Determination of Melting Parameters of Cyclodextrins Using Fast Scanning Calorimetry
Abstract
1. Introduction
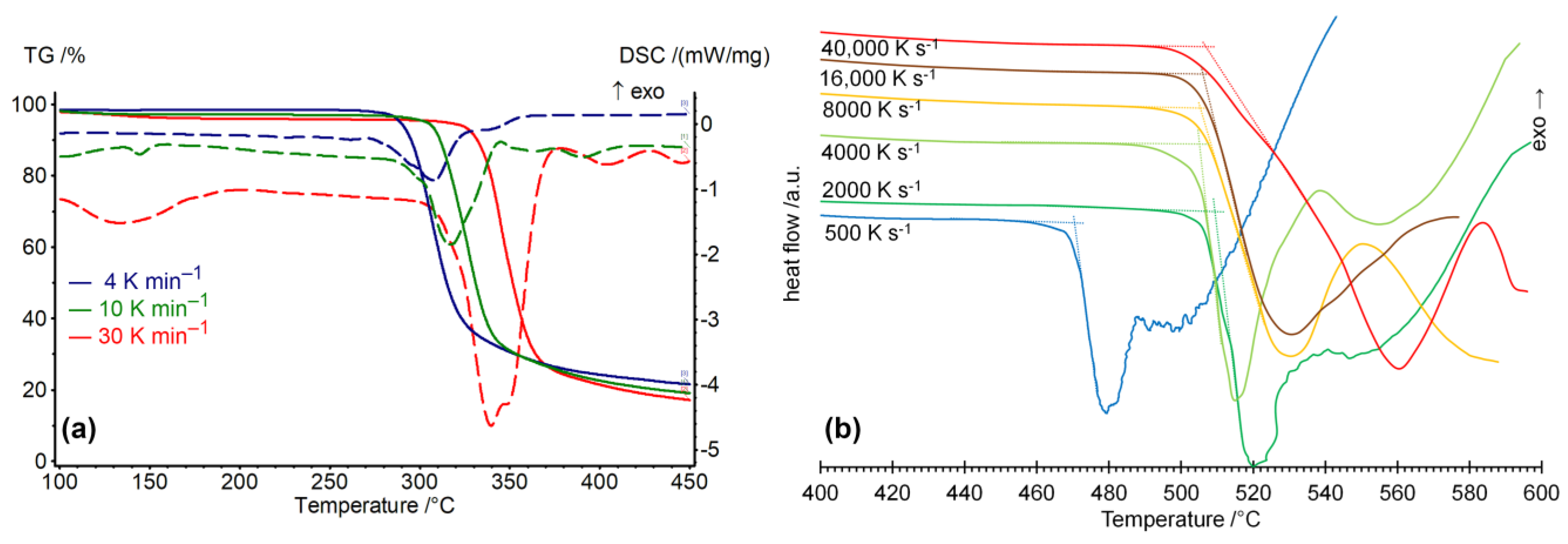
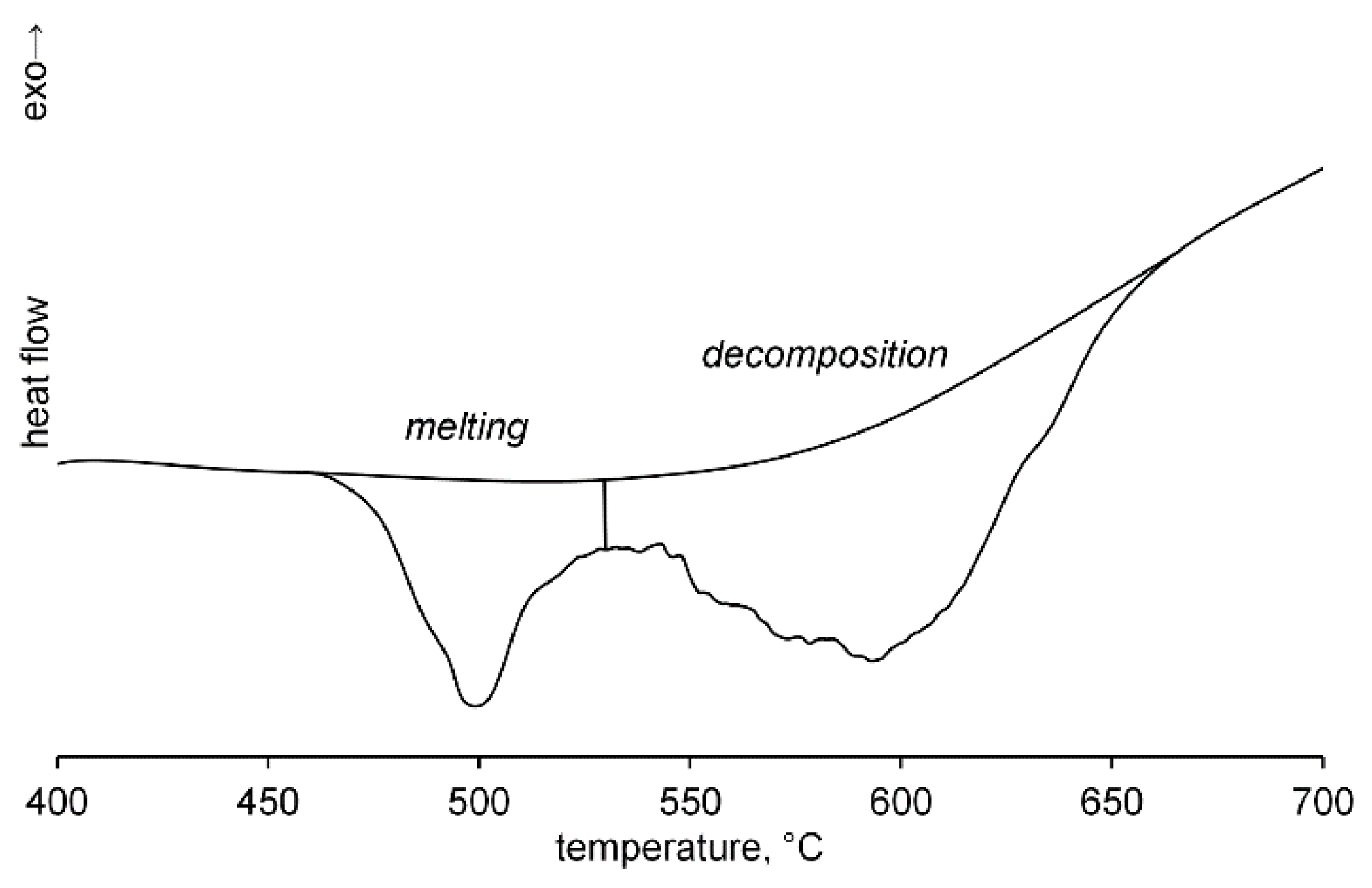
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Simultaneous TG/DSC Experiment
3.3. Model-Free Methods of Thermokinetic Analysis
3.4. DSC Experiment
3.5. Fast Scanning Calorimetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chua, Y.Z.; Do, H.T.; Kumar, A.; Hallermann, M.; Zaitsau, D.; Schick, C.; Held, C. The Melting Properties of D-α-Glucose, D-β-Fructose, D-Sucrose, D-α-Galactose, and D-α-Xylose and Their Solubility in Water: A Revision. Food Biophys. 2022, 17, 181–197. [Google Scholar] [CrossRef]
- Jain, N.; Yalkowsky, S.H. Estimation of the Aqueous Solubility I: Application to Organic Nonelectrolytes. J. Pharm. Sci. 2001, 90, 234–252. [Google Scholar] [CrossRef]
- Manson, A.; Sefcik, J.; Lue, L. Temperature Dependence of Solubility Predicted from Thermodynamic Data Measured at a Single Temperature: Application to α, β, and γ-Glycine. Cryst. Growth Des. 2022, 22, 1691–1706. [Google Scholar] [CrossRef] [PubMed]
- Yoshihashi, Y.; Kitano, H.; Yonemochi, E.; Terada, K. Quantitative Correlation between Initial Dissolution Rate and Heat of Fusion of Drug Substance. Int. J. Pharm. 2000, 204, 1–6. [Google Scholar] [CrossRef]
- Rimer, J.D.; Trofymluk, O.; Navrotsky, A.; Lobo, R.F.; Vlachos, D.G. Kinetic and Thermodynamic Studies of Silica Nanoparticle Dissolution. Chem. Mater. 2007, 19, 4189–4197. [Google Scholar] [CrossRef]
- Do, H.T.; Chua, Y.Z.; Kumar, A.; Pabsch, D.; Hallermann, M.; Zaitsau, D.; Schick, C.; Held, C. Melting Properties of Amino Acids and Their Solubility in Water. RSC Adv. 2020, 10, 44205–44215. [Google Scholar] [CrossRef]
