Valdecoxib Protects against Cell Apoptosis Induced by Endoplasmic Reticulum Stress via the Inhibition of PERK-ATF4-CHOP Pathway in Experimental Glaucoma
Abstract
1. Introduction
2. Results
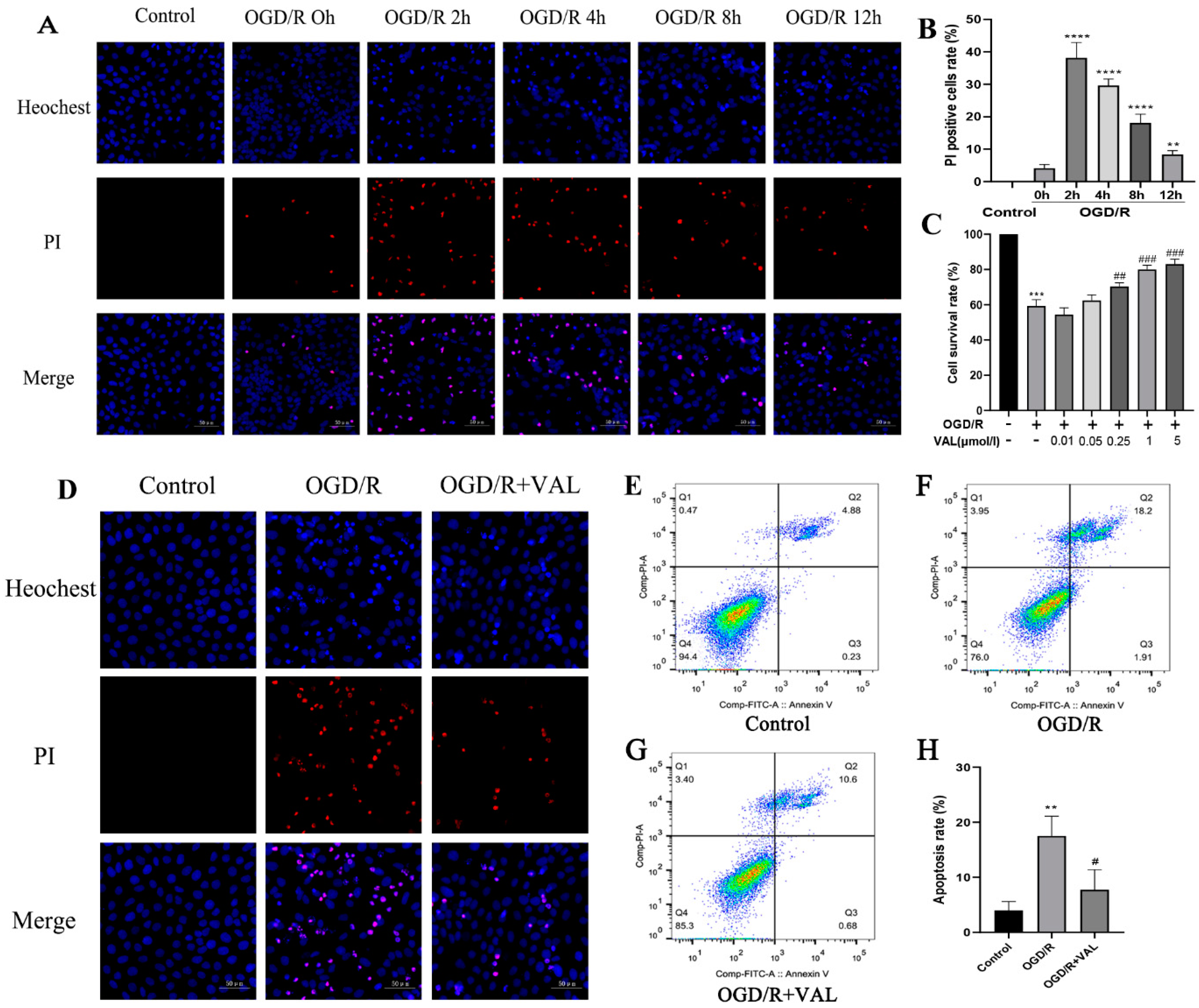
2.1. Valdecoxib Protects R28 from OGD/R Injury by Inhibiting Apoptosis In Vitro
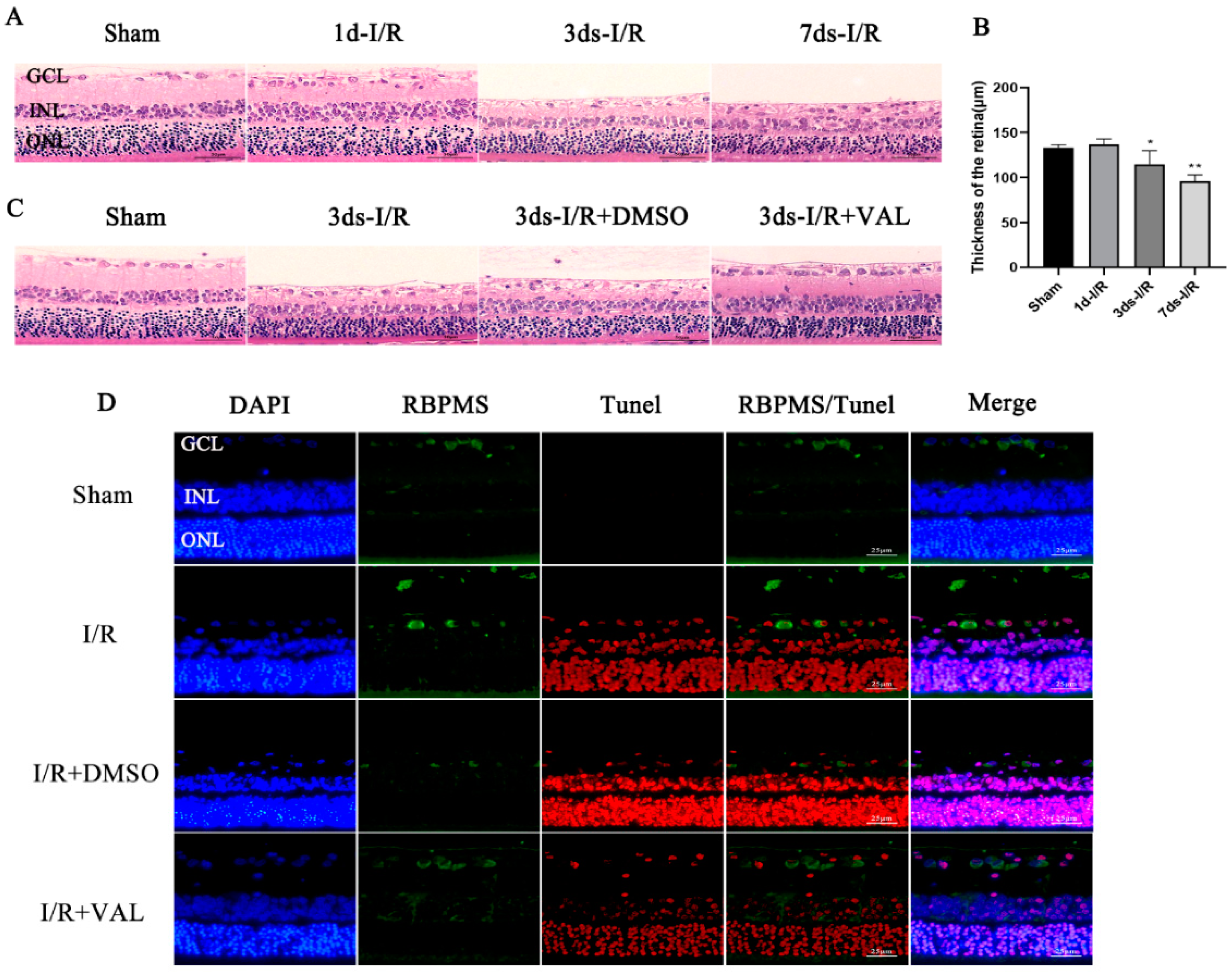
2.2. Valdecoxib Protects the Retina from Ischemia-Reperfusion Injury (IRI) by Inhibiting Apoptosis In Vivo
2.3. Valdecoxib Inhibits R28 Apoptosis by Alleviating PERK-ATF4-CHOP Pathway-Mediated ER Stress
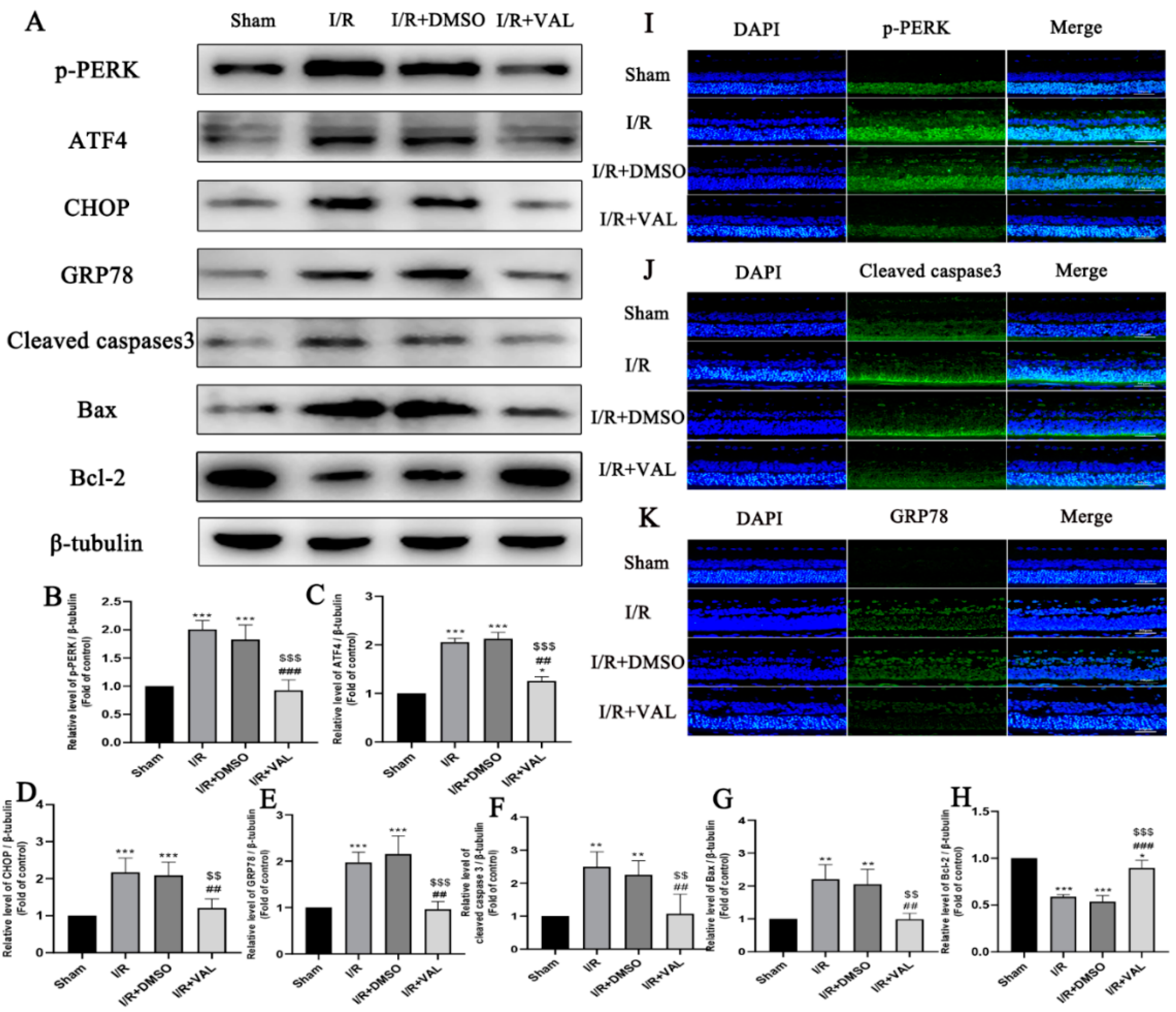
2.4. Valdecoxib Protects the Retina from Ischemia Reperfusion Injury (IRI)-Mediated Apoptosis by Alleviating PERK-ATF4-CHOP Pathway-Mediated ER Stress
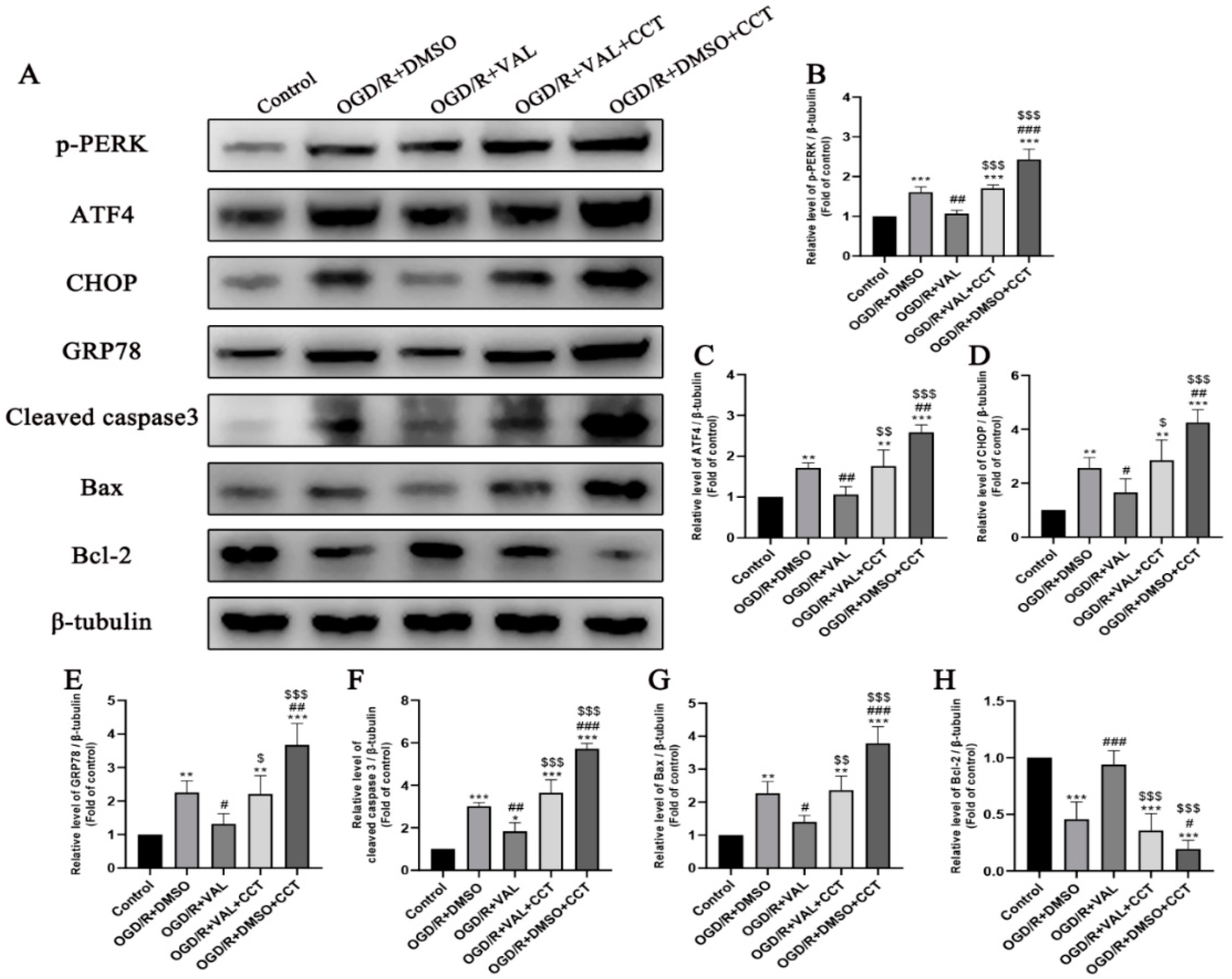
2.5. CCT020312 Reverses Valdecoxib’s Anti-Apoptosis Effect by Activating PERK-ATF4-CHOP Pathway-Mediated ER Stress In Vitro
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Animals
4.3. Reagents and Antibodies
4.4. I/R Injury Model (IRI)
Valdecoxib Treatment in I/R Model
4.5. R28 Cell OGD/R Injury Model
4.6. Hematoxylin and Eosin (HE) Staining
4.7. Terminal Deoxynucleotidyl Transferase-Mediated Nick End-Labeling (TUNEL)
4.8. Cell Counting Kit-8 (CCK-8)
4.9. Western Blot Assay
4.10. Annexin V-FITC/PI Flow Cytometry
4.11. Immunofluorescence Staining Assay
4.12. Propidium Iodide (PI) Staining
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, S.; Hodge, W.; Malvankar-Mehta, M. The Cost-Effectiveness Analysis of Teleglaucoma Screening Device. PLoS ONE 2015, 10, e0137913. [Google Scholar] [CrossRef] [PubMed]
