Aspirin Inhibits the Inflammatory Response of Protease-Activated Receptor 1 in Pregnancy Neutrophils: Implications for Treating Women with Preeclampsia
Abstract
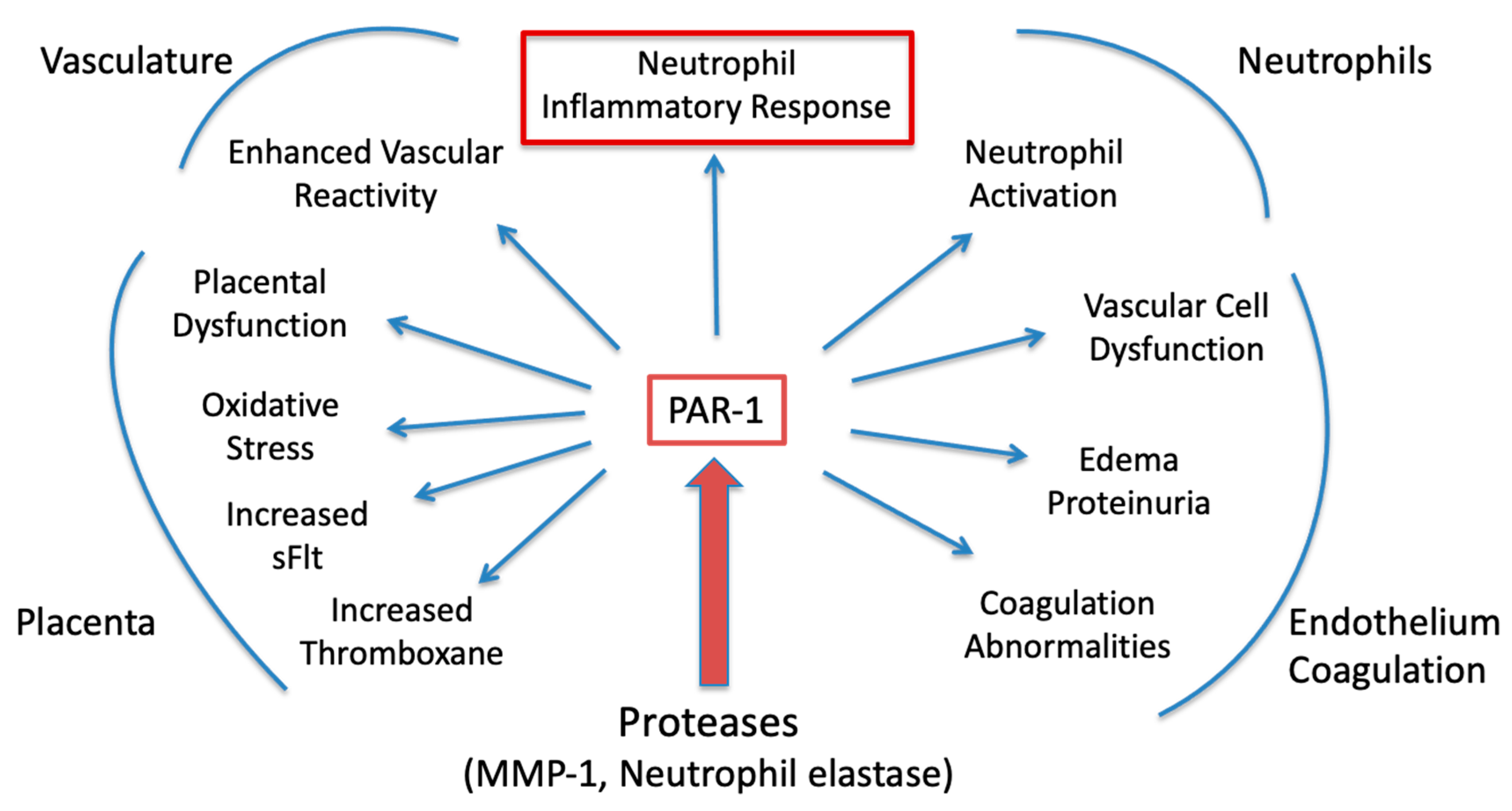
1. Introduction
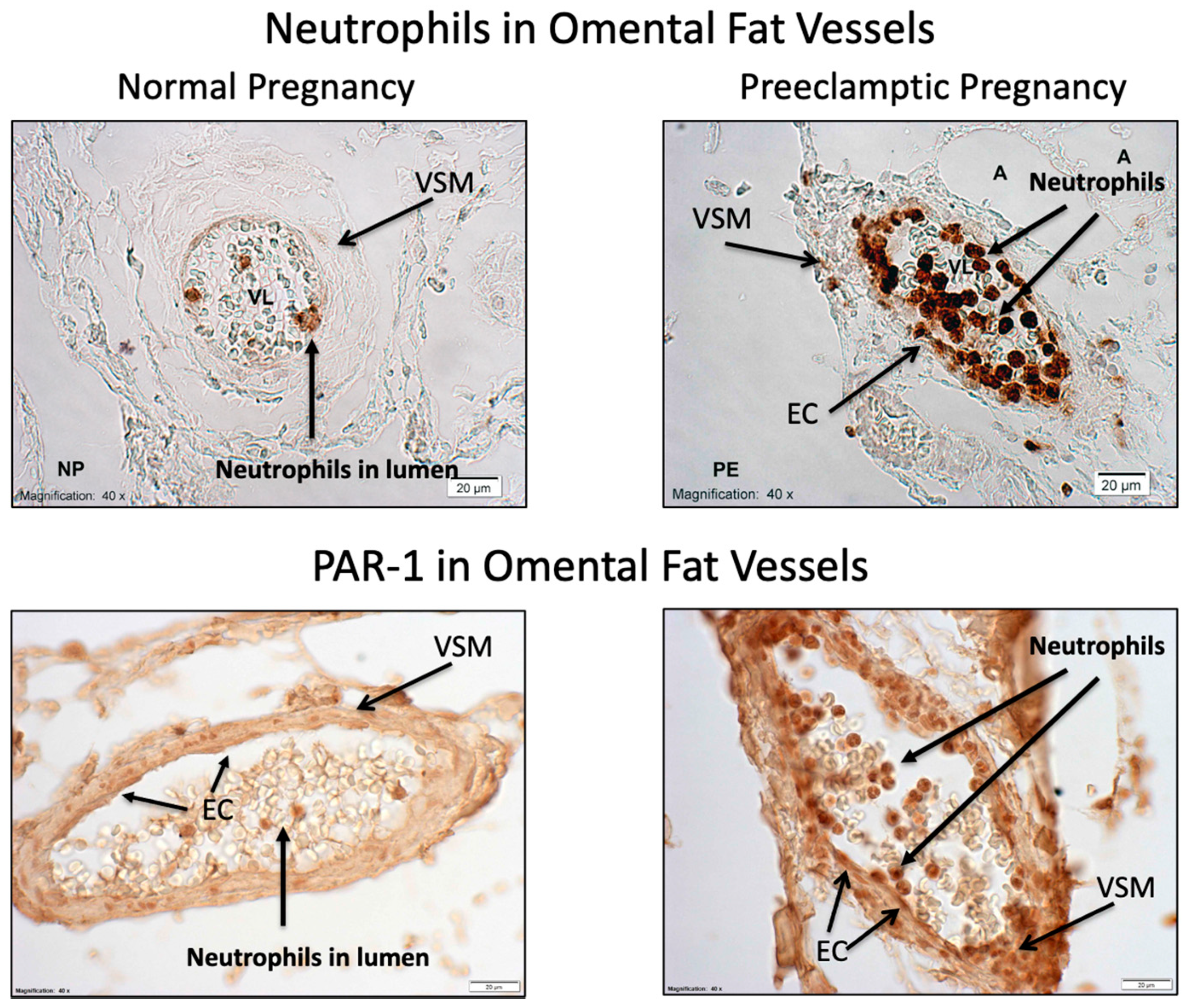
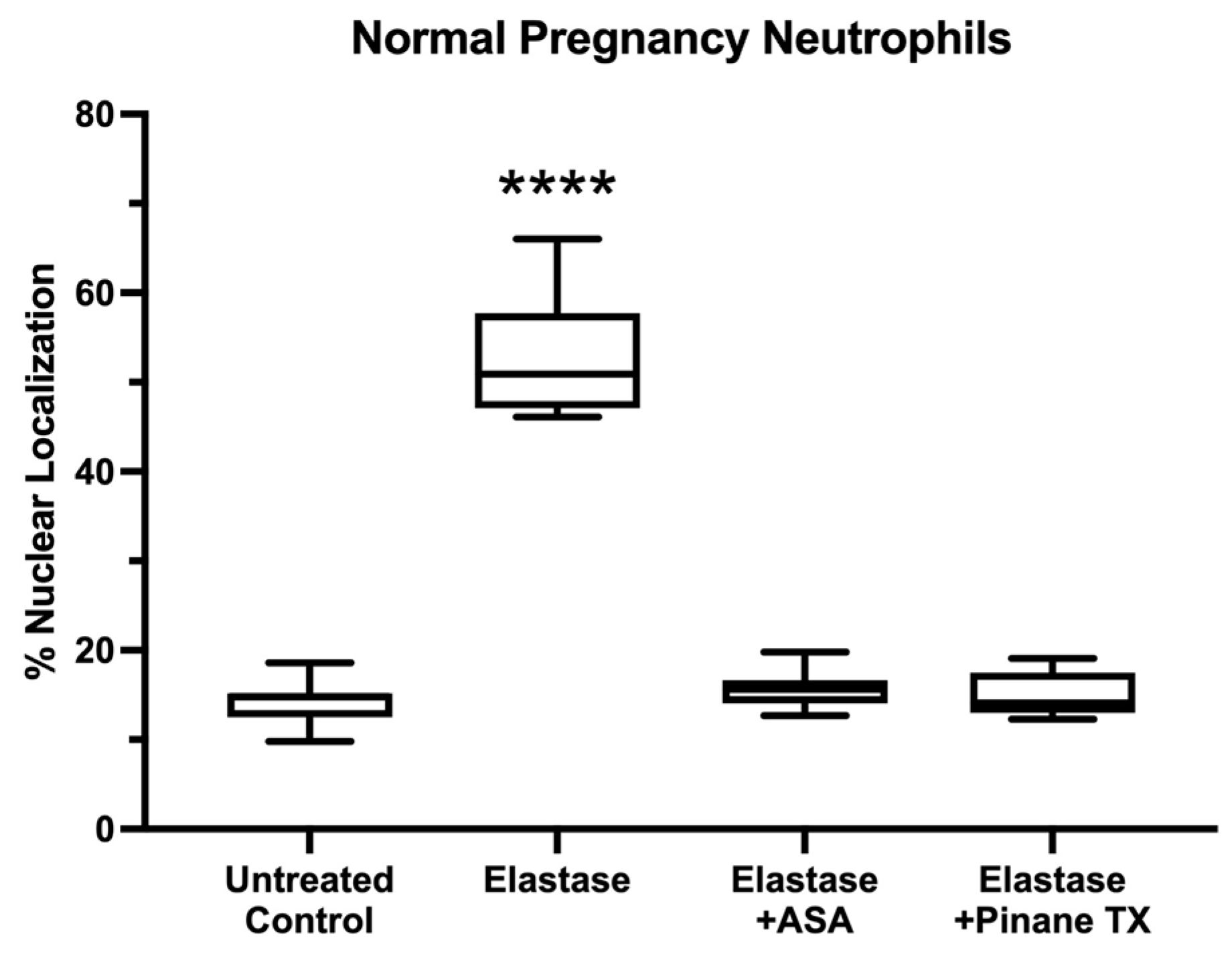
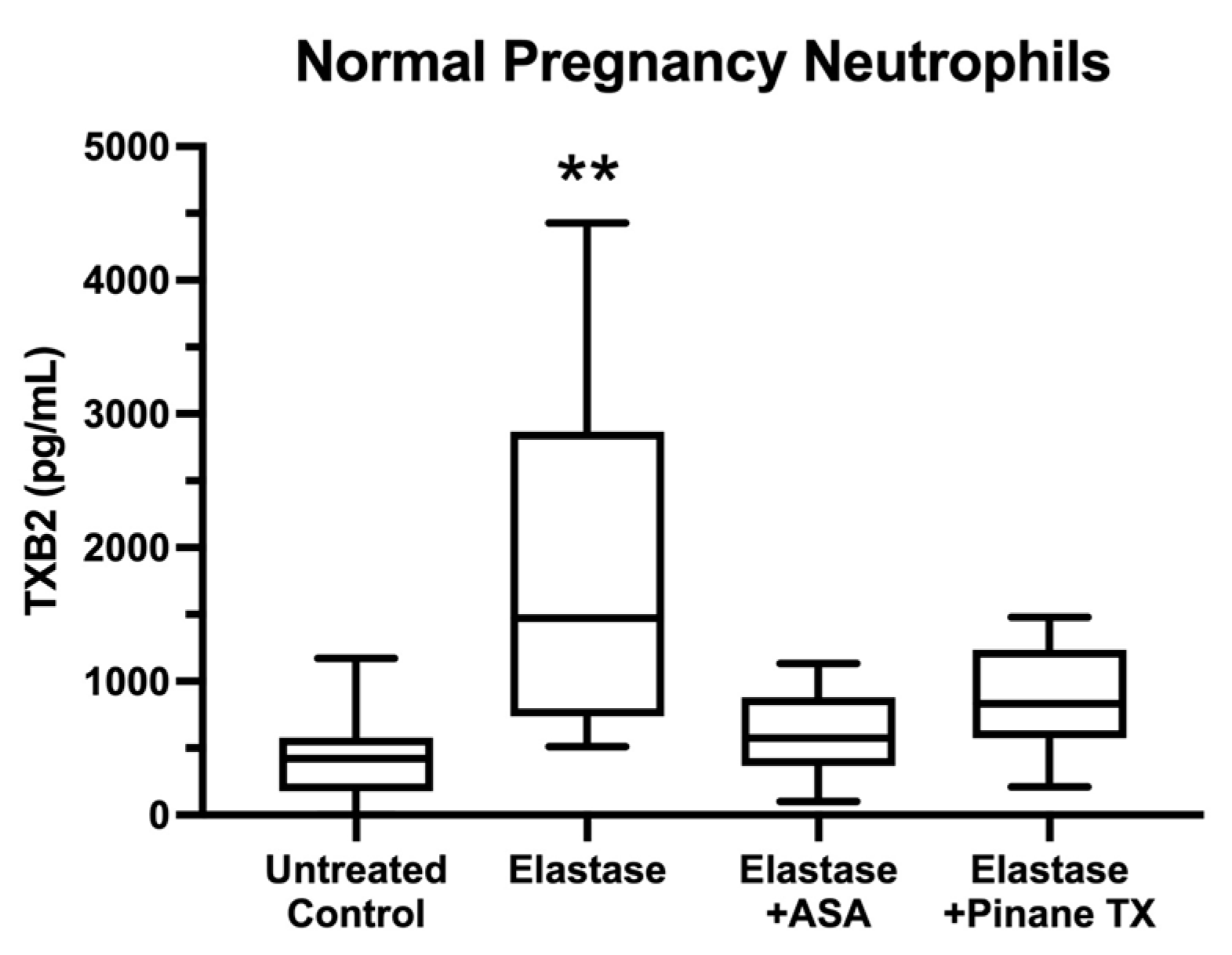
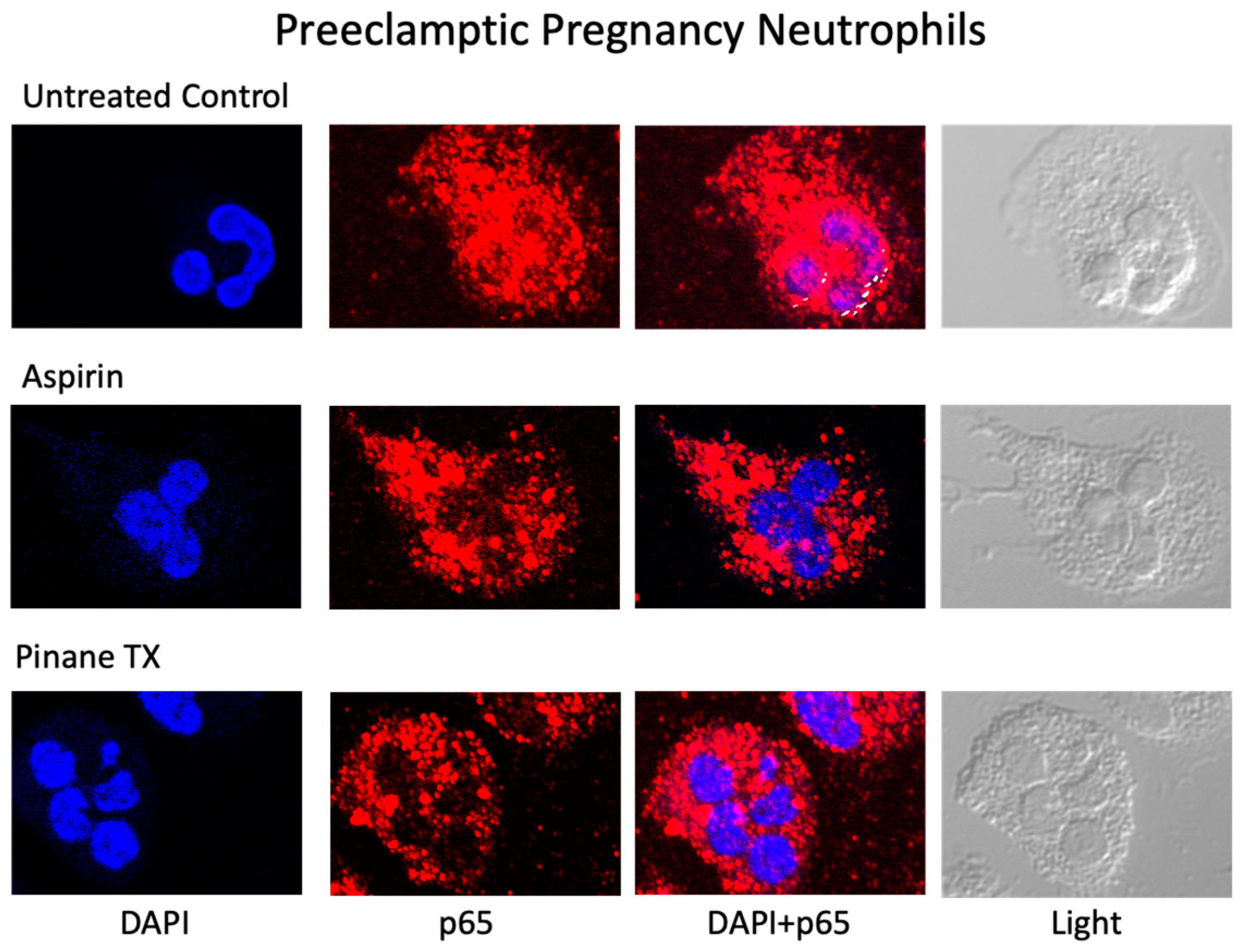
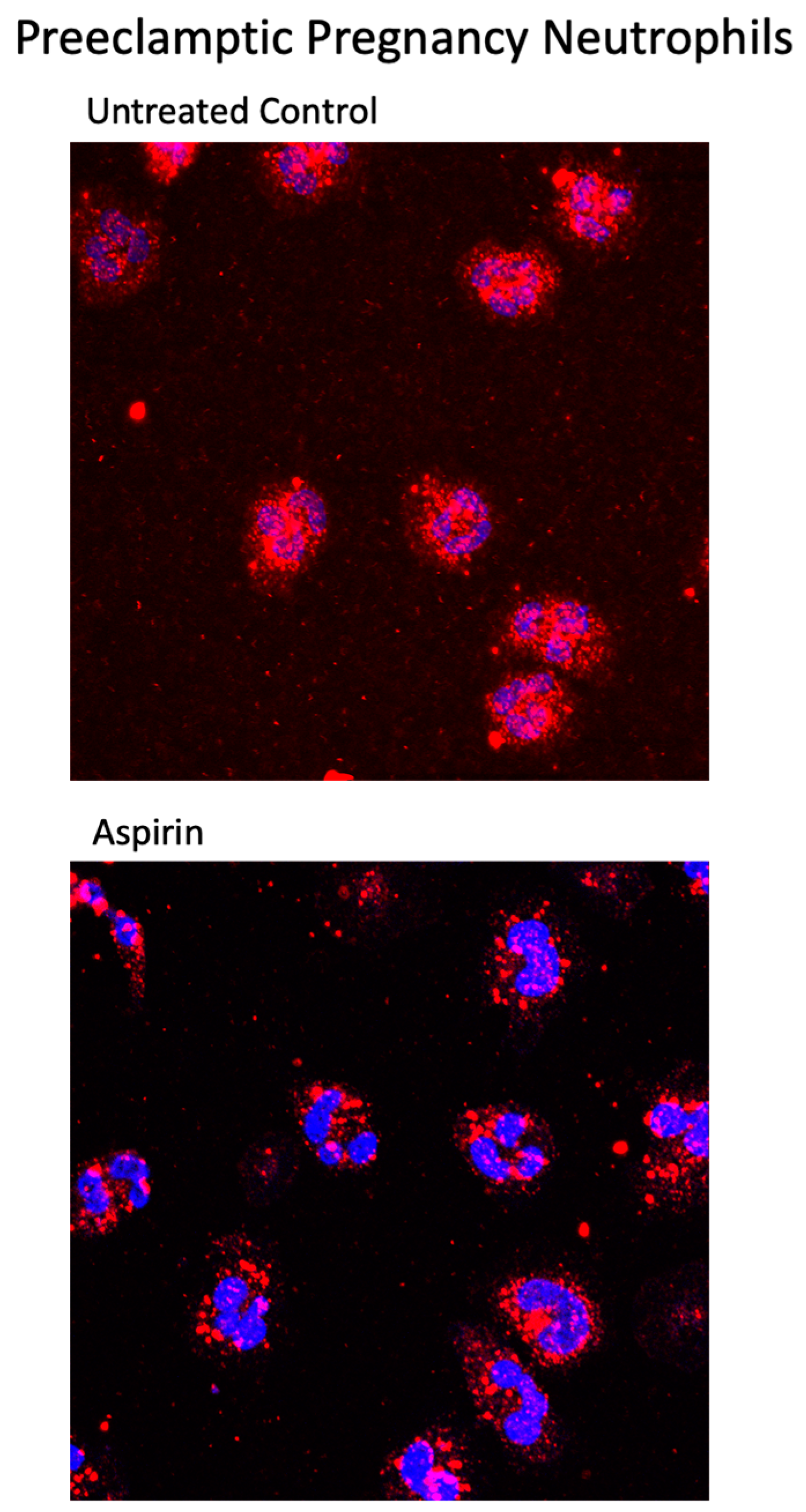
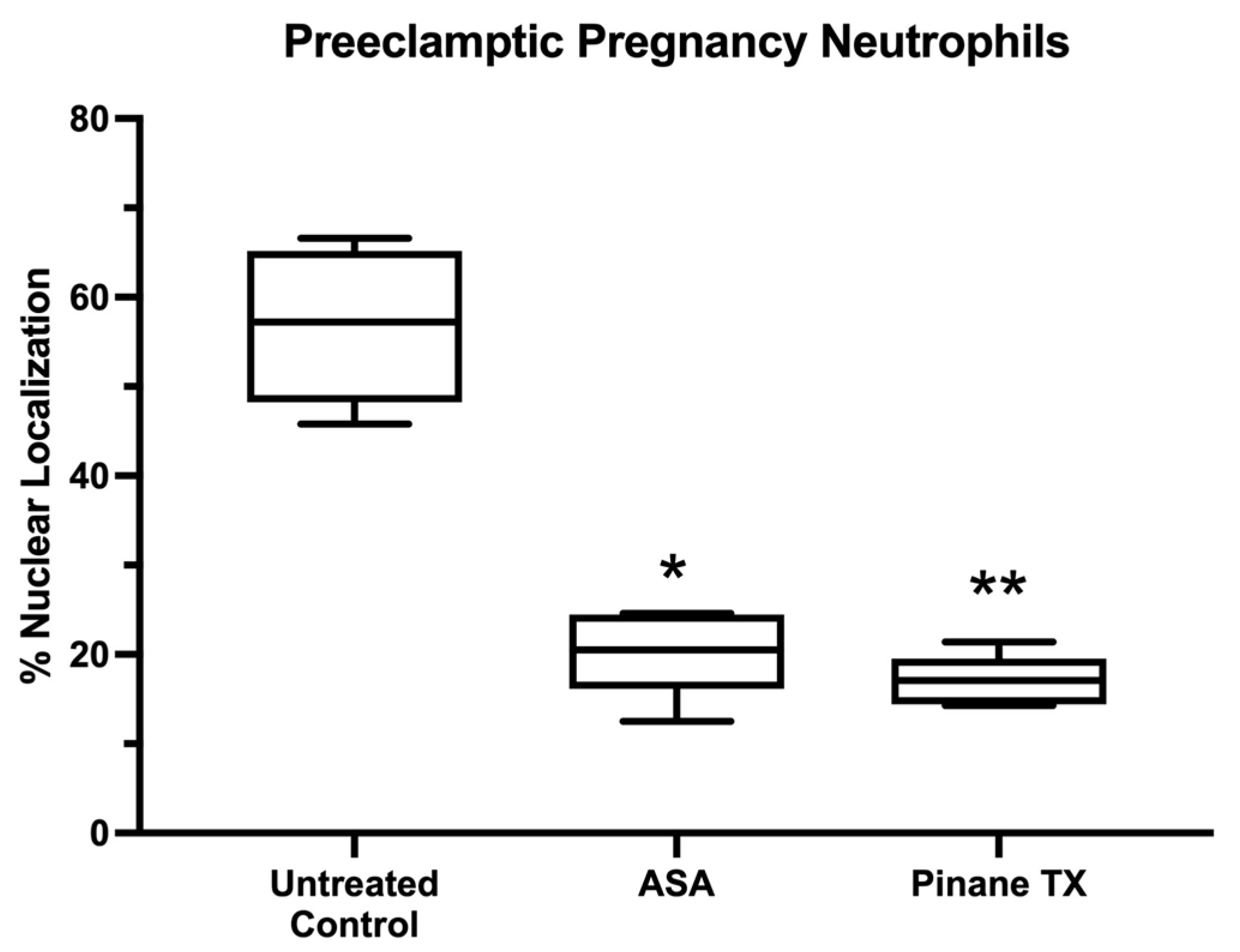
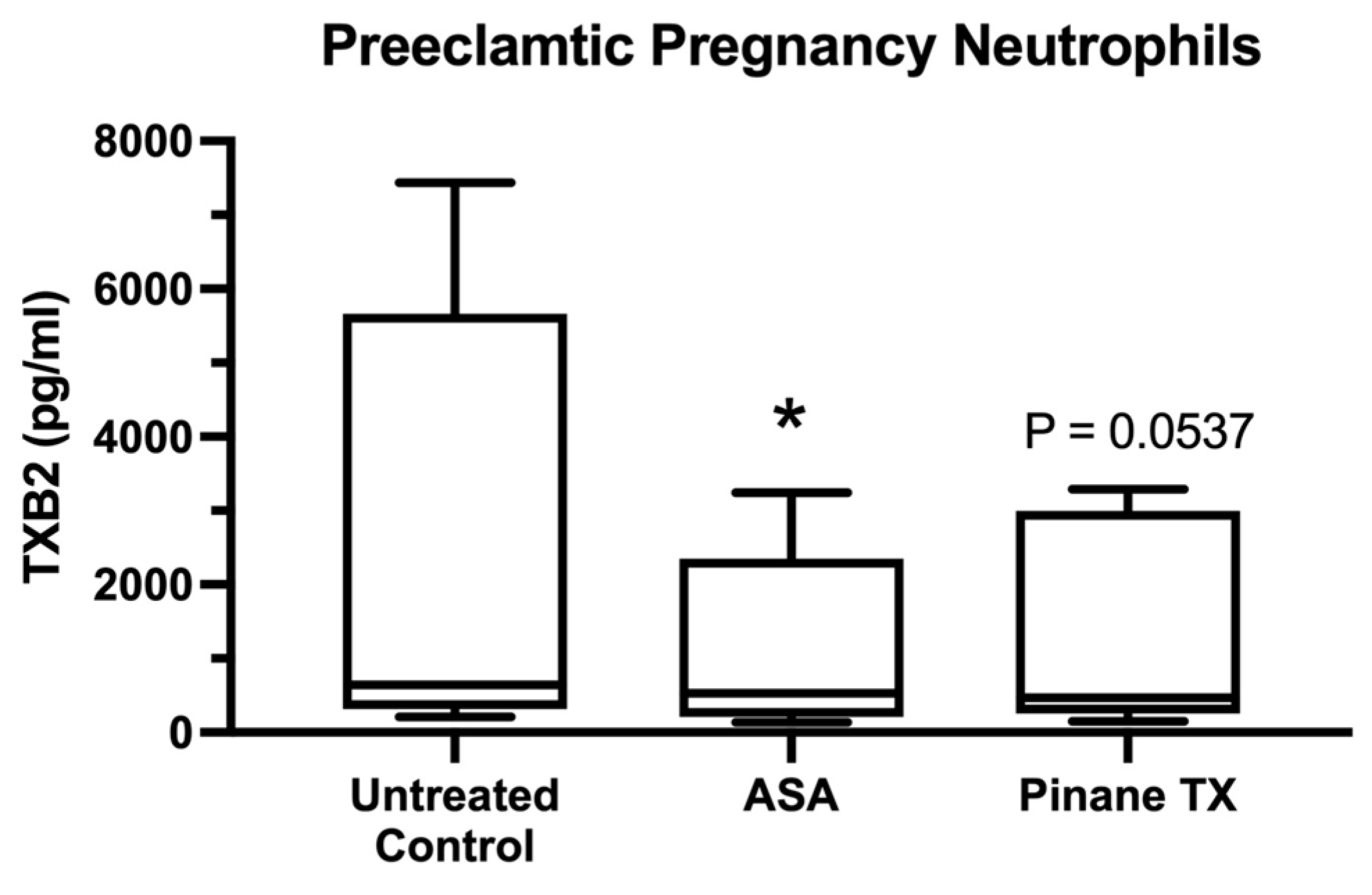
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Neutrophil Cell Culture
4.3. Confocal Microscopy Immunofluorescent Analysis
4.4. Measurement of Media Thromboxane B2 (TXB2)
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Walsh, S.W.; Strauss, J.F., III. The Road to Low-Dose Aspirin Therapy for the Prevention of Preeclampsia Began with the Placenta. Int. J. Mol. Sci. 2021, 22, 6985. [Google Scholar] [CrossRef] [PubMed]
- Goodlin, R.C.; Haesslein, H.O.; Fleming, J. Aspirin for the treatment of recurrent toxaemia. Lancet 1978, 312, 51. [Google Scholar] [CrossRef]
- Walsh, S.W.; Strauss, J.F., III. Pregnancy-specific expression of protease-activated receptor 1: A therapeutic target for prevention and treatment of preeclampsia? Am. J. Obstet. Gynecol. 2022, 226, S945–S953. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.W.; Reep, D.T.; Alam, S.M.K.; Washington, S.L.; Al Dulaimi, M.; Lee, S.M.; Springel, E.H.; Strauss, J.F., III; Stephenson, D.J.; Chalfant, C.E. Placental Production of Eicosanoids and Sphingolipids in Women Who Developed Preeclampsia on Low-Dose Aspirin. Reprod. Sci. 2020, 27, 2158–2169. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.E.; Walsh, S.W. Neutrophils from pregnant women produce thromboxane and tumor necrosis factor-alpha in response to linoleic acid and oxidative stress. Am J Obstet. Gynecol. 2005, 193, 830–835. [Google Scholar] [CrossRef]
