Research Progress on Graphitic Carbon Nitride/Metal Oxide Composites: Synthesis and Photocatalytic Applications
Abstract
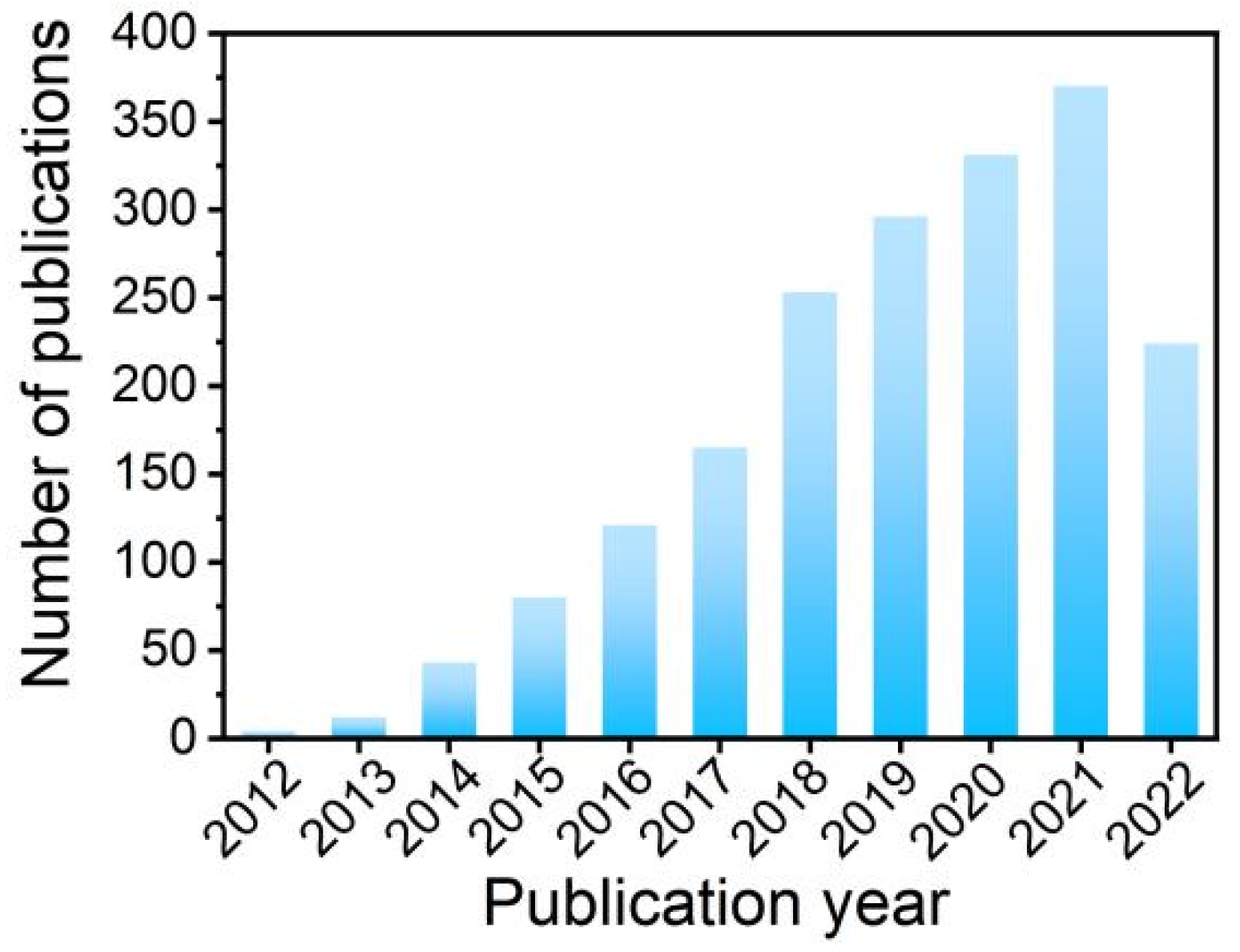
1. Introduction
2. Synthesis of g-C3N4 and Metal Oxides/g-C3N4 Composites
2.1. Synthesis of g-C3N4
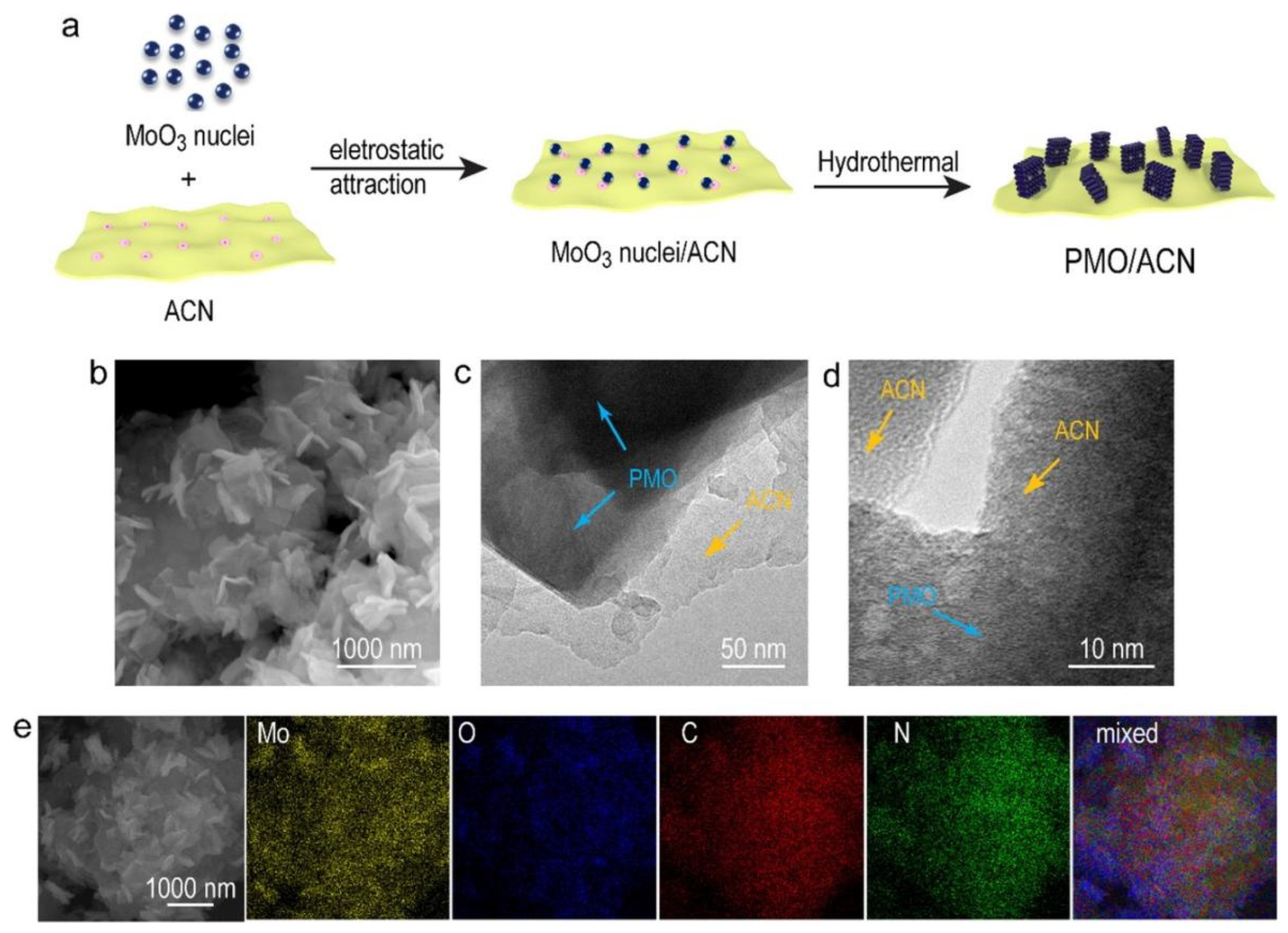
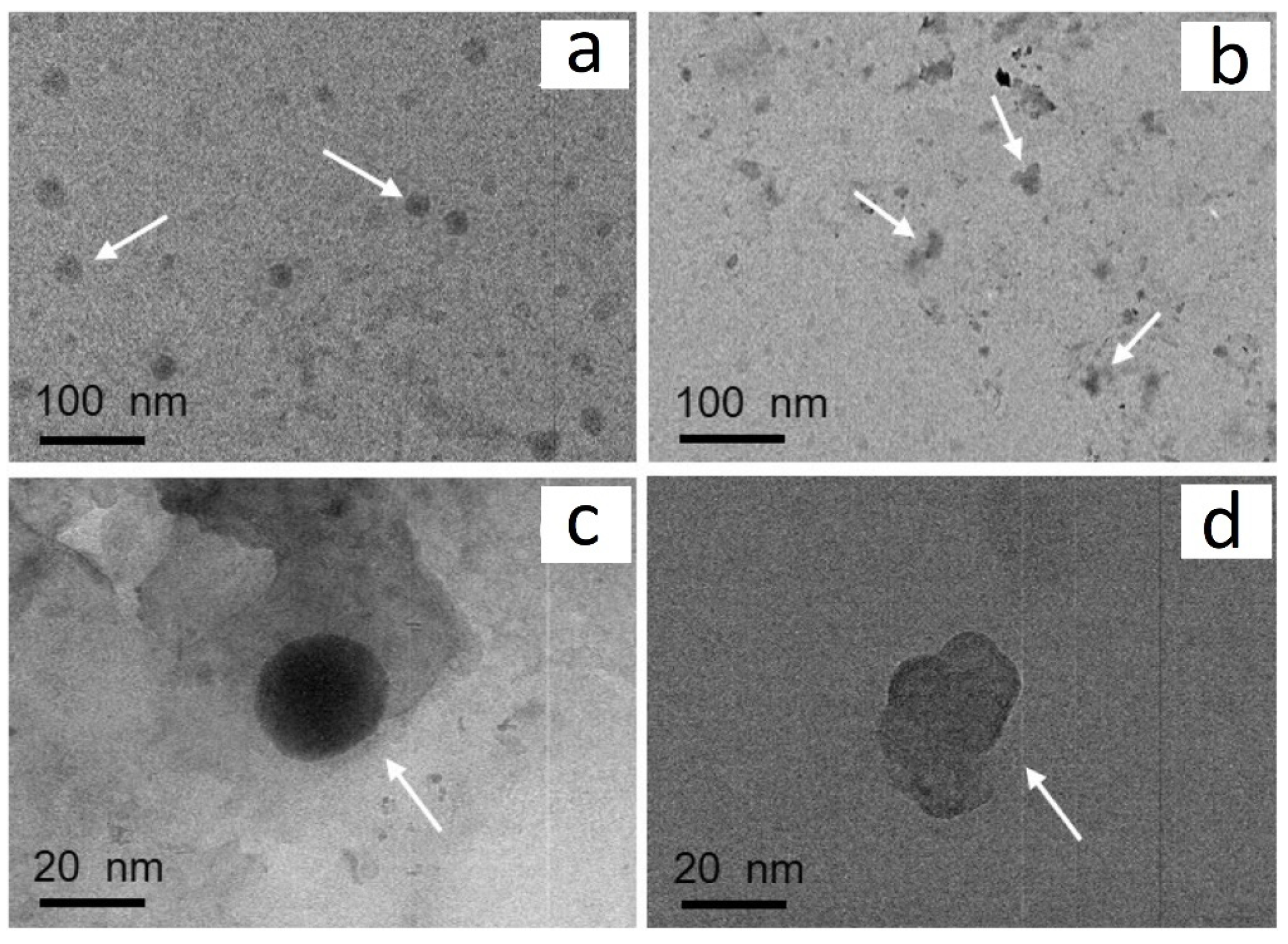
2.2. Synthesis of Single-Metal Oxide/g-C3N4 Heterojunctions
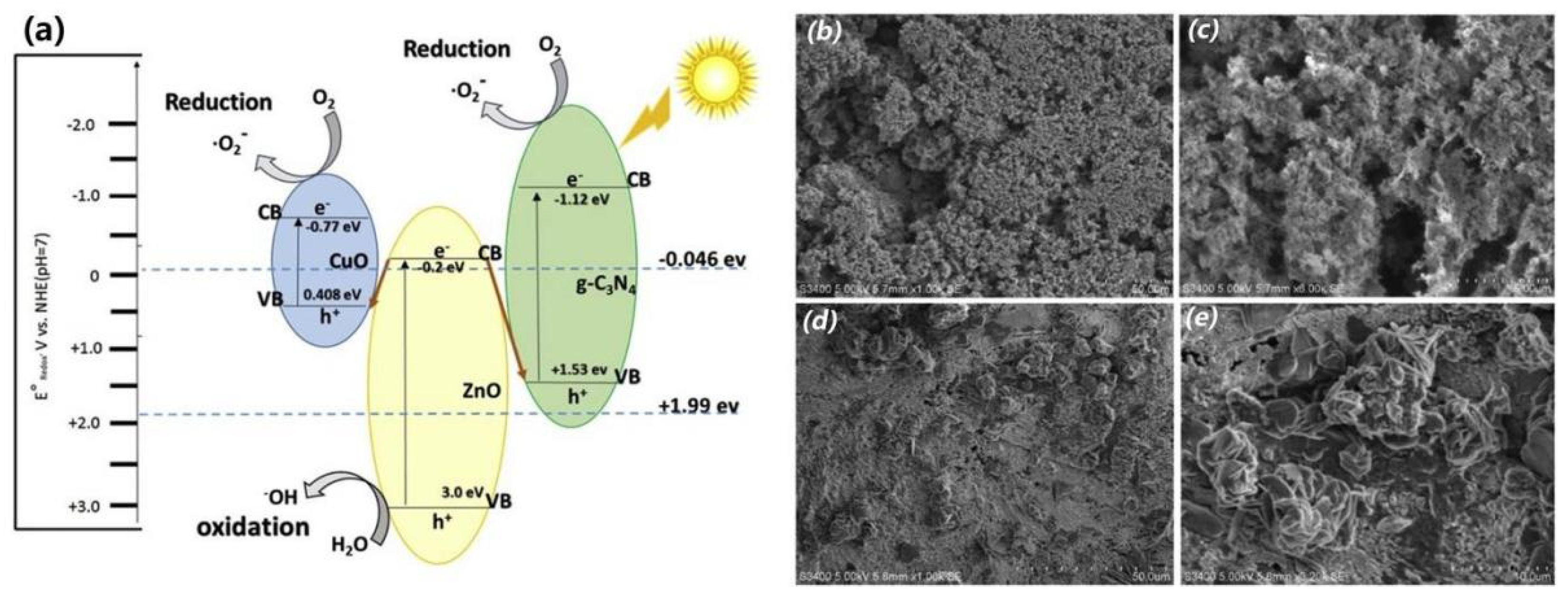
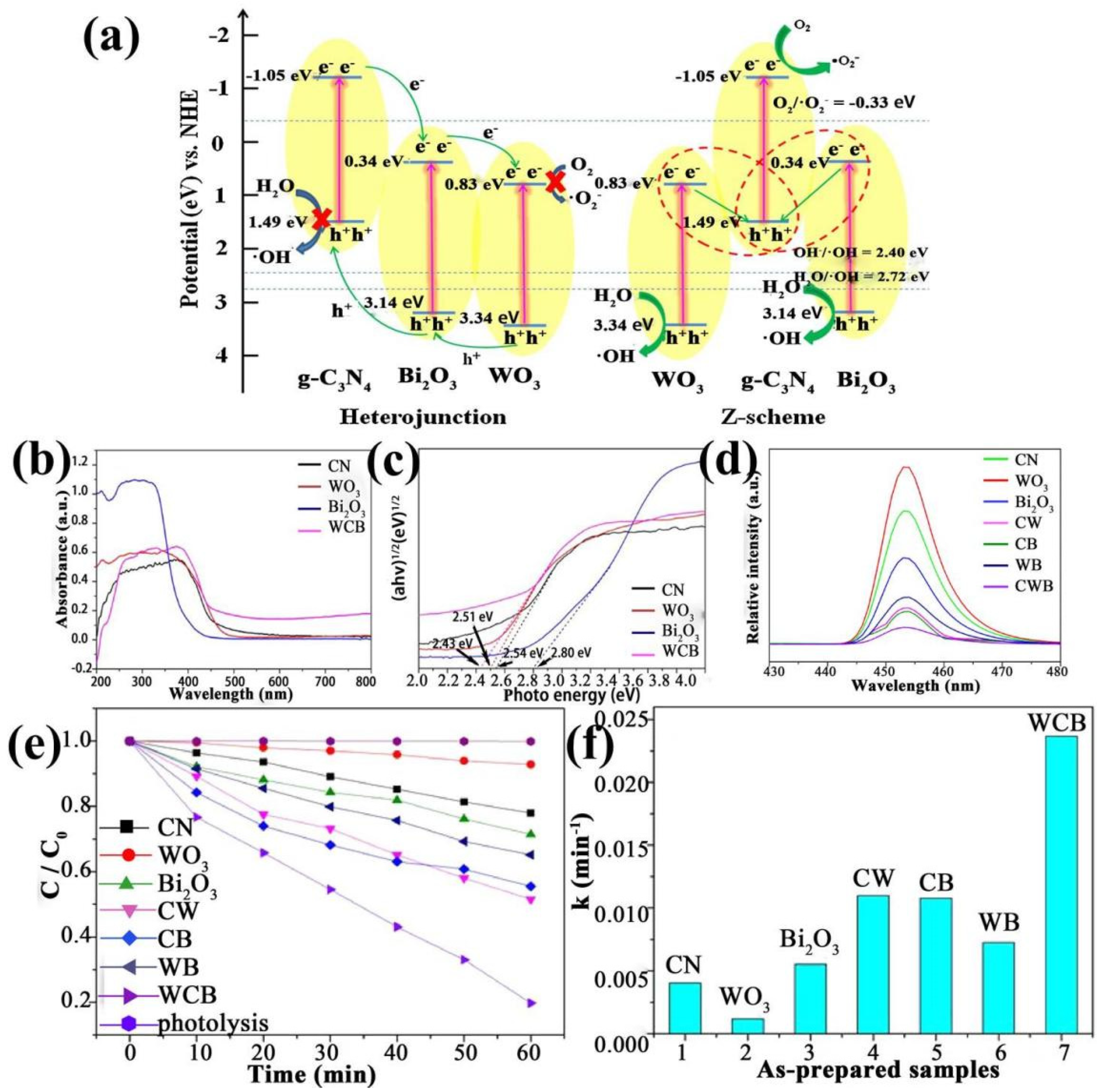
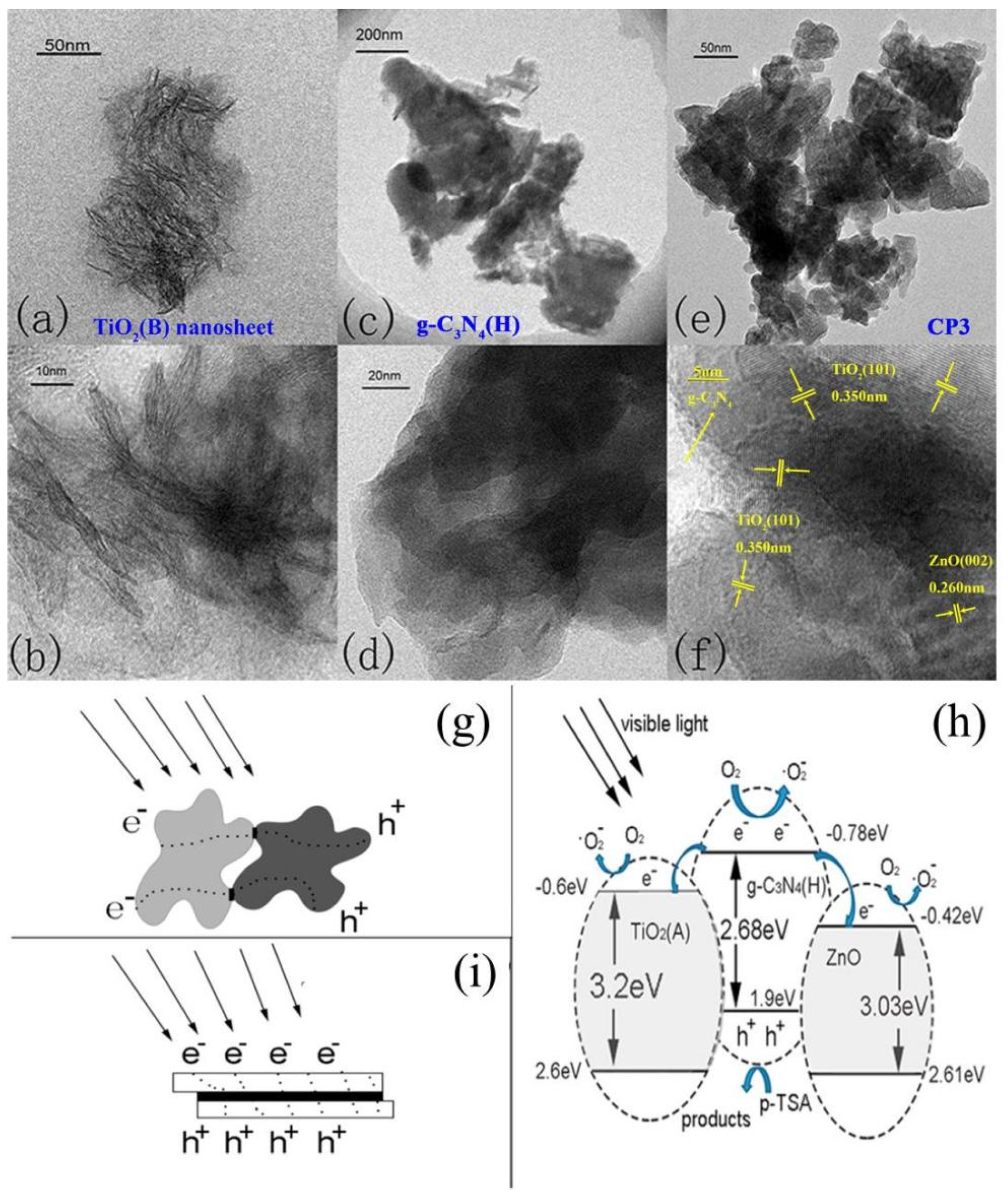
2.3. Multiple-Metal Oxide/g-C3N4 Heterojunctions
3. Applications of g-C3N4-Based Photocatalysts
3.1. Photocatalytic Water Splitting for H2
3.2. Photocatalytic Reduction of CO2 to Renewable Hydrocarbon Fuels
E0redox = −0.61V (vs. NHE at pH 7)
E0redox = −0.53V (vs. NHE at pH 7)
E0redox = −0.48V (vs. NHE at pH 7)
E0redox = −0.38V (vs. NHE at pH 7)
E0redox = −0.24V (vs. NHE at pH 7)
3.3. Photocatalytic Degradation of Pollutants
3.4. Sterilization and Disinfection
4. Summary and Outlook
Funding
Conflicts of Interest
References
- Ali, T.; Muhammad, N.; Qian, Y.; Liu, S.; Wang, S.; Wang, M.; Qian, T.; Yan, C. Recent advances in material design and reactor engineering for electrocatalytic ambient nitrogen fixation. Mater. Chem. Front. 2022, 6, 843–879. [Google Scholar] [CrossRef]
- Ali, T.; Qiao, W.; Zhang, D.; Liu, W.; Sajjad, S.; Yan, C.; Su, R. Surface Sulfur Vacancy Engineering of Metal Sulfides Promoted Desorption of Hydrogen Atoms for Enhanced Electrocatalytic Hydrogen Evolution. J. Phys. Chem. C 2021, 125, 12707–12712. [Google Scholar] [CrossRef]
- Ali, T.; Wang, X.; Tang, K.; Li, Q.; Sajjad, S.; Khan, S.; Farooqi, S.A.; Yan, C. SnS2 quantum dots growth on MoS2: Atomic-level heterostructure for electrocatalytic hydrogen evolution. Electrochim. Acta 2019, 300, 45–52. [Google Scholar] [CrossRef]
- Khan, S.; Ali, T.; Wang, X.; Iqbal, W.; Bashir, T.; Chao, W.; Sun, H.; Lu, H.; Yan, C.; Muhammad Irfan, R. Ni3S2@Ni5P4 nanosheets as highly productive catalyst for electrocatalytic oxygen evolution. Chem. Eng. Sci. 2022, 247, 117020. [Google Scholar] [CrossRef]
- Xu, X.; Liu, H.; Wang, J.; Chen, T.; Ding, X.; Chen, H. Insight into surface hydroxyl groups for environmental purification: Characterizations, applications and advances. Surf. Interfaces 2021, 25, 101272. [Google Scholar] [CrossRef]
- Yeganeh, M.; Sobhi, H.R.; Esrafili, A. Efficient photocatalytic degradation of metronidazole from aqueous solutions using Co/g-C3N4/Fe3O4 nanocomposite under visible light irradiation. Environ. Sci. Pollut. Res. 2022, 29, 25486–25495. [Google Scholar] [CrossRef] [PubMed]
- Palanivel, B.; Jayaraman, V.; Ayyappan, C.; Alagiri, M. Magnetic binary metal oxide intercalated g-C3N4: Energy band tuned p-n heterojunction towards Z-scheme photo-Fenton phenol reduction and mixed dye degradation. J. Water Process. Eng. 2019, 32, 100968. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Wu, G.C.; Liu, W.G.; Goddard, W.A.; Yang, C.M. Nanocomposites of Tantalum-Based Pyrochlore and Indium Hydroxide Showing High and Stable Photocatalytic Activities for Overall Water Splitting and Carbon Dioxide Reduction. Angew. Chem. Int. Edit. 2014, 53, 14216–14220. [Google Scholar] [CrossRef]
- Yang, X.; Xu, X.; Wang, J.; Chen, T.; Wang, S.; Ding, X.; Chen, H. Insights into the Surface/Interface Modifications of Bi2MoO6: Feasible Strategies and Photocatalytic Applications. Sol. RRL 2021, 5, 2000442. [Google Scholar] [CrossRef]
- Oshima, T.; Nishioka, S.; Kikuchi, Y.; Hirai, S.; Yanagisawa, K.I.; Eguchi, M.; Miseki, Y.; Yokoi, T.; Yui, T.; Kimoto, K.; et al. An Artificial Z-Scheme Constructed from Dye-Sensitized Metal Oxide Nanosheets for Visible Light-Driven Overall Water Splitting. J. Am. Chem. Soc. 2020, 142, 8412–8420. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.Z.; Xue, X.L.; Zhang, W.J.; Li, X.J.; Liu, J.; Yang, S.Y.; Hu, Y.; Chen, R.P.; Yan, Y.P.; Zhu, G.Y.; et al. Well-designed Te/SnS2/Ag artificial nanoleaves for enabling and enhancing visible-light driven overall splitting of pure water. Nano Energy 2017, 39, 539–545. [Google Scholar] [CrossRef]
- Sajjad, S.; Wang, C.; Wang, X.; Ali, T.; Qian, T.; Yan, C. In situ evolved NiMo/NiMoO4 nanorods as a bifunctional catalyst for overall water splitting. Nanotechnology 2020, 31, 495404. [Google Scholar] [CrossRef]
- Li, Y.H.; Gu, M.L.; Shi, T.; Cui, W.; Zhang, X.M.; Dong, F.; Cheng, J.S.; Fan, J.J.; Lv, K.L. Carbon vacancy in C3N4 nanotube: Electronic structure, photocatalysis mechanism and highly enhanced activity. Appl. Catal. B Environ. 2020, 262, 118281. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys. Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Pourahmad, A.; Radaee, E. Photocatalytic degradation of basic blue 9 by CoS nanoparticles supported on AlMCM-41 material as a catalyst. J. Hazard. Mater. 2009, 170, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.K.; Jana, N.R. Reduced Graphene Oxide-Silver Nanoparticle Composite as Visible Light Photocatalyst for Degradation of Colorless Endocrine Disruptors. ACS Appl. Mater. Interfaces 2014, 6, 20085–20092. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Yu, S.Y.; Zhang, X.X.; Wang, Y.; Liu, C.B.; Chen, G.; Dong, H.J. Insight into photocatalytic activity, universality and mechanism of copper/chlorine surface dual-doped graphitic carbon nitride for degrading various organic pollutants in water. J. Colloid Interf. Sci. 2019, 538, 462–473. [Google Scholar] [CrossRef]
- Vadaei, S.; Faghihian, H. Enhanced visible light photodegradation of pharmaceutical pollutant, warfarin by nano-sized SnTe, effect of supporting, catalyst dose, and scavengers. Environ. Toxicol. Phar. 2018, 58, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Danish, M.; Saud Athar, M.; Ahmad, I.; Warshagha, M.Z.A.; Rasool, Z.; Muneer, M. Highly efficient and stable Fe2O3/g-C3N4/GO nanocomposite with Z-scheme electron transfer pathway: Role of photocatalytic activity and adsorption isotherm of organic pollutants in wastewater. Appl. Surf. Sci. 2022, 604, 154604. [Google Scholar] [CrossRef]
- Palanivel, B.; Lallimathi, M.; Arjunkumar, B.; Shkir, M.; Alshahrani, T.; Al-Namshah, K.S.; Hamdy, M.S.; Shanavas, S.; Venkatachalam, M.; Ramalingam, G. rGO supported g-C3N4/CoFe2O4 heterojunction: Visible-light-active photocatalyst for effective utilization of H2O2 to organic pollutant degradation and OH radicals production. J. Environ. Chem. Eng. 2021, 9, 104698. [Google Scholar] [CrossRef]