- Wiggs, J.L.; Pasquale, L.R. Genetics of glaucoma. Hum. Mol. Genet. 2017, 26, R21–R27. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Hare, W.; WoldeMussie, E.; Lai, R.; Ton, H.; Ruiz, G.; Feldmann, B.; Wijono, M.; Chun, T.; Wheeler, L. Efficacy and safety of memantine, an NMDA-type open-channel blocker, for reduction of retinal injury associated with experimental glaucoma in rat and monkey. Surv. Ophthalmol. 2001, 45 (Suppl. 3), S284–S289; discussion S295–S296. [Google Scholar] [CrossRef]
- Ko, M.L.; Hu, D.N.; Ritch, R.; Sharma, S.C. The combined effect of brain-derived neurotrophic factor and a free radical scavenger in experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2967–2971. [Google Scholar]
- Bakalash, S.; Ben-Shlomo, G.; Aloni, E.; Shaked, I.; Wheeler, L.; Ofri, R.; Schwartz, M. T-cell-based vaccination for morphological and functional neuroprotection in a rat model of chronically elevated intraocular pressure. J. Mol. Med. 2005, 83, 904–916. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Vohra, R.; Tsai, J.C.; Kolko, M. The role of inflammation in the pathogenesis of glaucoma. Surv. Ophthalmol. 2013, 58, 311–320. [Google Scholar] [CrossRef]
- Doh, S.H.; Kim, J.H.; Lee, K.M.; Park, H.Y.; Park, C.K. Retinal ganglion cell death induced by endoplasmic reticulum stress in a chronic glaucoma model. Brain Res. 2010, 1308, 158–166. [Google Scholar] [CrossRef]
- Yang, L.; Li, S.; Miao, L.; Huang, H.; Liang, F.; Teng, X.; Xu, L.; Wang, Q.; Xiao, W.; Ridder, W.H., 3rd; et al. Rescue of Glaucomatous Neurodegeneration by Differentially Modulating Neuronal Endoplasmic Reticulum Stress Molecules. J. Neurosci. 2016, 36, 5891–5903. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef]