- Vaughan, J.E.; Walsh, S.W.; Ford, G.D. Thromboxane mediates neutrophil superoxide production in pregnancy. Am. J. Obstet. Gynecol. 2006, 195, 1415–1420. [Google Scholar] [CrossRef]
- Bachawaty, T.; Washington, S.L.; Walsh, S.W. Neutrophil Expression of Cyclooxygenase-2 in Preeclampsia. Reprod. Sci. 2010, 17, 465–470. [Google Scholar] [CrossRef][Green Version]
- Nelson, D.M.; Walsh, S.W. Aspirin differentially affects thromboxane and prostacyclin production by trophoblast and villous core compartments of human placental villi. Am. J. Obstet. Gynecol. 1989, 161, 1593–1598. [Google Scholar] [CrossRef]
- Walsh, S.W.; Wang, Y. Maternal Perfusion with Low-Dose Aspirin Preferentially Inhibits Placental Thromboxane While Sparing Prostacyclin. Hypertens. Pregnancy 1998, 17, 203–215. [Google Scholar] [CrossRef]
- Walsh, S.W.; Wang, Y.; Kay, H.H.; McCoy, M.C. Low-dose aspirin inhibits lipid peroxides and thromboxane but not prostacyclin in pregnant women. Am. J. Obstet. Gynecol. 1992, 167, 926–930. [Google Scholar] [CrossRef]
- Wang, Y.; Walsh, S.W. Aspirin inhibits both lipid peroxides and thromboxane in preeclamptic placentas. Free Radic. Biol. Med. 1995, 18, 585–591. [Google Scholar] [CrossRef]
- Leik, C.E.; Walsh, S.W. Neutrophils Infiltrate Resistance-Sized Vessels of Subcutaneous Fat in Women with Preeclampsia. Hypertension 2004, 44, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Nugent, W.H.; Mahavadi, S.; Walsh, S.W. Mechanisms of Enhanced Vascular