- Xu, X.; Ding, X.; Yang, X.; Wang, P.; Li, S.; Lu, Z.; Chen, H. Oxygen vacancy boosted photocatalytic decomposition of ciprofloxacin over Bi2MoO6: Oxygen vacancy engineering, biotoxicity evaluation and mechanism study. J. Hazard. Mater. 2018, 364, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, N.; Wang, P.; Wang, S.; Xiang, Y.; Zhang, X.; Ding, X.; Chen, H. Highly Intensified Molecular Oxygen Activation on Bi@Bi2MoO6 via a Metallic Bi-Coordinated Facet-Dependent Effect. ACS Appl. Mater. Interfaces 2020, 12, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.; Chen, T.; Yang, N.; Wang, S.; Ding, X.; Chen, H. Deep insight into ROS mediated direct and hydroxylated dichlorination process for efficient photocatalytic sodium pentachlorophenate mineralization. Appl. Catal. B Environ. 2021, 296, 120352. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Akira, F.; Tooru, I.; Tadashi, W.; Kenichi, H. Stabilization of photoanodes in electrochemical photocells for solar energy conversion. Chem. Lett. 1978, 7, 357–360. [Google Scholar]
- Gao, M.; Zhu, L.; Ong, W.L.; Wang, J.; Ho, G. Structural Design of TiO2-based Photocatalyst for H2 Production and Degradation Applications. Catal. Sci. Technol. 2015, 5, 4703–4726. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, Q.; Lei, W.; Zhang, T.; Qi, X.; Liu, G.; Deng, K.; Yu, J. Origin of Tunable Photocatalytic Selectivity of Well-Defined α-Fe2O3 Nanocrystals. Small 2014, 10, 674–679. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.-J.; Yin, L.; Li, B.; Hong, X.; Fan, Z.; Chen, B.; Xue, C.; Zhang, H. One-pot Synthesis of CdS Nanocrystals Hybridized with Single-Layer Transition-Metal Dichalcogenide Nanosheets for Efficient Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Edit. 2015, 54, 1210–1214. [Google Scholar] [CrossRef]
- Chen, S.; Shen, S.; Liu, G.; Qi, Y.; Zhang, F.; Li, C. Interface Engineering of a CoOx/Ta3N5 Photocatalyst for Unprecedented Water Oxidation Performance under Visible-Light-Irradiation. Angew. Chem. Int. Edit. 2015, 54, 3047–3051. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Lallimathi, M.; Kalisamy, P.; Suryamathi, M.; Alshahrani, T.; Shkir, M.; Venkatachalam, M.; Palanivel, B. Carbon Dot Loaded Integrative CoFe2O4/g-C3N4 P-N Heterojunction: Direct Solar Light-Driven Photocatalytic H2 Evolution and Organic Pollutant Degradation. ChemistrySelect 2020, 5, 10607–10617. [Google Scholar] [CrossRef]
- Palanivel, B.; Hossain, M.S.; Macadangdang, R.R.; Ayappan, C.; Krishnan, V.; Marnadu, R.; Kalaivani, T.; Alharthi, F.A.; Sreedevi, G. Activation of Persulfate for Improved Naproxen Degradation Using FeCo2O4@g-C3N4 Heterojunction Photocatalysts. ACS Omega 2021, 6, 34563–34571. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.F.; Song, J.L.; McGuire, A.F.; Kang, S.F.; Fang, X.Y.; Wang, J.J.; Yin, C.C.; Li, X.; Wang, Y.G.; Cui, B.X. Constructing Highly Uniform Onion-Ring-like Graphitic Carbon Nitride for Efficient Visible-Light-Driven Photocatalytic Hydrogen Evolution. ACS Nano 2018, 12, 5551–5558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Huang, Z.X.; Shi, J.W.; Guan, X.J.; Cheng, C.; Zong, S.C.; Huangfu, Y.L.; Ma, L.J.; Guo, L.J. Maleic hydrazide-based molecule doping in three-dimensional lettuce-like graphite carbon nitride towards highly efficient photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 272, 119009. [Google Scholar] [CrossRef]
- Zhang, M.L.; Yang, Y.; An, X.Q.; Zhao, J.J.; Bao, Y.P.; Hou, L.A. Exfoliation method matters: The microstructure-dependent photoactivity of g-C3N4 nanosheets for water purification. J. Hazard. Mater. 2022, 424, 127424. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Shi, J.W.; Huang, Z.X.; Guan, X.J.; Zong, S.C.; Cheng, C.; Zheng, B.T.; Guo, L.J. Synchronous construction of CoS2 in-situ loading and S doping for g-C3N4: Enhanced photocatalytic H2-evolution activity and mechanism insight. Chem. Eng. J. 2020, 401, 126135. [Google Scholar] [CrossRef]
- Xiong, T.; Cen, W.L.; Zhang, Y.X.; Dong, F. Bridging the g-C3N4 Interlayers for Enhanced Photocatalysis. ACS Catal. 2016, 6, 2462–2472. [Google Scholar] [CrossRef]
- Luo, L.; Gong, Z.; Ma, J.; Wang, K.; Zhu, H.; Li, K.; Xiong, L.; Guo, X.; Tang, J. Ultrathin sulfur-doped holey carbon nitride nanosheets with superior photocatalytic hydrogen production from water. Appl. Catal. B Environ. 2021, 284, 119742. [Google Scholar] [CrossRef]
- Guo, H.T.; Huang, H.; Li, Y.; Lu, S.K.; Xue, M.H.; Weng, W.; Zheng, T. Stepwise preparation of Ti-doped functionalized carbon nitride nanoparticles and hybrid TiO2/graphitic-C3N4 for detection of free residual chlorine and visible-light photocatalysis. Chem. Commun. 2019, 55, 13848–13851. [Google Scholar] [CrossRef]
- Liu, J.; Han, D.D.; Chen, P.J.; Zhai, L.P.; Wang, Y.J.; Chen, W.H.; Mi, L.W.; Yang, L.P. Positive roles of Br in g-C3N4/PTCDI-Br heterojunction for photocatalytic degrading chlorophenols. Chem. Eng. J. 2021, 418, 129492. [Google Scholar] [CrossRef]
- Palanivel, B.; Mani, A. Conversion of a Type-II to a Z-Scheme Heterojunction by Intercalation of a 0D Electron Mediator between the Integrative NiFe2O4/g-C3N4 Composite Nanoparticles: Boosting the Radical Production for Photo-Fenton Degradation. ACS Omega 2020, 5, 19747–19759. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Du, T.; Hu, C.; Chen, J.; Lu, J.; Lu, Z.; Han, H. Antibacterial Activity of Graphene Oxide/g-C3N4 Composite through Photocatalytic Disinfection under Visible Light. ACS Sustain. Chem. Eng. 2017, 5, 8693–8701. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Syrgiannis, Z.; La Parola, V.; Montini, T.; Petit, C.; Stathatos, E.; Godin, R.; Durrant, J.R.; Prato, M.; Fornasiero, P. Metal-free dual-phase full organic carbon nanotubes/g-C3N4 heteroarchitectures for photocatalytic hydrogen production. Nano Energy 2018, 50, 468–478. [Google Scholar] [CrossRef]
- Yu, F.T.; Wang, Z.Q.; Zhang, S.C.; Ye, H.N.; Kong, K.Y.; Gong, X.Q.; Hua, J.L.; Tian, H. Molecular Engineering of Donor-Acceptor Conjugated Polymer/g-C3N4 Heterostructures for Significantly Enhanced Hydrogen Evolution Under Visible-Light Irradiation. Adv. Funct. Mater. 2018, 28, 1804512. [Google Scholar] [CrossRef]
- Jo, W.-K.; Selvam, N.C.S. Z-scheme CdS/g-C3N4 composites with RGO as an electron mediator for efficient photocatalytic H2 production and pollutant degradation. Chem. Eng. J. 2017, 317, 913–924. [Google Scholar] [CrossRef]
- Mullakkattuthodi, S.; Haridas, V.; Sugunan, S.; Narayanan, B.N. Z-scheme mechanism for methylene blue degradation over Fe2O3/g-C3N4 nanocomposite prepared via one-pot exfoliation and magnetization of g-C3N4. Front. Mater. Sci. 2022, 16, 220612. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, S.; Chen, M.; Zhang, J.; Wang, X. Flower-like-flake Fe3O4/g-C3N4 nanocomposite: Facile synthesis, characterization, and enhanced photocatalytic performance. Colloid. Surf. A 2018, 537, 372–382. [Google Scholar] [CrossRef]