- Peters, J.C.; Bhattacharya, S.; Clark, A.F.; Zode, G.S. Increased Endoplasmic Reticulum Stress in Human Glaucomatous Trabecular Meshwork Cells and Tissues. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3860–3868. [Google Scholar] [CrossRef] [PubMed]
- Shimazawa, M.; Inokuchi, Y.; Ito, Y.; Murata, H.; Aihara, M.; Miura, M.; Araie, M.; Hara, H. Involvement of ER stress in retinal cell death. Mol. Vis. 2007, 13, 578–587. [Google Scholar] [PubMed]
- Lin, B.; Zhang, X.; Xu, X. Nerve Growth Factor Protects Retinal Ganglion Cells Related to Inhibiting Endoplasmic Reticulum Stress by Inhibiting IRE1-JNK-CHOP Signaling Pathway. Ocul. Immunol. Inflamm. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Park, K.K.; Yang, L.; Wei, X.; Yang, Q.; Cho, K.S.; Thielen, P.; Lee, A.H.; Cartoni, R.; Glimcher, L.H.; et al. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. Neuron 2012, 73, 445–452. [Google Scholar] [CrossRef]
- Kivitz, A.; Eisen, G.; Zhao, W.W.; Bevirt, T.; Recker, D.P. Randomized placebo-controlled trial comparing efficacy and safety of valdecoxib with naproxen in patients with osteoarthritis. J. Fam. Pract. 2002, 51, 530–537. [Google Scholar]
- Makarowski, W.; Zhao, W.W.; Bevirt, T.; Recker, D.P. Efficacy and safety of the COX-2 specific inhibitor valdecoxib in the management of osteoarthritis of the hip: A randomized, double-blind, placebo-controlled comparison with naproxen. Osteoarthritis Cartilage 2002, 10, 290–296. [Google Scholar] [CrossRef][Green Version]
- Bensen, W.; Weaver, A.; Espinoza, L.; Zhao, W.W.; Riley, W.; Paperiello, B.; Recker, D.P. Efficacy and safety of valdecoxib in treating the signs and symptoms of rheumatoid arthritis: A randomized, controlled comparison with placebo and naproxen. Rheumatology 2002, 41, 1008–1016. [Google Scholar] [CrossRef]
