega, J.; García-Villegas, M.R.; Valdés, J. El papel de los contactos celulares en la recuperación de canales iónicos de membranas de células epiteliales [The role of cellular contacts in the recovery of the ion channels of epithelial cell membranes]. Gac. Med. Mex. 1997, 133, 121–126. (In Spanish) [Google Scholar]
- Martinez-Palomo, A.; Meza, I.; Beaty, G.; Cereijido, M. Experimental modulation of occluding junctions in a cultured transporting epithelium. J. Cell Biol. 1980, 87, 736–745. [Google Scholar] [CrossRef]
- Meza, I.; Ibarra, G.; Sabanero, M.; Martinez-Palomo, A.; Cereijido, M. Occluding junctions and cytoskeletal components in a cultured transporting epithelium. J. Cell Biol. 1980, 87, 746–754. [Google Scholar] [CrossRef]
- Del Toro, A.O.; Jimenez, L.; Hinojosa, L.; Martínez-Rendón, J.; Castillo, A.; Cereijido, M.; Ponce, A. Influence of Endogenous Cardiac Glycosides, Digoxin, and Marinobufagenin in the Physiology of Epithelial Cells. Cardiol. Res. Pract. 2019, 2019, 8646787. [Google Scholar] [CrossRef]
- Soderberg, K.; Rossi, B.; Lazdunski, M.; Louvard, D. Characterization of ouabain-resistant mutants of a canine kidney cell line, MDCK. J. Biol. Chem. 1983, 258, 12300–12307. [Google Scholar] [CrossRef]
- Paramanathan, T.; Vladescu, I.; McCauley, M.J.; Rouzina, I.; Williams, M.C. Force spectroscopy reveals the DNA structural dynamics that govern the slow binding of Actinomycin D. Nucleic Acids Res. 2012, 40, 4925–4932. [Google Scholar] [CrossRef] [PubMed]
- Sobell, H.M. Actinomycin and DNA transcription. Proc. Natl. Acad. Sci. USA 1985, 82, 5328–5331. [Google Scholar] [CrossRef] [PubMed]
- Choong, M.L.; Yang, H.; Lee, M.A.; Lane, D.P. Specific activation of the p53 pathway by low dose actinomycin D: A new route to p53 based cyclotherapy. Cell Cycle 2009, 8, 2810–2818. [Google Scholar] [CrossRef]
- Hallen, H.E.; Luo, H.; Scott-Craig, J.S.; Walton, J.D. Gene family encoding the major toxins of lethal Amanita mushrooms. Proc. Natl. Acad. Sci. USA 2007, 104, 19097–19101. [Google Scholar] [CrossRef]
- Gu, W.; Powell, W.H.; Mote, J.; Reines, D. Nascent RNA cleavage by arrested RNA polymerase II does not require upstream translocation of the elongation complex on DNA. J. Biol. Chem. 1993, 268, 25604–25616. [Google Scholar] [CrossRef]