Reactivity in Preeclampsia. Hypertension 2011, 58, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.A.; Strauss, J.F., III; Walsh, S.W. Reduced methylation of the thromboxane synthase gene is correlated with its increased vascular expression in preeclampsia. Hypertension 2012, 59, 1249–1255. [Google Scholar] [CrossRef]
- Shah, T.J.; Walsh, S.W. Activation of NF-kappaB and expression of COX-2 in association with neutrophil infiltration in systemic vascular tissue of women with preeclampsia. Am. J. Obstet. Gynecol. 2007, 196, 48.e1. [Google Scholar] [CrossRef] [PubMed]
- Cadden, K.A.; Walsh, S.W. Neutrophils, but Not Lymphocytes or Monocytes, Infiltrate Maternal Systemic Vasculature in Women with Preeclampsia. Hypertens. Pregnancy 2008, 27, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.W.; Al Dulaimi, M.; Strauss, J.F., III. Gene Expression of Pregnancy Neutrophils Differs for Protease versus Lipopolysaccharide Stimulation. Int. J. Mol. Sci. 2022, 23, 4924. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Y.; Lucas, M.J. Expression of thrombin receptors in endothelial cells and neutrophils from normal and preeclamptic pregnancies. J. Clin. Endocrinol. Metab. 2002, 87, 3728–3734. [Google Scholar] [CrossRef][Green Version]
- Shpacovitch, V.; Feld, M.; Hollenberg, M.D.; Luger, T.A.; Steinhoff, M. Role of protease-activated receptors in inflammatory responses, innate and adaptive immunity. J. Leukoc. Biol. 2008, 83, 1309–1322. [Google Scholar] [CrossRef]
- Walsh, S.W.; Nugent, W.H.; Al Dulaimi, M.; Washington, S.L.; Dacha, P.; Strauss, J.F., III. Proteases Activate Pregnancy Neutrophils by a Protease-Activated Receptor 1 Pathway: Epigenetic Implications for Preeclampsia. Reprod. Sci. 2020, 27, 2115–2127. [Google Scholar] [CrossRef]
