Beneficial Effects of Oleosomes Fused with Human Fibroblast Growth Factor 1 on Wound Healing via the Promotion of Angiogenesis
Abstract
1. Introduction
2. Results
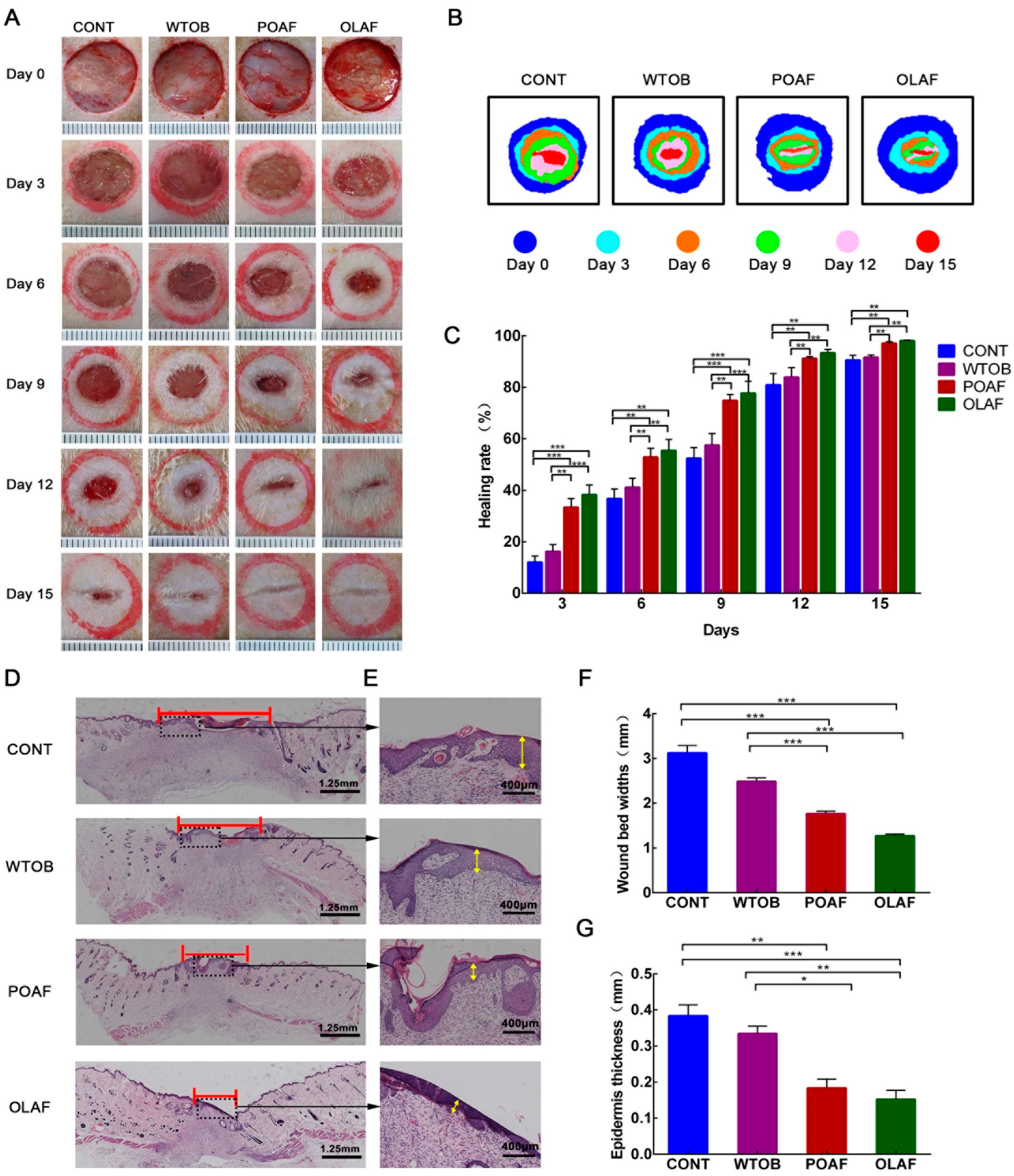
2.1. The Wound Healing Potential of Oleosomes Fused with Human Fibroblast Growth Factor 1 in Rats
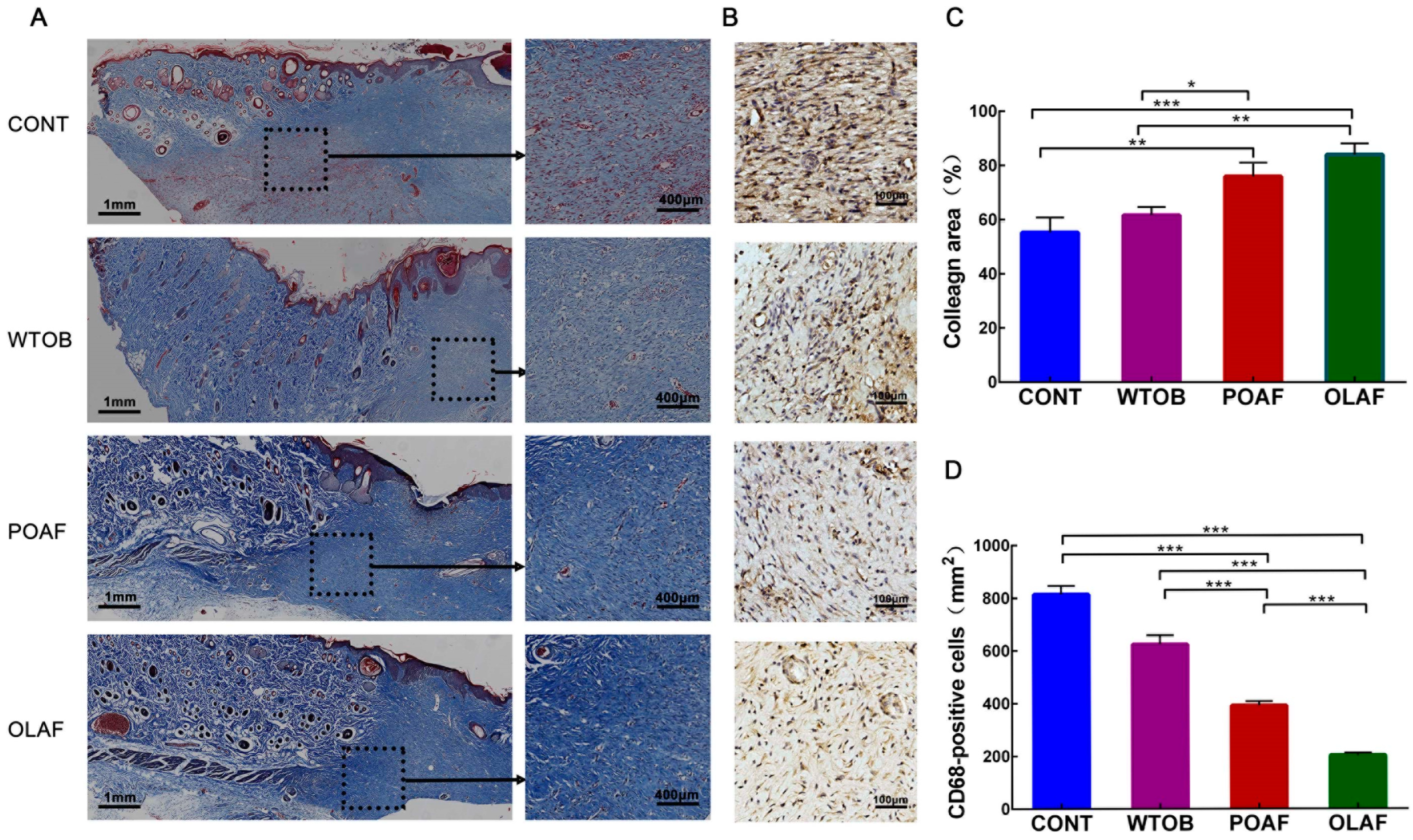
2.2. The Effects of Oleosomes Fused with Human Fibroblast Growth Factor 1 on the Collagen Deposition and CD68+ Macrophage Infiltration of Wounds
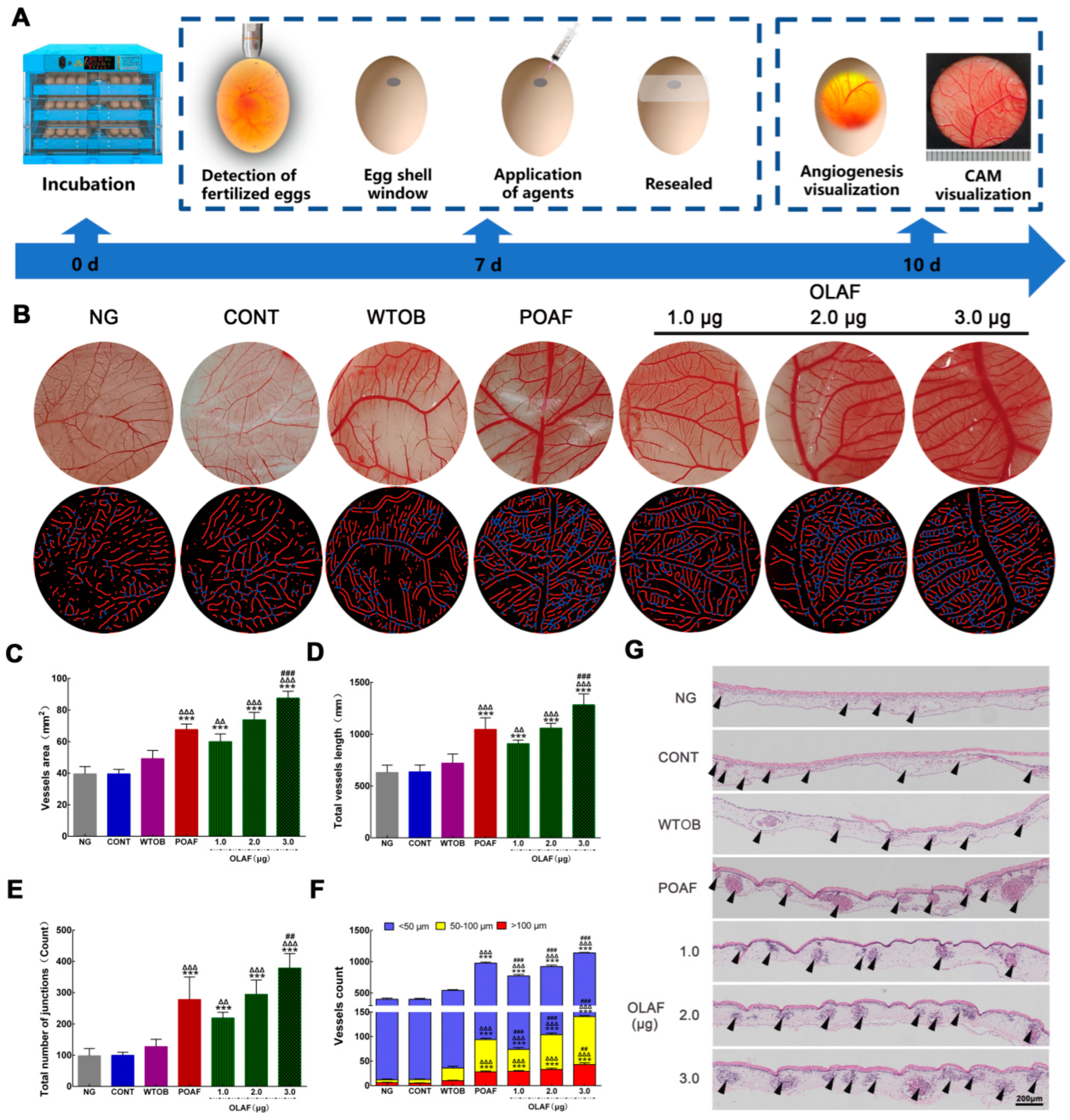
2.3. Oleosomes Fused with Human Fibroblast Growth Factor 1 Facilitated Angiogenesis at the Wound Sites
2.4. The Effect of Oleosomes Fused with Human Fibroblast Growth Factor 1 on Vascular Permeability in Rats
2.5. The Oleosomes Fused with Human Fibroblast Growth Factor 1 Stimulated Angiogenesis on CAM
2.6. OLAF-Induced Cell Proliferation, Scratch Wound Healing, Migration and Tube Formation in HUVECs
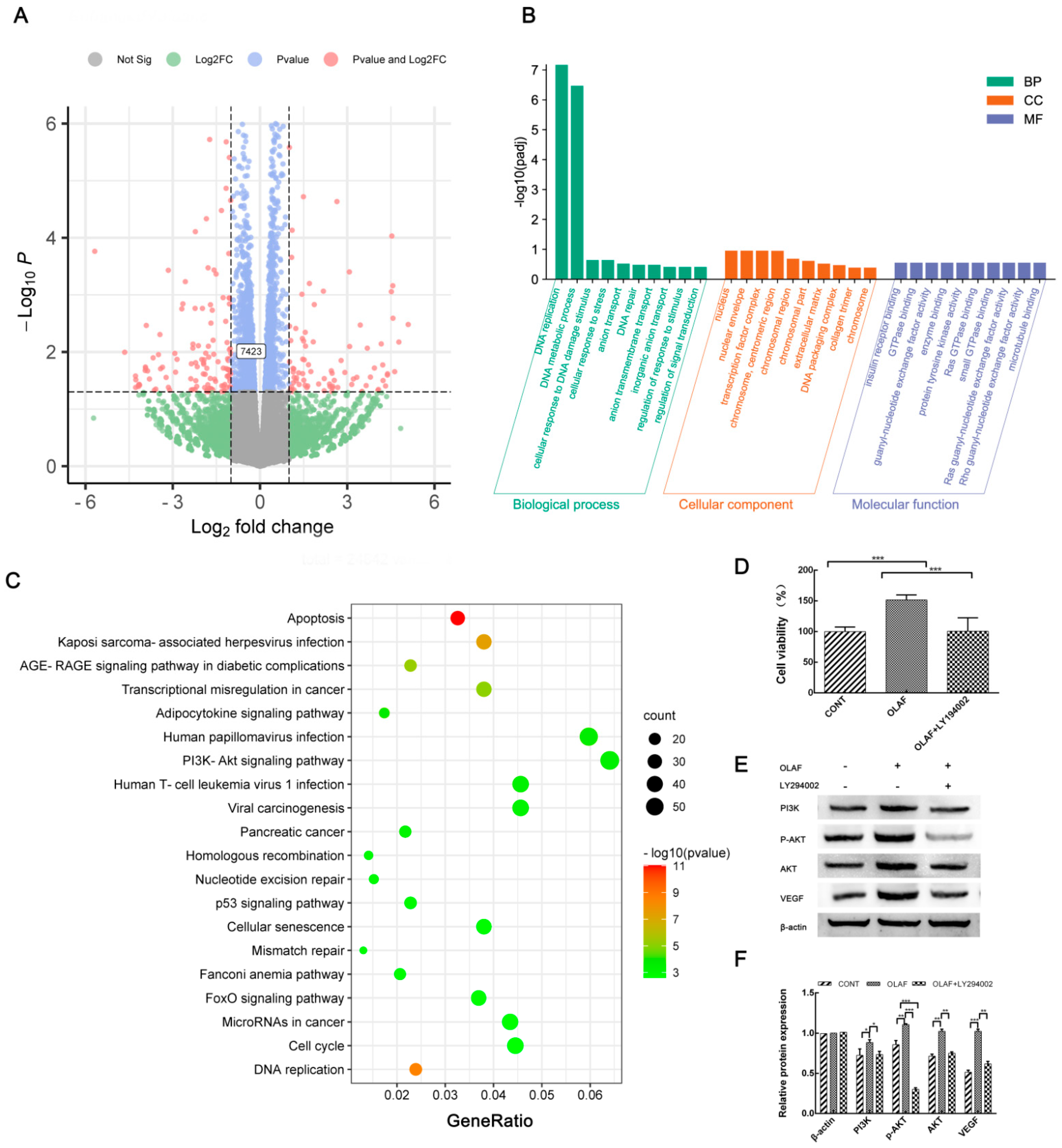
2.7. OLAF Improved Angiogenesis through the PI3K-AKT Pathway
3. Discussion
4. Methods and Materials
4.1. Oleosome Extraction Procedure
4.2. Wound Healing Potential of Oleosomes Fused with hFGF1 in Full-Thickness Skin Defect Rats
4.2.1. Creation and Treatment of Full-Thickness Skin Defect Rat Models
4.2.2. Monitoring of Wound Closure and Specimen Collection
4.2.3. Histopathological, Immunohistochemical and Immunofluorescence Assessment
4.2.4. Evan’s Blue Assay
4.3. Chick Embryo Chorioallantoic Membrane (CAM) Assay for the Pro-Angiogenic Potential of OLAF
4.4. In Vitro Evaluation of the Beneficial Effect of OLAF on Angiogenesis
4.4.1. Cell Culture
4.4.2. Cell Proliferation Vitality Assessment and Morphological Observation
4.4.3. Scratch Wound Assay and Transwell Migration Test
4.4.4. Tube Formation Assay
4.5. RNA-Seq-Based Transcriptome Analysis of HUVECs under OLAF Treatment
4.6. Western Blot and qRT-PCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lien, L.T.; Tho, N.T.; Ha, D.M.; Hang, P.L.; Nghia, P.T.; Thang, N.D. Influence of phytochemicals in piper betle linn leaf extract on wound healing. Burns Trauma 2015, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Nour, S.; Imani, R.; Chaudhry, G.R.; Sharifi, A.M. Skin wound healing assisted by angiogenic targeted tissue engineering: A comprehensive review of bioengineered approaches. J. Biomed. Mater. Res. 2021, 109, 453–478. [Google Scholar] [CrossRef] [PubMed]
- Pazyar, N.; Yaghoobi, R.; Rafiee, E.; Mehrabian, A.; Feily, A. Skin wound healing and phytomedicine: A review. Skin Pharmacol. Physiol. 2012, 27, 303–310. [Google Scholar] [CrossRef]
