Non-Mouse Models of Atherosclerosis: Approaches to Exploring the Translational Potential of New Therapies
Abstract
1. Introduction
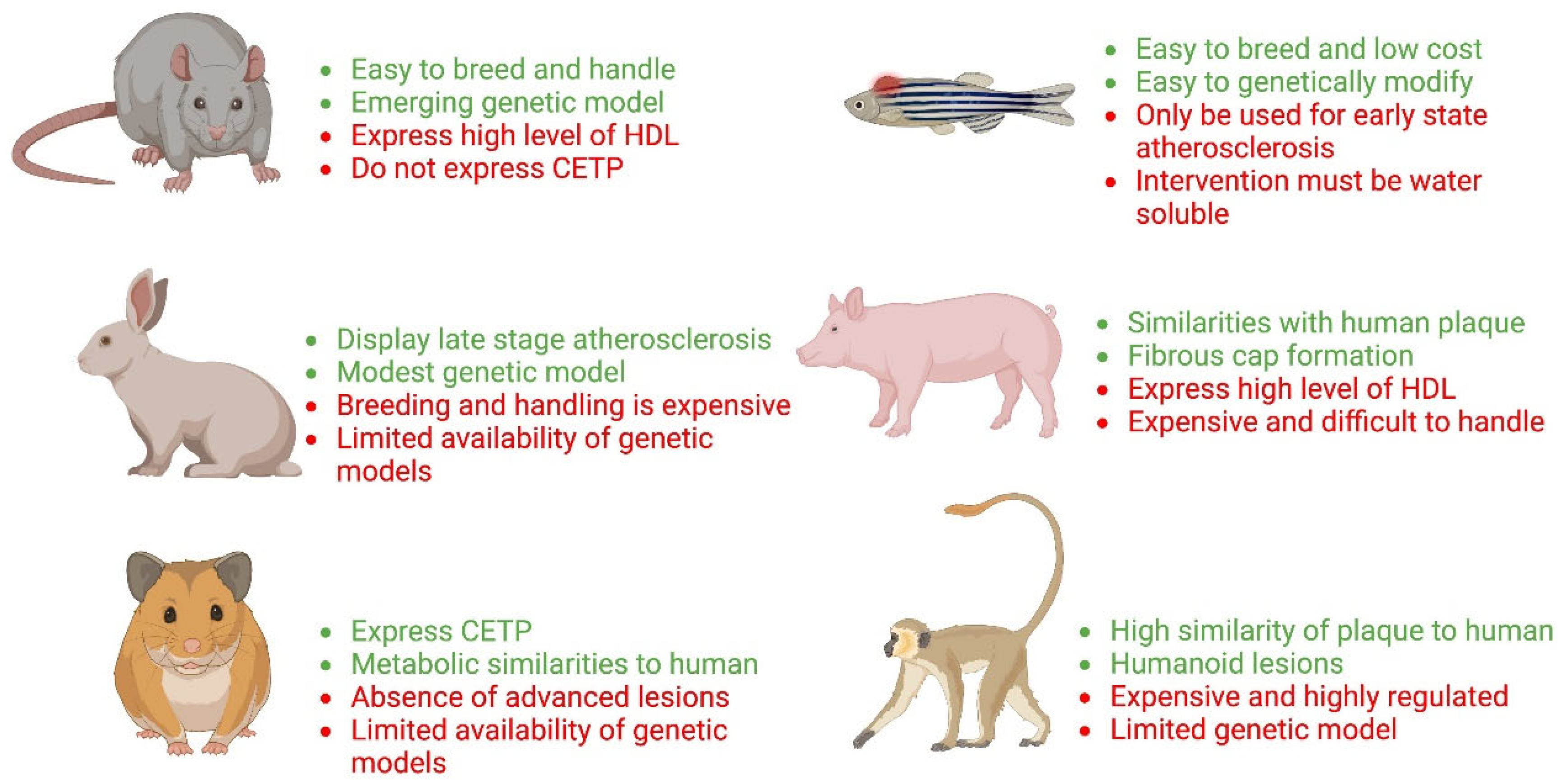
2. Animal Models of Atherosclerosis
2.1. Rat Models of Atherosclerosis
2.2. Hamster Models of Atherosclerosis
2.3. Rabbit Models of Atherosclerosis
2.4. Other Animal Models of Atherosclerosis
3. Interventional Strategies for Preclinical Therapeutic Studies of Atherosclerosis
3.1. Administration of Drugs or Compounds to Attenuate Atherosclerosis
3.2. Nucleic Acid-Based Drug Delivery
3.3. CRISPR-Cas9 Technology
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nigro, J.; Osman, N.; Dart, A.M.; Little, P.J. Insulin Resistance and Atherosclerosis. Endocr. Rev. 2006, 27, 242–259. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The biology of atherosclerosis comes full circle: Lessons for conquering cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 683–684. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. The pathogenesis of atherosclerosis. N. Engl. J. Med. 1986, 314, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Falk, E. Morphologic features of unstable atherothrombotic plaques underlying acute coronary syndromes. Am. J. Cardiol. 1989, 63, 114E–120E. [Google Scholar] [CrossRef]
- Ilyas, I.; Little, P.J.; Liu, Z.; Xu, Y.; Kamato, D.; Berk, B.C.; Weng, J.; Xu, S. Mouse models of atherosclerosis in translational research. Trends Pharmacol. Sci. 2022, 43, 920–939. [Google Scholar] [CrossRef]
- Ballinger, M.L.; Osman, N.; Hashimura, K.; de Hann, J.; Jandeleit-Dahm, K.; Allen, T.J.; Tannock, L.R.; Rutledge, J.C.; Little, P.J. Imatinib inhibits vascular smooth muscle proteoglycan synthesis and reduces LDL binding In Vitro and aortic lipid deposition In Vivo. J. Cell. Mol. Med. 2010, 14, 1408–1418. [Google Scholar] [CrossRef]
- Schaftenaar, F.; Frodermann, V.; Kuiper, J.; Lutgens, E. Atherosclerosis: The interplay between lipids and immune cells. Curr. Opin. Lipidol. 2016, 27, 209–215. [Google Scholar] [CrossRef]
- Little, P.J.; Osman, N.; O’Brien, K.D. Hyperelongated biglycan: The surreptitious initiator of atherosclerosis. Curr. Opin. Lipidol. 2008, 19, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Boren, J.; Gustafsson, M.; Skalen, K.; Flood, C.; Innerarity, T.L. Role of extracellular retention of low density lipoproteins in atherosclerosis. Curr. Opin. Lipidol. 2000, 11, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.J.; Tabas, I. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Anggraeni, V.Y.; Emoto, N.; Yagi, K.; Mayasari, D.S.; Nakayama, K.; Izumikawa, T.; Kitagawa, H.; Hirata, K. Correlation of C4ST-1 and ChGn-2 expression with chondroitin sulfate chain elongation in atherosclerosis. Biochem. Biophys. Res. Commun. 2011, 406, 36–41. [Google Scholar] [CrossRef]