- Cadroy, Y.; Grandjean, H.; Pichon, J.; Desprats, R.; Berrebi, A.; Fournie, A.; Boneu, B. Evaluation of six markers of haemostatic system in normal pregnancy and pregnancy complicated by hypertension or pre-eclampsia. Br. J. Obstet. Gynaecol. 1993, 100, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Chaiworapongsa, T.; Yoshimatsu, J.; Espinoza, J.; Kim, Y.M.; Berman, S.; Edwin, S.; Yoon, B.H.; Romero, R. Evidence of in vivo generation of thrombin in patients with small-for-gestational-age fetuses and pre-eclampsia. J. Matern. Neonatal Med. 2002, 11, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Greer, I.A.; Haddad, N.G.; Dawes, J.; Johnstone, F.D.; Calder, A.A. Neutrophil activation in pregnancy-induced hypertension. Br. J. Obstet. Gynaecol. 1989, 96, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gebhardt, S.; Hillermann, R.; Holzgreve, W.; Hahn, S. Analysis of plasma elastase levels in early and late onset preeclampsia. Arch. Gynecol. Obstet. 2005, 273, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Schjetlein, R.; Haugen, G.; Wisløff, F. Markers of intravascular coagulation and fibrinolysis in preeclampsia: Association with intrauterine growth retardation. Acta Obstet. Gynecol. Scand. 1997, 76, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, M.J.; Boer, K.; Berckmans, R.J.; Meijers, J.C.; van der Post, J.A.; Sturk, A.; VanBavel, E.; Nieuwland, R. Enhanced coagulation activation in preeclampsia: The role of APC resistance, microparticles and other plasma constituents. Thromb. Haemost. 2002, 88, 415–420. [Google Scholar]
- Barnes, P.J.; Karin, M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef]
