Metabolomic Differences between the Skin and Blood Sera of Atopic Dermatitis and Psoriasis
Abstract
1. Introduction
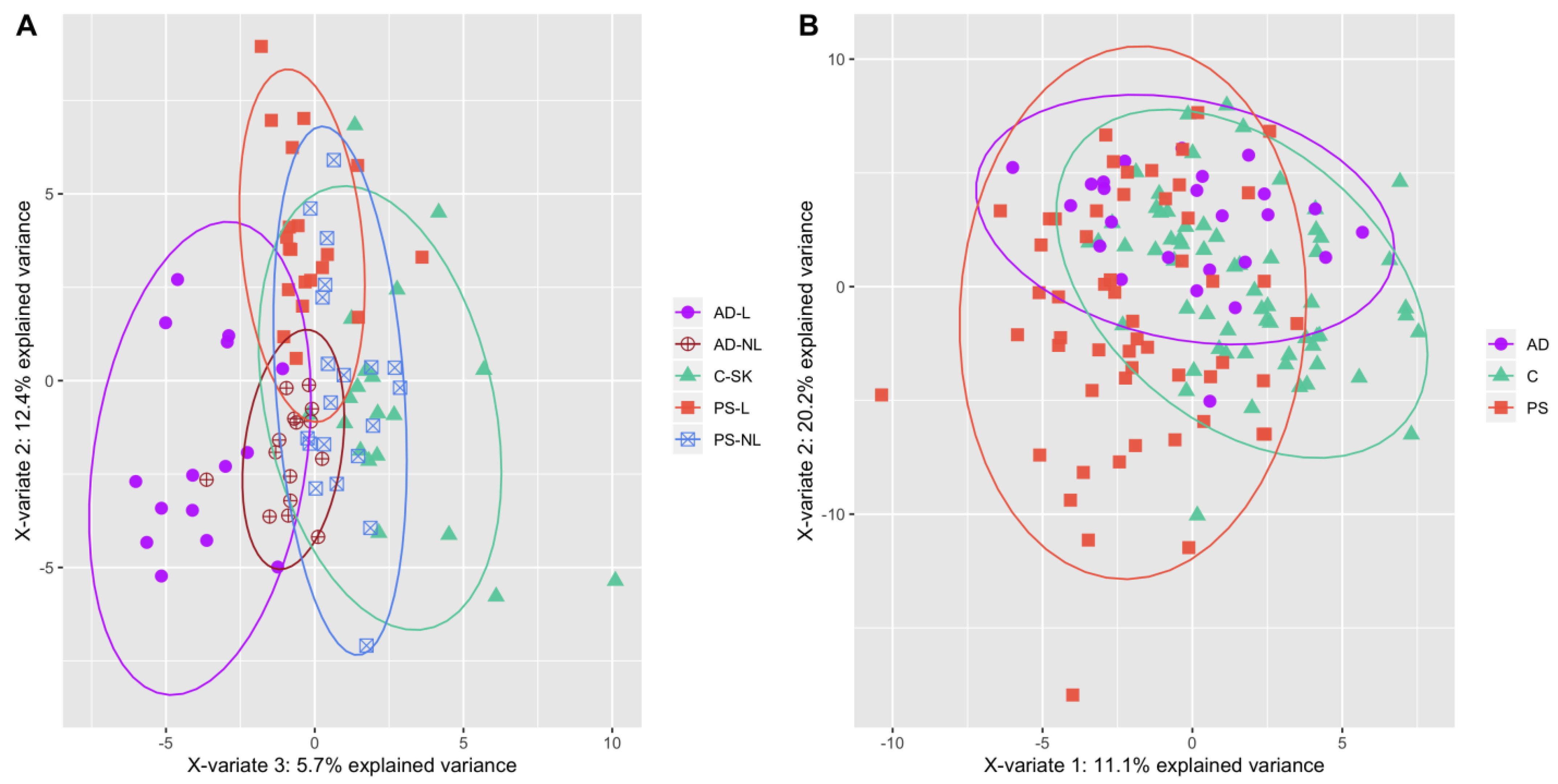
2. Results
3. Discussion
4. Materials and Methods
4.1. Volunteer Recruitment
4.2. Skin Biopsies
4.3. Blood Samples
4.4. Metabolomic Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffiths, C.E.; Barker, J.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Puig, L. Cardiometabolic Comorbidities in Psoriasis and Psoriatic Arthritis. Int. J. Mol. Sci. 2017, 19, 58. [Google Scholar] [CrossRef]
- Tokuyama, M.; Mabuchi, T. New Treatment Addressing the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 21, 7488. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496. [Google Scholar] [CrossRef] [PubMed]
- Nutten, S. Atopic Dermatitis: Global Epidemiology and Risk Factors. Ann. Nutr. Metab. 2015, 66 (Suppl. S1), 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sacotte, R.; Silverberg, J.I. Epidemiology of adult atopic dermatitis. Clin. Dermatol. 2018, 36, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Boothe, W.D.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 2017, 1027, 21–37. [Google Scholar]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Noda, S.; Suárez-Fariñas, M.; Ungar, B.; Kim, S.J.; de Guzman Strong, C.; Xu, H.; Peng, X.; Estrada, Y.D.; Nakajima, S.; Honda, T.; et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J. Allergy Clin. Immunol. 2015, 136, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.M.; Wong, E.; Mallet, A.I.; Olins, L.A.; Greaves, M.W. The analysis of arachidonic acid metabolites in normal, uninvolved and lesional psoriatic skin. Prostaglandins 1984, 28, 57–65. [Google Scholar] [CrossRef]
- Brain, S.; Camp, R.; Derm, F.F.; Dowd, P.; Black, A.K.; Greaves, M. The Release of Leukotriene B4-like Material in Biologically Active Amounts from the Lesional Skin of patients with Psoriasis. J. Investig. Dermatol. 1984, 83, 70. [Google Scholar] [CrossRef]