- Wyttenbach, N.; Niederquell, A.; Kuentz, M. Machine Estimation of Drug Melting Properties and Influence on Solubility Prediction. Mol. Pharm. 2020, 17, 2660–2671. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Viernstein, H.; Wolschann, P. Cyclodextrin Inclusion Complexation and Pharmaceutical Applications. ScienceAsia 2020, 46, 254. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Bandur, G.N.; David, I.; Hădărugă, D.I. A Review on Thermal Analyses of Cyclodextrins and Cyclodextrin Complexes. Environ. Chem. Lett. 2019, 17, 349–373. [Google Scholar] [CrossRef]
- Abdelaziz, A.; Zaitsau, D.H.; Mukhametzyanov, T.A.; Solomonov, B.N.; Cebe, P.; Verevkin, S.P.; Schick, C. Melting Temperature and Heat of Fusion of Cytosine Revealed from Fast Scanning Calorimetry. Thermochim. Acta 2017, 657, 47–55. [Google Scholar] [CrossRef]
- Safiullina, A.S.; Buzyurov, A.V.; Ziganshina, S.A.; Gerasimov, A.V.; Schick, C.; Gorbatchuk, V.V.; Ziganshin, M.A. Using Fast Scanning Calorimetry to Study Solid-State Cyclization of Dipeptide L-Leucyl-L-Leucine. Thermochim. Acta 2020, 692, 178748. [Google Scholar] [CrossRef]
- Gabdulkhaev, M.N.; Ziganshin, M.A.; Buzyurov, A.V.; Schick, C.; Solovieva, S.E.; Popova, E.V.; Gubaidullin, A.T.; Gorbatchuk, V.V. Smart Control of Calixarene Polymorphic States. CrystEngComm 2020, 22, 7002–7015. [Google Scholar] [CrossRef]
- Cebe, P.; Thomas, D.; Merfeld, J.; Partlow, B.P.; Kaplan, D.L.; Alamo, R.G.; Wurm, A.; Zhuravlev, E.; Schick, C. Heat of Fusion of Polymer Crystals by Fast Scanning Calorimetry. Polymer 2017, 126, 240–247. [Google Scholar] [CrossRef]
- Do, H.T.; Chua, Y.Z.; Habicht, J.; Klinksiek, M.; Hallermann, M.; Zaitsau, D.; Schick, C.; Held, C. Melting Properties of Peptides and Their Solubility in Water. Part 1: Dipeptides Based on Glycine or Alanine. RSC Adv. 2019, 9, 32722–32734. [Google Scholar] [CrossRef]
- Corvis, Y.; Wurm, A.; Schick, C.; Espeau, P. Vitreous State Characterization of Pharmaceutical Compounds Degrading upon Melting by Using Fast Scanning Calorimetry. J. Phys. Chem. B 2015, 119, 6848–6851. [Google Scholar] [CrossRef]