- Obrig, T.G.; Culp, W.J.; McKeehan, W.L.; Hardesty, B. The Mechanism by which Cycloheximide and Related Glutarimide Antibiotics Inhibit Peptide Synthesis on Reticulocyte Ribosomes. J. Biol. Chem. 1971, 246, 174–181. [Google Scholar] [CrossRef]
- Baskić, D.; Popović, S.; Ristić, P.; Arsenijević, N.N. Analysis of cycloheximide-induced apoptosis in human leukocytes: Fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol. Int. 2006, 30, 924–932. [Google Scholar] [CrossRef]
- Lee, S.; Liu, B.; Lee, S.; Huang, S.X.; Shen, B.; Qian, S.B. Global mapping of translation initiation sites in mammalian cells at single-nucleotide resolution. Proc. Natl. Acad. Sci. USA 2012, 109, E2424–E2432. [Google Scholar] [CrossRef]
- Azzam, M.E.; Algranati, I.D. Mechanism of Puromycin Action: Fate of Ribosomes after Release of Nascent Protein Chains from Polysomes. Proc. Natl. Acad. Sci. USA 1973, 70, 3866–3869. [Google Scholar] [CrossRef]
- Rodriguez-Fonseca, C.R.; Phan, H.; Long, K.S.; Porse, B.T.; Kirillov, S.V.; Amils, R.; Garrett, R.A. Puromycin-rRNA interaction sites at the peptidyl transferase center. RNA 2000, 6, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Lührmann, R.; Bald, R.; Stöffler-Meilicke, M.; Stöffler, G. Localization of the puromycin binding site on the large ribosomal subunit of Escherichia coli by immunoelectron microscopy. Proc. Natl. Acad. Sci. USA 1981, 78, 7276–7280. [Google Scholar] [CrossRef]
- Fujiwara, T.; Oda, K.; Yokota, S.; Takatsuki, A.; Ikehara, Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J. Biol. Chem. 1988, 263, 18545–18552. [Google Scholar] [CrossRef]
- Ayala, J. Transport and internal organization of membranes: Vesicles, membrane networks and GTP-binding proteins. J. Cell Sci. 1994, 107, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Thyberg, J.; Moskalewski, S. Role of Microtubules in the Organization of the Golgi Complex. Exp. Cell Res. 1999, 246, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Takatsuki, A.; Arima, K.; Tamura, G. Tunicamycin, a new antibiotic. I. Isolation and characterization of tunicamycin. J. Antibiot. 1971, 24, 215–223. [Google Scholar] [CrossRef]
- Price, N.P.; Hartman, T.M.; Li, J.; Velpula, K.K.; A Naumann, T.; Guda, M.R.; Yu, B.; Bischoff, K.M. Modified tunicamycins with reduced eukaryotic toxicity that enhance the antibacterial activity of β-lactams. J. Antibiot. 2017, 70, 1070–1077. [Google Scholar] [CrossRef]