- Hammarström, S.; Hamberg, M.; Samuelsson, B.; Duell, E.A.; Stawiski, M.; Voorhees, J.J. Increased concentrations of nonesterified arachidonic acid, 12L-hydroxy-5,8,10,14-eicosatetraenoic acid, prostaglandin E2, and prostaglandin F2alpha in epidermis of psoriasis. Proc. Natl. Acad. Sci. USA 1975, 72, 5130–5134. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A.V.; Domenichiello, A.F.; Dey, A.K.; Yuan, Z.-X.; Goyal, A.; Rose, S.M.; Playford, M.P.; Ramsden, C.E.; Mehta, N.N. Bioactive Lipid Mediator Profiles in Human Psoriasis Skin and Blood. J. Investig. Dermatol. 2018, 138, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Sitter, B.; Johnsson, M.K.; Halgunset, J.; Bathen, T.F. Metabolic changes in psoriatic skin under topical corticosteroid treatment. BMC Dermatol. 2013, 13, 8. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Wu, J.; Johnson, M.A.; Grapov, D.; Azizi, B.; Dhillon, J.; Fiehn, O. Metabolomics in psoriatic disease: Pilot study reveals metabolite differences in psoriasis and psoriatic arthritis. F1000Research 2014, 3, 248. [Google Scholar] [CrossRef]
- Chen, C.; Hou, G.; Zeng, C.; Ren, Y.; Chen, X.; Peng, C. Metabolomic profiling reveals amino acid and carnitine alterations as metabolic signatures in psoriasis. Theranostics 2021, 11, 754–767. [Google Scholar] [CrossRef]
- Zeng, C.; Wen, B.; Hou, G.; Lei, L.; Mei, Z.; Jia, X.; Chen, X.; Zhu, W.; Li, J.; Kuang, Y.; et al. Lipidomics profiling reveals the role of glycerophospholipid metabolism in psoriasis. GigaScience 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Berdyshev, E.; Goleva, E.; Bronova, I.; Dyjack, N.; Rios, C.; Jung, J.; Taylor, P.; Jeong, M.; Hall, C.F.; Richers, B.N.; et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight 2018, 3, e98006. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, G.; Liu, X.; Shao, Y.; Gao, P.; Xin, C.; Cui, Z.; Zhao, X.; Xu, G. Serum Metabolomics Study and Eicosanoid Analysis of Childhood Atopic Dermatitis Based on Liquid Chromatography-Mass Spectrometry. J. Proteome Res. 2014, 13, 5715–5723. [Google Scholar] [CrossRef] [PubMed]
- Assfalg, M.; Bortoletti, E.; D’Onofrio, M.; Pigozzi, R.; Molinari, H.; Boner, A.; Peroni, D.; Piacentini, G. An exploratory 1H-nuclear magnetic resonance metabolomics study reveals altered urine spectral profiles in infants with atopic dermatitis. Br. J. Dermatol. 2011, 166, 1123–1125. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. 2018. Available online: https://www.R-project.org/ (accessed on 21 April 2021).
