Selective Oxidation of Toluene to Benzaldehyde Using Co-ZIF Nano-Catalyst
Abstract
1. Introduction
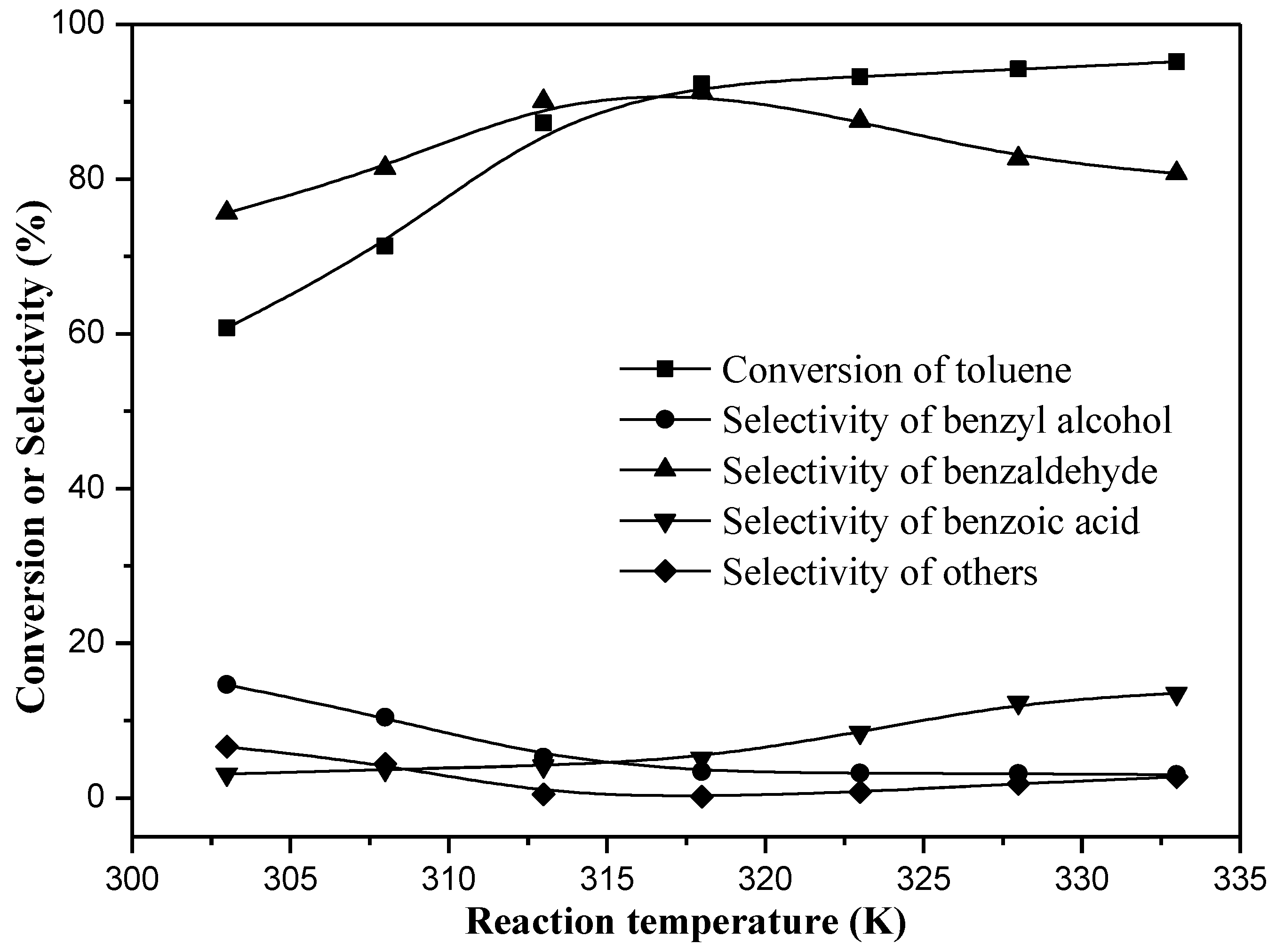
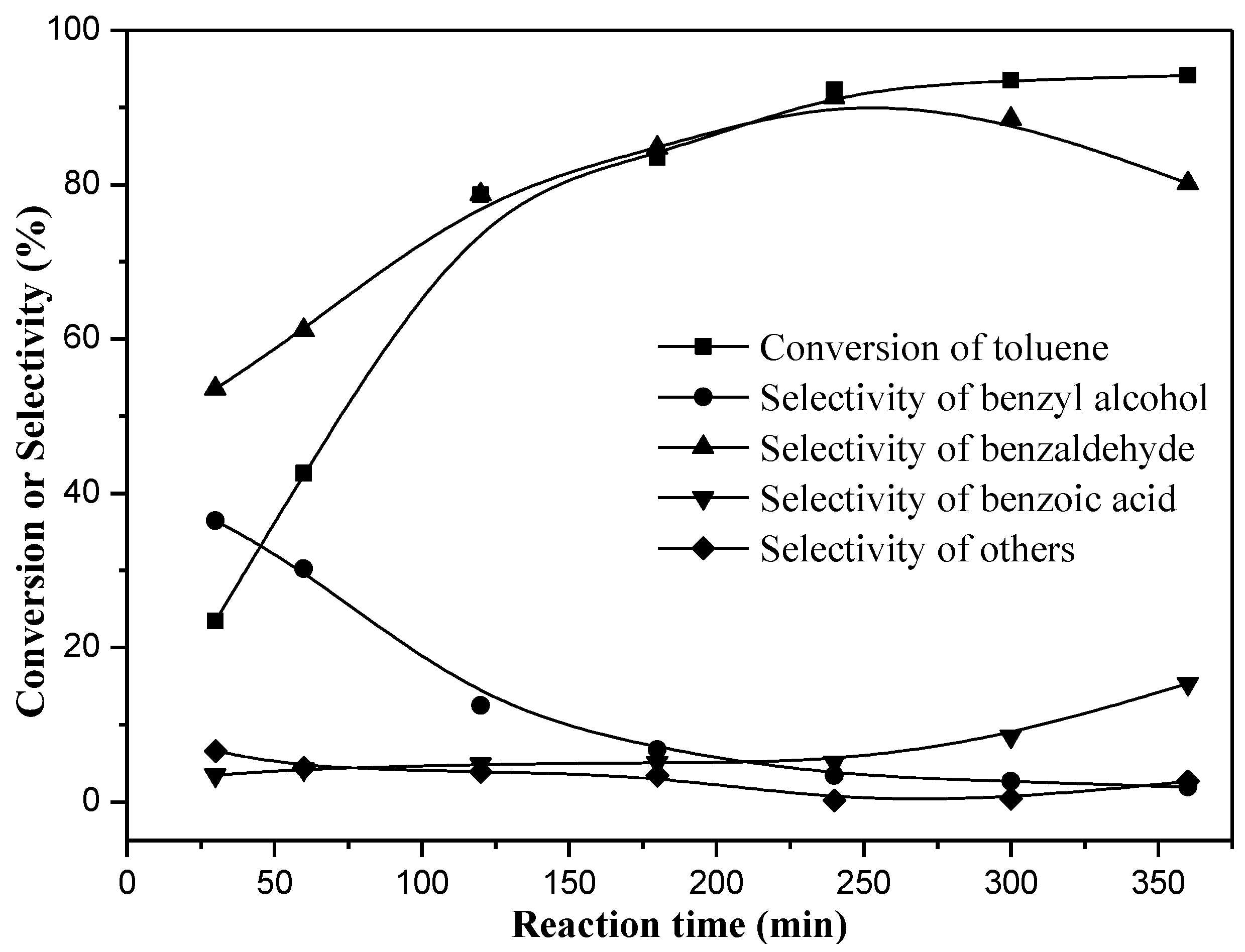
2. Results
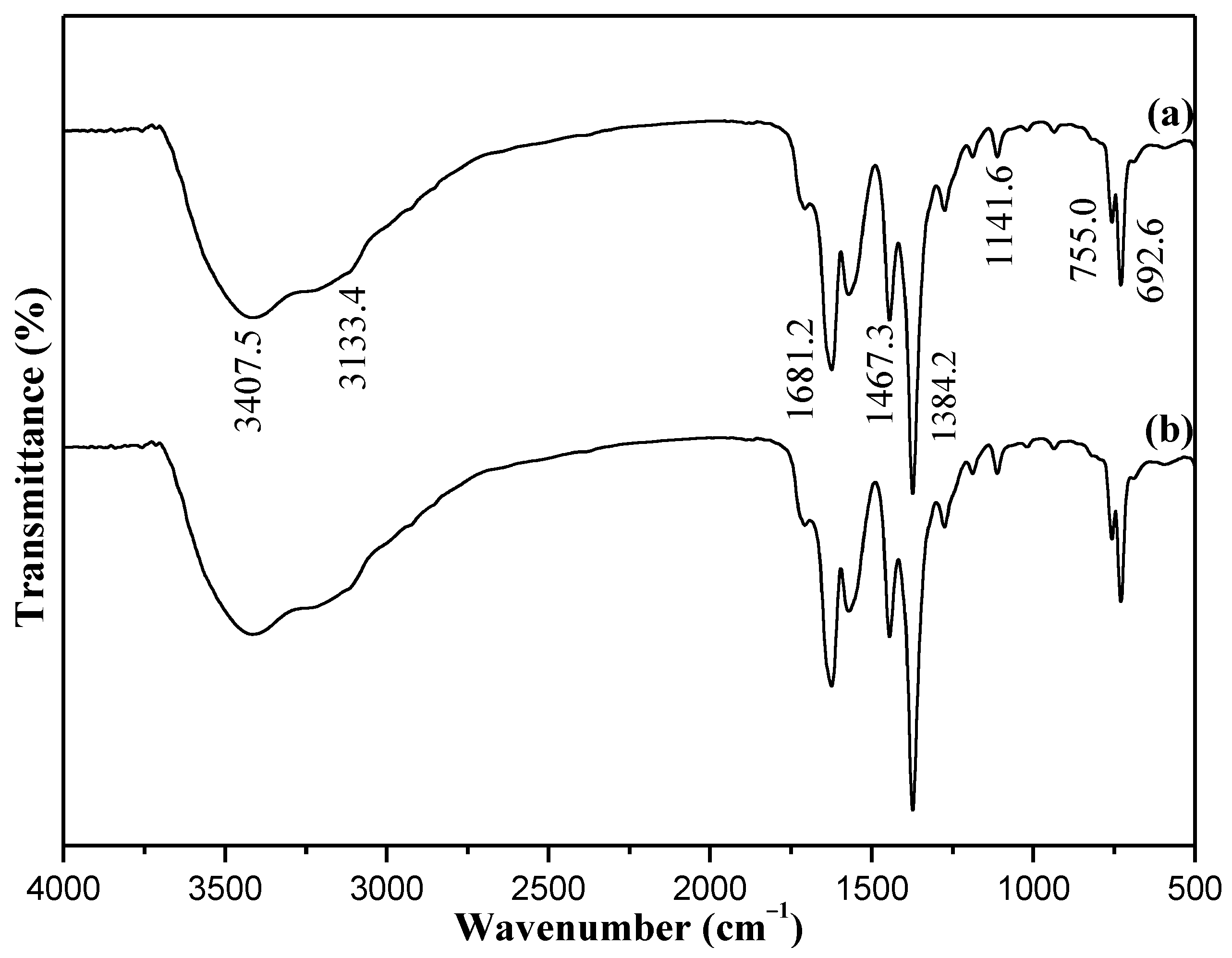
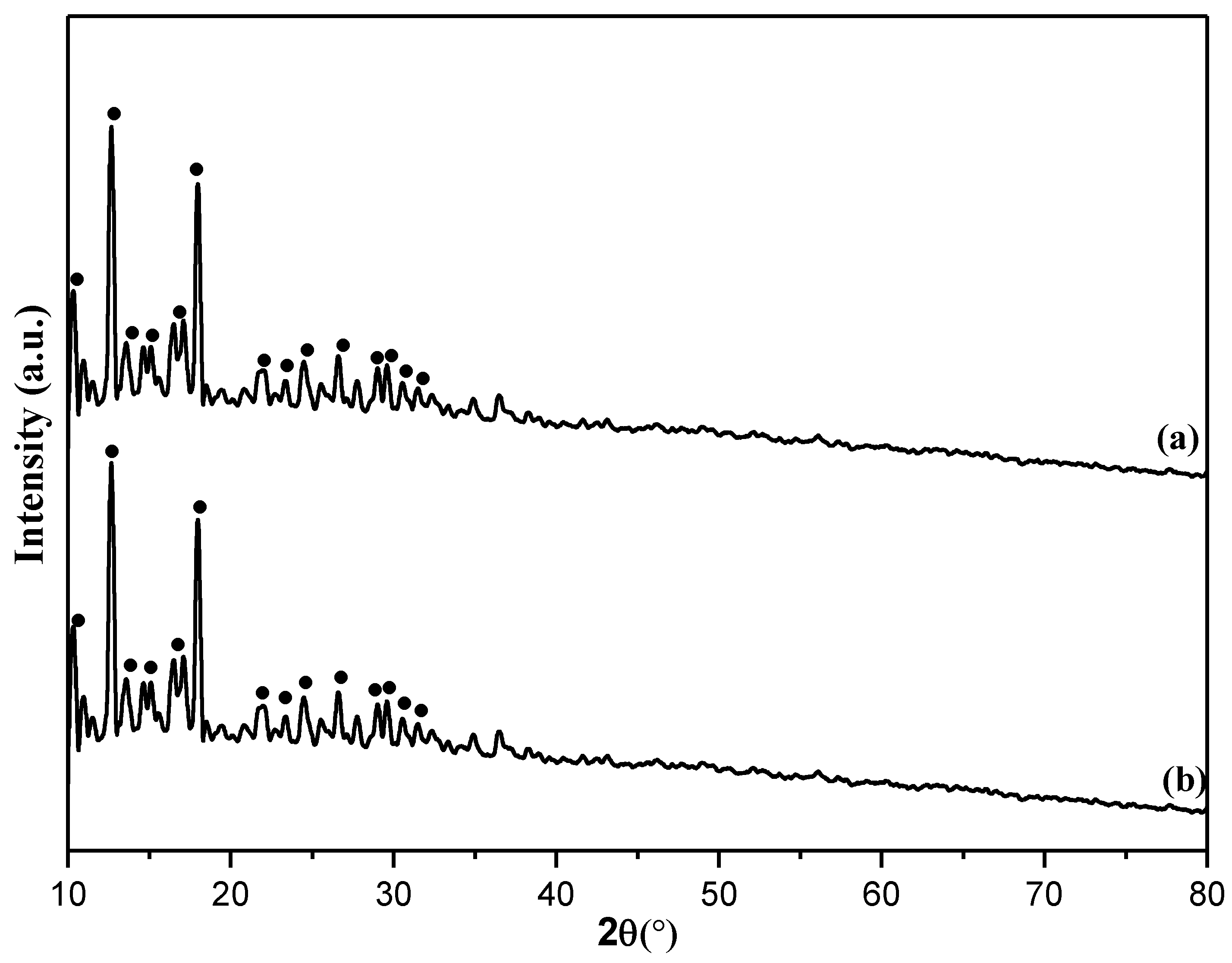
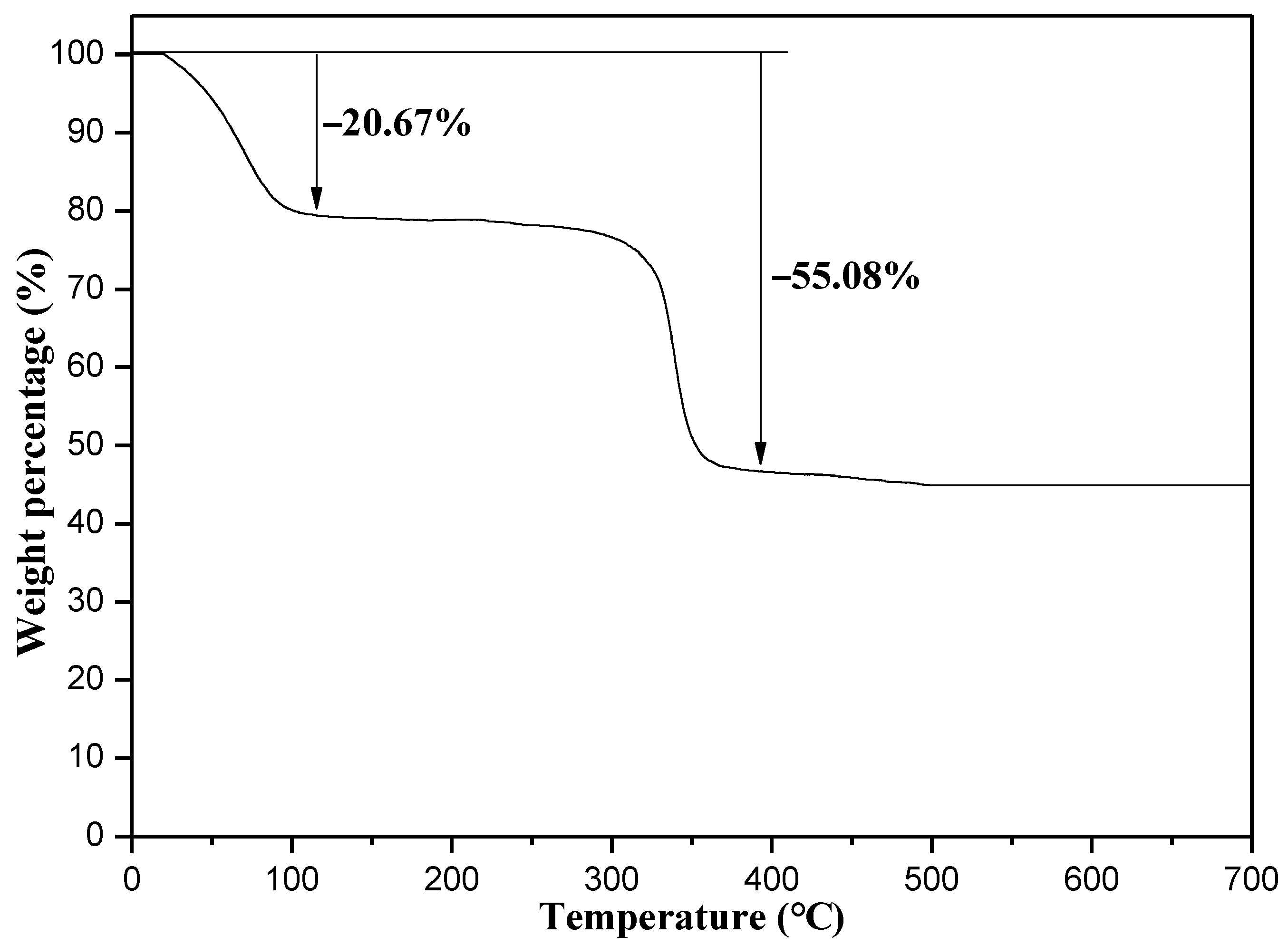
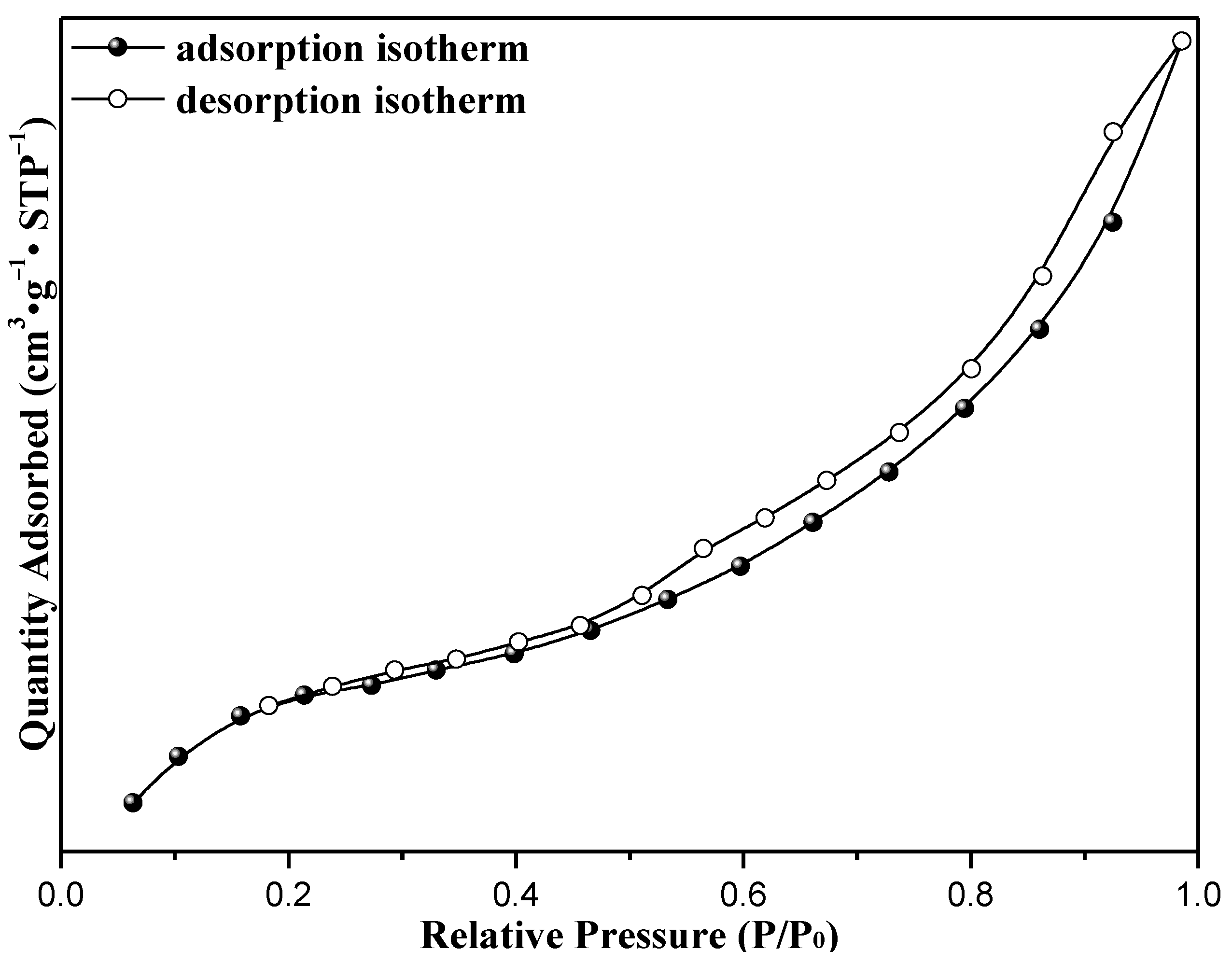
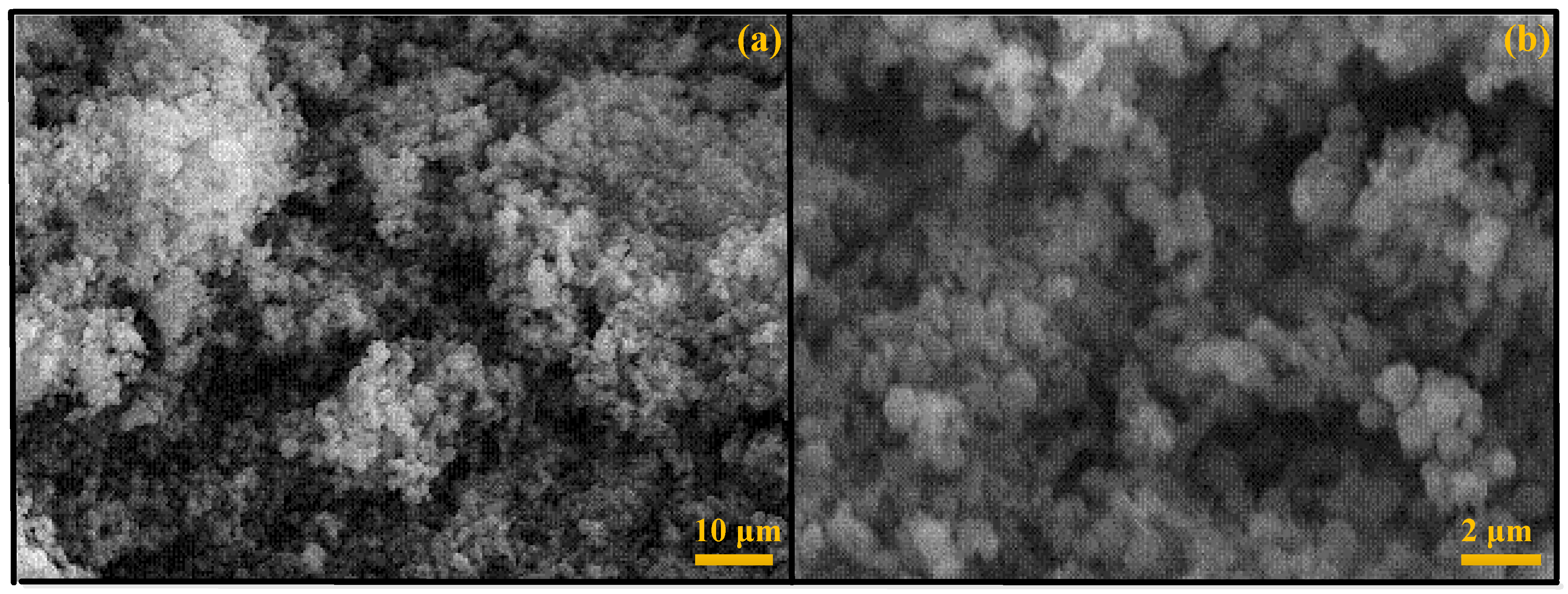
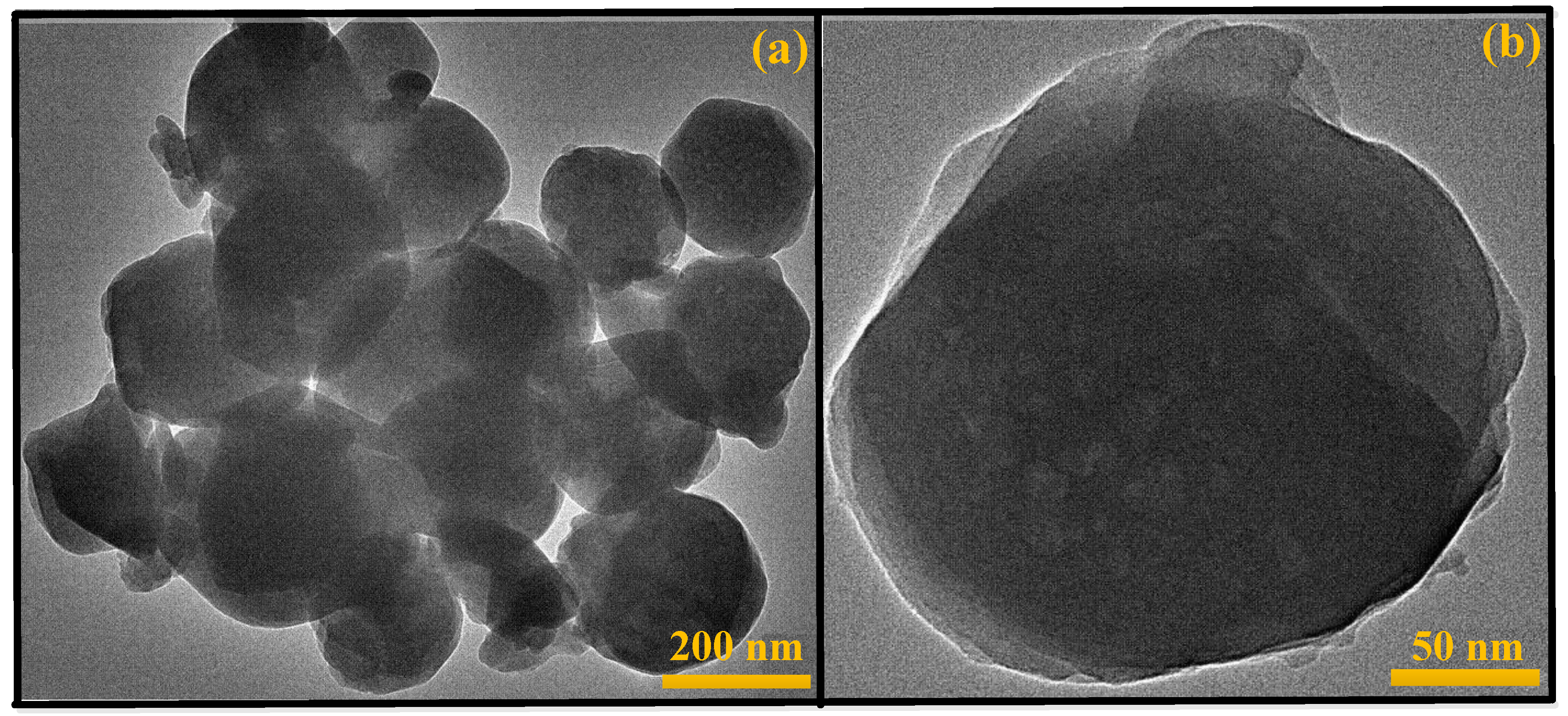
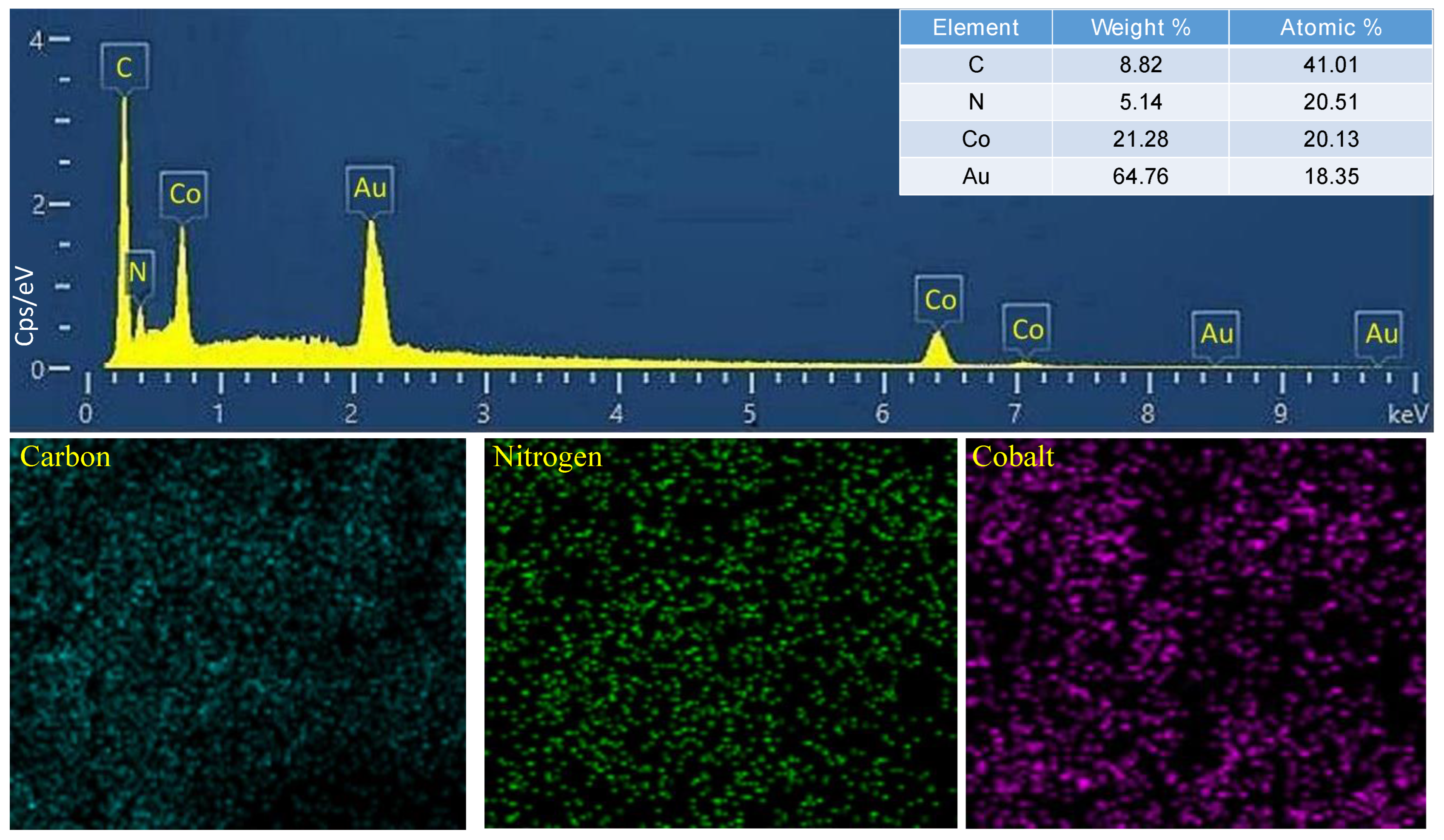
Characterization of Catalysts
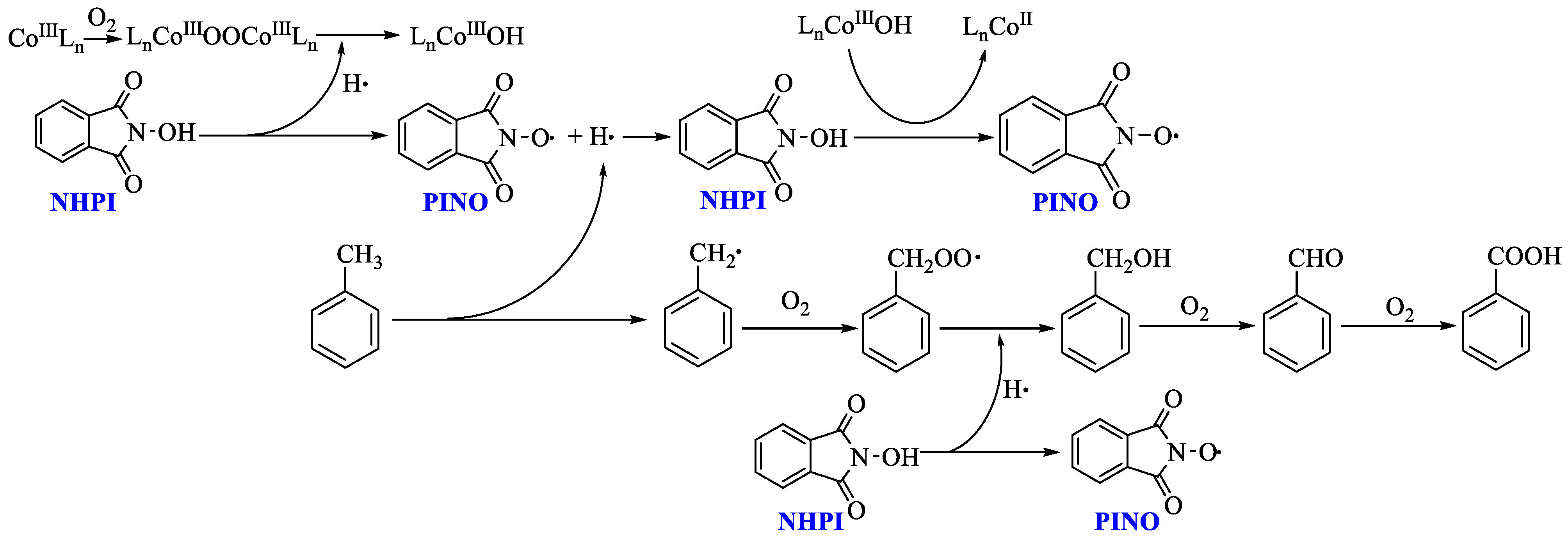
3. Discussion
3.1. Catalytic Performance
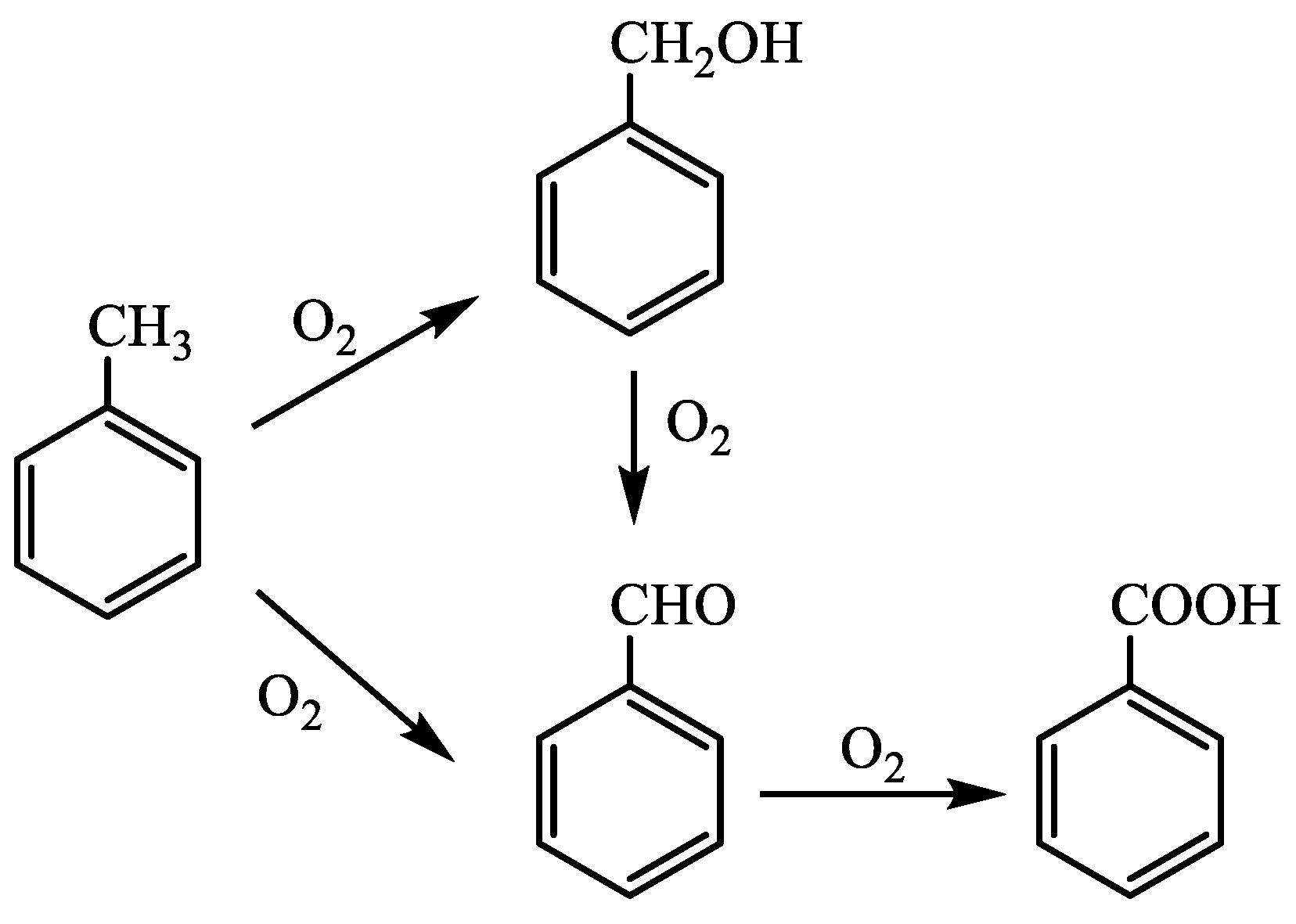
3.2. Probable Catalytic Mechanism
4. Materials and Methods
4.1. Materials
4.2. Catalyst Preparation
4.3. Catalyst Characterization
4.4. Catalytic Test Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qi, Y.; Shen, L.; Zhang, J.; Yao, J.; Lu, R.; Miyakoshi, T. Species and release characteristics of VOCs in furniture coating process. Environ. Pollut. 2019, 245, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Lerner, J.C.; Sanchez, E.Y.; Sambeth, J.E.; Porta, A.A. Characterization and health risk assessment of VOCs in occupational environments in buenos airs, argantina. Atmos. Environ. 2012, 55, 440–447. [Google Scholar] [CrossRef]
- Carriero, G.; Neri, L.; Famulari, D.; Di Lonardo, S.; Piscitelli, D.; Manco, A.; Baraldi, R. Composition and emission of VOC from biogas produced by illegally managed waste landfills in giugliano (Campania, Italy) and potential impact on the local population. Sci. Total Environ. 2018, 640–641, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bai, P.; Wei, Y.; Liu, W.; Ren, X.; Bai, J.; Yu, J. Three-Dimensional-Printed core-shell structured MFI-type zeolite monoliths for volatile organic compound capture under humid conditions. ACS Appl. Mater. Interfaces 2019, 11, 38955–38963. [Google Scholar] [CrossRef]