- Tkacz, J.S.; Lampen, O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem. Biophys. Res. Commun. 1975, 65, 248–257. [Google Scholar] [CrossRef]
- Berger, E.G.; Buddecke, E.; Kamerling, J.P.; Kobata, A.; Paulson, J.C.; Vliegenthart, J.F.G. Structure, biosynthesis and functions of glycoprotein glycans. Experientia 1982, 38, 1129–1162. [Google Scholar] [CrossRef][Green Version]
- Theodoropoulos, P.A.; Gravanis, A.; Tsapara, A.; Margioris, A.N.; Papadogiorgaki, E.; Galanopoulos, V.; Stournaras, C. Cytochalasin B may shorten actin filaments by a mechanism independent of barbed end capping. Biochem. Pharmacol. 1994, 47, 1875–1881. [Google Scholar] [CrossRef]
- Steinberg, M.S.; Wiseman, L.L. Do morphogenetic tissue rearrangements require active cell movements? The reversible inhibition of cell sorting and tissue spreading by cytochalasin B. J. Cell Biol. 1972, 55, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yu, S.; Lasell, T.K.R.; Jadhav, A.P.; Macia, E.; Chardin, P.; Melancon, P.; Roth, M.; Mitchison, T.; Kirchhausen, T. Exo1: A new chemical inhibitor of the exocytic pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 6469–6474. [Google Scholar] [CrossRef] [PubMed]
- Hanke, J.H.; Gardner, J.P.; Dow, R.L.; Changelian, P.S.; Brissette, W.H.; Weringer, E.J.; Pollok, B.A.; Connelly, P.A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 1996, 271, 695–701. [Google Scholar] [CrossRef]
- Barwe, S.P.; Anilkumar, G.; Moon, S.Y.; Zheng, Y.; Whitelegge, J.P.; Rajasekaran, S.A.; Rajasekaran, A.K. Novel Role for Na,K-ATPase in Phosphatidylinositol 3-Kinase Signaling and Suppression of Cell Motility. Mol. Biol. Cell 2005, 16, 1082–1094. [Google Scholar] [CrossRef]
- Vilchis-Nestor, C.A.; Roldán, M.L.; Leonardi, A.; Navea, J.G.; Padilla-Benavides, T.; Shoshani, L. Ouabain Enhances Cell-Cell Adhesion Mediated by β1 Subunits of the Na+,K+-ATPase in CHO Fibroblasts. Int. J. Mol. Sci. 2019, 20, 2111. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Meyers, R.E.; Cantley, L.C. Phosphoinositide kinases. Ann. Rev. Biochem. 2008, 67, 481–507. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shreder, K.R.; Gai, W.; Corral, S.; Ferris, D.K.; Rosenblum, J. Wortmannin, a Widely Used Phosphoinositide 3-Kinase Inhibitor, also Potently Inhibits Mammalian Polo-like Kinase. Chem. Biol. 2005, 12, 99–107. [Google Scholar] [CrossRef]