- Okur, M.E.; Ayla, Ş.; Polat, D.Ç.; Günal, M.Y.; Yoltaş, A.; Biçeroğlu, Ö. Novel insight into wound healing properties of methanol extract of Capparis ovata Desf. var. palaestina Zohary fruits. J. Pharm. Pharmacol. 2018, 70, 1401–1413. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Chattopadhyay, P.; Islam, J.; Ray, S.; Raju, P.S.; Mazumder, B. Aspects of nanomaterials in wound healing. Curr. Drug Deliv. 2019, 16, 26–41. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.H.; Xue, Y.Y.; Cheng, X.T.; Zhao, W.F.; Wang, J.; He, R.; Wan, Q.B.; Pei, X.B. Tazarotene released from aligned electrospun membrane facilitates cutaneous wound healing by promoting angiogenesis. ACS Appl. Mater. Interfaces 2019, 11, 36141–36153. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, D.B.; Severn, C.E.; Twomey, C.; Greenhough, A.; Cash, J.; Toye, A.M.; Mellor, H.; Martin, P. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J. 2018, 37, e97786. [Google Scholar] [CrossRef]
- Liu, X.A.; Yang, Y.F.; Guo, X.J.; Liu, L.L.; Wu, K.L.; Yu, M.B. The antiangiogenesis effect of pirfenidone in wound healing in vitro. J. Ocul. Pharmacol. Ther. 2017, 33, 693–703. [Google Scholar] [CrossRef]
- Guerra, A.; Belinha, J.; Jorge, R.N. Modelling skin wound healing angiogenesis: A review. J. Theor. Biol. 2018, 459, 1–17. [Google Scholar] [CrossRef]
- Almalki, W.H.; Shahid, I.; Mehdi, A.Y.; Hafeez, M.H. Assessment methods for angiogenesis and current approaches for its quantification. Indian J. Pharmacol. 2014, 46, 251–256. [Google Scholar] [CrossRef]
- Schiffmann, L.M.; Werthenbach, J.P.; Heintges-Kleinhofer, F.; Seeger, J.M.; Fritsch, M.; Günther, S.D.; Willenborg, S.; Brodesser, S.; Lucas, C.; Jüngst, C.; et al. Mitochondrial respiration controls neoangiogenesis during wound healing and tumor growth. Nat. Commun. 2020, 11, 3653. [Google Scholar] [CrossRef]
- Odorisio, T.; Cianfarani, F.; Failla, C.M.; Zambruno, G. The placenta growth factor in skin angiogenesis. J. Dermatol. Sci. 2006, 41, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Kwon, Y.W.; Park, G.T.; Do, E.K.; Yoon, J.W.; Kim, S.C.; Ko, H.C.; Kim, M.B.; Kim, J.H. Atrial natriuretic peptide accelerates human endothelial progenitor cell-stimulated cutaneous wound healing and angiogenesis. Wound Repair Regen. 2018, 26, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Zhao, Y.; Zhang, Q.; Gao, G.Y.; Zhang, S.L.; Gao, Y.; Chen, X.F.; Qian, X.P. Alkaloid extract of Corydalis yanhusuo inhibits angiogenesis via targeting vascular endothelial growth factor receptor signaling. BMC Complement. Altern. Med. 2019, 19, 359. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.N.; Zhu, N.; Liu, C.; Wu, H.T.; Gui, Y.; Liao, D.F.; Qin, L. Wnt5a and its signaling pathway in angiogenesis. Clin. Chim. Acta. 2017, 471, 263–269. [Google Scholar] [CrossRef]