- Schiavon, M.; Scapinello, M.; Tosi, P.; Ragazzi, M.; Torretta, V.; Rada, E.C. Potentical of non-thermal plasmas for helping the biodegradation of volatile organic compounds(VOCs) released by waste management plants. J. Clean. Prod. 2015, 104, 211–219. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Jiang, Z.; Shangguan, W.F. Low-temperature catalysis for VOCs removal in technology and application: A state of the art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Park, M.J.; Kim, S.B.; Kim, H.J.; Baik, L.J.; Jo, Y.M. Effective dielectric barrier discharge reactor operation for decomposition of volatile organic compounds. J. Clean. Prod. 2018, 198, 1232–1238. [Google Scholar] [CrossRef]
- Tidahy, H.L.; Hosseni, M.; Siffert, S.; Cousin, R.; Lamonier, J.F.; Aboukaïs, A.; Leclercq, G. Nanostructured macro-mesoporous zirconia impregnated by noble metal for catalytic total oxidation of toluene. Catal. Today 2008, 137, 335–339. [Google Scholar] [CrossRef]
- Lin, T.; Yu, L.; Sun, M.; Cheng, G.; Lan, B.; Fu, Z. Mesoporous α-MnO2 microspheres with high specific surface area: Controlled synthesis and catalytic activities. Chem. Eng. J. 2016, 286, 114–121. [Google Scholar] [CrossRef]
- Maira, A.J.; Yeung, K.L.; Soria, J.; Coronado, J.M.; Belver, C.; Lee, C.Y.; Augugliaro, V. Gas-phase photo-oxidation of toluene using nanometer-size TiO2 catalysts. Appl. Catal. B Environ. 2001, 29, 327–336. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Fu, J.L.; Dong, N.; Ye, Q.; Cheng, S.Y.; Kang, T.F.; Dai, H.X. Enhanced performance of the OMS-2 catalyst by Ag Loading for the oxidation of benzene, toluene, and formaldehyde. New J. Chem. 2018, 42, 18117–18127. [Google Scholar] [CrossRef]