- Klein, P.J.; Schmidt, C.M.; Wiesenauer, C.A.; Choi, J.N.; Gage, E.A.; Yip-Schneider, M.T.; Wiebke, E.A.; Wang, Y.; Omer, C.; Sebolt-Leopold, J.S. The Effects of a Novel MEK Inhibitor PD184161 on MEK-ERK Signaling and Growth in Human Liver Cancer. Neoplasia 2006, 8, 1–8. [Google Scholar] [CrossRef]
- Ohori, M.; Kinoshita, T.; Okubo, M.; Sato, K.; Yamazaki, A.; Arakawa, H.; Nishimura, S.; Inamura, N.; Nakajima, H.; Neya, M.; et al. Identification of a selective ERK inhibitor and structural determination of the inhibitor-ERK2 complex. Biochem. Biophys. Res. Commun. 2005, 336, 357–363. [Google Scholar] [CrossRef]
- Orellana, A.M.; Leite, J.A.; Kinoshita, P.F.; Vasconcelos, A.R.; Andreotti, D.Z.; Lima, L.D.S.; Xavier, G.F.; Kawamoto, E.M.; Scavone, C. Ouabain increases neuronal branching in hippocampus and improves spatial memory. Neuropharmacology 2018, 140, 260–274. [Google Scholar] [CrossRef]
- Kawamoto, E.; Lima, L.; Munhoz, C.; Yshii, L.; Kinoshita, P.; Amara, F.; Pestana, R.; Orellana, A.; Cipolla-Neto, J.; Britto, L.; et al. Influence of N-methyl-D-aspartate receptors on ouabain activation of nuclear factor-κB in the rat hippocampus. J. Neurosci. Res. 2011, 90, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Waelchli, R.; Bollbuck, B.; Bruns, C.; Buhl, T.; Eder, J.; Feifel, R.; Hersperger, R.; Janser, P.; Revesz, L.; Zerwes, H.-G.; et al. Design and preparation of 2-benzamido-pyrimidines as inhibitors of IKK. Bioorganic Med. Chem. Lett. 2005, 16, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Coldewey, S.M.; Rogazzo, M.; Collino, M.; Patel, N.S.; Thiemermann, C. Inhibition of I k B kinase reduces the multiple organ dysfunction caused by sepsis in the mouse. Dis. Model. Mech. 2013, 6, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Thein, S.; Pham, A.; Bayer, K.U.; Tao-Cheng, J.H.; Dosemeci, A. IKK regulates the deubiquitinase CYLD at the postsynaptic density. Biochem. Biophys. Res. Commun. 2014, 450, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Handeli, S.; Simon, J.A. A small-molecule inhibitor of Tcf/β-catenin signaling down-regulates PPARγ and PPARδ activities. Mol. Cancer Ther. 2008, 7, 521–529. [Google Scholar] [CrossRef]