- Norrby, K. In vivo models of angiogenesis. J. Cell. Mol. Med. 2006, 10, 588–612. [Google Scholar] [CrossRef]
- Cho, W.C.; Jour, G.; Aung, P.P. Role of angiogenesis in melanoma progression: Update on key angiogenic mechanisms and other associated components. Semin. Cancer Biol. 2019, 59, 175–186. [Google Scholar] [CrossRef]
- Li, R.; Wang, B.N.; Wu, C.B.; Li, D.H.; Wu, Y.Q.; Ye, L.P.; Ye, L.X.; Chen, X.J.; Li, P.F.; Yuan, Y.; et al. Acidic fibroblast growth factor attenuates type 2 diabetes-induced demyelination via suppressing oxidative stress damage. Cell Death Dis. 2021, 12, 107. [Google Scholar] [CrossRef]
- Liu, Y.M.; Yu, F.L.; Zhang, B.B.; Zhou, M.; Bei, Y. Improving the protective effects of aFGF for peripheral nerve injury repair using sulfated chitooligosaccharides. Asian J. Pharm. Sci. 2019, 14, 511–520. [Google Scholar] [CrossRef]
- Zhou, N.Q.; Fang, Z.X.; Huang, N.; Zuo, Y.; Qiu, Y.; Guo, L.J.; Song, P.; Xu, J.; Wan, G.R.; Tian, X.Q.; et al. aFGF targeted mediated by novel nanoparticles-microbubble complex combined with ultrasound-targeted microbubble destruction attenuates doxorubicin-induced heart failure via anti-apoptosis and promoting cardiac angiogenesis. Front. Pharmacol. 2021, 12, 607785. [Google Scholar] [CrossRef]
- Ma, J.K.; Barros, E.; Bock, R.; Christou, P.; Dale, P.J.; Fischer, R.; Irwin, J.; Mahoney, R.; Pezzotti, M.; Schillberg, S.; et al. Molecular farming for new drugs and vaccines. Current perspectives on the production of pharmaceuticals in transgenic plants. EMBO Rep. 2005, 6, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, J.; Guan, L.L.; Yi, S.Y.; Du, L.N.; Tian, H.S.; Guo, Y.X.; Zhai, F.; Lu, Z.; Li, H.Y.; et al. Expression of bioactive recombinant human fibroblast growth factor 10 in Carthamus tinctorius L. seeds. Protein Expr. Purif. 2017, 138, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.T.; Wang, F.W.; Hu, X.L.; Li, Y.L.; Zhang, Y.; Carther, K.F.I.; Wang, B.; Min, F.; Wang, X.; Wu, H.; et al. Camelina lipid droplets as skin delivery system promotes wound repair by enhancing the absorption of hFGF2. Int. J. Pharm. 2021, 598, 120327. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, S.C.; Kaushik, V.; Yadav, M.K. Use of oil bodies and oleosins in recombinant protein production and other biotechnological applications. Biotechnol. Adv. 2010, 28, 293–300. [Google Scholar] [CrossRef]