- Ilves, L.; Ottas, A.; Kaldvee, B.; Abram, K.; Soomets, U.; Zilmer, M.; Jaks, V.; Kingo, K. Metabolomic Analysis of Skin Biopsies from Patients with Atopic Dermatitis Reveals Hallmarks of Inflammation, Disrupted Barrier Function and Oxidative Stress. Acta Derm. Venereol. 2021, 101, adv00407. [Google Scholar] [CrossRef] [PubMed]
- Pohla, L.; Ottas, A.; Kaldvee, B.; Abram, K.; Soomets, U.; Zilmer, M.; Reemann, P.; Jaks, V.; Kingo, K. Hyperproliferation is the main driver of metabolomic changes in psoriasis lesional skin. Sci. Rep. 2020, 10, 3081. [Google Scholar] [CrossRef]
- Choi, M.J.; Maibach, H.I. Role of Ceramides in Barrier Function of Healthy and Diseased Skin. Am. J. Clin. Dermatol. 2005, 6, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Hara, J.; Higuchi, K.; Okamoto, R.; Kawashima, M.; Imokawa, G. High-Expression of Sphingomyelin Deacylase is an Important Determinant of Ceramide Deficiency Leading to Barrier Disruption in Atopic Dermatitis1. J. Investig. Dermatol. 2000, 115, 406–413. [Google Scholar] [CrossRef]
- Imokawa, G.; Hattori, M. A possible function of structural lipids in the water-holding properties of the stratum corneum. J. Investig. Dermatol. 1985, 84, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Abe, A.; Jin, K.; Higaki, Y.; Kawashima, M.; Hidano, A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin? J. Investig. Dermatol. 1991, 96, 523–526. [Google Scholar] [CrossRef]
- Van Smeden, J.; Bouwstra, J. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Curr. Probl. Dermatol. 2016, 49, 8–26. [Google Scholar]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef]
- Gibellini, F.; Smith, T.K. The Kennedy pathway-De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 2010, 62, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Luberto, C.; Argraves, K.M. Enzymes of Sphingolipid Metabolism: From Modular to Integrative Signaling. Biochemistry 2001, 40, 4893–4903. [Google Scholar] [CrossRef]
- Imokawa, G. A possible mechanism underlying the ceramide deficiency in atopic dermatitis: Expression of a deacylase enzyme that cleaves the N-acyl linkage of sphingomyelin and glucosylceramide. J. Dermatol. Sci. 2009, 55, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.-M.; Fölster-Holst, R.; Baranowsky, A.; Schunck, M.; Winoto-Morbach, S.; Neumann, C.; Schütze, S.; Proksch, E. Impaired Sphingomyelinase Activity and Epidermal Differentiation in Atopic Dermatitis. J. Investig. Dermatol. 2004, 122, 1423–1431. [Google Scholar] [CrossRef]
- Sawada, E.; Yoshida, N.; Sugiura, A.; Imokawa, G. Th1 cytokines accentuate but Th2 cytokines attenuate ceramide production in the stratum corneum of human epidermal equivalents: An implication for the disrupted barrier mechanism in atopic dermatitis. J. Dermatol. Sci. 2012, 68, 25–35. [Google Scholar] [CrossRef]
- Dutkiewicz, E.P.; Hsieh, K.T.; Wang, Y.S.; Chiu, H.Y.; Urban, P.L. Hydrogel Micropatch and Mass Spectrometry—Assisted Screening for Psoriasis-Related Skin Metabolites. Clin. Chem. 2016, 62, 1120–1128. [Google Scholar] [CrossRef]