- Hu, S.; Ouyang, W.; Guo, L.; Lin, Z.; Jiang, X.; Qiu, B.; Chen, G. Facile synthesis of Fe3O4/g-C3N4/HKUST-1 composites as a novel biosensor platform for ochratoxin A. Bios. Bioelectron. 2017, 92, 718–723. [Google Scholar] [CrossRef]
- Li, K.; Gao, S.; Wang, Q.; Xu, H.; Wang, Z.; Huang, B.; Dai, Y.; Lu, J. In-Situ-Reduced Synthesis of Ti3+ Self-Doped TiO2/g-C3N4 Heterojunctions with High Photocatalytic Performance under LED Light Irradiation. ACS Appl. Mater. Interfaces 2015, 7, 9023–9030. [Google Scholar] [CrossRef]
- Tang, L.; Liu, Y.; Wang, J.; Zeng, G.; Deng, Y.; Dong, H.; Feng, H.; Wang, J.; Peng, B. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism. Appl. Catal. B Environ. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Ahilandeswari, G.; Arivuoli, D. Investigation of Ce2(WO4)3/g-C3N4 nanocomposite for degradation of industrial pollutants through sunlight-driven photocatalysis. Appl. Phys. A 2022, 128, 705. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, S.; Yang, J. Preparation of Ag2O/g-C3N4/Fe3O4 composites and the application in the photocatalytic degradation of Rhodamine B under visible light. J. Alloy. Compd. 2017, 708, 1141–1149. [Google Scholar] [CrossRef]
- Huang, S.; Xu, Y.; Xie, M.; Xu, H.; He, M.; Xia, J.; Huang, L.; Li, H. Synthesis of magnetic CoFe2O4/g-C3N4 composite and its enhancement of photocatalytic ability under visible-light. Colloid. Surf. A 2015, 478, 71–80. [Google Scholar] [CrossRef]
- Xu, Z.; Guan, L.; Li, H.; Sun, J.; Ying, Z.; Wu, J.; Xu, N. Structure Transition Mechanism of Single-Crystalline Silicon, g-C3N4, and Diamond Nanocone Arrays Synthesized by Plasma Sputtering Reaction Deposition. J. Phys. Chem. C 2015, 119, 29062–29070. [Google Scholar] [CrossRef]
- Cui, Y.; Tang, Y.; Wang, X. Template-free synthesis of graphitic carbon nitride hollow spheres for photocatalytic degradation of organic pollutants. Mater. Lett. 2015, 161, 197–200. [Google Scholar] [CrossRef]
- Fang, W.; Xing, M.; Zhang, J. Modifications on reduced titanium dioxide photocatalysts: A review. J. Photoch. Photobio. C 2017, 32, 21–39. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Zuo, Y.; Zhang, X.; Cui, L.F. Simple synthesis of ordered cubic mesoporous graphitic carbon nitride by chemical vapor deposition method using melamine. Mater. Lett. 2014, 136, 271–273. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kofuji, Y.; Sakamoto, H.; Tanaka, S.; Ichikawa, S.; Hirai, T. Effects of Surface Defects on Photocatalytic H2O2 Production by Mesoporous Graphitic Carbon Nitride under Visible Light Irradiation. ACS Catal. 2015, 5, 3058–3066. [Google Scholar] [CrossRef]
- Yang, L.; Liu, X.; Liu, Z.; Wang, C.; Liu, G.; Li, Q.; Feng, X. Enhanced photocatalytic activity of g-C3N4 2D nanosheets through thermal exfoliation using dicyandiamide as precursor. Ceram. Int. 2018, 44, 20613–20619. [Google Scholar] [CrossRef]
- Papailias, I.; Giannakopoulou, T.; Todorova, N.; Demotikali, D.; Vaimakis, T.; Trapalis, C. Effect of processing temperature on structure and photocatalytic properties of g-C3N4. Appl. Surf. Sci. 2015, 358, 278–286. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, E.; Shi, J.; Lin, X.; Sheng, L.; Zhang, M.; Wang, L.; Chen, J. A direct one-step synthesis of ultrathin g-C3N4 nanosheets from thiourea for boosting solar photocatalytic H2 evolution. Int. J. Hydrogen Energy 2019, 44, 7194–7204. [Google Scholar] [CrossRef]
- Paul, D.R.; Sharma, R.; Nehra, S.P.; Sharma, A. Effect of calcination temperature, pH and catalyst loading on photodegradation efficiency of urea derived graphitic carbon nitride towards methylene blue dye solution. RSC Adv. 2019, 9, 15381–15391. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, G.; Lin, Z.; Wang, X. Condensed and low-defected graphitic carbon nitride with enhanced photocatalytic hydrogen evolution under visible light irradiation. Appl. Catal. B: Environ. 2016, 181, 413–419. [Google Scholar] [CrossRef]
- Kumru, B.; Antonietti, M. Colloidal properties of the metal-free semiconductor graphitic carbon nitride. Adv. Colloid Interface Sci. 2020, 283, 102229. [Google Scholar] [CrossRef]
- Hasija, V.; Raizada, P.; Sudhaik, A.; Sharma, K.; Kumar, A.; Singh, P.; Jonnalagadda, S.B.; Thakur, V.K. Recent advances in noble metal free doped graphitic carbon nitride based nanohybrids for photocatalysis of organic contaminants in water: A review. Appl. Mater. Today 2019, 15, 494–524. [Google Scholar] [CrossRef]
- Groenewolt, M.; Antonietti, M. Synthesis of g-C3N4 Nanoparticles in Mesoporous Silica Host Matrices. Adv. Mater. 2005, 17, 1789–1792. [Google Scholar] [CrossRef]
- Ge, L. Synthesis and photocatalytic performance of novel metal-free g-C3N4 photocatalysts. Mater. Lett. 2011, 65, 2652–2654. [Google Scholar] [CrossRef]
- Samanta, S.; Yadav, R.; Kumar, A.; Kumar Sinha, A.; Srivastava, R. Surface modified C, O co-doped polymeric g-C3N4 as an efficient photocatalyst for visible light assisted CO2 reduction and H2O2 production. Appl. Catal. B Environ. 2019, 259, 118054. [Google Scholar] [CrossRef]
- Wang, T.; Nie, C.; Ao, Z.; Wang, S.; An, T. Recent progress in g-C3N4 quantum dots: Synthesis, properties and applications in photocatalytic degradation of organic pollutants. J. Mater. Chem. A 2020, 8, 485–502. [Google Scholar] [CrossRef]
- Schaber, P.M.; Colson, J.; Higgins, S.; Thielen, D.; Anspach, B.; Brauer, J. Thermal decomposition (pyrolysis) of urea in an open reaction vessel. Thermochim. Acta 2004, 424, 131–142. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Wang, Z.; Dawson, G.; Chen, W. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 2011, 21, 14398–14401. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhang, M.; Wang, X. Polycondensation of thiourea into carbon nitride semiconductors as visible light photocatalysts. J. Mater. Chem. 2012, 22, 8083–8091. [Google Scholar] [CrossRef]
- Chen, X.; Jun, Y.S.; Takanabe, K.; Maeda, K.; Domen, K.; Fu, X.; Antonietti, M.; Wang, X. Ordered Mesoporous SBA-15 Type Graphitic Carbon Nitride: A Semiconductor Host Structure for Photocatalytic Hydrogen Evolution with Visible Light. Chem. Mater. 2009, 21, 4093–4095. [Google Scholar] [CrossRef]
- Tian, N.; Xiao, K.; Zhang, Y.; Lu, X.; Ye, L.; Gao, P.; Ma, T.; Huang, H. Reactive sites rich porous tubular yolk-shell g-C3N4 via precursor recrystallization mediated microstructure engineering for photoreduction. Appl. Catal. B Environ. 2019, 253, 196–205. [Google Scholar] [CrossRef]
- Wu, X.; Gao, D.; Wang, P.; Yu, H.; Yu, J. NH4Cl-induced low-temperature formation of nitrogen-rich g-C3N4 nanosheets with improved photocatalytic hydrogen evolution. Carbon 2019, 153, 757–766. [Google Scholar] [CrossRef]