- Daniels, S.E.; Torri, S.; Desjardins, P.J. Valdecoxib for treatment of primary dysmenorrhea. A randomized, double-blind comparison with placebo and naproxen. J. Gen. Intern. Med. 2005, 20, 62–67. [Google Scholar] [CrossRef]
- Camu, F.; Beecher, T.; Recker, D.P.; Verburg, K.M. Valdecoxib, a COX-2-specific inhibitor, is an efficacious, opioid-sparing analgesic in patients undergoing hip arthroplasty. Am. J. Ther. 2002, 9, 43–51. [Google Scholar] [CrossRef]
- Daniels, S.E.; Desjardins, P.J.; Talwalker, S.; Recker, D.P.; Verburg, K.M. The analgesic efficacy of valdecoxib vs. oxycodone/acetaminophen after oral surgery. J. Am. Dent. Assoc. 2002, 133, 611–621; quiz 625. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, P.J.; Shu, V.S.; Recker, D.P.; Verburg, K.M.; Woolf, C.J. A single preoperative oral dose of valdecoxib, a new cyclooxygenase-2 specific inhibitor, relieves post-oral surgery or bunionectomy pain. Anesthesiology 2002, 97, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Lee, H.J.; Pyun, D.H.; Abd El-Aty, A.M.; Jeong, J.H.; Jung, T.W. Valdecoxib improves lipid-induced skeletal muscle insulin resistance via simultaneous suppression of inflammation and endoplasmic reticulum stress. Biochem. Pharmacol. 2021, 188, 114557. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Xiang, P.; Luo, W.; Tan, X.; Gu, C.; Liu, M.; Chen, M.; Wang, Q.; Yang, J. CTRP1 Attenuates Cerebral Ischemia/Reperfusion Injury via the PERK Signaling Pathway. Front. Cell Dev. Biol. 2021, 9, 700854. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Seghal, N.; Padi, S.V.; Naidu, P.S. Differential effects of cyclooxygenase inhibitors on intracerebroventricular colchicine-induced dysfunction and oxidative stress in rats. Eur. J. Pharmacol. 2006, 551, 58–66. [Google Scholar] [CrossRef]