- Kons, A.; Mishnev, A.; Mukhametzyanov, T.A.; Buzyurov, A.V.; Lapuk, S.E.; Bērziņš, A. Hexamorphism of Dantrolene: Insight into the Crystal Structures, Stability, and Phase Transformations. Cryst. Growth Des. 2021, 21, 1190–1201. [Google Scholar] [CrossRef]
- Abdelaziz, A.; Zaitsau, D.H.; Kuratieva, N.V.; Verevkin, S.P.; Schick, C. Melting of Nucleobases. Getting the Cutting Edge of “Walden’s Rule”. Phys. Chem. Chem. Phys. 2019, 21, 12787–12797. [Google Scholar] [CrossRef]
- Mathot, V.; Pyda, M.; Pijpers, T.; Vanden Poel, G.; van de Kerkhof, E.; van Herwaarden, S.; van Herwaarden, F.; Leenaers, A. The Flash DSC 1, a Power Compensation Twin-Type, Chip-Based Fast Scanning Calorimeter (FSC): First Findings on Polymers. Thermochim. Acta 2011, 522, 36–45. [Google Scholar] [CrossRef]
- Petitprez, J.; Legrand, F.-X.; Tams, C.; Pipkin, J.D.; Antle, V.; Kfoury, M.; Fourmentin, S. Huge Solubility Increase of Poorly Water-Soluble Pharmaceuticals by Sulfobutylether-β-Cyclodextrin Complexation in a Low-Melting Mixture. Environ. Chem. Lett. 2022, 20, 1561–1568. [Google Scholar] [CrossRef]
- Thomas, A.; Moinuddin, K.; Tretsiakova-McNally, S.; Joseph, P. A Kinetic Analysis of the Thermal Degradation Behaviours of Some Bio-Based Substrates. Polymers 2020, 12, 1830. [Google Scholar] [CrossRef] [PubMed]
- Danil de Namor, A.F.; Traboulssi, R.; Lewis, D.F.V. Host Properties of Cyclodextrins towards Anion Constituents of Antigenic Determinants. A Thermodynamic Study in Water and in N,N-Dimethylformamide. J. Am. Chem. Soc. 1990, 112, 8442–8447. [Google Scholar] [CrossRef]
- Venkatesh, M.; Ravi, P.; Tewari, S.P. Isoconversional Kinetic Analysis of Decomposition of Nitroimidazoles: Friedman Method vs Flynn–Wall–Ozawa Method. J. Phys. Chem. A 2013, 117, 10162–10169. [Google Scholar] [CrossRef] [PubMed]