- Bertinchamps, F.; Gregoire, C.; Gaineaux, E.M. Systematic investigation of supported transition metal oxide based formulations for the catalytic oxidative elimination of (chloro)-aromatics. Part II. Influence of the nature and addition protocol of secondary phases to VOx/TiO2. Appl. Catal. B. 2006, 66, 10–22. [Google Scholar] [CrossRef]
- Dos Santos, A.A.; Lima, K.M.; Figueiredo, R.T.; Egues, S.M.d.S.; Ramos, A.L.D. Toluene deep oxidation over nble metals, Copper and Vanadium Oxides. Catal. Lett. 2007, 114, 59–63. [Google Scholar] [CrossRef]
- Gaur, V.; Sharma, A.; Verma, N. Catalytic oxidation of toluene and m-xylene by activated carbon fiber impregnated with transition metals. Carbon 2005, 43, 3041–3053. [Google Scholar] [CrossRef]
- Wu, J.C.S.; Chang, T.Y. VOC deep oxidation over Pt catalysts using hydrophobic supports. Catal. Today 1998, 44, 111–118. [Google Scholar]
- Chen, C.; Wu, Q.; Chen, F.; Zhang, L.; Pan, S.; Bian, C.; Xiao, F.S. Aluminium-rich beta zeolite-supported platinum nanoparticles for the low-temperature catalytic removal of toluene. J. Mater. Chem. A 2015, 3, 5556–5562. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Influence of physicochemical treatments on iron-based spent catalyst for catalytic oxidation of toluene. J. Hazard. Mater. 2007, 154, 310–316. [Google Scholar] [CrossRef]
- Zou, X.; Rui, Z.; Ji, H. Core-Shell NiO@PdO nanoparticles supported on alumina as an advanced catalyst for methane oxidation. ACS Catal. 2017, 7, 1615–1625. [Google Scholar] [CrossRef]
- Feijen-Jeurissen, M.M.R.; Joma, J.J.; Nieuwenhuys, B.E.; Sinquin, G.; Petit, C.; Hindermann, J.P. Mechanism of catalytic destruction of 1,2-dichloroethane and trichloroethylene over g-Al2O3 and g-Al2O3 supported chromium and palladium catalysts. Catal. Today 1999, 54, 65–79. [Google Scholar] [CrossRef]