- Walsh, S.W.; Vaughan, J.E.; Wang, Y.; Roberts, L.J., II. Placental isoprostane is significantly increased in preeclampsia. FASEB J. 2000, 14, 1289–1296. [Google Scholar] [CrossRef]
- Walsh, S.W.; Wang, Y. Secretion of lipid peroxides by the human placenta. Am. J. Obstet. Gynecol. 1993, 169, 1462–1466. [Google Scholar] [CrossRef]
- Walsh, S.W.; Wang, Y.; Jesse, R. Placental Production of Lipid Peroxides, Thromboxane, and Prostacyclin in Preeclampsia. Hypertens. Pregnancy 1996, 15, 101–111. [Google Scholar] [CrossRef]
- Estrada-Gutierrez, G.; Cappello, R.E.; Mishra, N.; Romero, R.; Strauss, J.F., III; Walsh, S.W. Increased expression of matrix metalloproteinase-1 in systemic vessels of preeclamptic women: A critical mediator of vascular dysfunction. Am. J. Pathol. 2011, 178, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.R. Thrombin signalling and protease-activated receptors. Nature 2000, 407, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.R. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 2005, 3, 1800–1814. [Google Scholar] [CrossRef]
- Riento, K.; Ridley, A.J. Rocks: Multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 2003, 4, 446–456. [Google Scholar] [CrossRef]
- Yao, L.; Romero, M.J.; Toque, H.A.; Yang, G.; Caldwell, R.B.; Caldwell, R.W. The role of RhoA/Rho kinase pathway in endothelial dysfunction. J. Cardiovasc. Dis. Res. 2010, 1, 165–170. [Google Scholar] [PubMed]