- Furse, S.; de Kroon, A.I.P.M. Phosphatidylcholine’s functions beyond that of a membrane brick. Mol. Membr. Biol. 2015, 32, 117–119. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y. The sphingomyelin synthase family: Proteins, diseases, and inhibitors. Biol. Chem. 2017, 398, 1319–1325. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, W.; Chen, C.; Yan, B.; Zhu, L.; Chen, X.; Peng, C. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020, 247, 117443. [Google Scholar] [CrossRef]
- Bilgic, O.; Altinyazar, H.C.; Baran, H.; Unlu, A. Serum homocysteine, asymmetric dimethyl arginine (ADMA) and other arginine-NO pathway metabolite levels in patients with psoriasis. Arch. Dermatol. Res. 2015, 307, 439–444. [Google Scholar] [CrossRef]
- Dowsett, L.; Higgins, E.; Alanazi, S.; Alshuwayer, N.A.; Leiper, F.C.; Leiper, J. ADMA: A Key Player in the Relationship between Vascular Dysfunction and Inflammation in Atherosclerosis. J. Clin. Med. 2020, 9, 3026. [Google Scholar] [CrossRef]
- Mangiacapra, F.; Conte, M.; Demartini, C.; Muller, O.; Delrue, L.; Dierickx, K.; Di Sciascio, G.; Trimarco, B.; De Bruyne, B.; Wijns, W.; et al. Relationship of asymmetric dimethylarginine (ADMA) with extent and functional severity of coronary atherosclerosis. Int. J. Cardiol. 2016, 220, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.N.; Azfar, R.S.; Shin, D.B.; Neimann, A.L.; Troxel, A.B.; Gelfand, J.M. Patients with severe psoriasis are at increased risk of cardiovascular mortality: Cohort study using the General Practice Research Database. Eur. Heart J. 2010, 31, 1000–1006. [Google Scholar] [CrossRef]
- Reuter, S.E.; Evans, A.M. Carnitine and Acylcarnitines: Pharmacokinetic, Pharmacological and Clinical Aspects. Clin. Pharm. 2012, 51, 553–572. [Google Scholar] [CrossRef]
- Ottas, A.; Fishman, D.; Okas, T.L.; Kingo, K.; Soomets, U. The metabolic analysis of psoriasis identifies the associated metabolites while providing computational models for the monitoring of the disease. Arch. Dermatol. Res. 2017, 309, 519–528. [Google Scholar] [CrossRef]
- Ottas, A.; Fishman, D.; Okas, T.-L.; Püssa, T.; Toomik, P.; Martson, A.; Kingo, K.; Soomets, U. Blood serum metabolome of atopic dermatitis: Altered energy cycle and the markers of systemic inflammation. PLoS ONE 2017, 12, e0188580. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. Glutamate: A truly functional amino acid. Amino Acids 2013, 45, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Carli, F.; Bugianesi, E.; Gastaldelli, A.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and comorbid diseases: Implications for management. J. Am. Acad. Dermatol. 2017, 76, 393–403. [Google Scholar] [CrossRef]
- Lehn-Stefan, A.; Peter, A.; Machann, J.; Schick, F.; Randrianarisoa, E.; Heni, M.; Wagner, R.; Birkenfeld, A.L.; Fritsche, A.; Häring, H.-U.; et al. Elevated Circulating Glutamate Is Associated with Subclinical Atherosclerosis Independently of Established Risk Markers: A Cross-Sectional Study. J. Clin. Endocrinol. Metab. 2021, 106, e982–e989. [Google Scholar] [CrossRef]
- KEGG PATHWAY: Arginine and Proline Metabolism—Homo Sapiens. Available online: https://www.genome.jp/kegg/pathway/map/hsa00330.html (accessed on 10 June 2021).