- Yang, H.; Deng, J.; Liu, Y.; Xie, S.; Wu, Z.; Dai, H. Preparation and catalytic performance of Ag, Au, Pd or Pt nanoparticles supported on 3dom CeO2-Al2O3 for toluene oxidation. J. Mol. Catal. A Chem. 2016, 414, 9–18. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z.P. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xia, L.; Liu, Y.; Yang, T.; Deng, J.; Dai, H. Concurrent catlytic removal of typical volatile organic compound mixtures over Au-Pd/α-MnO2 nanotubes. J. Environ. Sci. 2018, 64, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Xia, Q.; Liu, Z.; Li, Z. Catalytic oxidation of toluene over copper and manganese based catalysts: Effect of water vapor. Catal. Commun. 2011, 14, 15–19. [Google Scholar] [CrossRef]
- Yang, H.; Deng, J.; Liu, Y.; Xie, S.; Xu, P.; Dai, H. Pt/Co3O4/3DOM Al2O3: Highly effective catalysts for toluene combustion. Chin. J. Catal. 2016, 37, 934–946. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, J.; Chen, F.; Meng, X.; Zheng, X.; Gao, X.; Xiao, F.S. Enhanced performance in catalytic combustion of toluene over mesoporous beta zeolite-supported platinum catalyst. Appl. Catal. B Environ. 2013, 140–141, 199–205. [Google Scholar] [CrossRef]
- Zhao, S.; Hu, F.; Li, J. Hierarchical core-shell Al2O3@Pd-CoAlO microspheres for low-temperature toluene combustion. ACS Catal. 2016, 6, 3433–3441. [Google Scholar] [CrossRef]
- Deng, J.G.; Ultra, H.X. Low loading of silver nanoparticles on Mn2O3 nanowires derived with molten salts: A high-efficiency catalyst for the oxidative removal of toluene. Environ. Sci. Technol. 2015, 49, 11089–11095. [Google Scholar] [CrossRef]
- Xie, S.H.; Deng, J.G.; Zang, S.M.; Yang, H.G.; Guo, G.S.; Arandiyan, H.; Dai, H.X. Au-Pd/3DOM Co3O4: Highly active and stable nanocatalysts for toluene oxidation. J. Catal. 2015, 322, 38–48. [Google Scholar] [CrossRef]
- Dong, C.; Wang, H.; Ren, Y.W.; Qu, Z.P. Layer MnO2 with oxygen vacancy for improved toluene oxidation activity. Surf. Interfaces 2021, 22, 100897–100905. [Google Scholar] [CrossRef]