- Nykiforuk, C.L. Liquid-liquid phase separation of oil bodies from seeds. Methods Mol. Biol. 2016, 1385, 173–188. [Google Scholar] [CrossRef]
- Julien, J.A.; Pellett, A.L.; Shah, S.S.; Wittenberg, N.J.; Glover, K.J. Preparation and characterization of neutrally-buoyant oleosin-rich synthetic lipid droplets. Biochim. Biophys. Acta. Biomembr. 2021, 1863, 183624. [Google Scholar] [CrossRef]
- Adams, G.G.; Imran, S.; Wang, S.; Mohammad, A.; Kok, M.S.; Gray, D.A.; Channell, G.A.; Harding, S.E. Extraction, isolation and characterisation of oil bodies from pumpkin seeds for therapeutic use. Food Chem. 2012, 134, 1919–1925. [Google Scholar] [CrossRef]
- Xu, J.F.; Dolan, M.C.; Medrano, G.; Cramer, C.L.; Weathers, P.J. Green factory: Plants as bioproduction platforms for recombinant proteins. Biotechnol. Adv. 2012, 30, 1171–1184. [Google Scholar] [CrossRef]
- Lundquist, P.K.; Shivaiah, K.K.; Espinoza-Corral, R. Lipid droplets throughout the evolutionary tree. Prog. Lipid Res. 2020, 78, 101029. [Google Scholar] [CrossRef]
- Yang, J.; Guan, L.L.; Guo, Y.X.; Du, L.N.; Wang, F.W.; Wang, Y.F.; Lu, Z.; Wang, Q.M.; Zou, D.Y.; Chen, W.; et al. Expression of biologically recombinant human acidic fibroblast growth factor in Arabidopsis thaliana seeds via oleosin fusion technology. Gene 2015, 566, 89–94. [Google Scholar] [CrossRef]
- Guo, Y.X.; Li, Y.Y.; Wu, Q.; Lan, X.X.; Chu, G.D.; Qiang, W.D.; Noman, M.; Gao, T.T.; Guo, J.N.; Han, L.; et al. Optimization of the extraction conditions and dermal toxicity of oil body fused with acidic fibroblast growth factor (OLAF). Cutan. Ocul. Toxicol. 2021, 40, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Y.; Liang, J.; Xu, Y.Q.; Liu, J.; Liu, Y. The wound healing effects of the Tilapia collagen peptide mixture TY001 in streptozotocin diabetic mice. J. Sci. Food Agric. 2020, 100, 2848–2858. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, B.; Vadalà, M.; Laurino, C. Nutrition in wound healing: Investigation of the molecular mechanisms, a narrative review. J. Wound Care 2019, 28, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, D.S.; Kim, C.E.; Kim, C.E.; Shin, M.S.; Seo, C.S.; Shin, H.K.; Hwang, G.S.; An, J.M.; Kim, S.N. Effects of fermented black ginseng on wound healing mediated by angiogenesis through the mitogen-activated protein kinase pathway in human umbilical vein endothelial cells. J. Ginseng Res. 2018, 42, 524–531. [Google Scholar] [CrossRef]