- Qi, H.; Liu, Y.; Li, C.; Zou, X.; Huang, Y.; Wang, Y. Precursor-reforming protocol to synthesis of porous N-doped g-C3N4 for highly improved photocatalytic water treatments. Mater. Lett. 2020, 264, 127329. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, G.; Wang, M.; Li, B.; Dang, M.; Ren, H.; Xia, A. The modulation of g-C3N4 energy band structure by excitons capture and dissociation. Mater. Res. Bull. 2020, 122, 110685. [Google Scholar] [CrossRef]
- Sun, S.; Fan, E.; Xu, H.; Cao, W.; Shao, G.; Fan, B.; Wang, H.; Zhang, R. Enhancement of photocatalytic activity of g-C3N4 by hydrochloric acid treatment of melamine. Nanotechnology 2019, 30, 315601. [Google Scholar] [CrossRef]
- Yin, J.-T.; Li, Z.; Cai, Y.; Zhang, Q.-F.; Chen, W. Ultrathin graphitic carbon nitride nanosheets with remarkable photocatalytic hydrogen production under visible LED irradiation. Chem. Commun. 2017, 53, 9430–9433. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Z.; Feng, J.; Yan, S.; Zhang, H.; Li, Z.; Zou, Z. Improvement in photocatalytic H2 evolution over g-C3N4 prepared from protonated melamine. Appl. Surf. Sci. 2014, 295, 253–259. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Y.; Xu, S. Synthesis of graphitic carbon nitride by directly heating sulfuric acid treated melamine for enhanced photocatalytic H2 production from water under visible light. Int. J. Hydrogen Energy 2012, 37, 125–133. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Cao, L.Y.; Xing, G.X.; Bai, Z.Q.; Huang, J.F.; Zhang, Z.P. Microscale flower-like magnesium oxide for highly efficient photocatalytic degradation of organic dyes in aqueous solution. RSC Adv. 2019, 9, 7338–7348. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, Z.F.; Ji, H.; Tang, H.; Tang, G.G.; Jiang, H.B. g-C3N4 anchored with MoS2 ultrathin nanosheets as high performance anode material for supercapacitor. Mater. Lett. 2019, 241, 35–38. [Google Scholar] [CrossRef]
- Wu, J.; Xie, Y.; Ling, Y.; Dong, Y.Y.; Li, J.; Li, S.Q.; Zhao, J.S. Synthesis of Flower-Like g-C3N4/BiOBr and Enhancement of the Activity for the Degradation of Bisphenol A Under Visible Light Irradiation. Front. Chem. 2019, 7, 649. [Google Scholar] [CrossRef]
- Bai, X.J.; Li, J.; Cao, C.B.; Hussain, S. Solvothermal synthesis of the special shape (deformable) hollow g-C3N4 nanospheres. Mater. Lett. 2011, 65, 1101–1104. [Google Scholar] [CrossRef]
- Lv, S.; Ng, Y.H.; Zhu, R.; Li, S.; Wu, C.; Liu, Y.; Zhang, Y.; Jing, L.; Deng, J.; Dai, H. Phosphorus vapor assisted preparation of P-doped ultrathin hollow g-C3N4 sphere for efficient solar-to-hydrogen conversion. Appl. Catal. B Environ. 2021, 297, 120438. [Google Scholar] [CrossRef]
- Huang, Z.W.; Zhang, Y.W.; Dai, H.Y.; Wang, Y.Y.; Qin, C.C.; Chen, W.X.; Zhou, Y.M.; Yuan, S.H. Highly dispersed Pd nanoparticles hybridizing with 3D hollow-sphere g-C3N4 to construct 0D/3D composites for efficient photocatalytic hydrogen evolution. J. Catal. 2019, 378, 331–340. [Google Scholar] [CrossRef]
- Li, Y.N.; Chen, Z.Y.; Wang, M.Q.; Zhang, L.Z.; Bao, S.J. Interface engineered construction of porous g-C3N4/TiO2 heterostructure for enhanced photocatalysis of organic pollutants. Appl. Surf. Sci. 2018, 440, 229–236. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Shi, Y.B.; Du, Z.Y.; He, J.J.; Zhong, J.B.; Zhao, L.C.; She, H.D.; Liu, G.; Su, B.T. Synthesis of Rod-Like g-C3N4/ZnS Composites with Superior Photocatalytic Activity for the Degradation of Methyl Orange. Eur. J. Inorg. Chem. 2015, 2015, 4108–4115. [Google Scholar] [CrossRef]
- Wu, M.; Li, Q.; Chen, C.; Su, G.; Song, M.; Sun, B.; Meng, J.; Shi, B. Constructed palladium-anchored hollow-rod-like graphitic carbon nitride created rapid visible-light-driven debromination of hexabromocyclododecane. Appl. Catal. B Environ. 2021, 297, 120409. [Google Scholar] [CrossRef]
- Niu, P.; Liu, G.; Cheng, H.M. Nitrogen Vacancy-Promoted Photocatalytic Activity of Graphitic Carbon Nitride. J. Phys. Chem. C 2012, 116, 11013–11018. [Google Scholar] [CrossRef]
- Liang, Q.; Li, Z.; Huang, Z.-H.; Kang, F.; Yang, Q.-H. Holey Graphitic Carbon Nitride Nanosheets with Carbon Vacancies for Highly Improved Photocatalytic Hydrogen Production. Adv. Funct. Mater. 2015, 25, 6885–6892. [Google Scholar] [CrossRef]
- Xu, J.; Fujitsuka, M.; Kim, S.; Wang, Z.; Majima, T. Unprecedented effect of CO2 calcination atmosphere on photocatalytic H2 production activity from water using g-C3N4 synthesized from triazole polymerization. Appl. Catal. B Environ. 2019, 241, 141–148. [Google Scholar] [CrossRef]
- Maeda, K.; Wang, X.; Nishihara, Y.; Lu, D.; Antonietti, M.; Domen, K. Photocatalytic Activities of Graphitic Carbon Nitride Powder for Water Reduction and Oxidation under Visible Light. J. Phys. Chem. C 2009, 113, 4940–4947. [Google Scholar] [CrossRef]
- Long, B.; Lin, J.; Wang, X. Thermally-induced desulfurization and conversion of guanidine thiocyanate into graphitic carbon nitride catalysts for hydrogen photosynthesis. J. Mater. Chem. A 2014, 2, 2942–2951. [Google Scholar] [CrossRef]
- Tian, N.; Zhang, Y.; Li, X.; Xiao, K.; Huang, H. Precursor-reforming protocol to 3D mesoporous g-C3N4 established by ultrathin self-doped nanosheets for superior hydrogen evolution. Nano Energy 2017, 38, 72–81. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zhang, G.; Wang, X. Synthesis of Carbon Nitride Semiconductors in Sulfur Flux for Water Photoredox Catalysis. ACS Catal. 2012, 2, 940–948. [Google Scholar] [CrossRef]
- Duan, Y.; Li, X.; Lv, K.; Zhao, L.; Liu, Y. Flower-like g-C3N4 assembly from holy nanosheets with nitrogen vacancies for efficient NO abatement. Appl. Surf. Sci. 2019, 492, 166–176. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Wu, L.; Fu, C.; Huang, W. Surface chemistry of TiO2 connecting thermal catalysis and photocatalysis. Phys. Chem. Chem. Phys. 2020, 22, 9875–9909. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.C.; Bromley, S.T.; Lee, J.Y.; Illas, F. Size-Dependent Level Alignment between Rutile and Anatase TiO2 Nanoparticles: Implications for Photocatalysis. J. Phys. Chem. Lett. 2017, 8, 5593–5598. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Huang, W.; Yang, S.; Zhou, X.; Ge, C.; Gao, Q.; Fang, Y.; Zhang, S. Facile synthesis of anatase/rutile TiO2/g-C3N4 multi-heterostructure for efficient photocatalytic overall water splitting. Int. J. Hydrogen Energy 2020, 45, 17378–17387. [Google Scholar] [CrossRef]
- Chen, H.; Xie, Y.; Sun, X.; Lv, M.; Xu, X.X. Efficient charge separation based on type-II g-C3N4/TiO2-B nanowire/tube heterostructure photocatalysts. Dalton Trans. 2015, 44, 13030–13039. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, M.; Zhu, Y. Photocatalytic enhancement of hybrid C3N4/TiO2 prepared via ball milling method. Phys. Chem. 2015, 17, 3647–3652. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, C.; Fu, X.; Raziq, F.; Qu, Y.; Jing, L. Synthesis of silicate-bridged ZnO/g-C3N4 nanocomposites as efficient photocatalysts and its mechanism. RSC Adv. 2015, 5, 37275–37280. [Google Scholar] [CrossRef]