- Yuan, W.H.; Li, Y.Y.; Dagaut, P.; Yang, J.Z.; Qi, F. Investigation on the pyrolysis and oxidation of toluene over a wide range conditions. II. A comprehensive kinetic modeling study. Combust. Flame 2015, 162, 22–40. [Google Scholar] [CrossRef]
- Usman, M.; Iqbal, N.; Noor, T.; Zaman, N.; Asghar, A.; Abdelnaby, M.M.; Galadima, A.; Helal, A. Advanced strategies in metal-organic-frameworks for CO2 capture and separation. Chem. Rec. 2022, 22, e202100230–e202100258. [Google Scholar] [CrossRef] [PubMed]
- Helal, A.; Shah, S.S.; Usman, M.; Khan, M.Y.; Aziz, M.A.; Rahman, M.M. Potential applications of Nickel-based metal-organic-frameworks and their derivatives. Chem. Rec. 2022, 22, e202200055. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Zeb, Z.; Ullah, H.; Suliman, M.H.; Humayun, M.; Ullash, L.; Shah, S.N.Z.; Ahmed, U.; Saeed, M. A review of metal-organic frameworks/graphitic carbon nitride composites for solar-driven green H2 production, CO2 reduction, and water purification. J. Environ. Chem. Eng. 2022, 10, 107548–107574. [Google Scholar] [CrossRef]
- Shafiq, S.; Al-Maythalony, B.A.; Usman, M.; Ba-Shammakh, M.S.; Al-Shammari, A.A. ZIF-95 as a filler for enhanced gas separation performance of polysulfone membrane. RSC Adv. 2021, 11, 34319–34328. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Y.W.; Gao, J.H.; Fu, M.L.; Zou, S.B.; Li, Y.X.; Ye, D.Q. Interfaces in MOF-derived CeO2-MnOx composites as high-activity catalysts for toluene oxidation: Monolayer dispersion threshold. Catalysts 2020, 10, 681. [Google Scholar] [CrossRef]
- Zhou, K.; Mousavi, B.; Luo, Z.; Phatanasri, S.; Chaemchuen, S.; Verpoort, F. Characterization and properties of Zn/Co zeolitic imidazolate frameworks vs. ZIF-8 and ZIF-67. J. Mater. Chem. A 2017, 5, 952–957. [Google Scholar] [CrossRef]