- Faisy, C.; Grassin-Delyle, S.; Blouquit-Laye, S.; Brollo, M.; Naline, E.; Chapelier, A.; Devillier, P. Wnt/β-catenin signaling modulates human airway sensitization induced by β2-adrenoceptor stimulation. PLoS ONE 2014, 9, e111350. [Google Scholar] [CrossRef]
- Davies, S.P.; Reddy, H.; Caivano, M.; Cohen, P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000, 351, 95–105. [Google Scholar] [CrossRef]
- Uehata, M.; Ishizaki, T.; Satoh, H.; Ono, T.; Kawahara, T.; Morishita, T.; Tamakawa, H.; Yamagami, K.; Inui, J.; Maekawa, M.; et al. Calcium sensitization of smooth muscle mediated by a Rho-assocciated protein kinase in hypertension. Nature 1997, 389, 990–994. [Google Scholar] [CrossRef]
- Lingrel, J.B. The Physiological Significance of the Cardiotonic Steroid/Ouabain-Binding Site of the Na,K-ATPase. Annu. Rev. Physiol. 2010, 72, 395–412. [Google Scholar] [CrossRef]
- Casteels, R. The action of ouabain on the smooth muscle cells of the guinea-pig’s taenia coli. J. Physiol. 1966, 184, 131–142. [Google Scholar] [CrossRef]
- Arnon, A.; Hamlyn, J.M.; Blaustein, M.P. Ouabain augments Ca2+ transients in arterial smooth muscle without raising cytosolic Na+. Am. J. Physiol. Circ. Physiol. 2000, 279, H679–H691. [Google Scholar] [CrossRef] [PubMed]
- Charlemagne, D. Molecular and cellular level of action of digitalis. Herz 1993, 18, 79–85. [Google Scholar] [PubMed]
- Smith, T.W. The basic mechanism of inotropic action of digitalis glycosides. J. Pharmacol. 1984, 15 (Suppl. S1), 35–51. [Google Scholar] [PubMed]
- A Langer, G. Relationship between myocardial contractility and the effects of digitalis on ionic exchange. Fed. Proc. 1977, 36, 2231–2334. [Google Scholar]
- Chase, H.S. Does calcium couple the apical and basolateral membrane permeabilities in epithelia? Am. J. Physiol.-Ren. Physiol. 1984, 247, F869–F876. [Google Scholar] [CrossRef]
- Simonini, M.; Casanova, P.; Citterio, L.; Messaggio, E.; Lanzani, C.; Manunta, P. Endogenous Ouabain and Related Genes in the Translation from Hypertension to Renal Diseases. Int. J. Mol. Sci. 2018, 19, 1948. [Google Scholar] [CrossRef]
- Hamlyn, J.M.; Blaustein, M.P. Endogenous Ouabain. Hypertension 2016, 68, 526–532. [Google Scholar] [CrossRef]