- Guo, Y.; Chang, B.; Wen, T.; Zhang, S.; Yang, B. A Z-scheme photocatalyst for enhanced photocatalytic H2 evolution, constructed by growth of 2D plasmonic MoO3-x nanoplates onto 2D g-C3N4 nanosheets. J. Colloid Interf. Sci. 2020, 567, 213–223. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Zhang, L.; Gao, R.; Dai, W.-L. Construction of Highly Efficient 3D/2D MnO2/g-C3N4 Nanocomposite in the Epoxidation of Styrene with TBHP. ACS Sustain. Chem. Eng. 2019, 7, 17008–17019. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, J.; Hu, Z. Nano-sized g-C3N4 thin layer @ CeO2 sphere core-shell photocatalyst combined with H2O2 to degrade doxycycline in water under visible light irradiation. Sep. Purif. Technol. 2019, 227, 115665. [Google Scholar] [CrossRef]
- Cheng, R.; Zhang, L.; Fan, X.; Wang, M.; Shi, J. One-step construction of FeOx modified g-C3N4 for largely enhanced visible-light photocatalytic hydrogen evolution. Carbon 2016, 101, 62–70. [Google Scholar] [CrossRef]
- Mou, H.; Wang, J.; Yu, D.; Zhang, D.; Chen, W.; Wang, Y.; Wang, D.; Mu, T. Fabricating Amorphous g-C3N4/ZrO2 Photocatalysts by One-Step Pyrolysis for Solar-Driven Ambient Ammonia Synthesis. ACS Appl. Mater. Interfaces 2019, 11, 44360–44365. [Google Scholar] [CrossRef] [PubMed]
- Ramacharyulu, P.V.R.K.; Abbas, S.J.; Ke, S.-C. Enhanced charge separation and photoactivity in heterostructured g-C3N4: A synergistic interaction in environmental friendly CaO/g-C3N4. Catal. Sci. Technol. 2017, 7, 4940–4943. [Google Scholar] [CrossRef]
- Prakash, K.; Senthil, K.P.; Latha, P.; Shanmugam, R.; Karuthapandian, S. Dry synthesis of water lily flower like SrO2/g-C3N4 nanohybrids for the visible light induced superior photocatalytic activity. Mater. Res. Bull. 2017, 93, 112–122. [Google Scholar]
- Guan, R.; Li, J.; Zhang, J.; Zhao, Z.; Wang, D.; Zhai, H.; Sun, D. Photocatalytic Performance and Mechanistic Research of ZnO/g-C3N4 on Degradation of Methyl Orange. ACS Omega 2019, 4, 20742–20747. [Google Scholar] [CrossRef]
- Huang, L.; Xu, H.; Zhang, R.; Cheng, X.; Xia, J.; Xu, Y.; Li, H. Synthesis and characterization of g-C3N4/MoO3 photocatalyst with improved visible-light photoactivity. Appl. Surf. Sci. 2013, 283, 25–32. [Google Scholar] [CrossRef]
- Shen, W.; Wang, X.; Ge, Y.; Feng, H.; Feng, L. Synthesis and characterization of AgO/g-C3N4 hybrids with enhanced visible-light photocatalytic activity for Rhodamine B degradation and bactericidal inactivation. Colloid. Surf. A 2019, 575, 102–110. [Google Scholar] [CrossRef]
- Munusamy, T.D.; Yee, C.S.; Khan, M.M.R. Construction of hybrid g-C3N4/CdO nanocomposite with improved photodegradation activity of RhB dye under visible light irradiation. Adv. Powder Technol. 2020, 31, 2921–2931. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Zhang, W.-D. In2O3/g-C3N4 composite photocatalysts with enhanced visible light driven activity. Appl Surf Sci 2014, 301, 428–435. [Google Scholar] [CrossRef]
- Zhang, T.; Chang, F.; Qi, Y.; Zhang, X.; Yang, J.; Liu, X.; Li, S. A facile one-pot and alkali-free synthetic procedure for binary SnO2/g-C3N4 composites with enhanced photocatalytic behavior. Mat. Sci. Semicon. Proc. 2020, 115, 105112. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, Z.; Yang, Q.; Dong, X.; Zhang, J.; Qin, L. In-situ construction of all-solid-state Z-scheme g-C3N4/TiO2 nanotube arrays photocatalyst with enhanced visible-light-induced properties. Sol. Energ. Mat. Sol. C. 2016, 157, 399–405. [Google Scholar] [CrossRef]
- Li, B.; Nengzi, L.-C.; Guo, R.; Cui, Y.; Zhang, Y.; Cheng, X. Novel synthesis of Z-scheme α-Bi2O3/g-C3N4 composite photocatalyst and its enhanced visible light photocatalytic performance: Influence of calcination temperature. Chin. Chem. Lett. 2020, 31, 2705–2711. [Google Scholar] [CrossRef]
- Idrees, F.; Dillert, R.; Bahnemann, D.; Butt, F.K.; Tahir, M. In-Situ Synthesis of Nb2O5/g-C3N4 Heterostructures as Highly Efficient Photocatalysts for Molecular H2 Evolution under Solar Illumination. Catalysts 2019, 9, 169. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, J.; Yao, J. Shuttle-like CeO2/g-C3N4 composite combined with persulfate for the enhanced photocatalytic degradation of norfloxacin under visible light. Ecotox. Environ. Safe. 2020, 190, 110062. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Renu; Kaur, J.; Kaur, P.; Nitansh; Kumar, V.; Tikoo, K.; Kaushik, A.; Singhal, S. An innovative Z-scheme g-C3N4/ZnO/NiFe2O4 heterostructure for the concomitant photocatalytic removal and real-time monitoring of noxious fluoroquinolones. Chem. Eng. J. 2022, 443, 136441. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Boroumand, Z. Synthesis and characterization of a g-C3N4/TiO2-ZnO nanostructure for Photocatalytic degradation of methylene blue. Nano Futures 2022, 6, 035001. [Google Scholar]
- Preeyanghaa, M.; Dhileepan, M.D.; Madhavan, J.; Neppolian, B. Revealing the charge transfer mechanism in magnetically recyclable ternary g-C3N4/BiOBr/Fe3O4 nanocomposite for efficient photocatalytic degradation of tetracycline antibiotics. Chemosphere 2022, 303, 135070. [Google Scholar] [CrossRef]
- Hanif, M.A.; Akter, J.; Kim, Y.S.; Kim, H.G.; Hahn, J.R.; Kwac, L.K. Highly efficient and sustainable ZnO/CuO/g-C3N4 photocatalyst for wastewater treatment under visible light through heterojunction development. Catalysts 2022, 12, 151. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Hossaini, H.; Asadi, A.; Jafari, Z. Enhanced degradation of diazinon with a WO3-Fe3O4/g-C3N4-persulfate system under visible light: Pathway, intermediates toxicity and mechanism. Process Saf. Environ. Prot. 2022, 162, 1107–1123. [Google Scholar] [CrossRef]
- Swedha, M.; Alatar, A.A.; Okla, M.K.; Alaraidh, I.A.; Mohebaldin, A.; Aufy, M.; Raju, L.L.; Thomas, A.M.; Abdel-Maksoud, M.A.; Sudheer Khan, S. Graphitic carbon nitride embedded Ni3(VO4)2/ZnCr2O4 Z-scheme photocatalyst for efficient degradation of p-chlorophenol and 5-fluorouracil, and genotoxic evaluation in Allium cepa. J. Ind. Eng. Chem. 2022, 112, 244–257. [Google Scholar] [CrossRef]
- Li, F.-T.; Liu, S.-J.; Xue, Y.-B.; Wang, X.-J.; Hao, Y.-J.; Zhao, J.; Liu, R.-H.; Zhao, D. Structure Modification Function of g-C3N4 for Al2O3 in the In Situ Hydrothermal Process for Enhanced Photocatalytic Activity. Chem. Eur. J. 2015, 21, 10149–10159. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Zhu, C.; Li, H.; Kang, Z. CoO and g-C3N4 complement each other for highly efficient overall water splitting under visible light. Appl. Catal. B Environ. 2018, 226, 412–420. [Google Scholar] [CrossRef]