- Wei, G.; Zhou, Z.; Zhao, X.; Zhang, W.; An, C. Ultrathin metal-organic framework nanosheet-derived ultrathin Co3O4 nanomeshes with robust oxygen-evolving performance and asymmetric supercapacitors. ACS Appl. Mater. Inter. 2018, 10, 23721–23730. [Google Scholar] [CrossRef]
- Shah, S.S.; Qasem, M.A.A.; Berni, R.; Casino, C.D.; Cai, G.; Contal, S.; Ahmad, I.; Siddiqui, K.S.; Gatti, E.; Predieri, S.; et al. Physico-chemical properties and toxicological effects on plant and algal models of carbon nanosheets from a nettle fibreclone. Sci. Rep. 2021, 11, 6945–6960. [Google Scholar] [CrossRef]
- Shah, S.S.; Cevik, E.; Aziz, M.A.; Qahtan, T.F.; Bozkurt, A.; Yamani, Z.H. Jute sticks derived and commercially available activated carbons for symmetric supercapacitors with bio-electrolyte: A comparative study. Synth. Met. 2021, 277, 116765–116780. [Google Scholar] [CrossRef]
- Gardner, K.A.; Mayer, J.M. Understanding C-H bond oxidations:H· and H- transfer in the oxidation of toluene by permanganate. Science 1995, 269, 1849–1851. [Google Scholar] [CrossRef]
- Grasselli, R.K. Fundamental principles of selective heterogeneous oxidation catalysis. Top. Catal. 2002, 21, 79–88. [Google Scholar] [CrossRef]
- Li, W.B.; Chu, W.B.; Zhuang, M.; Hua, J. Catalytic oxidation of toluene on Mn-containing mixed oxides prepared in reverse microemulsions. Catal. Today 2004, 93–95, 205–209. [Google Scholar] [CrossRef]
- Guo, C.C.; Liu, Q.; Wang, X.T.; Hu, H.Y. Selective liquid phase oxidation of toluene with air. Appl. Catal. A: Gen. 2005, 282, 55–59. [Google Scholar] [CrossRef]
- Florea, M.; Alifanti, M.; Parvulescu, V.I.; Mihaila-Tarabasanu, D.; Diamandescu, L.; Feder, M.; Negrila, C.; Frunza, L. Total oxidation of toluene on ferrite-type catalysts. Catal. Today 2009, 141, 361–366. [Google Scholar] [CrossRef]