- Acevedo, F.; Rubilar, M.; Jofré, I.; Villarroel, M.; Navarrete, P.; Esparza, M.; Romero, F.; Vilches, E.A.; Acevedo, V.; Shene, C. Oil bodies as a potential microencapsulation carries for astaxanthin stabilisation and safe delivery. J. Microencapsul. 2014, 31, 488–500. [Google Scholar] [CrossRef]
- Caillon, L.; Nieto, V.; Gehan, P.; Omrane, M.; Rodriguez, N.; Monticelli, L.; Thiam, A.R. Triacylglycerols sequester monotopic membrane proteins to lipid droplets. Nat. Commun. 2020, 11, 3944. [Google Scholar] [CrossRef]
- Shao, Q.; Liu, X.F.; Su, T.; Ma, C.L.; Wang, P.P. New insights into the role of seed oil body proteins in metabolism and plant development. Front. Plant Sci. 2019, 10, 1568. [Google Scholar] [CrossRef]
- Vargo, K.B.; Zaki, A.A.; Warden-Rothman, R.; Tsourkas, A.; Hammer, D.A. Superparamagnetic iron oxide nanoparticle micelles stabilized by recombinant oleosin for targeted magnetic resonance imaging. Small 2015, 11, 1409–1413. [Google Scholar] [CrossRef]
- Makkhun, S.; Khosla, A.; Foster, T.; McClements, D.J.; Grundy, M.M.; Gray, D.A. Impact of extraneous proteins on the gastrointestinal fate of sunflower seed (Helianthus annuus) oil bodies: A simulated gastrointestinal tract study. Food Funct. 2015, 6, 125–135. [Google Scholar] [CrossRef]
- Chaudhary, S.; Parmenter, D.L.; Moloney, M.M. Transgenic Brassica carinata as a vehicle for the production of recombinant proteins in seeds. Plant Cell Rep. 1998, 17, 195–200. [Google Scholar] [CrossRef]
- Gujral, G.S.; Askari, S.N.; Ahmad, S.; Zakir, S.M.; Saluja, K. Topical vitamin C, vitamin E, and acetylcysteine as corneal wound healing agents: A comparative study. Indian J. Ophthalmol. 2020, 68, 2935–2939. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.R.; Tang, Y.; Pang, L.B.; Lin, C.L.; Huang, W.H.; Wang, D.P.; Jia, W.T. Angiogenesis and full-thickness wound healing efficiency of copper-doped borate bioactive glass/poly (lactic-co-glycolic acid) dressing loaded with vitamin E in vivo and in vitro. ACS Appl. Mater. Interfaces 2018, 10, 22939–22950. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.N.; Huang, X.; Yang, X.Q.; Guo, J.; Yin, S.W.; He, X.T.; Wang, L.J.; Zhu, J.H.; Qi, J.R.; Zheng, E.L. In vitro assessment of the bioaccessibility of fatty acids and tocopherol from soybean oil body emulsions stabilized with ι-carrageenan. J. Agric. Food Chem. 2012, 61, 1567–1575. [Google Scholar] [CrossRef]