- Kalra, J.; Kumar, P.; Majeed, A.B.; Prakash, A. Modulation of LOX and COX pathways via inhibition of amyloidogenesis contributes to mitoprotection against beta-amyloid oligomer-induced toxicity in an animal model of Alzheimer’s disease in rats. Pharmacol. Biochem. Behav. 2016, 146–147, 1–12. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, A.; Kulkarni, S.K. Targeting oxidative stress, mitochondrial dysfunction and neuroinflammatory signaling by selective cyclooxygenase (COX)-2 inhibitors mitigates MPTP-induced neurotoxicity in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 974–981. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, C.K. Retinal ganglion cell death is delayed by activation of retinal intrinsic cell survival program. Brain Res. 2005, 1057, 17–28. [Google Scholar] [CrossRef]
- Quigley, H.A.; Nickells, R.W.; Kerrigan, L.A.; Pease, M.E.; Thibault, D.J.; Zack, D.J. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Investig. Ophthalmol. Vis. Sci. 1995, 36, 774–786. [Google Scholar]
- Kerrigan, L.A.; Zack, D.J.; Quigley, H.A.; Smith, S.D.; Pease, M.E. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch. Ophthalmol. 1997, 115, 1031–1035. [Google Scholar] [CrossRef]
- Garcia-Valenzuela, E.; Shareef, S.; Walsh, J.; Sharma, S.C. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995, 61, 33–44. [Google Scholar] [CrossRef]