- Kang, H.; Li, X.; Zhou, Q.; Quan, C.; Xue, F.; Zheng, J.; Yu, Y. Exploration of candidate biomarkers for human psoriasis based on gas chromatography-mass spectrometry serum metabolomics. Br. J. Dermatol. 2017, 176, 713–722. [Google Scholar] [CrossRef]
- Kamleh, M.A.; Snowden, S.G.; Grapov, D.; Blackburn, G.J.; Watson, D.G.; Xu, N.; Ståhle, M.; Wheelock, C.E. LC-MS Metabolomics of Psoriasis Patients Reveals Disease Severity-Dependent Increases in Circulating Amino Acids That Are Ameliorated by Anti-TNFα Treatment. J. Proteome Res. 2014, 14, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Neubert, R.H.H.; Ziegler, J.; Hause, G.; Wohlrab, J. Quantitative Analysis of Free Amino Acids and Urea Derived from Isolated Corneocytes of Healthy Young, Healthy Aged, and Diseased Skin. Skin Pharmacol. Physiol. 2019, 32, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.; Sun, Y.; Xu, Z.; Niu, L.; Wang, Z.; Deng, S.; Liu, Z.; Zhou, H.; Bai, J.; Yin, Q.; et al. Excessive Polyamine Generation in Keratinocytes Promotes Self-RNA Sensing by Dendritic Cells in Psoriasis. Immunity 2020, 53, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Sánchez, B.; Priego-Capote, F.; Luque de Castro, M.D. Metabolomics analysis I. Selection of biological samples and practical aspects preceding sample preparation. Trends Anal. Chem. 2010, 29, 111–119. [Google Scholar] [CrossRef]
| Metabolite | AD-L Median Value | PS-L Median Value | AD-L vs. PS-L MWW p-Value FDR 5% Corrected | Median Fold Change (AD-L over PS-L) | Median Fold Change (PS-L over AD-L) |
|---|---|---|---|---|---|
| Biogenic amines | |||||
| ADMA | 0.1622 | 0.4483 | 0.0043 | 2.8 | |
| Acylcarnitines | |||||
| C2 | 0.1504 | 0.2529 | 0.0086 | 1.7 | |
| C18.2 | 0.2241 | 0.4397 | 0.0318 | 2 | |
| Sphingolipids | |||||
| SM.C26.0 | 0.5119 | 0.2083 | 0.0028 | 2.5 | |
| SM.C26.1 | 0.4966 | 0.2109 | 0.0035 | 2.4 | |
| SM.OH.C22.1 | 0.6539 | 0.3365 | 0.0095 | 1.9 | |
| SM.C18.1 | 0.5165 | 0.2443 | 0.0095 | 2.1 | |
| SM.C24.1 | 0.6115 | 0.3218 | 0.0095 | 1.9 | |
| SM.C24.0 | 0.6181 | 0.3434 | 0.0294 | 1.8 | |
| SM.OH.C22.2 | 0.5429 | 0.3222 | 0.0328 | 1.7 | |
| SM.C16.1 | 0.4554 | 0.3275 | 0.044 | 1.4 | |
| SM.C16.0 | 0.5086 | 0.3374 | 0.044 | 1.5 | |
| Glycerophospholipids | |||||
| PC.ae.C38.1 | 0.1106 | 0.241 | 0.0095 | 2.2 | |
| PC.ae.C36.0 | 0.0722 | 0.1618 | 0.0184 | 2.2 | |
| lysoPC.a.C16.1 | 0.1331 | 0.2108 | 0.0274 | 1.6 | |
| PC.ae.C38.0 | 0.1751 | 0.3319 | 0.0365 | 1.9 | |
| PC.ae.C38.2 | 0.1615 | 0.2585 | 0.044 | 1.6 | |
| PC.ae.C36.1 | 0.1518 | 0.2338 | 0.044 | 1.5 | |
| Metabolite ratios | |||||
| Orn…Arg | 0.0063 | 0.0025 | 0.0285 | 2.5 | |
| Metabolite | AD Serum Median Value | PS Serum Median Value | AD Serum vs. PS Serum MWW p-Value FDR 5% Corrected | Median Fold Change (PS Serum over AD Serum) |