- Kim, E.J.; Jin, X.J.; Kim, Y.K.; Oh, I.K.; Kim, J.E.; Park, C.H.; Chung, J.H. UV decreases the synthesis of free fatty acids and triglycerides in the epidermis of human skin in vivo, contributing to development of skin photoaging. J. Dermatol. Sci. 2010, 57, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Korntner, S.; Lehner, C.; Gehwolf, R.; Wagner, A.; Grütz, M.; Kunkel, N.; Tempfer, H.; Traweger, A. Limiting angiogenesis to modulate scar formation. Adv. Drug Deliv. Rev. 2019, 146, 170–189. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM) assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Vacca, A.; Roncali, L.; Presta, M. Endogenous and exogenous fibroblast growth factor-2 modulate wound healing in the chick embryo chorioallantoic membrane. Angiogenesis 1999, 3, 89–95. [Google Scholar] [CrossRef]
- Forough, R.; Weylie, B.; Patel, C.; Ambrus, S.; Singh, U.S.; Zhu, J. Role of AKT/PKB signaling in fibroblast growth factor-1 (FGF-1)-induced angiogenesis in the chicken choriallantoic membrane (CAM). J. Cell. Biochem. 2005, 94, 109–116. [Google Scholar] [CrossRef]
- Nawara, H.M.; Afify, S.M.; Hassan, G.; Zahra, M.H.; Atallah, M.N.; Seno, A.; Seno, M. An assay for cancer stem cell-induced angiogenesis on chick chorioallantoic membrane. Cell Biol. Int. 2021, 45, 749–756. [Google Scholar] [CrossRef]
- Bai, Y.; Bai, L.J.; Zhou, J.; Chen, H.L.; Zhang, L.K. Sequential delivery of VEGF, FGF-2 and PDGF from the polymeric system enhance HUVECs angiogenesis in vitro and CAM angiogenesis. Cell Immunol. 2018, 323, 19–32. [Google Scholar] [CrossRef]
- Belvedere, R.; Bizzarro, V.; Parente, L.; Petrella, F.; Petrella, A. The pharmaceutical device prisma skin promotes in vitro angiogenesis through endothelial to mesenchymal transition during skin wound healing. Int. J. Mol. Sci. 2017, 18, 1614. [Google Scholar] [CrossRef] [PubMed]
- Somanath, P.R.; Chen, J.; Byzova, T.V. Akt1 is necessary for the vascular maturation and angiogenesis during cutaneous wound healing. Angiogenesis 2008, 11, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.W.; Jiang, J.S.; Lu, W.Q. Ferulic acid exerts anti-angiogenic and anti-tumor activity by targeting fibroblast growth factor receptor 1-mediated angiogenesis. Int. J. Mol. Sci. 2015, 16, 24011–24031. [Google Scholar] [CrossRef] [PubMed]