- Hamlyn, J.M.; Manunta, P. Endogenous Cardiotonic Steroids in Kidney Failure: A Review and an Hypothesis. Adv. Chronic Kidney Dis. 2015, 22, 232–244. [Google Scholar] [CrossRef]
- Lang, F.; Friedrich, F.; Paulmichl, M.; Schobersberger, W.; Jungwirth, A.; Ritter, M.; Steidl, M.; Weiss, H.; Wöll, E.; Tschernko, E.; et al. Ion Channels in Madin-Darby Canine Kidney Cells. Kidney Blood Press. Res. 1990, 13, 82–93. [Google Scholar] [CrossRef]
- Coetzee, W.A.; Amarillo, Y.; Chiu, J.; Chow, A.; Lau, D.; McCormack, T.; Moreno, H.; Nadal, M.S.; Ozaita, A.; Pountney, D.; et al. Molecular diversity of K+ channels. Ann. N. Y. Acad. Sci. 1999, 868, 233–285. [Google Scholar] [CrossRef]
- Gaush, C.R.; Hard, W.L.; Smith, T.F. Characterization of an Established Line of Canine Kidney Cells (MDCK). Exp. Biol. Med. 1966, 122, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Rindler, M.J.; Chuman, L.M.; Shaffer, L.; Saier, M.H. Retention of differentiated properties in an established dog kidney epithelial cell line (MDCK). J. Cell Biol. 1979, 81, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Saier, M.H., Jr.; Boerner, P.; Grenier, F.C.; McRoberts, J.A.; Rindler, M.J.; Taub, M.; HS, U. Sodium entry pathways in renal epithelial cell lines. Miner. Electrolyte Metab. 1986, 12, 42–50. [Google Scholar] [PubMed]
- Vander, A.J. Potassium secretion and reabsorption in distal nephron. Am. J. Physiol. Content 1961, 201, 505–510. [Google Scholar] [CrossRef]
- Malnic, G.; Klose, R.M.; Giebisch, G. Micropuncture study of distal tubular potassium and sodium transport in rat nephron. Am. J. Physiol. Content 1966, 211, 529–547. [Google Scholar] [CrossRef]
- Wasserstein, A.G.; Agus, Z.S. Potassium secretion in the rabbit proximal straight tubule. Am. J. Physiol. Physiol. 1983, 245, F167–F174. [Google Scholar] [CrossRef]
- Welling, P.A. Regulation of Renal Potassium Secretion: Molecular Mechanisms. Semin. Nephrol. 2013, 33, 215–228. [Google Scholar] [CrossRef]
- Bailey, M.A.; Cantone, A.; Yan, Q.; MacGregor, G.G.; Leng, Q.; Amorim, J.B.; Wang, T.; Hebert, S.C.; Giebisch, G.; Malnic, G. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of type II Bartter’s syndrome and in adaptation to a high-K diet. Kidney Int. 2006, 70, 51–59. [Google Scholar] [CrossRef]