|---|---|---|---|---|
| Amino acids | ||||
| Cit | 0.1843 | 0.2806 | 0.0392 | 1.5 |
| Glu | 0.1146 | 0.2282 | 0.0392 | 2 |
| Pro | 0.1793 | 0.3073 | 0.0392 | 1.7 |
| Acylcarnitines | ||||
| C0 | 0.154 | 0.4292 | 0.0184 | 2.8 |
| C18.1 | 0.2312 | 0.3417 | 0.0392 | 1.5 |
| Patients’ Characteristics | ||||
|---|---|---|---|---|
| Psoriasis (Skin) | Atopic Dermatitis (Skin) | Psoriasis (Serum) | Atopic Dermatitis (Serum) | |
| Age, mean (y) (range) | 46.5 (20–75) | 32.1 (20–50) | 46.3 (20–75) | 33.4 (19–54) |
| Sex, no. (%) | ||||
| Male | 13 (65) | 4 (26.7) | 37 (67.3) | 6 (24) |
| Female | 7 (35) | 11 (73.3) | 18 (32.7) | 19 (76) |
| Ethnicity | Caucasian | Caucasian | Caucasian | Caucasian |
| PASI score, mean (range) | 8.455 (2.8–22) | 9.675 (1–34) | ||
| Psoriasis, no. (%) | ||||
| Psoriatic associated joint involvement | 5 (25) | 15 (27.3) | ||
| Psoriatic associated nail involvement | 7 (35) | 22 (40) | ||
| Atopic dermatitis, no. (%) | ||||
| AD associated rhinitis | 10 (66.7) | 13 (52) | ||
| AD associated asthma | 6 (40) | 9 (36) | ||
| AD type, no. (%) | ||||
| normal IgE–intrinsic | 2 (13.3) | 5 (20) | ||
| elevated IgE–extrinsic | 13 (86.7) | 18 (72) | ||
| unknown | 2 (8) | |||
| Family history | ||||
| positive | 9 (45) | 7 (46.7) | 23 (41.8) | 12 (48) |
| negative | 10 (50) | 8 (53.3) | 24 (43.6) | 10 (40) |
| unknown | 1 (5) | 8 (14.6) | 3 (12) | |
| Age of onset (%) | ||||
| ≥40 years of age (PS) | 7 (35) | 12 (21.8) | ||
| <18 years of age (AD) | 14 (93.3) | 23 (92) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilves, L.; Ottas, A.; Kaldvee, B.; Abram, K.; Soomets, U.; Zilmer, M.; Jaks, V.; Kingo, K. Metabolomic Differences between the Skin and Blood Sera of Atopic Dermatitis and Psoriasis. Int. J. Mol. Sci. 2022, 23, 13001. https://doi.org/10.3390/ijms232113001
Ilves L, Ottas A, Kaldvee B, Abram K, Soomets U, Zilmer M, Jaks V, Kingo K. Metabolomic Differences between the Skin and Blood Sera of Atopic Dermatitis and Psoriasis. International Journal of Molecular Sciences. 2022; 23(21):13001. https://doi.org/10.3390/ijms232113001
Chicago/Turabian StyleIlves, Liis, Aigar Ottas, Bret Kaldvee, Kristi Abram, Ursel Soomets, Mihkel Zilmer, Viljar Jaks, and Külli Kingo. 2022. "Metabolomic Differences between the Skin and Blood Sera of Atopic Dermatitis and Psoriasis" International Journal of Molecular Sciences 23, no. 21: 13001. https://doi.org/10.3390/ijms232113001
APA StyleIlves, L., Ottas, A., Kaldvee, B., Abram, K., Soomets, U., Zilmer, M., Jaks, V., & Kingo, K. (2022). Metabolomic Differences between the Skin and Blood Sera of Atopic Dermatitis and Psoriasis. International Journal of Molecular Sciences, 23(21), 13001. https://doi.org/10.3390/ijms232113001