- Shi, G.; Xu, S.; Bao, Y.; Xu, j.; Liang, Y. Selective aerobic oxidation of toluene to benzaldehyde on immobilized CoOx on SiO2 catalyst in the presence of N-hydroxyph thalimide and hexafluoropropan-2-ol. Catal. Commun. 2019, 123, 73–78. [Google Scholar] [CrossRef]
- Xiao, Y.P.; Song, B.C.; Chen, Y.J.; Cheng, L.H.; Ren, Q.G. ZIF-67 with precursor concentration-dependence morphology for aerobic oxidation of toluene. J. Organomet. Chem. 2020, 930, 121597–121602. [Google Scholar] [CrossRef]
- Wu, Z.L.; Zhu, D.D.; Chen, Z.Z.; Yao, S.L.; Li, J.; Gao, E.; Wang, W. Enhanced energy efficiency and reduced nanoparticle emission on plasma catalytic oxidation of toluene using Au/γ-Al2O3 nanocatalyst. Chem. Eng. J. 2022, 427, 130983–130992. [Google Scholar] [CrossRef]
- Gaster, E.; Kozuch, S.; Pappo, D. Selective aerobic oxidation of methylarenes to benzaldehydes catalyzed by N-hydroxyphthalimi de and cobalt(II) acetate in hexafluoropropanol. Angew. Chem. Int. Ed. 2017, 56, 5912–5915. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Du, R.; Yuan, H.; Wang, Y.; Yao, J.; Li, H. Selective one-step aerobic oxidation of cyclohexane to e-caprolactone mediated by N-hydroxyphthalimide (NHPI). Chem. Cat Chem. 2019, 11, 2260–2264. [Google Scholar]
- Zakzeski, J.; Dczak, A.; Bruijnincx, P.C.A. Catalytic oxidation of aromatic oxygenates by the heterogene ous catalyst Co-ZIF-9. Appl. Catal. A Gen. 2011, 394, 79–85. [Google Scholar] [CrossRef]


| O2 Pressure (MPa) | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| Benzyl Alcohol | Benzaldehyde | Benzoic Acid | Others | ||
| 0.06 | 79.35 | 8.14 | 82.17 | 2.17 | 7.52 |
| 0.08 | 86.32 | 6.42 | 86.58 | 3.24 | 3.76 |
| 0.10 | 90.11 | 4.13 | 89.32 | 4.39 | 2.16 |
| 0.12 | 92.30 | 3.42 | 91.31 | 5.13 | 0.14 |
| 0.14 | 92.56 | 2.98 | 89.14 | 6.57 | 1.31 |
| 0.16 | 92.88 | 2.67 | 86.34 | 8.46 | 2.53 |
| Toluene Concentration (mmol/L) | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| Benzyl Alcohol | Benzaldehyde | Benzoic Acid | Others | ||