- Pluznick, J.L.; Sansom, S.C. BK channels in the kidney: Role in K+ secretion and localization of molecular components. Am. J. Physiol.-Ren. Physiol. 2006, 291, F517–F529. [Google Scholar] [CrossRef]
- Rieg, T.; Vallon, V.; Sausbier, M.; Sausbier, U.; Kaissling, B.; Ruth, P.; Osswald, H. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int. 2007, 72, 566–573. [Google Scholar] [CrossRef]
- Bolívar, J.J.; Cereijido, M. Voltage and Ca2+-Activated K+ channel in cultured epithelial cells (MDCK). J. Membr. Biol. 1987, 97, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Urrego, D.; Tomczak, A.P.; Zahed, F.; Stühmer, W.; Pardo, L.A. Potassium channels in cell cycle and cell proliferation. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130094. [Google Scholar] [CrossRef] [PubMed]
- Stühmer, W.; Alves, F.; Hartung, F.; Zientkowska, M.; Pardo, L.A. Potassium channels as tumour markers. FEBS Lett. 2006, 580, 2850–2852. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.; Contreras-Jurado, C.; Zientkowska, M.; Alves, F.; Stühmer, W. Role of Voltage-gated Potassium Channels in Cancer. J. Membr. Biol. 2005, 205, 115–124. [Google Scholar] [CrossRef]
- Bejček, J.; Spiwok, V.; Kmoníčková, E.; Rimpelová, S. Na+/K+-ATPase Revisited: On Its Mechanism of Action, Role in Cancer, and Activity Modulation. Molecules 2021, 26, 1905. [Google Scholar] [CrossRef]
- Osman, M.H.; Farrag, E.; Selim, M.; Osman, M.S.; Hasanine, A.; Selim, A. Cardiac glycosides use and the risk and mortality of cancer; systematic review and meta-analysis of observational studies. PLoS ONE 2017, 12, e0178611. [Google Scholar] [CrossRef]
- Newman, R.A.; Yang, P.; Pawlus, A.D.; Block, K.I. Cardiac Glycosides as Novel Cancer Therapeutic Agents. Mol. Interv. 2008, 8, 36–49. [Google Scholar] [CrossRef]
- Prassas, I.; Diamandis, E.P. Novel therapeutic applications of cardiac glycosides. Nat. Rev. Drug Discov. 2008, 7, 926–935. [Google Scholar] [CrossRef]
- Ponce, A.; Castillo, A.; Hinojosa, L.; Martínez-Rendón, J.; Cereijido, M. The expression of endogenous voltage-gated potassium channels in HEK293 cells is affected by culture conditions. Physiol. Rep. 2018, 6, e13663. [Google Scholar] [CrossRef]
- Ponce, A. Expression of Voltage Dependent Potassium Currents in Freshly Dissociated Rat Articular Chondrocytes. Cell. Physiol. Biochem. 2006, 18, 35–46. [Google Scholar] [CrossRef]