- Peng, H.; Sun, Y.B.; Hao, J.L.; Lu, C.W.; Bi, M.C.; Song, E. Neuroprotective effects of overexpressed microRNA-200a on activation of glaucoma-related retinal glial cells and apoptosis of ganglion cells via downregulating FGF7-mediated MAPK signaling pathway. Cell Signal. 2019, 54, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.J.; Zhang, S.H.; Xu, P.; Yang, B.Q.; Zhang, R.; Cheng, Y.; Zhou, X.J.; Huang, W.J.; Wang, M.; Chen, J.Y.; et al. Quercetin Declines Apoptosis, Ameliorates Mitochondrial Function and Improves Retinal Ganglion Cell Survival and Function in In Vivo Model of Glaucoma in Rat and Retinal Ganglion Cell Culture In Vitro. Front. Mol. Neurosci. 2017, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; Zhang, Y.J.; Guo, H.F.; Hao, J.; Liu, Q.J.; Liu, J.R.; Guo, J.W.; Liu, J.H.; Zuo, L.F. The regulatory effect of the p38 signaling pathway on valdecoxib-induced apoptosis of the Eca109 cell line. Oncol. Rep. 2009, 22, 313–319. [Google Scholar]
- Majchrzak-Celinska, A.; Misiorek, J.O.; Kruhlenia, N.; Przybyl, L.; Kleszcz, R.; Rolle, K.; Krajka-Kuzniak, V. COXIBs and 2,5-dimethylcelecoxib counteract the hyperactivated Wnt/beta-catenin pathway and COX-2/PGE2/EP4 signaling in glioblastoma cells. BMC Cancer 2021, 21, 493. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, S.H.; Narko, K.; Trifan, O.C.; Wu, M.T.; Smith, E.; Haudenschild, C.; Lane, T.F.; Hla, T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J. Biol. Chem. 2001, 276, 18563–18569. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.N.; Wang, K.; Zhang, L.; Huang, Z.; Liu, J.; Zhang, Z.; Luo, M.; Lei, Y.; Peng, Y.; et al. Ketoconazole exacerbates mitophagy to induce apoptosis by downregulating cyclooxygenase-2 in hepatocellular carcinoma. J. Hepatol. 2019, 70, 66–77. [Google Scholar] [CrossRef]