- Briggner, L.-E.; Wadsö, I. Heat Capacities of Maltose, Maltotriose, Maltotetrose and α-, β-, and γ-Cyclodextrin in the Solid State and in Dilute Aqueous Solution. J. Chem. Thermodyn. 1990, 22, 1067–1074. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of Thermal Degradation of Char-Forming Plastics from Thermogravimetry. Application to a Phenolic Plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General Treatment of the Thermogravimetry of Polymers. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1966, 70A, 487–523. [Google Scholar] [CrossRef]
- Ozawa, T. Estimation of Activation Energy by Isoconversion Methods. Thermochim. Acta 1992, 203, 159–165. [Google Scholar] [CrossRef]
- Gatiatulin, A.K.; Osel’skaya, V.Y.; Ziganshin, M.A.; Gorbatchuk, V.V. Smart Control of Guest Inclusion by α-Cyclodextrin Using Its Hydration History. RSC Adv. 2019, 9, 37778–37787. [Google Scholar] [CrossRef]
- Buzyurov, A.V.; Nagrimanov, R.N.; Zaitsau, D.H.; Mukhametzyanov, T.A.; Abdelaziz, A.; Solomonov, B.N.; Schick, C. Application of the Flash DSC 1 and 2+ for Vapor Pressure Determination above Solids and Liquids. Thermochim. Acta 2021, 706, 179067. [Google Scholar] [CrossRef]
- Gatiatulin, A.K.; Osel’skaya, V.Y.; Ziganshin, M.A.; Gorbatchuk, V.V. Size Exclusion Effect in Binary Inclusion Compounds of α-Cyclodextrin. Phys. Chem. Chem. Phys. 2018, 20, 26105–26116. [Google Scholar] [CrossRef]
- Gorbatchuk, V.V.; Gatiatulin, A.K.; Ziganshin, M.A.; Gubaidullin, A.T.; Yakimova, L.S. Unusually High Efficiency of β-Cyclodextrin Clathrate Preparation by Water-Free Solid-Phase Guest Exchange. J. Phys. Chem. B 2013, 117, 14544–14556. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.; Wang, W.; Chen, C.; Jiao, L.; Wu, W. Preparation, Characterization, and Bioavailability of Host-Guest Inclusion Complex of Ginsenoside Re with Gamma-Cyclodextrin. Molecules 2021, 26, 7227. [Google Scholar] [CrossRef]