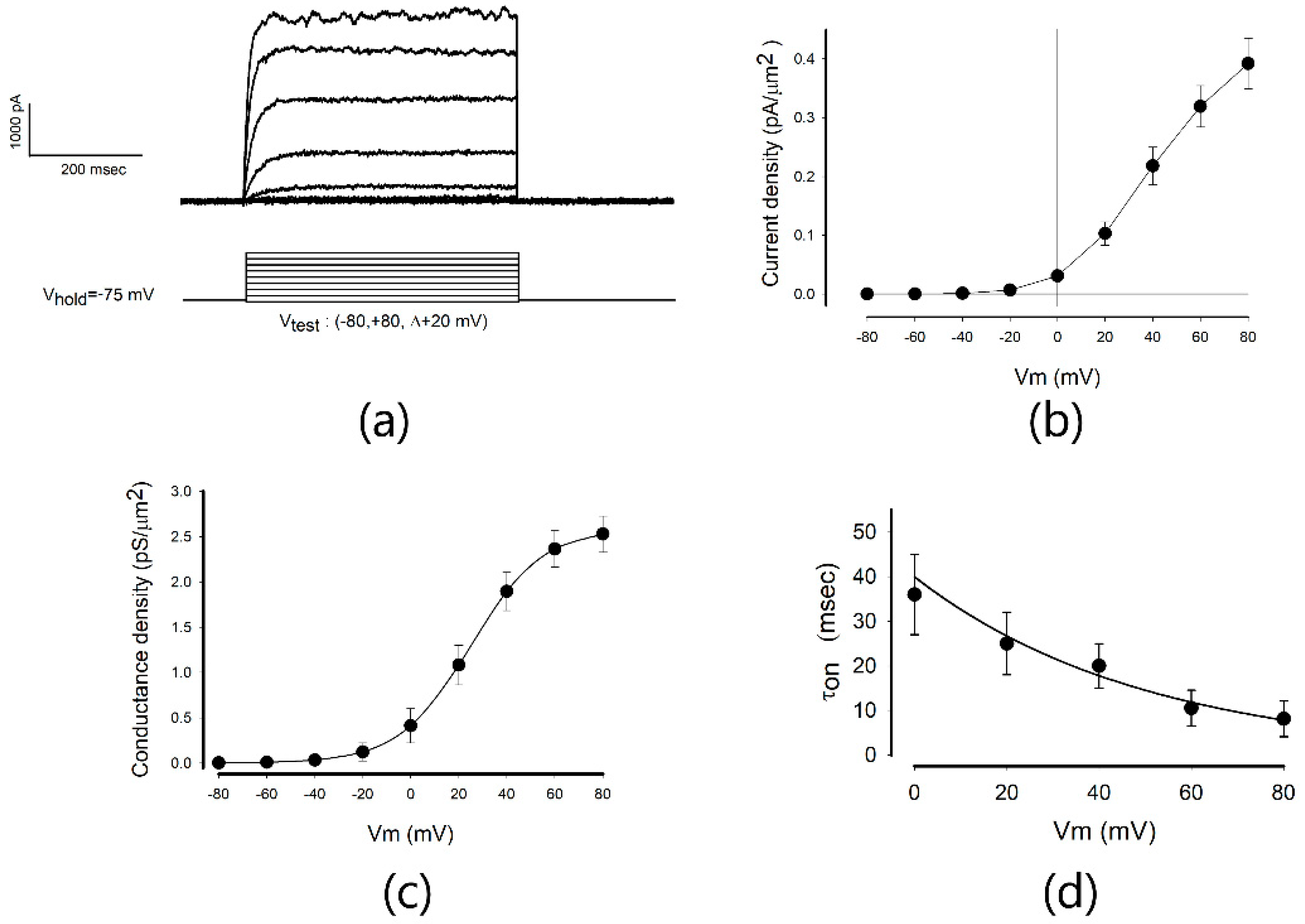
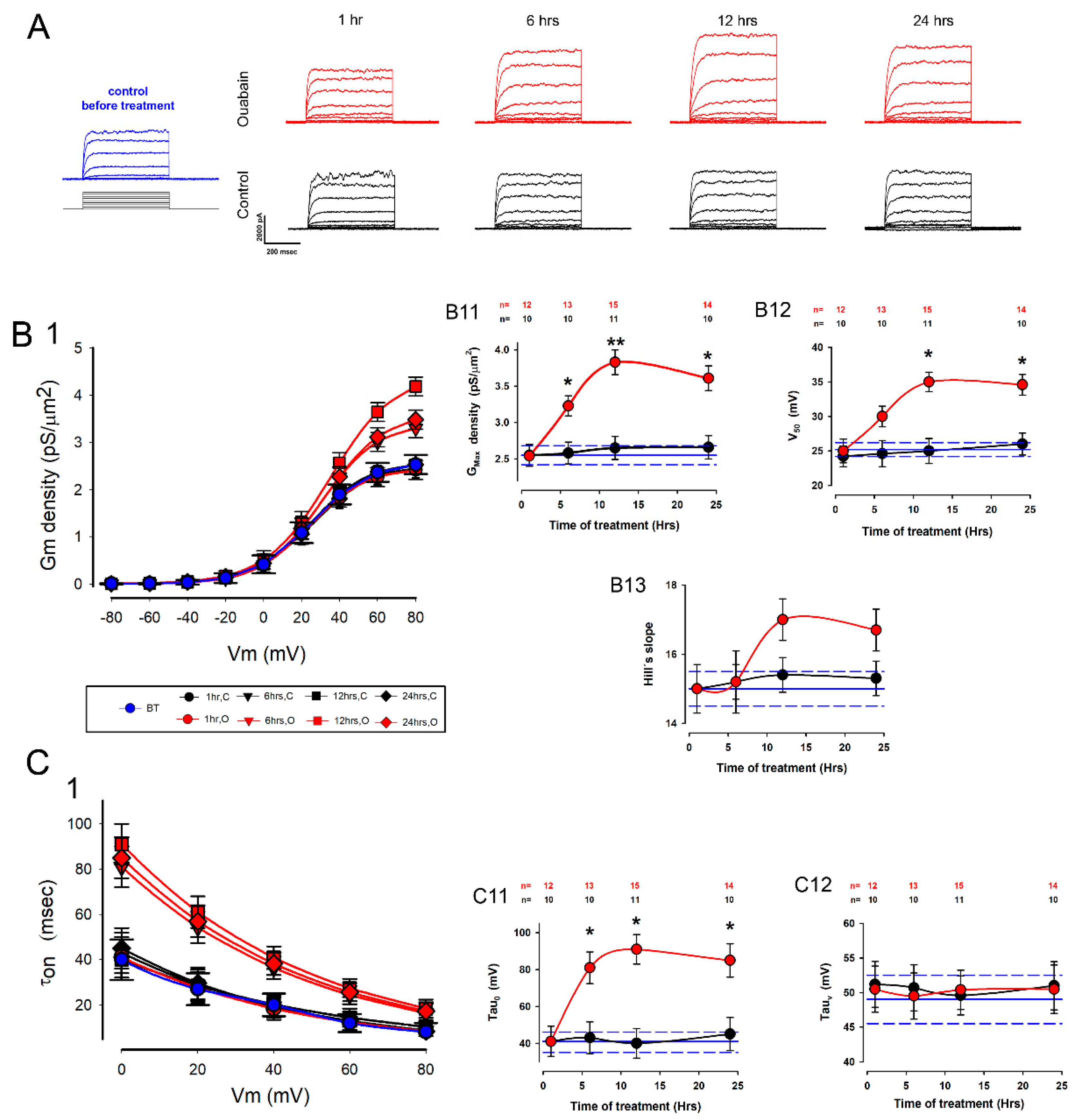
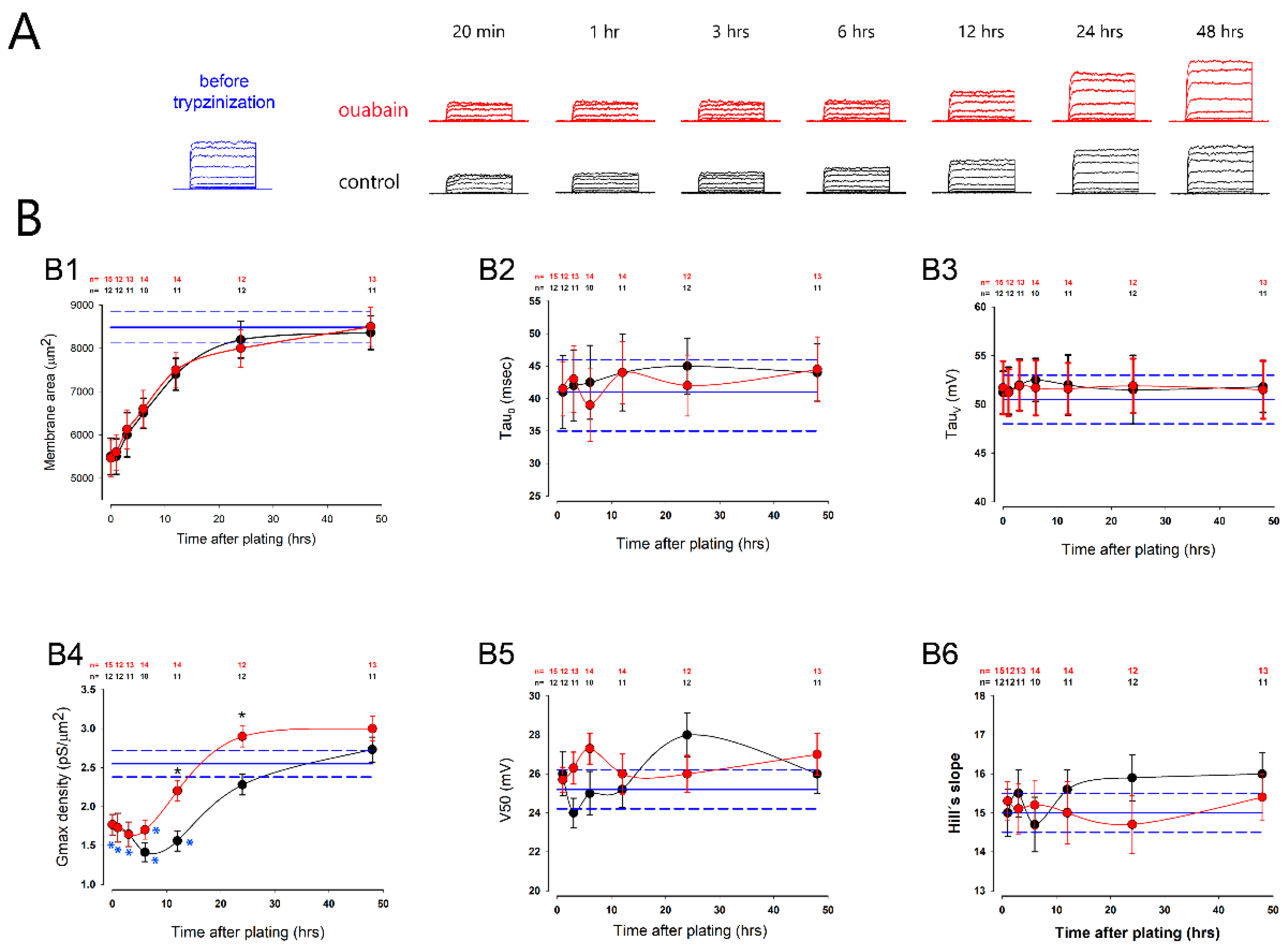
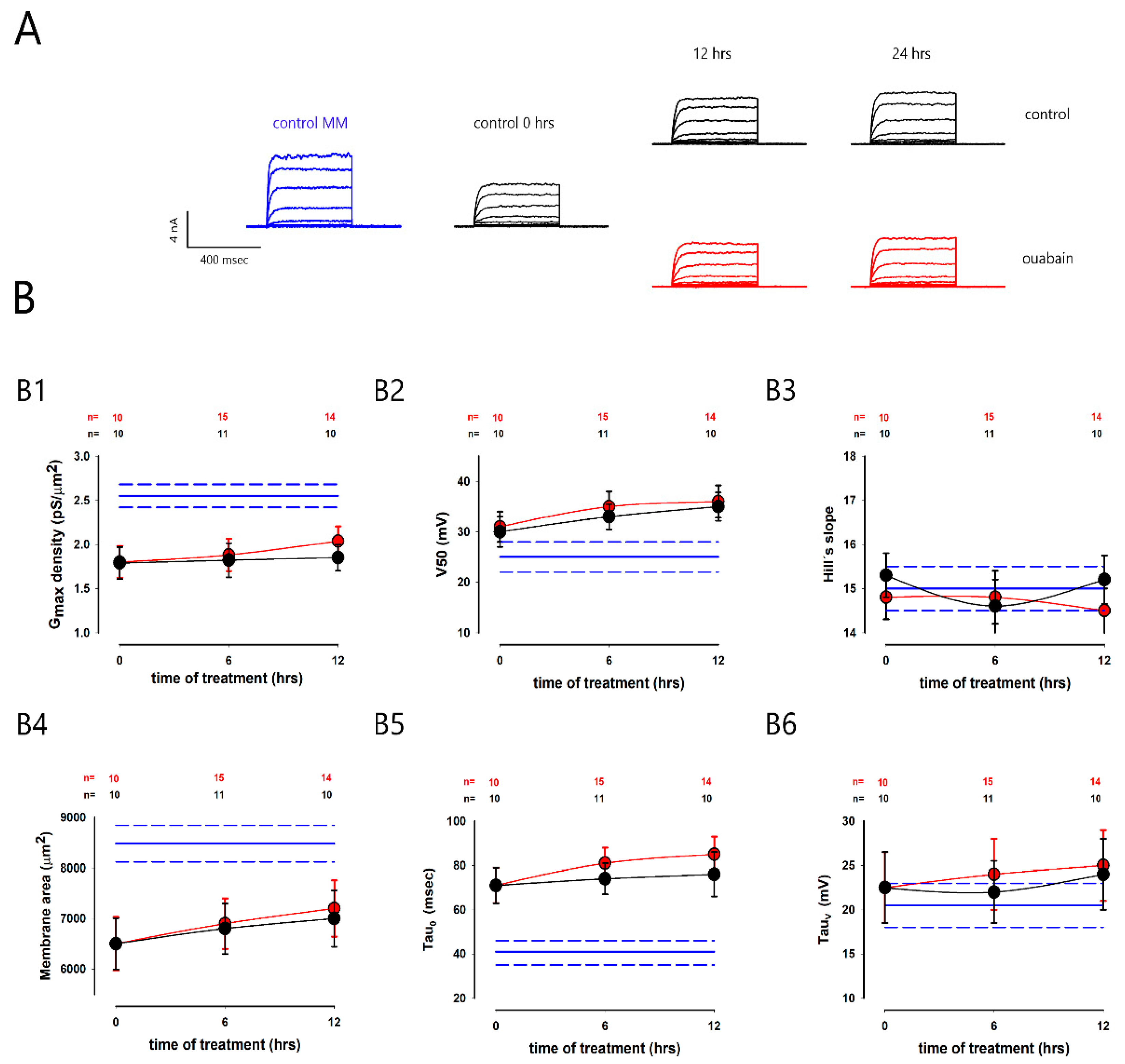
| Parameter | Mean | SE | Units |
|---|---|---|---|
| δGmax | 2.55 | 0.13 | pS/µm2 |
| V50 | 25.2 | 1.0 | mV |
| Hs | 15 | 0.5 | mV−1 |
| τ0 | 39.9 | 1.1 | ms |
| τv | 49.0 | 3.5 | mV |
| Name | Cat. No. | Stock mg/mL | Dose |
|---|---|---|---|
| Actinomycin D | A1410 | 10 | 50 nM |
| α-amanitin | A2263 | 1 | 10 μg/mL |
| Cycloheximide | C7698 | 25 | 50 μM |
| Puromycin | P8833 | 13 | 10 μM |
| Brefeldin A | B7651 | 10 | 20 μM |
| Tunicamycin | T7765 | 20 | 10 μg/mL |
| Cytochalasin B | C6762 | 20 | 5 μg/mL |
| Exo1 | E8280 | 100 | 50 μM |
| FR180204 | SML0320 | 25 | 400 nM |
| Y-27632 | Y0503 | 30 | 140 nM |
| PD 098059 | P215 | 30 | 7 µM |
| FH535 | F5682 | 10 | 15 µM |
| IKK-16 | SML1138 | 10 | 200 nM |
| Wortmannin | W1628 | 14 | 10 nM |
| PP2 | P0042 | 1.4 | 100 nM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cereijido, M.; Jimenez, L.; Hinojosa, L.; Castillo, A.; Martínez-Rendon, J.; Ponce, A. Ouabain-Induced Changes in the Expression of Voltage-Gated Potassium Channels in Epithelial Cells Depend on Cell–Cell Contacts. Int. J. Mol. Sci. 2022, 23, 13257. https://doi.org/10.3390/ijms232113257
Cereijido M, Jimenez L, Hinojosa L, Castillo A, Martínez-Rendon J, Ponce A. Ouabain-Induced Changes in the Expression of Voltage-Gated Potassium Channels in Epithelial Cells Depend on Cell–Cell Contacts. International Journal of Molecular Sciences. 2022; 23(21):13257. https://doi.org/10.3390/ijms232113257
Chicago/Turabian StyleCereijido, Marcelino, Lidia Jimenez, Lorena Hinojosa, Aida Castillo, Jacqueline Martínez-Rendon, and Arturo Ponce. 2022. "Ouabain-Induced Changes in the Expression of Voltage-Gated Potassium Channels in Epithelial Cells Depend on Cell–Cell Contacts" International Journal of Molecular Sciences 23, no. 21: 13257. https://doi.org/10.3390/ijms232113257
APA StyleCereijido, M., Jimenez, L., Hinojosa, L., Castillo, A., Martínez-Rendon, J., & Ponce, A. (2022). Ouabain-Induced Changes in the Expression of Voltage-Gated Potassium Channels in Epithelial Cells Depend on Cell–Cell Contacts. International Journal of Molecular Sciences, 23(21), 13257. https://doi.org/10.3390/ijms232113257