- Toth, A.; Nickson, P.; Mandl, A.; Bannister, M.L.; Toth, K.; Erhardt, P. Endoplasmic reticulum stress as a novel therapeutic target in heart diseases. Cardiovasc. Hematol. Disord Drug Targets 2007, 7, 205–218. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, C.; Shen, X. LncRNA GAS5 suppresses ER stressinduced apoptosis and inflammation by regulating SERCA2b in HGtreated retinal epithelial cell. Mol. Med. Rep. 2020, 22, 1072–1080. [Google Scholar] [CrossRef]
- Mo, J.S.; Choi, D.; Han, Y.R.; Kim, N.; Jeong, H.S. Morin has protective potential against ER stress induced apoptosis in renal proximal tubular HK-2 cells. Biomed. Pharmacother. 2019, 112, 108659. [Google Scholar] [CrossRef]
- Kroeger, H.; Chiang, W.C.; Felden, J.; Nguyen, A.; Lin, J.H. ER stress and unfolded protein response in ocular health and disease. FEBS J. 2019, 286, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zong, S.; Wang, Z.; Du, P.; Wen, Y.; Li, H.; Wu, N.; Xiao, H. The PERK/ATF4/CHOP signaling branch of the unfolded protein response mediates cisplatin-induced ototoxicity in hair cells. Drug Chem. Toxicol. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, L.; Shen, Y.; Xu, J. Rifampicin-induced injury in L02 cells is alleviated by 4-PBA via inhibition of the PERK-ATF4-CHOP pathway. Toxicol. In Vitro 2016, 36, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, S.; Huang, F.; Zhang, Q.; Qin, B.; Liao, L.; Wang, M.; Wan, H.; Yan, W.; Chen, D.; et al. c-FLIP regulates pyroptosis in retinal neurons following oxygen-glucose deprivation/recovery via a GSDMD-mediated pathway. Ann. Anat. 2021, 235, 151672. [Google Scholar] [CrossRef]
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014, 2014, 655–658. [Google Scholar] [CrossRef]