- Liu, J.; Jia, Q.; Long, J.; Wang, X.; Gao, Z.; Gu, Q. Amorphous NiO as co-catalyst for enhanced visible-light-driven hydrogen generation over g-C3N4 photocatalyst. Appl. Catal. B Environ. 2018, 222, 35–43. [Google Scholar] [CrossRef]
- Chen, J.; Shen, S.; Guo, P.; Wang, M.; Wu, P.; Wang, X.; Guo, L. In-situ reduction synthesis of nano-sized Cu2O particles modifying g-C3N4 for enhanced photocatalytic hydrogen production. Appl. Catal. B: Environ. 2014, 152–153, 335–341. [Google Scholar] [CrossRef]
- Mao, N.; Jiang, J.X. MgO/g-C3N4 Nanocomposites as Efficient Water Splitting Photocatalysts under Visible Light Irradiation. Appl. Surf. Sci. 2019, 476, 144–150. [Google Scholar] [CrossRef]
- Bajiri, M.A.; Hezam, A.; Namratha, K.; Viswanath, R.; Drmosh, Q.A.; Bhojya Naik, H.S.; Byrappa, K. CuO/ZnO/g-C3N4 heterostructures as efficient visible light-driven photocatalysts. J. Environ. Chem. Eng. 2019, 7, 103412. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Chen, X.; Yu, H.; Wang, H.; Wu, Z.; Zhang, J.; Xiong, T. In-situ synthesis of direct solid-state dual Z-scheme WO3/g-C3N4/Bi2O3 photocatalyst for the degradation of refractory pollutant. Appl. Catal. B Environ. 2018, 227, 376–385. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, G.-F.; Hu, W.-Y.; Xiong, D.-N.; Zhou, B.-X.; Chang, S.; Huang, W.-Q. Construction of g-C3N4/CeO2/ZnO ternary photocatalysts with enhanced photocatalytic performance. J. Phys. Chem. Solids 2017, 106, 1–9. [Google Scholar] [CrossRef]
- Jiang, D.; Yu, H.; Yu, H. Modified g-C3N4/TiO2 nanosheets/ZnO ternary facet coupled heterojunction for photocatalytic degradation of p-toluenesulfonic acid (p-TSA) under visible light. Physica E 2017, 85, 1–6. [Google Scholar] [CrossRef]
- Liu, S.J.; Li, F.T.; Li, Y.L.; Hao, Y.J.; Wang, X.J.; Li, B.; Liu, R.H. Fabrication of ternary g-C3N4/Al2O3/ZnO heterojunctions based on cascade electron transfer toward molecular oxygen activation. Appl. Catal. B Environ. 2017, 212, 115–128. [Google Scholar] [CrossRef]
- Ren, Y.; Zhan, W.; Tang, L.; Zheng, H.; Liu, H.; Tang, K. Constructing a ternary H2SrTa2O7/g-C3N4/Ag3PO4 heterojunction based on cascade electron transfer with enhanced visible light photocatalytic activity. CrystEngComm 2020, 22, 6485–6494. [Google Scholar] [CrossRef]
- Raza, A.; Shen, H.; Haidry, A.A.; Cui, S. Hydrothermal synthesis of Fe3O4 /TiO2/g-C3N4: Advanced photocatalytic application. Appl. Surf. Sci. 2019, 488, 887–895. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Hierarchical magnetic petal-like Fe3O4-ZnO@g-C3N4 for removal of sulfamethoxazole, suppression of photocorrosion, by-products identification and toxicity assessment. Chemosphere 2018, 205, 463. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, X.; Liu, X.; Yang, X.; Yang, Y. A Ternary Magnetic Recyclable ZnO/Fe3O4/g-C3N4 Composite Photocatalyst for Efficient Photodegradation of Monoazo Dye. Nanoscale Res. Lett. 2019, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Balu, S.; Velmurugan, S.; Palanisamy, S.; Chen, S.-W.; Velusamy, V.; Yang, T.C.K.; El-Shafey, E.-S.I. Synthesis of α-Fe2O3 decorated g-C3N4/ZnO ternary Z-scheme photocatalyst for degradation of tartrazine dye in aqueous media. J. Taiwan Inst. Chem. E. 2019, 99, 258–267. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Krishnan, V. Perovskite Oxide Based Materials for Energy and Environment-Oriented Photocatalysis. ACS Catal. 2020, 10, 10253–10315. [Google Scholar] [CrossRef]
- Grabowska, E. Selected perovskite oxides: Characterization, preparation and photocatalytic properties-A review. Appl. Catal. B Environ. 2016, 186, 97–126. [Google Scholar] [CrossRef]
- Shi, R.; Waterhouse, G.I.N.; Zhang, T.R. Recent Progress in Photocatalytic CO2 Reduction Over Perovskite Oxides. Sol. RRL 2017, 1, 1700126. [Google Scholar] [CrossRef]
- Kumar, A.; Schuerings, C.; Kumar, S.; Kumar, A.; Krishnan, V. Perovskite-structured CaTiO3 coupled with g-C3N4 as a heterojunction photocatalyst for organic pollutant degradation. Beilstin. J. Nanotechnol. 2018, 9, 671–685. [Google Scholar] [CrossRef]
- Ye, R.; Fang, H.; Zheng, Y.-Z.; Li, N.; Wang, Y.; Tao, X. Fabrication of CoTiO3/g-C3N4 Hybrid Photocatalysts with Enhanced H2 Evolution: Z-Scheme Photocatalytic Mechanism Insight. ACS Appl. Mater. Interfaces 2016, 8, 13879–13889. [Google Scholar] [CrossRef]
- Yao, Y.; Lu, F.; Zhu, Y.; Wei, F.; Liu, X.; Lian, C.; Wang, S. Magnetic core–shell CuFe2O4@C3N4 hybrids for visible light photocatalysis of Orange II. J. Hazard. Mater. 2015, 297, 224–233. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, K.; Yu, J.; Jin, J.; Qi, Y.; Li, H.; Hou, X.; Liu, G. Photocatalytic water splitting for hydrogen generation on cubic, orthorhombic, and tetragonal KNbO3 microcubes. Nanoscale 2013, 5, 8375–8383. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, Z.; Wang, Y.; Ma, Y.; Feng, Z.; Lin, H.; Wu, Y.; Zhao, L.; He, Y. Synthesis of KNbO3/g-C3N4 composite and its new application in photocatalytic H2 generation under visible light irradiation. J. Mater. Sci. 2018, 53, 7453–7465. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Tu, W.; Liu, Y.; Tan, Y.Z.; Yuan, X.; Chew, J.W. Quasi-polymeric construction of stable perovskite-type LaFeO3/g-C3N4 heterostructured photocatalyst for improved Z-scheme photocatalytic activity via solid p-n heterojunction interfacial effect. J. Hazard. Mater. 2018, 347, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Deng, B.; Pu, Y.; Liu, A.; Wang, J.; Ma, K.; Gao, F.; Gao, B.; Zou, W.; Dong, L. Interfacial coupling effects in g-C3N4/SrTiO3 nanocomposites with enhanced H2 evolution under visible light irradiation. Appl. Catal. B Environ. 2019, 247, 1–9. [Google Scholar] [CrossRef]
- Luo, J.; Chen, J.; Guo, R.; Qiu, Y.; Li, W.; Zhou, X.; Ning, X.; Zhan, L. Rational construction of direct Z-scheme LaMnO3/g-C3N4 hybrid for improved visible-light photocatalytic tetracycline degradation. Sep. Purif. Technol. 2019, 211, 882–894. [Google Scholar] [CrossRef]
- Wang, D.F.; Zou, Z.G.; Ye, J.H. A new spinel-type photocatalyst BaCr2O4 for H2 evolution under UV and visible light irradiation. Chem. Phys. Lett. 2003, 373, 191–196. [Google Scholar] [CrossRef]
- Jia, Y.F.; Ma, H.X.; Zhang, W.B.; Zhu, G.Q.; Yang, W.; Son, N.; Kang, M.; Liu, C.L. Z-scheme SnFe2O4-graphitic carbon nitride: Reusable, magnetic catalysts for enhanced photocatalytic CO2 reduction. Chem. Eng. J. 2020, 383, 123172. [Google Scholar] [CrossRef]