- Little, P.J.; Ballinger, M.L.; Burch, M.L.; Osman, N. Biosynthesis of natural and hyperelongated chondroitin sulfate glycosaminoglycans: New insights into an elusive process. Open Biochem. J. 2008, 2, 135–142. [Google Scholar] [CrossRef][Green Version]
- Emini Veseli, B.; Perrotta, P.; De Meyer, G.R.A.; Roth, L.; Van der Donckt, C.; Martinet, W.; De Meyer, G.R.Y. Animal models of atherosclerosis. Eur. J. Pharmacol. 2017, 816, 3–13. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Pello, O.M.; Silvestre, C.; De Pizzol, M.; Andrés, V. A glimpse on the phenomenon of macrophage polarization during atherosclerosis. Immunobiology 2011, 216, 1172–1176. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Lei, Y.; Tzvetkov, N.T.; Liu, X.; Yeung, A.W.K.; Xu, S.; Atanasov, A.G. Targeting Foam Cell Formation in Atherosclerosis: Therapeutic Potential of Natural Products. Pharmacol. Rev. 2019, 71, 596–670. [Google Scholar] [CrossRef]
- Tian, K.; Ogura, S.; Little, P.J.; Xu, S.W. Targeting LOX-1 in atherosclerosis and vasculopathy: Current knowledge and future perspectives. Ann. N. Y. Acad. Sci. 2019, 1443, 34–53. [Google Scholar] [CrossRef]
- Sakakura, K.; Nakano, M.; Otsuka, F.; Ladich, E.; Kolodgie, F.D.; Virmani, R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013, 22, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.J.; Castillo, A.I.; Zaragoza, C.; Ibáñez, B.; Andrés, V. Animal models of atherosclerosis. Prog. Mol. Biol. Transl. Sci. 2012, 105, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef]
- Hafiane, A. Vulnerable Plaque, Characteristics, Detection, and Potential Therapies. J. Cardiovasc. Dev. Dis. 2019, 6, 26. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis—From experimental insights to the clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef]
- Kasikara, C.; Doran, A.C.; Cai, B.; Tabas, I. The role of non-resolving inflammation in atherosclerosis. J. Clin. Investig. 2018, 128, 2713–2723. [Google Scholar] [CrossRef]
- Bäck, M.; Yurdagul, A., Jr.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Kay, A.M.; Simpson, C.L.; Stewart, J.A., Jr. The Role of AGE/RAGE Signaling in Diabetes-Mediated Vascular Calcification. J. Diabetes Res. 2016, 2016, 6809703. [Google Scholar] [CrossRef]
- Rajamannan, N.M.; Subramaniam, M.; Caira, F.; Stock, S.R.; Spelsberg, T.C. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation 2005, 112 (Suppl. S9), I229–I234. [Google Scholar] [CrossRef]
- Shioi, A.; Nishizawa, Y.; Jono, S.; Koyama, H.; Hosoi, M.; Morii, H. Beta-Glycerophosphate accelerates calcification in cultured bovine vascular smooth muscle cells. Atheroscler. Thromb. Vasc. Biol. 1995, 15, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Albanese, I.; Khan, K.; Barratt, B.; Al-Kindi, H.; Schwertani, A. Atherosclerotic Calcification: Wnt Is the Hint. J. Am. Heart Assoc. 2018, 7, e007356. [Google Scholar] [CrossRef]