| 6.25 | 100.00 | 1.03 | 72.69 | 22.46 | 3.82 |
| 12.5 | 100.00 | 2.37 | 83.79 | 12.37 | 1.47 |
| 25.0 | 92.30 | 3.42 | 91.31 | 5.13 | 0.14 |
| 37.5 | 73.48 | 12.49 | 80.49 | 4.16 | 2.86 |
| 50.0 | 52.87 | 23.17 | 70.34 | 3.38 | 3.11 |
| Catalysts | w (%) | Major Product | Si (%) | Years | Ref. |
|---|---|---|---|---|---|
| Co(OAc)2·4H2O | 91.0 | Benzaldehyde | 90.0 | 2002 | [42] |
| Mn0.67-Cu0.33 | 99.0 | Carbon dioxide | - | 2004 | [43] |
| Cobalt tetraphenylporphyrin | 8.9 | Benzaldehyde and benzyl alcohol | 60.0 | 2005 | [44] |
| Fe2O3-Mn2O3 | 100.0 | Carbon monoxide and carbon dioxide | - | 2009 | [45] |
| CoOx/SiO2 | 91.2 | Benzaldehyde | 68.8 | 2019 | [46] |
| ZIF-67-24 | 87.9 | Benzaldehyde | 66.9 | 2020 | [47] |
| Au/γ-Al2O3 | 99.0 | Carbon dioxide | - | 2022 | [48] |
| Co-ZIF | 92.3 | Benzaldehyde | 91.3 | in this work | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, W.; Chen, Z.; Huang, Y.; Kang, X. Selective Oxidation of Toluene to Benzaldehyde Using Co-ZIF Nano-Catalyst. Int. J. Mol. Sci. 2022, 23, 12881. https://doi.org/10.3390/ijms232112881
Long W, Chen Z, Huang Y, Kang X. Selective Oxidation of Toluene to Benzaldehyde Using Co-ZIF Nano-Catalyst. International Journal of Molecular Sciences. 2022; 23(21):12881. https://doi.org/10.3390/ijms232112881
Chicago/Turabian StyleLong, Wei, Zhilong Chen, Yinfei Huang, and Xinping Kang. 2022. "Selective Oxidation of Toluene to Benzaldehyde Using Co-ZIF Nano-Catalyst" International Journal of Molecular Sciences 23, no. 21: 12881. https://doi.org/10.3390/ijms232112881
APA StyleLong, W., Chen, Z., Huang, Y., & Kang, X. (2022). Selective Oxidation of Toluene to Benzaldehyde Using Co-ZIF Nano-Catalyst. International Journal of Molecular Sciences, 23(21), 12881. https://doi.org/10.3390/ijms232112881