- Chang, W.; Xue, W.; Liu, E.; Fan, J.; Zhao, B. Highly efficient H2 production over NiCo2O4 decorated g-C3N4 by photocatalytic water reduction. Chem. Eng. J. 2019, 362, 392–401. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B: Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Sivasakthi, S.; Gurunathan, K. Graphitic carbon nitride bedecked with CuO/ZnO hetero-interface microflower towards high photocatalytic performance. Renew. Energy 2020, 159, 786–800. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, W.; Li, H.; Yuan, S.; Zhao, H.; Wong, P.K. A Hierarchical Z-Scheme α-Fe2O3/g-C3N4 Hybrid for Enhanced Photocatalytic CO2 Reduction. Adv. Mater. 2018, 30, 1706108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Xiong, W.; Wang, L. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: A review. Chemosphere 2019, 214, 462–479. [Google Scholar] [PubMed]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zheng, B.; Mao, L.; Cheng, C.; Hu, Y.; Wang, H.; Li, G.; Jing, D.; Liang, X. MoO3/g-C3N4 Z-scheme (S-scheme) system derived from MoS2/melamine dual precursors for enhanced photocatalytic H2 evolution driven by visible light. Int. J. Hydrogen Energy 2021, 46, 2927–2935. [Google Scholar] [CrossRef]
- Li, W.; Da, P.; Zhang, Y.; Wang, Y.; Zheng, G. WO nanoflakes for enhanced photoelectrochemical conversion. ACS Nano 2014, 8, 11770–11777. [Google Scholar] [CrossRef]
- Bai, Y.; Ye, L.; Wang, L.; Shi, X.; Wang, P.; Bai, W. A dual-cocatalyst-loaded Au/BiOI/MnOx system for enhanced photocatalytic greenhouse gas conversion into solar fuels. Environ. Sci. Nano. 2016, 3, 902–909. [Google Scholar]
- Chen, X.; Zhu, K.; Wang, P.; Sun, G.; Yao, Y.; Luo, W.; Zou, Z. Reversible Charge Transfer and Adjustable Potential Window in Semiconductor/Faradaic Layer/Liquid Junctions. iScience 2020, 23, 100949. [Google Scholar] [CrossRef]
- Jahurul Islam, M.; Amaranatha Reddy, D.; Han, N.S.; Choi, J.; Song, J.K.; Kim, T.K. An oxygen-vacancy rich 3D novel hierarchical MoS2/BiOI/AgI ternary nanocomposite: Enhanced photocatalytic activity through photogenerated electron shuttling in a Z-scheme manner. Phys. Chem. Chem. Phys. 2016, 18, 24984–24993. [Google Scholar]
- Wang, N.; Wu, L.; Li, J.; Mo, J.; Peng, Q.; Li, X. Construction of hierarchical Fe2O3@MnO2 core/shell nanocube supported C3N4 for dual Z-scheme photocatalytic water splitting. Sol. Energy Mater. Sol. Cells 2020, 215, 110624. [Google Scholar]
- Chang, X.; Wang, T.; Gong, J. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Zhang, L.; Teng, B.; Fan, M. High-efficiency conversion of CO2 to fuel over ZnO/g-C3N4 photocatalyst. Appl. Catal. B Environ. 2015, 168–169, 1–8. [Google Scholar] [CrossRef]
- Nie, N.; Zhang, L.; Fu, J.; Cheng, B.; Yu, J. Self-assembled hierarchical direct Z-scheme g-C3N4/ZnO microspheres with enhanced photocatalytic CO2 reduction performance. Appl. Surf. Sci. 2018, 441, 12–22. [Google Scholar] [CrossRef]
- Bhosale, R.; Jain, S.; Vinod, C.P.; Kumar, S.; Ogale, S. Direct Z-Scheme g-C3N4/FeWO4 Nanocomposite for Enhanced and Selective Photocatalytic CO2 Reduction under Visible Light. ACS Appl. Mater. Interfaces 2019, 11, 6174–6183. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Peng, Z.; Wang, W.; Tan, F.; Wang, X.; Su, B.; Qiao, X.; Wong, P.K. g-C3N4/MgO nanosheets: Light-independent, metal-poisoning-free catalysts for the activation of hydrogen peroxide to degrade organics. J. Mater. Chem. A 2018, 6, 16421–16429. [Google Scholar] [CrossRef]
- Fan, J.; Qin, H.; Jiang, S. Mn-doped g-C3N4 composite to activate peroxymonosulfate for acetaminophen degradation: The role of superoxide anion and singlet oxygen. Chem. Eng. J. 2019, 359, 723–732. [Google Scholar] [CrossRef]
- Ding, X.; Xiao, D.; Ji, L.; Jin, D.; Dai, K.; Yang, Z.; Wang, S.; Chen, H. Simple fabrication of Fe3O4/C/g-C3N4 two-dimensional composite by hydrothermal carbonization approach with enhanced photocatalytic performance under visible light. Catal. Sci. Technol. 2018, 8, 3484–3492. [Google Scholar] [CrossRef]
- Zou, X.; Dong, Y.; Li, S.; Ke, J.; Cui, Y.; Ou, X. Fabrication of V2O5/g-C3N4 heterojunction composites and its enhanced visible light photocatalytic performance for degradation of gaseous ortho-dichlorobenzene. J. Taiwan Inst. Chem. E. 2018, 93, 158–165. [Google Scholar] [CrossRef]
- Katsumata, K.-i.; Motoyoshi, R.; Matsushita, N.; Okada, K. Preparation of graphitic carbon nitride (g-C3N4)/WO3 composites and enhanced visible-light-driven photodegradation of acetaldehyde gas. J. Hazard. Mater. 2013, 260, 475–482. [Google Scholar] [CrossRef]
- Wang, S.; Ding, X.; Zhang, X.; Pang, H.; Hai, X.; Zhan, G.; Zhou, W.; Song, H.; Zhang, L.; Chen, H.; et al. In Situ Carbon Homogeneous Doping on Ultrathin Bismuth Molybdate: A Dual-Purpose Strategy for Efficient Molecular Oxygen Activation. Adv. Funct. Mater. 2017, 27, 1703923. [Google Scholar] [CrossRef]
- Xu, X.; Geng, A.; Yang, C.; Carabineiro, S.A.C.; Lv, K.; Zhu, J.; Zhao, Z. One-pot synthesis of La–Fe–O@CN composites as photo-Fenton catalysts for highly efficient removal of organic dyes in wastewater. Ceram. Int. 2020, 46, 10740–10747. [Google Scholar] [CrossRef]
- Xu, X.; Lin, H.; Xiao, P.; Zhu, J.; Bi, H.; Carabineiro, S.A.C. Construction of Ag-Bridged Z-Scheme LaFe0.5Co0.5O3/Ag10/Graphitic Carbon Nitride Heterojunctions for Photo-Fenton Degradation of Tetracycline Hydrochloride: Interfacial Electron Effect and Reaction Mechanism. Adv. Mater. Interfaces 2022, 9, 2101902. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, P.; Shi, Y.-K.; Li, J.-S.; You, W.-S.; Zhang, L.-C.; Sang, X.-J. Preparation of a g-C3N4/Co3O4/Ag2O ternary heterojunction nanocomposite and its photocatalytic activity and mechanism. New J. Chem. 2020, 44, 6261–6268. [Google Scholar] [CrossRef]
- She, X.; Xu, H.; Wang, H.; Xia, J.; Song, Y.; Yan, J.; Xu, Y.; Zhang, Q.; Du, D.; Li, H. Controllable synthesis of CeO2/g-C3N4 composites and their applications in the environment. Dalton T. 2015, 44, 7021–7031. [Google Scholar] [CrossRef]
- Chen, H.Y.; Qiu, L.G.; Xiao, J.D.; Ye, S.; Jiang, X.; Yuan, Y.P. Inorganic–organic hybrid NiO–g-C3N4 photocatalyst for efficient methylene blue degradation using visible light. RSC Adv. 2014, 4, 22491. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, D.; Yan, Z.; Liu, D.; Qian, K.; Xie, J. A new visible light active multifunctional ternary composite based on TiO2–In2O3 nanocrystals heterojunction decorated porous graphitic carbon nitride for photocatalytic treatment of hazardous pollutant and H2 evolution. Appl. Catal. B Environ. 2015, 170–171, 195–205. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Li, H.; Ge, H.; Bian, Z. The enhanced photoreduction of Cr(VI) to Cr(III) using carbon dots coupled TiO2 mesocrystals. Appl. Catal. B Environ. 2017, 226, 213–219. [Google Scholar] [CrossRef]
- Liang, Z.-Y.; Wei, J.-X.; Wang, X.; Yu, Y.; Xiao, F.-X. Elegant Z-scheme-dictated g-C3N4 enwrapped WO3 superstructures: A multifarious platform for versatile photoredox catalysis. J. Mater. Chem. A 2017, 5, 15601–15612. [Google Scholar] [CrossRef]