- Naranjo, A.; Pirakitikulr, N.; Pelaez, D.; Sabater, A.L.; Monsalve, P.; Amescua, G.; Galor, A.; Dubovy, S.R. Clinicopathologic Correlations of Retrocorneal Membranes Associated With Endothelial Corneal Graft Failure. Am. J. Ophthalmol. 2021, 222, 24–33. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Li, M.; Yao, F.; Xia, X.; Duan, T.; Meng, J.; Huang, Y.; He, Y.; Saro, A.; Huang, J. Valdecoxib Protects against Cell Apoptosis Induced by Endoplasmic Reticulum Stress via the Inhibition of PERK-ATF4-CHOP Pathway in Experimental Glaucoma. Int. J. Mol. Sci. 2022, 23, 12983. https://doi.org/10.3390/ijms232112983
Gao Z, Li M, Yao F, Xia X, Duan T, Meng J, Huang Y, He Y, Saro A, Huang J. Valdecoxib Protects against Cell Apoptosis Induced by Endoplasmic Reticulum Stress via the Inhibition of PERK-ATF4-CHOP Pathway in Experimental Glaucoma. International Journal of Molecular Sciences. 2022; 23(21):12983. https://doi.org/10.3390/ijms232112983
Chicago/Turabian StyleGao, Zhaolin, Min Li, Fei Yao, Xiaobo Xia, Tianqi Duan, Jingzhuo Meng, Yanxia Huang, Ye He, Adonira Saro, and Jufang Huang. 2022. "Valdecoxib Protects against Cell Apoptosis Induced by Endoplasmic Reticulum Stress via the Inhibition of PERK-ATF4-CHOP Pathway in Experimental Glaucoma" International Journal of Molecular Sciences 23, no. 21: 12983. https://doi.org/10.3390/ijms232112983
APA StyleGao, Z., Li, M., Yao, F., Xia, X., Duan, T., Meng, J., Huang, Y., He, Y., Saro, A., & Huang, J. (2022). Valdecoxib Protects against Cell Apoptosis Induced by Endoplasmic Reticulum Stress via the Inhibition of PERK-ATF4-CHOP Pathway in Experimental Glaucoma. International Journal of Molecular Sciences, 23(21), 12983. https://doi.org/10.3390/ijms232112983