- Forough, R.; Weylie, B.; Collins, C.; Parker, J.L.; Zhu, J.; Barhoumi, R.; Watson, D.K. Transcription factor Ets-1 regulates fibroblast growth factor-1-mediated angiogenesis in vivo: Role of Ets-1 in the regulation of the PI3K/AKT/MMP-1 pathway. J. Vasc. Res. 2006, 43, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Manjunathan, R.; Devarajan, N.; Ragunathan, M. Possible mechanism of human recombinant leptin-induced VEGF A synthesis via PI3K/Akt/mTOR/S6 kinase signaling pathway while inducing angiogenesis: An analysis using chicken chorioallantoic membrane model. J. Vasc. Res. 2021, 58, 343–360. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.; Liang, F.; Chen, Y.; Yang, G. Integrin alpha x stimulates cancer angiogenesis through PI3K/Akt signaling-mediated VEGFR2/VEGF-A overexpression in blood vessel endothelial cells. J. Cell. Biochem. 2019, 120, 1807–1818. [Google Scholar] [CrossRef]
- Di, J.; Gao, K.; Qu, D.; Yang, J.; Zheng, J. Rap2B promotes angiogenesis via PI3K/AKT/VEGF signaling pathway in human renal cell carcinoma. Tumour Biol. 2017, 39, 1010428317701653. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Kheirandish, R.; Salarpoor, M. Topical effect of allogenous serum rich in growth factors (SRGF) on diabetic skin wound in rat. Transfus. Aphe. Sci. 2019, 58, 498–504. [Google Scholar] [CrossRef]
- Deng, Z.H.; Yin, J.J.; Luo, W.; Kotian, R.N.; Gao, S.S.; Yi, Z.Q.; Xiao, W.F.; Li, W.P.; Li, Y.S. The effect of earthworm extract on promoting skin wound healing. Biosci. Rep. 2018, 38, BSR20171366. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, J.J.; He, C.C.; Xiao, Z.C.; Ye, J.J.; Li, Y.; Chen, A.Q.; Zhang, H.Y.; Li, X.K.; Lin, L.; et al. Comparative study of heparin-poloxamer hydrogel modified bFGF and aFGF for in vivo wound healing efficiency. ACS Appl. Mater. Interfaces 2016, 8, 18710–18721. [Google Scholar] [CrossRef]
- Arasteh, S.; Khanjani, S.; Golshahi, H.; Mobini, S.; Jahed, M.T.; Heidari-Vala, H.; Edalatkhan, H.; Kazemnejad, S. Efficient wound healing using synthetic nanofibrous bilayer skin substitute in murine model. J. Surg. Res. 2020, 245, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, S.; Bal, A.; Singh, G.; Joshi, K. Tumor angiogenesis in breast cancer: Pericytes and maturation dose not correlate with lymph node metastasis and molecular subtypes. Clin. Breast Cancer 2016, 16, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.F.; Liu, Y.S.; Zhu, L.L.; Tang, J.B.; Huang, W.H.; Cheng, B. Lumbar sympathectomy reduces vascular permeability, possibly through decreased adenosine receptor A2a expression in the hind plantar skin of rats. Clin. Hemorheol. Microcirc. 2018, 68, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.H.; Liu, X.H.; Yang, Y.; Wei, B.; Li, Q.M.; Mao, G.Q.; He, Y.J.; Li, Y.Y.; Zheng, L.Y.; Zhang, Q.Q.; et al. Decylubiquinone suppresses breast cancer growth and metastasis by inhibiting angiogenesis via the ROS/p53/BAl1 signaling pathway. Angiogenesis 2020, 23, 325–338. [Google Scholar] [CrossRef]