- Song, X.-Y.; Chen, Q.-L. Facile preparation of g-C3N4/Bi2WO6 hybrid photocatalyst with enhanced visible light photoreduction of Cr(VI). J. Nanopart. Res. 2019, 21, 183. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, Y.; Chen, W.; Zhao, Z.; Thomas, A. Graphitic carbon nitride as a metal-free catalyst for NO decomposition. Chem. Commun. 2010, 46, 6965–6967. [Google Scholar] [CrossRef]
- Sano, T.; Tsutsui, S.; Koike, K.; Hirakawa, T.; Teramoto, Y.; Negishi, N.; Takeuchi, K. Activation of graphitic carbon nitride (g-C3N4) by alkaline hydrothermal treatment for photocatalytic NO oxidation in gas phase. J. Mater. Chem. A 2013, 1, 6489–6496. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, W.; Sun, Y.; Dong, F.; Zhou, Y.; Ho, W.-K. Water-assisted production of honeycomb-like g-C3N4 with ultralong carrier lifetime and outstanding photocatalytic activity. Nanoscale 2015, 7, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liang, L.; Wang, F.; Ma, J.; Sun, J. Polycondensation of guanidine hydrochloride into a graphitic carbon nitride semiconductor with a large surface area as a visible light photocatalyst. Catal. Sci. Technol. 2014, 4, 3235–3243. [Google Scholar] [CrossRef]
- Wang, W.; Huang, G.; Yu, J.C.; Wong, P.K. Advances in photocatalytic disinfection of bacteria: Development of photocatalysts and mechanisms. J. Environ. Sci. 2015, 34, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Tadashi, M.; Ryozo, T.; Toshiaki, N.; Hitoshi, W. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar]
- Huang, J.; Ho, W.; Wang, X. Metal-free disinfection effects induced by graphitic carbon nitride polymers under visible light illumination. Chem. Commun. 2014, 50, 4338–4340. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Zhu, Y. Enhanced Visible-Light-Driven Photocatalytic Disinfection Performance and Organic Pollutant Degradation Activity of Porous g-C3N4 Nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 27727–27735. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Shuai, D.; Naraginti, S.; Wang, D.; Zhang, W. Visible-light-driven photocatalytic inactivation of MS2 by metal-free g-C3N4: Virucidal performance and mechanism. Water Res. 2016, 106, 249–258. [Google Scholar] [CrossRef]
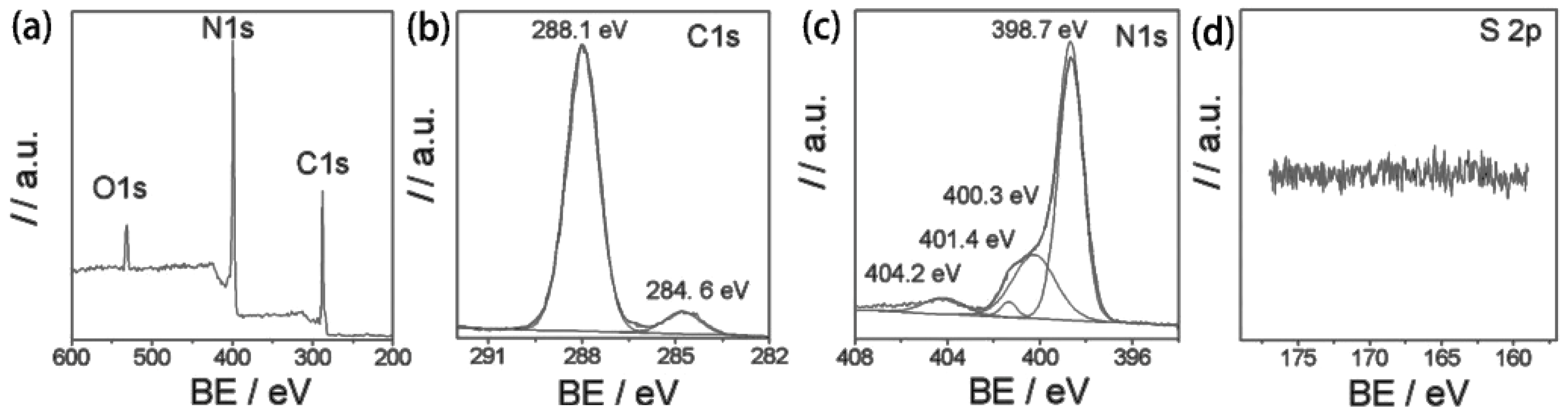

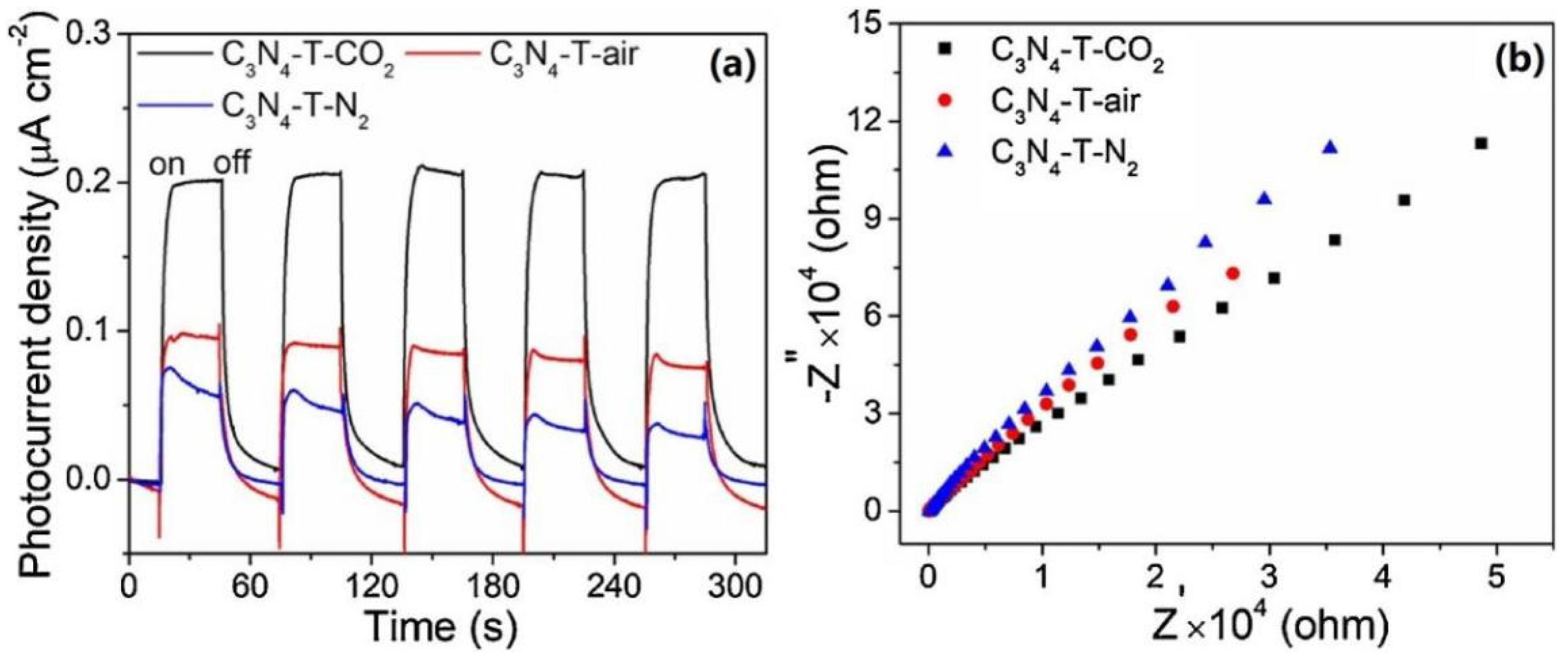
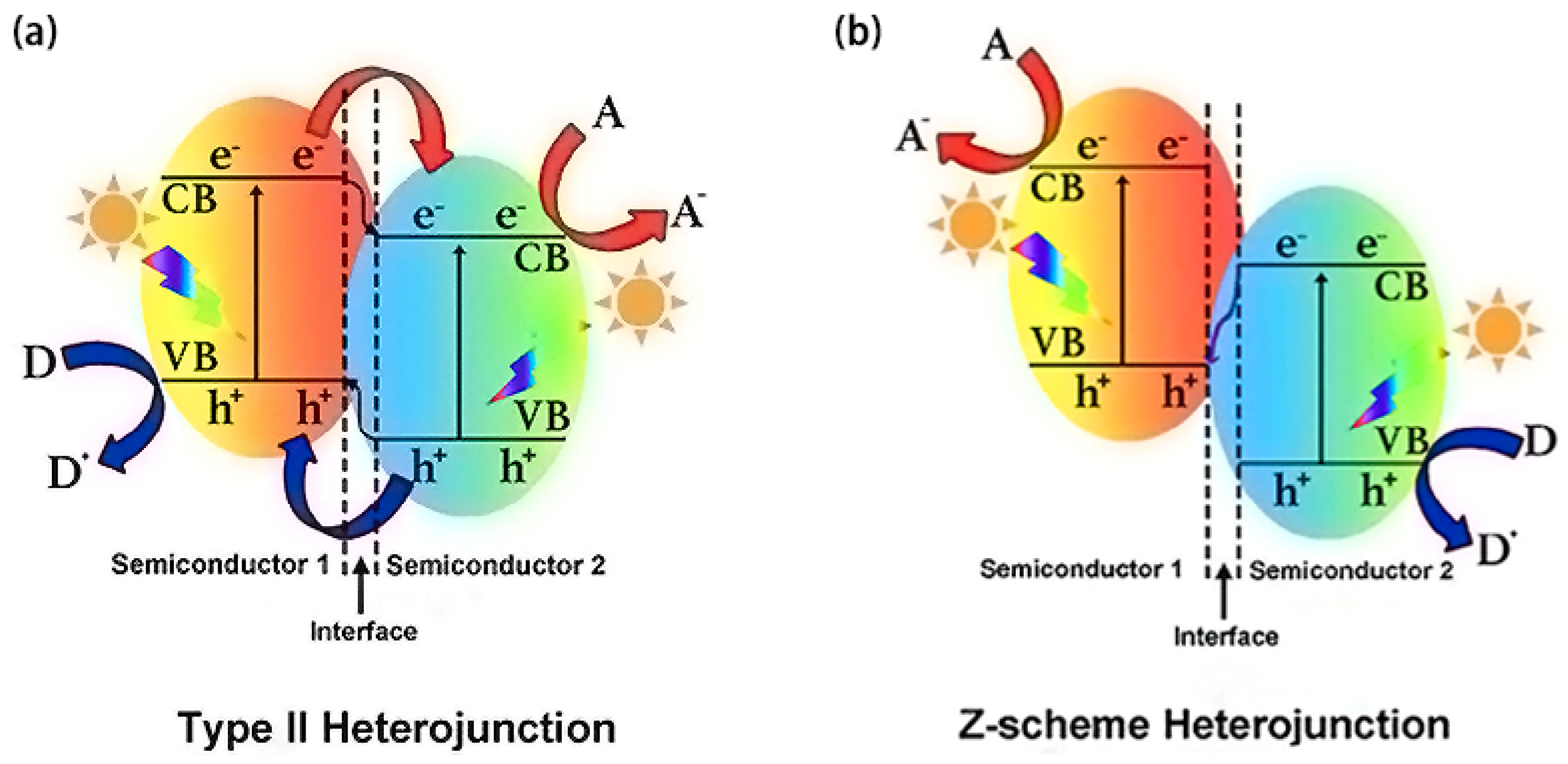


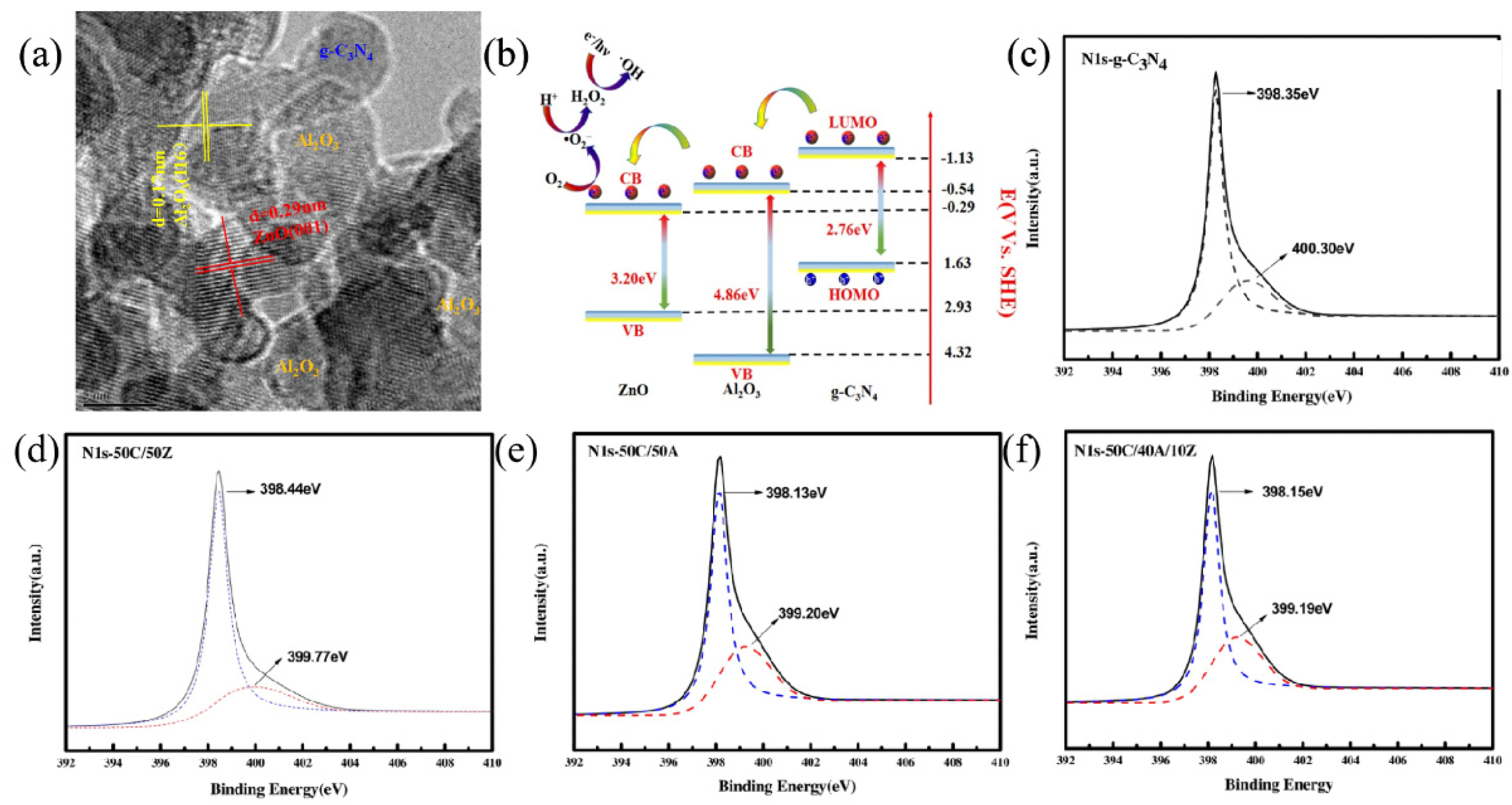
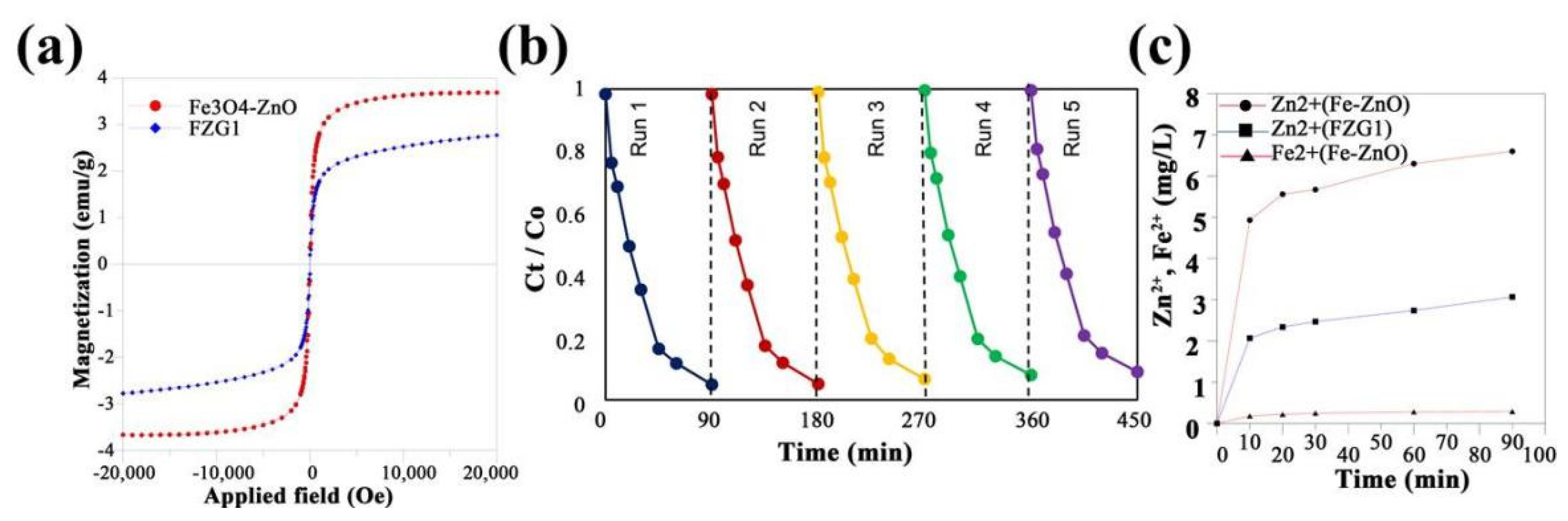
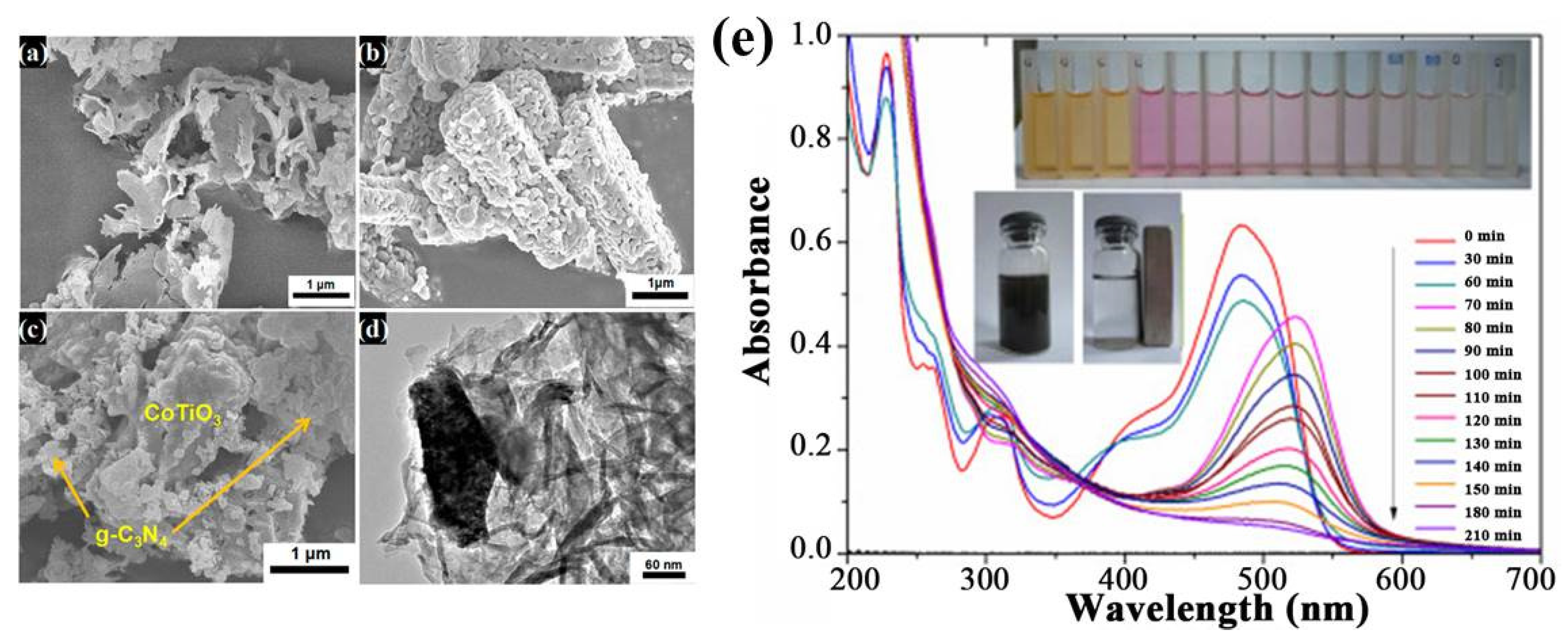
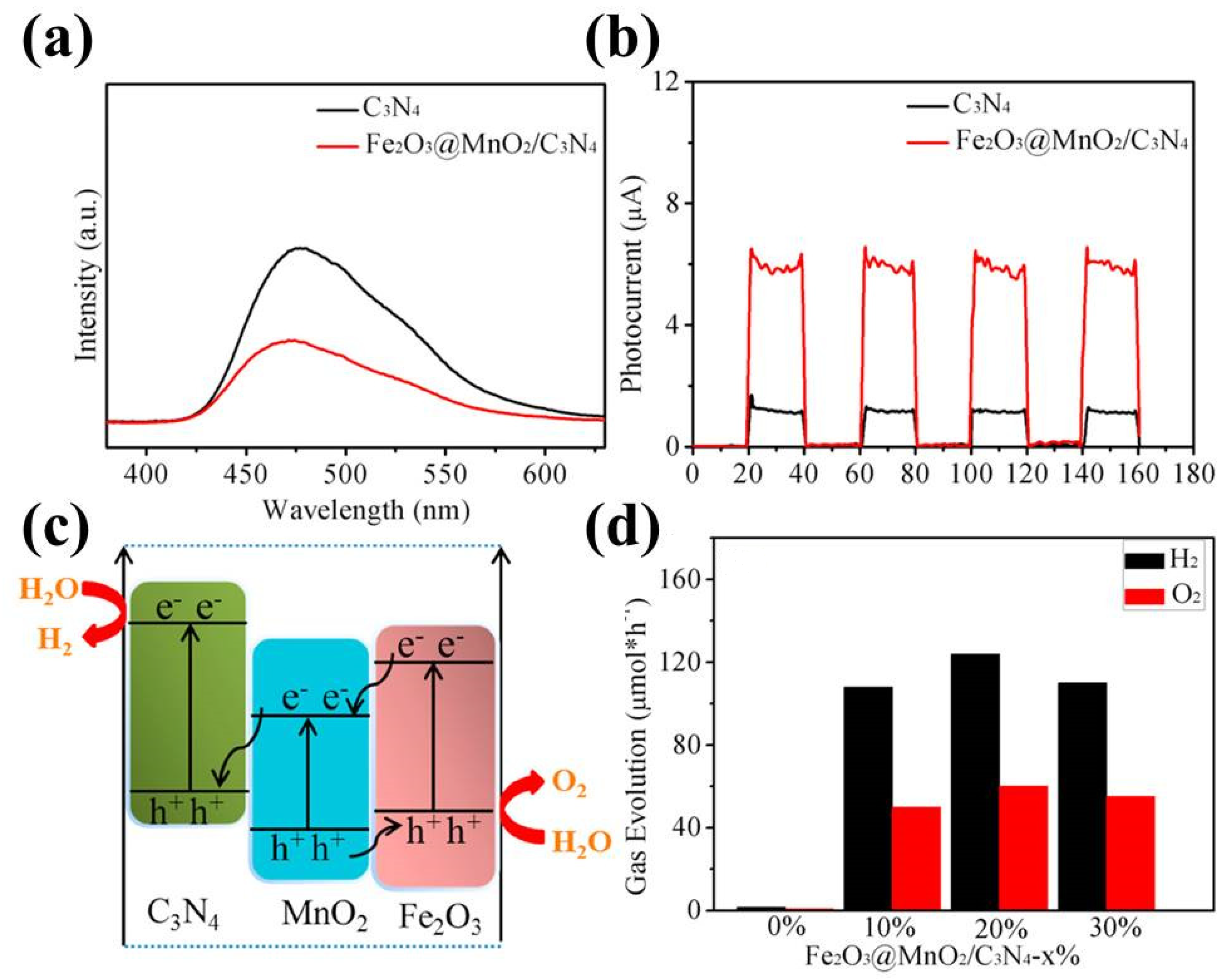
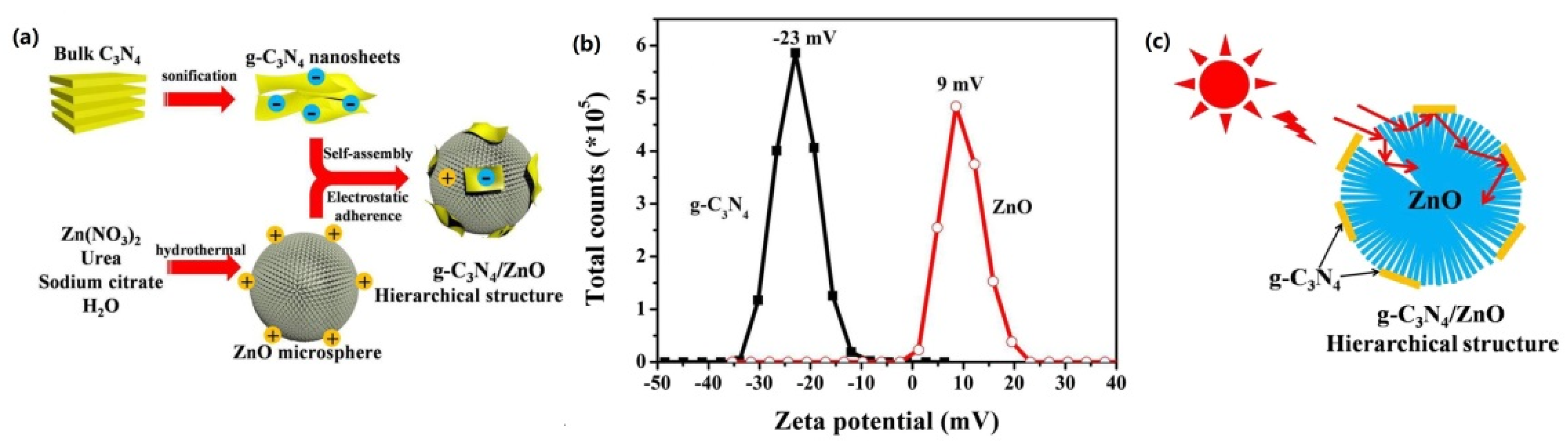

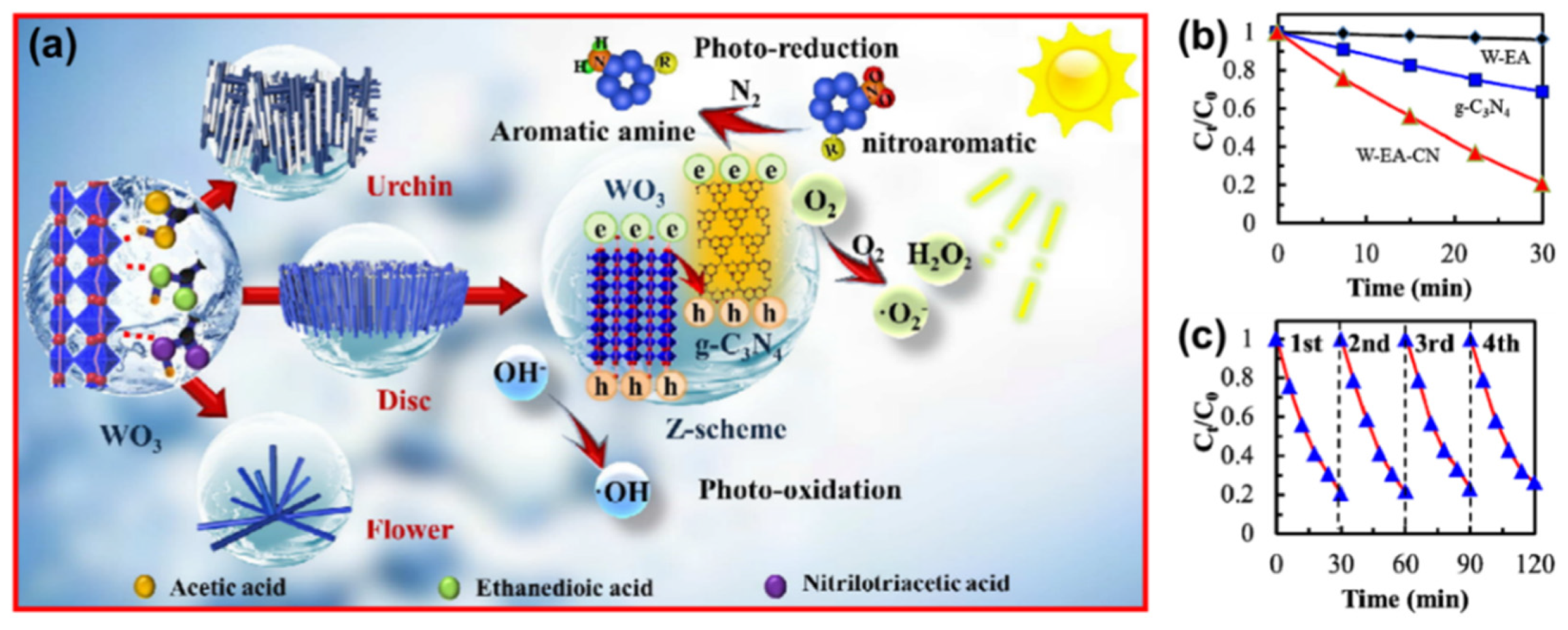
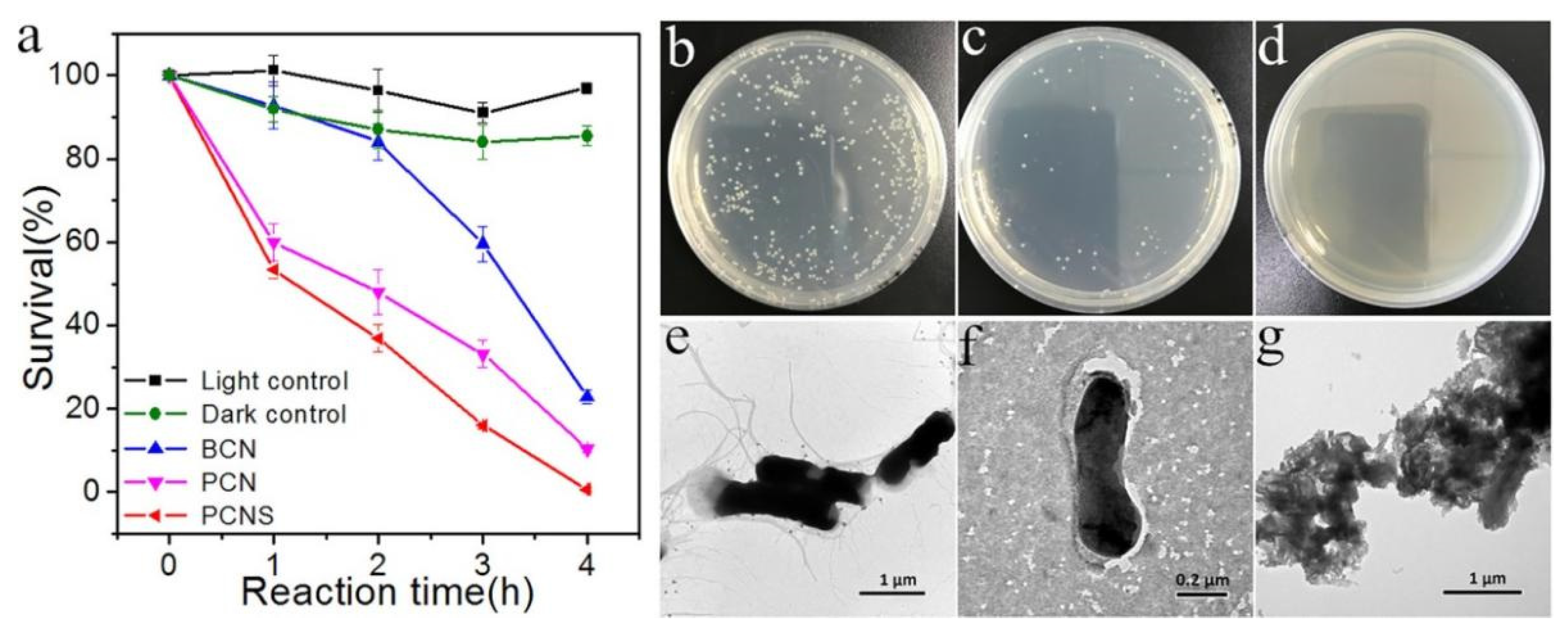
| Precursor | Reaction Conditions | Band Gap [eV] | Surface Area [m2/g] | Ref. |
|---|---|---|---|---|
| Cyanamide | 550 °C, 4 h, N2 | 2.62 | 10 | [95] |
| Dicyandiamide | 550 °C, 3 h, air | 2.64 | 40.5 | [38] |
| Melamine | 550 °C, 3 h, air | 2.66 | 28.2 | [38] |
| Urea | 550 °C, 3 h, air | 2.72 | 67.1 | [38] |
| Urea | 550 °C, 2 h, air | 2.76 | 58 | [39] |
| Thiourea | 550 °C, 2 h, air | 2.58 | 18 | [39] |
| 3-amino-1, 2, 4-triazole | 550 °C, 4 h, CO2 | 2.05 | 7.2 | [56] |
| Ammonium thiocyanate | 550 °C, 2 h, NH3 | 2.87 | 46 | [55] |
| Guanidine hydrochlorides | 550 °C, 3 h, air | 2.70 | 16.08 | [58] |
| Guanidine thiocyanate | 550 °C, 2 h, N2 | 2.74 | 8 | [96] |
| Urea Melamine | 520 °C, 4 h, air | 2.47 | 39.06 | [97] |
| Imidazole-mixed urea | 550 °C, 4 h, air | 2.26 | 105.28 | [77] |
| Sulfur-mixed melamine | 650 °C, 2 h, N2 | 2.65 | 26 | [98] |
| Melamine–cyanuric acid | 550 °C, 10 h, air | 2.72 | 142.8 | [99] |
| H2SO4-treated melamine | 600 °C, 4 h, Ar | 2.69 | 15.6 | [82] |
| HCl-treated melamine | 550 °C, 2 h, air | 2.66 | 24.7 | [79] |
| HNO3-treated melamine | 550 °C, 2 h, air | 2.65 | 59.3 | [81] |
| Sample | Model Reaction | Reaction Activity (mol/g/min) | Refs. |
|---|---|---|---|
| CaO/g-C3N4 | Degradation of MB | 2.6 × 10−5 | [112] |
| SrO2/g-C3N4 | Degradation of RhB | 4.5 × 10−7 | [113] |
| MnO2/g-C3N4 | Degradation of MO | 9.4 × 10−7 | [108] |
| ZnO/g-C3N4 | Degradation of MO | 3.3 × 10−7 | [114] |
| MoO3/g-C3N4 | Degradation of MB | 9.7 × 10−7 | [115] |
| AgO/g-C3N4 | Degradation of RhB | 5.2 × 10−6 | [116] |
| CdO/g-C3N4 | Degradation of RhB | 1.1 × 10−7 | [117] |
| In2O3/g-C3N4 | Degradation of RhB | 5.2 × 10−10 | [118] |
| SnO2/g-C3N4 | Degradation of MO | 7.9 × 10−8 | [119] |
| TiO2/g-C3N4 | Degradation of RhB | 9.3 × 10−9 | [120] |
| Bi2O3/g-C3N4 | Degradation of Amido black 10B dye | 3.3 × 10−7 | [121] |
| Nb2O5/g-C3N4 | Degradation of tetracycline | 5.3 × 10−7 | [122] |
| CeO2/g-C3N4 | Degradation of Norfloxacin | 4.6 × 10−7 | [123] |
| ZnO/NiFe2O4/g-C3N4 | Degradation of LVX | 1.7 × 10−7 | [124] |
| TiO2/ZnO/g-C3N4 | Degradation of MB | 4.9 × 10−7 | [125] |
| Fe3O4/BiOBr/g-C3N4 | Degradation of TC | 8.8 × 10−7 | [126] |
| ZnO/CuO/g-C3N4 | Degradation of MB | 6.1 × 10−6 | [127] |
| WO3/Fe3O4/g-C3N4 | Degradation of diazinon | 6.5 × 10−7 | [128] |
| Ni3(VO4)2/ZnCr2O4/g-C3N4 | Degradation of p-CP | 4.8 × 10−4 | [129] |
| Sample | Reaction Activity (mol/g/min) | Refs. |
|---|---|---|
| Al2O3/g-C3N4 | Al2O3/g-C3N4: 8.7 × 10−6 g-C3N4: 3.5 × 10−6 | [130] |
| CoO/g-C3N4 | CoO/g-C3N4: 8.4 × 10−7 CoO: 4.9 × 10−8 g-C3N4: 8.3 × 10−8 | [131] |
| NiO/g-C3N4 | NiO/g-C3N4: 2.5 × 10−7 g-C3N4: 2.7 × 10−9 | [132] |
| Cu2O/g-C3N4 | Cu2O/g-C3N4: 4.0 × 10−6 g-C3N4: 2.4 × 10−6 | [133] |
| MgO/g-C3N4 | MgO/g-C3N4: 5.0 × 10−6 g-C3N4: 9.7 × 10−7 | [134] |
| FEOX/G-C3N4 | FeOx/g-C3N4: 1.8 × 10−5 g-C3N4: 4.3 × 10−6 | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Xiao, Y.; Geng, A.; Bi, H.; Xu, X.; Xu, X.; Zhu, J. Research Progress on Graphitic Carbon Nitride/Metal Oxide Composites: Synthesis and Photocatalytic Applications. Int. J. Mol. Sci. 2022, 23, 12979. https://doi.org/10.3390/ijms232112979
Lin H, Xiao Y, Geng A, Bi H, Xu X, Xu X, Zhu J. Research Progress on Graphitic Carbon Nitride/Metal Oxide Composites: Synthesis and Photocatalytic Applications. International Journal of Molecular Sciences. 2022; 23(21):12979. https://doi.org/10.3390/ijms232112979
Chicago/Turabian StyleLin, Hao, Yao Xiao, Aixia Geng, Huiting Bi, Xiao Xu, Xuelian Xu, and Junjiang Zhu. 2022. "Research Progress on Graphitic Carbon Nitride/Metal Oxide Composites: Synthesis and Photocatalytic Applications" International Journal of Molecular Sciences 23, no. 21: 12979. https://doi.org/10.3390/ijms232112979
APA StyleLin, H., Xiao, Y., Geng, A., Bi, H., Xu, X., Xu, X., & Zhu, J. (2022). Research Progress on Graphitic Carbon Nitride/Metal Oxide Composites: Synthesis and Photocatalytic Applications. International Journal of Molecular Sciences, 23(21), 12979. https://doi.org/10.3390/ijms232112979