- Erez, O.; Romero, R.; Kim, S.-S.; Kim, J.-S.; Kim, Y.M.; Wildman, D.E.; Than, N.G.; Mazaki-Tovi, S.; Gotsch, F.; Pineles, B.; et al. Over-expression of the thrombin receptor (PAR-1) in the placenta in preeclampsia: A mechanism for the intersection of coagulation and inflammation. J. Matern.-Fetal Neonatal Med. 2008, 21, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.T.; Chen, J.H.; Hang, L.L.; Liu, S.S.; Zhong, M. Activation of PAR-1/NADPH oxidase/ROS signaling pathways is crucial for the thrombin-induced sFlt-1 production in extravillous trophoblasts: Possible involvement in the pathogenesis of preeclampsia. Cell Physiol Biochem. 2015, 35, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Koga, K.; Osuga, Y.; Nagai, M.; Izumi, G.; Takamura, M.; Harada, M.; Hirota, Y.; Yoshino, O.; Taketani, Y. Thrombin enhances soluble Fms-like tyrosine kinase 1 expression in trophoblasts; possible involvement in the pathogenesis of preeclampsia. Fertil. Steril. 2012, 98, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Even-Ram, S.C.; Grisaru-Granovsky, S.; Pruss, D.; Maoz, M.; Salah, Z.; Yong-Jun, Y.; Bar-Shavit, R. The pattern of expression of protease-activated receptors (PARs) during early trophoblast development. J. Pathol. 2003, 200, 47–52. [Google Scholar] [CrossRef]
- Lockwood, C.J.; Toti, P.; Arcuri, F.; Norwitz, E.; Funai, E.F.; Huang, S.-T.J.; Buchwalder, L.F.; Krikun, G.; Schatz, F. Thrombin Regulates Soluble fms-Like Tyrosine Kinase-1 (sFlt-1) Expression in First Trimester Decidua: Implications for Preeclampsia. Am. J. Pathol. 2007, 170, 1398–1405. [Google Scholar] [CrossRef]
- Walsh, S.W. Eicosanoids in preeclampsia. Prostaglandins Leukot Essent Fat. Acids 2004, 70, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Austin, K.M.; Covic, L.; Kuliopulos, A. Matrix metalloproteases and PAR1 activation. Blood 2013, 121, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Goerge, T.; Barg, A.; Schnaeker, E.