- Pan, J.; Wang, X.L.; Li, D.Q.; Li, J.M.; Jiang, Z.P. MSCs inhibits the angiogenesis of HUVECs through the miR-211/Prox1 pathway. J. Biochem. 2019, 166, 107–133. [Google Scholar] [CrossRef]
- Miao, X.; Xiang, Y.W.; Mao, W.W.; Chen, Y.R.; Li, Q.; Fan, B. TRIM27 promotes the proliferation and inflammation factor production induced by IL-6 by activating STAT3 signaling in HaCaT cells. Am. J. Physiol. Cell Physiol. 2020, 318, C272–C281. [Google Scholar] [CrossRef]
- Aquino, F.L.T.; Silva, J.P.D.; Ferro, J.N.S.; Lagente, V.; Barreto, E. trans-Cinnamic acid, but not p-coumaric acid or methyl cinnamate, induces fibroblast migration through PKA- and p38-MAPK signalling pathways. J. Tissue Viability 2021, 30, 363–371. [Google Scholar] [CrossRef]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer 2019, 19, 137. [Google Scholar] [CrossRef]
- Zhao, S.C.; Song, G.X.; Yuan, J.; Qiao, S.; Xu, S.; Si, Z.H.; Yang, Y.; Xu, X.X.; Wang, A.H. Circular RNA circ_0003204 inhibits proliferation, migration and tube formation of endothelial cell in atherosclerosis via miR-370-3p/TGFβR2/phosph-SMAD3 axis. J. Biomed. Sci. 2020, 27, 11. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Chu, G.; Cai, W.; Li, Y.; Lan, X.; Li, J.; Du, L.; Yang, J. Beneficial Effects of Oleosomes Fused with Human Fibroblast Growth Factor 1 on Wound Healing via the Promotion of Angiogenesis. Int. J. Mol. Sci. 2022, 23, 13152. https://doi.org/10.3390/ijms232113152
Guo Y, Chu G, Cai W, Li Y, Lan X, Li J, Du L, Yang J. Beneficial Effects of Oleosomes Fused with Human Fibroblast Growth Factor 1 on Wound Healing via the Promotion of Angiogenesis. International Journal of Molecular Sciences. 2022; 23(21):13152. https://doi.org/10.3390/ijms232113152
Chicago/Turabian StyleGuo, Yongxin, Guodong Chu, Weijia Cai, Yaying Li, Xinxin Lan, Jing Li, Linna Du, and Jing Yang. 2022. "Beneficial Effects of Oleosomes Fused with Human Fibroblast Growth Factor 1 on Wound Healing via the Promotion of Angiogenesis" International Journal of Molecular Sciences 23, no. 21: 13152. https://doi.org/10.3390/ijms232113152
APA StyleGuo, Y., Chu, G., Cai, W., Li, Y., Lan, X., Li, J., Du, L., & Yang, J. (2022). Beneficial Effects of Oleosomes Fused with Human Fibroblast Growth Factor 1 on Wound Healing via the Promotion of Angiogenesis. International Journal of Molecular Sciences, 23(21), 13152. https://doi.org/10.3390/ijms232113152