- Konstantinov, I.E.; Jankovic, G.M.; Alexander, I. Ignatowski: A pioneer in the study of atherosclerosis. Tex. Heart Inst. J. 2013, 40, 246–249. [Google Scholar] [PubMed]
- Anitschkow, N.; Chalatow, S. Ueber experimentelle Cholester-insteatose und ihre Bedeutung fuer die Entstehung einiger pathologischer Prozesse. Zentrbl. Allg. Pathol. Anat. 1913, 24, 1–9. [Google Scholar]
- Getz, G.S.; Reardon, C.A. Animal models of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Asrafuzzaman, M.; Cao, Y.; Afroz, R.; Kamato, D.; Gray, S.; Little, P.J. Animal models for assessing the impact of natural products on the aetiology and metabolic pathophysiology of Type 2 diabetes. Biomed. Pharmacother. 2017, 89, 1242–1251. [Google Scholar] [CrossRef]
- Getachew, R.; Ballinger, M.L.; Burch, M.L.; Little, P.J.; Osman, N. Characterisation of Ki11502 as a potent inhibitor of PDGF beta receptor-mediated proteoglycan synthesis in vascular smooth muscle cells. Eur. J. Pharmacol. 2010, 626, 186–192. [Google Scholar] [CrossRef]
- Getachew, R.; Ballinger, M.L.; Burch, M.L.; Reid, J.J.; Khachigian, L.M.; Wight, T.N.; Little, P.J.; Osman, N. PDGF beta-receptor kinase activity and ERK1/2 mediate glycosaminoglycan elongation on biglycan and increases binding to LDL. Endocrinology 2010, 151, 4356–4367. [Google Scholar] [CrossRef]
- Heinonen, S.E.; Genové, G.; Bengtsson, E.; Hübschle, T.; Åkesson, L.; Hiss, K.; Benardeau, A.; Ylä-Herttuala, S.; Jönsson-Rylander, A.C.; Gomez, M.F. Animal models of diabetic macrovascular complications: Key players in the development of new therapeutic approaches. J. Diabetes Res. 2015, 2015, 404085. [Google Scholar] [CrossRef]
- Ekuni, D.; Yoneda, T.; Endo, Y.; Kasuyama, K.; Irie, K.; Mizutani, S.; Azuma, T.; Tomofuji, T.; Morita, M. Occlusal disharmony accelerates the initiation of atherosclerosis in apoE knockout rats. Lipids Health Dis. 2014, 13, 144. [Google Scholar] [CrossRef][Green Version]
- Wei, S.; Zhang, Y.; Su, L.; He, K.; Wang, Q.; Zhang, Y.; Yang, D.; Yang, Y.; Ma, S. Apolipoprotein E-deficient rats develop atherosclerotic plaques in partially ligated carotid arteries. Atherosclerosis 2015, 243, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Sithu, S.D.; Malovichko, M.V.; Riggs, K.A.; Wickramasinghe, N.S.; Winner, M.G.; Agarwal, A.; Hamed-Berair, R.E.; Kalani, A.; Riggs, D.W.; Bhatnagar, A.; et al. Atherogenesis and metabolic dysregulation in LDL receptor-knockout rats. JCI Insight 2017, 2, e86442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Y.; Xing, R.; Cui, X.; Xiao, Y.; Xie, L.; You, P.; Wang, T.; Zeng, L.; Peng, W.; et al. Hyperlipidemia induces typical atherosclerosis development in Ldlr and Apoe deficient rats. Atherosclerosis 2018, 271, 26–35. [Google Scholar] [CrossRef]