-M.; Poppelmann, B.; Shpacovitch, V.; Rattenholl, A.; Maaser, C.; Luger, T.A.; Steinhoff, M.; Schneider, S.W. Tumor-Derived Matrix Metalloproteinase-1 Targets Endothelial Proteinase-Activated Receptor 1 Promoting Endothelial Cell Activation. Cancer Res. 2006, 66, 7766–7774. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.R.; Seatter, M.J.; Kanke, T.; Hunter, G.D.; Plevin, R. Proteinase-activated receptors. Pharmacol. Rev. 2001, 53, 245–282. [Google Scholar]
- Shapiro, S.; Siskind, V.; Monson, R.R.; Heinonen, O.P.; Kaufman, D.W.; Slone, D. Perinatal mortality and birth-weight in relation to aspirin taken during pregnancy. Lancet 1976, 1, 1375–1376. [Google Scholar] [CrossRef]
- Hastie, R.; Tong, S.; Wikström, A.K.; Sandström, A.; Hesselman, S.; Bergman, L. Aspirin use during pregnancy and the risk of bleeding complications: A Swedish population-based cohort study. Am. J. Obstet. Gynecol. 2021, 224, 95.e1–95.e12. [Google Scholar] [CrossRef]
- Nagelschmitz, J.; Blunck, M.; Kraetzschmar, J.; Ludwig, M.; Wensing, G.; Hohlfeld, T. Pharmacokinetics and pharmacodynamics of acetylsalicylic acid after intravenous and oral administration to healthy volunteers. Clin. Pharmacol. Adv. Appl. 2014, 6, 51–59. [Google Scholar] [CrossRef]

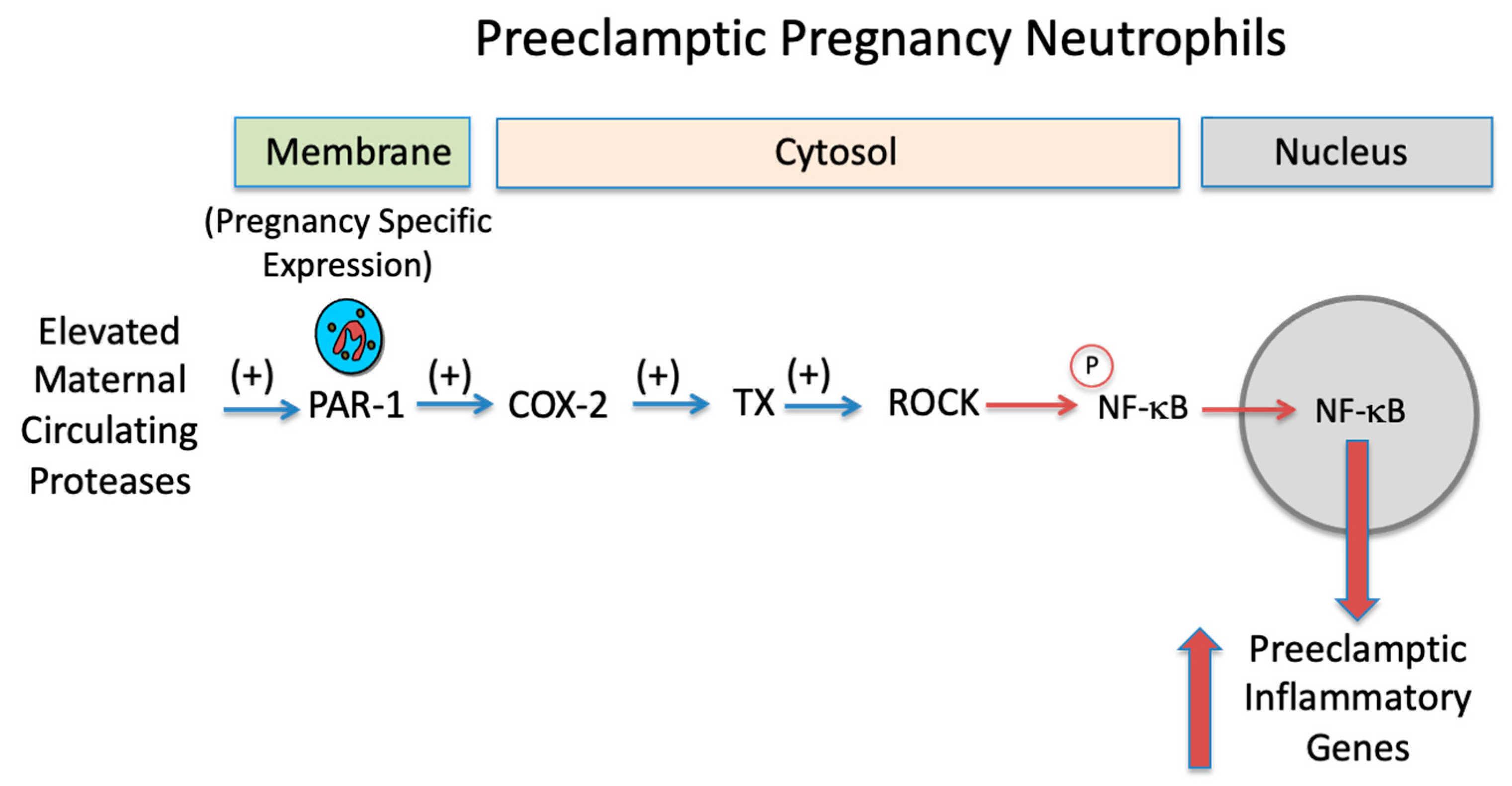
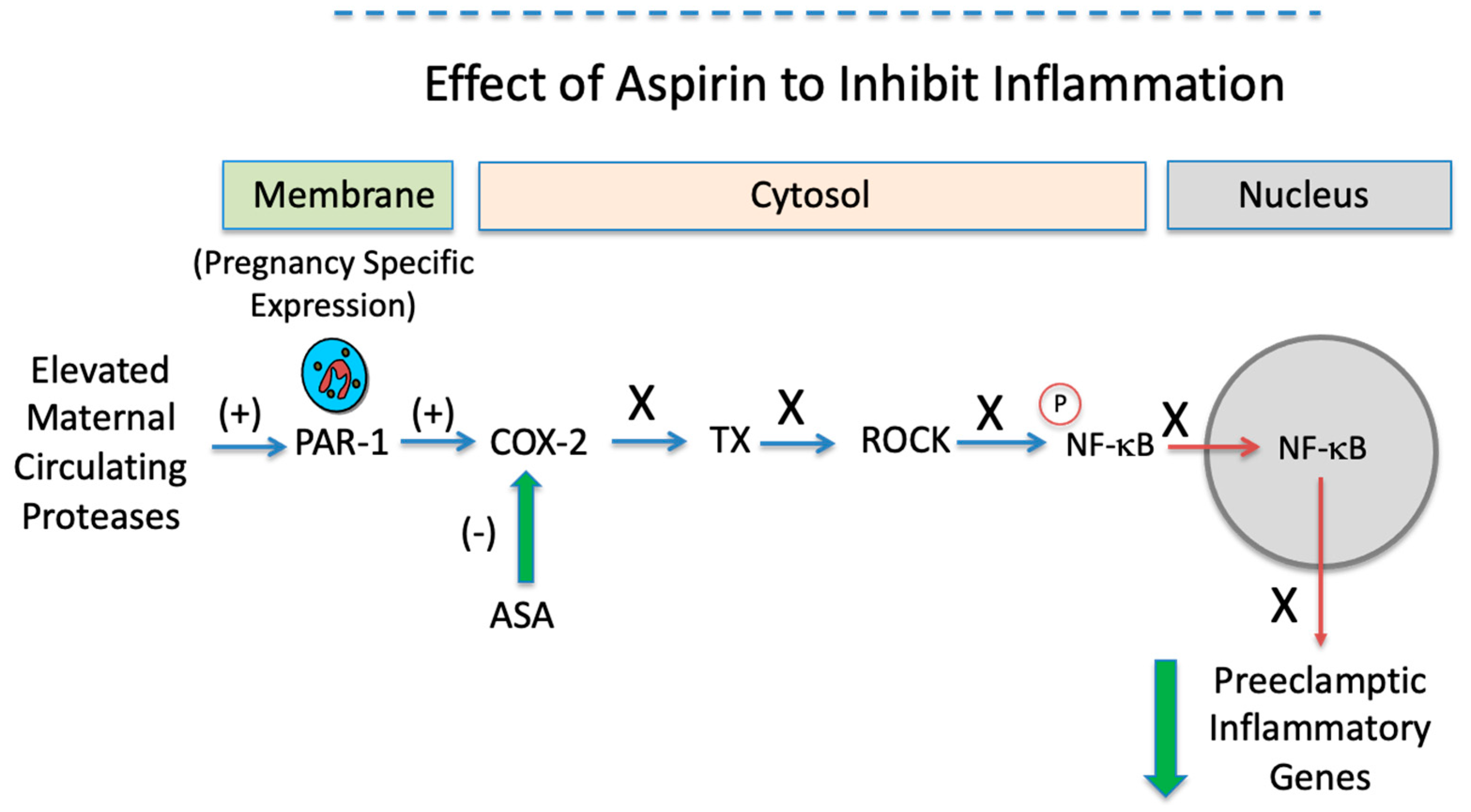
| Variable | Normal Pregnancy n = 11 | Preeclamptic Pregnancy n = 5 |
|---|---|---|
| Maternal age (years) | 27 ± 3 | 33 ± 6 |
| Pre-pregnancy BMI (kg/m2) | 25 ± 5 | 31 ± 10 |
| BMI at sample collection (kg/m2) | 30 ± 6 | 37 ± 10 |
| Systolic blood pressure at 30 weeks (mmHg) | 109 ± 12 | 157 ± 11 **** |
| Diastolic blood pressure at 30 weeks (mmHg) | 70 ± 10 | 92 ± 12 *** |