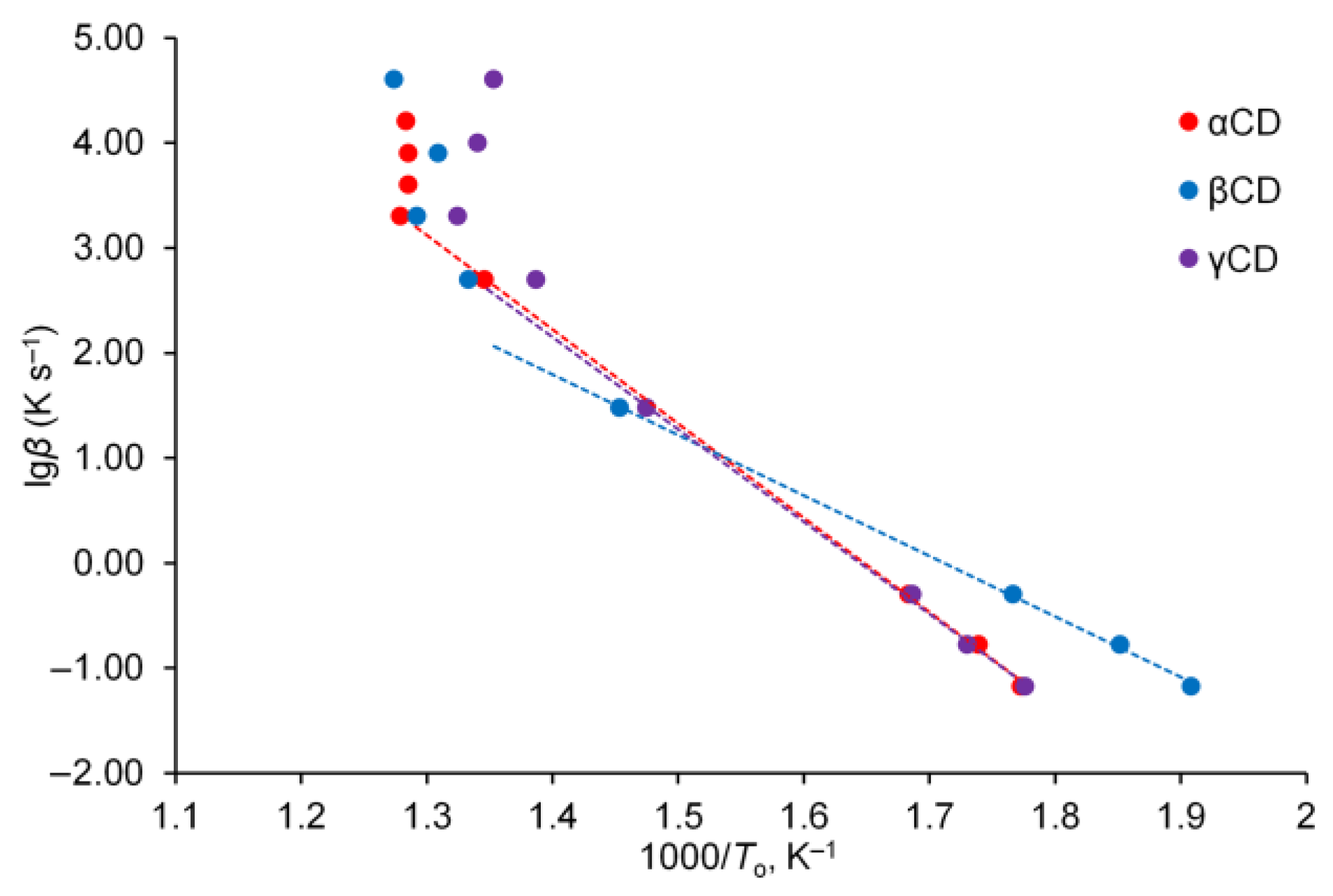
| α-Cyclodextrin | β-Cyclodextrin | γ-Cyclodextrin | |||
|---|---|---|---|---|---|
| β/K s−1 | To/°C | β/K s−1 | To/°C | β/K s−1 | To/°C |
| 0.0667 | 291 | 0.0667 | 251 | 0.0667 | 290 |
| 0.1667 | 302 | 0.1667 | 267 | 0.1667 | 305 |
| 0.5 | 321 | 0.5 | 293 | 0.5 | 320 |
| 500 | 470 | 30 | 415 | 30 | 405 |
| 2000 | 509 | 500 | 477 | 500 | 448 |
| 4000 | 505 | 2000 | 501 | 2000 | 482 |
| 8000 | 506 | 8000 | 491 | 10,000 | 473 |
| 16,000 | 506 | 40,000 | 512 | 40,000 | 466 |
| 40,000 | 508 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatiatulin, A.K.; Grishin, I.A.; Buzyurov, A.V.; Mukhametzyanov, T.A.; Ziganshin, M.A.; Gorbatchuk, V.V. Determination of Melting Parameters of Cyclodextrins Using Fast Scanning Calorimetry. Int. J. Mol. Sci. 2022, 23, 13120. https://doi.org/10.3390/ijms232113120
Gatiatulin AK, Grishin IA, Buzyurov AV, Mukhametzyanov TA, Ziganshin MA, Gorbatchuk VV. Determination of Melting Parameters of Cyclodextrins Using Fast Scanning Calorimetry. International Journal of Molecular Sciences. 2022; 23(21):13120. https://doi.org/10.3390/ijms232113120
Chicago/Turabian StyleGatiatulin, Askar K., Ivan A. Grishin, Aleksey V. Buzyurov, Timur A. Mukhametzyanov, Marat A. Ziganshin, and Valery V. Gorbatchuk. 2022. "Determination of Melting Parameters of Cyclodextrins Using Fast Scanning Calorimetry" International Journal of Molecular Sciences 23, no. 21: 13120. https://doi.org/10.3390/ijms232113120
APA StyleGatiatulin, A. K., Grishin, I. A., Buzyurov, A. V., Mukhametzyanov, T. A., Ziganshin, M. A., & Gorbatchuk, V. V. (2022). Determination of Melting Parameters of Cyclodextrins Using Fast Scanning Calorimetry. International Journal of Molecular Sciences, 23(21), 13120. https://doi.org/10.3390/ijms232113120