- Lee, J.G.; Ha, C.H.; Yoon, B.; Cheong, S.A.; Kim, G.; Lee, D.J.; Woo, D.C.; Kim, Y.H.; Nam, S.Y.; Lee, S.W.; et al. Knockout rat models mimicking human atherosclerosis created by Cpf1-mediated gene targeting. Sci. Rep. 2019, 9, 2628. [Google Scholar] [CrossRef]
- He, K.; Wang, J.; Shi, H.; Yu, Q.; Zhang, X.; Guo, M.; Sun, H.; Lin, X.; Wu, Y.; Wang, L.; et al. An interspecies study of lipid profiles and atherosclerosis in familial hypercholesterolemia animal models with low-density lipoprotein receptor deficiency. Am. J. Transl. Res. 2019, 11, 3116–3127. [Google Scholar] [PubMed]
- Wang, J.; He, K.; Yang, C.; Lin, X.; Zhang, X.; Wang, Y.; Liu, G.; Xian, X. Dietary Cholesterol Is Highly Associated with Severity of Hyperlipidemia and Atherosclerotic Lesions in Heterozygous LDLR-Deficient Hamsters. Int. J. Mol. Sci. 2019, 20, 3515. [Google Scholar] [CrossRef]
- Guo, X.; Gao, M.; Wang, Y.; Lin, X.; Yang, L.; Cong, N.; An, X.; Wang, F.; Qu, K.; Yu, L.; et al. LDL Receptor Gene-ablated Hamsters: A Rodent Model of Familial Hypercholesterolemia with Dominant Inheritance and Diet-induced Coronary Atherosclerosis. EBioMedicine 2018, 27, 214–224. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, M.J.; Cao, Z.; Yang, C.; Wang, J.; Wang, B.; Liu, J.; Wang, Y.; Xian, X. Heterozygous Ldlr-Deficient Hamster as a Model to Evaluate the Efficacy of PCSK9 Antibody in Hyperlipidemia and Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 5936. [Google Scholar] [CrossRef]
- Guo, M.; Xu, Y.; Dong, Z.; Zhou, Z.; Cong, N.; Gao, M.; Huang, W.; Wang, Y.; Liu, G.; Xian, X. Inactivation of ApoC3 by CRISPR/Cas9 Protects Against Atherosclerosis in Hamsters. Circ. Res. 2020, 127, 1456–1458. [Google Scholar] [CrossRef]
- Niimi, M.; Chen, Y.; Yan, H.; Wang, Y.; Koike, T.; Fan, J. Hyperlipidemic Rabbit Models for Anti-Atherosclerotic Drug Development. Appl. Sci. 2020, 10, 8681. [Google Scholar] [CrossRef]
- Jain, M.; Frobert, A.; Valentin, J.; Cook, S.; Giraud, M.N. The Rabbit Model of Accelerated Atherosclerosis: A Methodological Perspective of the Iliac Artery Balloon Injury. J. Vis. Exp. JoVE 2017, 128, e55295. [Google Scholar] [CrossRef] [PubMed]
- Niimi, M.; Yang, D.; Kitajima, S.; Ning, B.; Wang, C.; Li, S.; Liu, E.; Zhang, J.; Eugene Chen, Y.; Fan, J. ApoE knockout rabbits: A novel model for the study of human hyperlipidemia. Atherosclerosis 2016, 245, 187–193. [Google Scholar] [CrossRef]