| Protein/creatinine Ratio | ND | 0.56 ± 0.28 |
| Primiparous | 4 | 1 |
| Multiparous | 7 | 4 |
| Race | ||
| White | 6 | 3 |
| Black | 5 | 2 |
| Type of Delivery C-section Vaginal | 1 10 | 3 2 |
| Gestational age at sample collection (weeks) | 30 ± 3 | 32 ± 4 |
| Gestational age at delivery (weeks) | 39 ± 1 | 33 ± 4 **** |
| Infant birth weight (grams) | 3326 ± 371 | 1904 ± 672 **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walsh, S.W.; Al Dulaimi, M.; Strauss, J.F., III. Aspirin Inhibits the Inflammatory Response of Protease-Activated Receptor 1 in Pregnancy Neutrophils: Implications for Treating Women with Preeclampsia. Int. J. Mol. Sci. 2022, 23, 13218. https://doi.org/10.3390/ijms232113218
Walsh SW, Al Dulaimi M, Strauss JF III. Aspirin Inhibits the Inflammatory Response of Protease-Activated Receptor 1 in Pregnancy Neutrophils: Implications for Treating Women with Preeclampsia. International Journal of Molecular Sciences. 2022; 23(21):13218. https://doi.org/10.3390/ijms232113218
Chicago/Turabian StyleWalsh, Scott W., Marwah Al Dulaimi, and Jerome F. Strauss, III. 2022. "Aspirin Inhibits the Inflammatory Response of Protease-Activated Receptor 1 in Pregnancy Neutrophils: Implications for Treating Women with Preeclampsia" International Journal of Molecular Sciences 23, no. 21: 13218. https://doi.org/10.3390/ijms232113218
APA StyleWalsh, S. W., Al Dulaimi, M., & Strauss, J. F., III. (2022). Aspirin Inhibits the Inflammatory Response of Protease-Activated Receptor 1 in Pregnancy Neutrophils: Implications for Treating Women with Preeclampsia. International Journal of Molecular Sciences, 23(21), 13218. https://doi.org/10.3390/ijms232113218