- Yamada, S.; Koike, T.; Nakagawa, T.; Kuniyoshi, N.; Ying, Y.; Itabe, H.; Yamashita, A.; Asada, Y.; Shiomi, M. Morphological features of coronary plaques in WHHLMI rabbits (Oryctolagus cuniculus), an animal model for familial hypercholesterolemia. Exp. Anim. 2017, 66, 145–157. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Niimi, M.; Yang, D.; Liang, J.; Xu, J.; Kimura, T.; Mathew, A.V.; Guo, Y.; Fan, Y.; Zhu, T.; et al. Deficiency of Cholesteryl Ester Transfer Protein Protects Against Atherosclerosis in Rabbits. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, M.; Chen, S.; Deng, J.; Song, Y.; Lai, L.; Li, Z. Highly efficient RNA-guided base editing in rabbit. Nat. Commun. 2018, 9, 2717. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Q.; Shi, H.; Xie, J.; Zhang, Q.; Ouyang, Z.; Li, N.; Yang, Y.; Liu, Z.; Zhao, Y.; et al. Engineering CRISPR/Cpf1 with tRNA promotes genome editing capability in mammalian systems. Cell. Mol. Life Sci. CMLS 2018, 75, 3593–3607. [Google Scholar] [CrossRef]
- Lu, R.; Yuan, T.; Wang, Y.; Zhang, T.; Yuan, Y.; Wu, D.; Zhou, M.; He, Z.; Lu, Y.; Chen, Y.; et al. Spontaneous severe hypercholesterolemia and atherosclerosis lesions in rabbits with deficiency of low-density lipoprotein receptor (LDLR) on exon 7. EBioMedicine 2018, 36, 29–38. [Google Scholar] [CrossRef]
- Yuan, T.; Zhong, Y.; Wang, Y.; Zhang, T.; Lu, R.; Zhou, M.; Lu, Y.; Yan, K.; Chen, Y.; Hu, Z.; et al. Generation of hyperlipidemic rabbit models using multiple sgRNAs targeted CRISPR/Cas9 gene editing system. Lipids Health Dis. 2019, 18, 69. [Google Scholar] [CrossRef]
- Yang, D.; Xu, J.; Chen, Y.E. Generation of Rabbit Models by Gene Editing Nucleases. Methods Mol. Biol. 2019, 1874, 327–345. [Google Scholar] [CrossRef]
- Liu, Z.; Shan, H.; Chen, S.; Chen, M.; Song, Y.; Lai, L.; Li, Z. Highly efficient base editing with expanded targeting scope using SpCas9-NG in rabbits. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 588–596. [Google Scholar] [CrossRef]
- Yan, H.; Niimi, M.; Matsuhisa, F.; Zhou, H.; Kitajima, S.; Chen, Y.; Wang, C.; Yang, X.; Yao, J.; Yang, D.; et al. Apolipoprotein CIII Deficiency Protects Against Atherosclerosis in Knockout Rabbits. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2095–2107. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Koike, Y.; Yang, D.; Guo, Y.; Rom, O.; Song, J.; Xu, J.; Chen, Y.; Wang, Y.; Zhu, T.; et al. Human apolipoprotein A-II reduces atherosclerosis in knock-in rabbits. Atherosclerosis 2021, 316, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Chen, Y.; Waqar, A.B.; Yan, H.; Shiomi, M.; Zhang, J.; Chen, Y.E.; Wang, Y.; Itabe, H.; Liang, J.; et al. Hypertension Enhances Advanced Atherosclerosis and Induces Cardiac Death in Watanabe Heritable Hyperlipidemic Rabbits. Am. J. Pathol. 2018, 188, 2936–2947. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Waqar, A.B.; Yan, H.; Wang, Y.; Liang, J.; Fan, J. Renovascular Hypertension Aggravates Atherosclerosis in Cholesterol-Fed Rabbits. J. Vasc. Res. 2019, 56, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wang, Y.; Chen, Y.E. Genetically Modified Rabbits for Cardiovascular Research. Front. Genet. 2021, 12, 614379. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Kitajima, S.; Watanabe, T.; Xu, J.; Zhang, J.; Liu, E.; Chen, Y.E. Rabbit models for the study of human atherosclerosis: From pathophysiological mechanisms to translational medicine. Pharmacol. Ther. 2015, 146, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kim, Y.S.; Kim, J.; Pattison, J.; Kamaid, A.; Miller, Y.I. Modeling hypercholesterolemia and vascular lipid accumulation in LDL receptor mutant zebrafish. J. Lipid Res. 2018, 59, 391–399. [Google Scholar] [CrossRef]
- Liu, C.; Gates, K.P.; Fang, L.; Amar, M.J.; Schneider, D.A.; Geng, H.; Huang, W.; Kim, J.; Pattison, J.; Zhang, J.; et al. Apoc2 loss-of-function zebrafish mutant as a genetic model of hyperlipidemia. Dis. Model. Mech. 2015, 8, 989–998. [Google Scholar] [CrossRef]
- Pinto, C.L.; Kalasekar, S.M.; McCollum, C.W.; Riu, A.; Jonsson, P.; Lopez, J.; Swindell, E.C.; Bouhlatouf, A.; Balaguer, P.; Bondesson, M.; et al. Lxr regulates lipid metabolic and visual perception pathways during zebrafish development. Mol. Cell. Endocrinol. 2016, 419, 29–43. [Google Scholar] [CrossRef]
- Tang, D.; Geng, F.; Yu, C.; Zhang, R. Recent Application of Zebrafish Models in Atherosclerosis Research. Front. Cell Dev. Biol. 2021, 9, 643697. [Google Scholar] [CrossRef]
- Vedder, V.L.; Aherrahrou, Z.; Erdmann, J. Dare to Compare. Development of Atherosclerotic Lesions in Human, Mouse, and Zebrafish. Front. Cardiovasc. Med. 2020, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, X.; Zhao, M.; Wang, X. Accelerated miniature swine models of advanced atherosclerosis: A review based on morphology. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2020, 49, 107241. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Guo, L.; Park, K.H.; Woollard, J.R.; Taek-Geun, K.; Jiang, K.; Melkamu, T.; Zang, B.; Smith, S.L.; Fahrenkrug, S.C.; et al. Ossabaw Pigs with a PCSK9 Gain-of-Function Mutation Develop Accelerated Coronary Atherosclerotic Lesions: A Novel Model for Preclinical Studies. J. Am. Heart Assoc. 2018, 7, e006207. [Google Scholar] [CrossRef] [PubMed]
- Hedayat, A.F.; Park, K.H.; Kwon, T.G.; Woollard, J.R.; Jiang, K.; Carlson, D.F.; Lerman, A.; Lerman, L.O. Peripheral vascular atherosclerosis in a novel PCSK9 gain-of-function mutant Ossabaw miniature pig model. Transl. Res. J. Lab. Clin. Med. 2018, 192, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Ren, X.; Wang, Y.; Li, Z. Apolipoprotein E deficiency accelerates atherosclerosis development in miniature pigs. Dis. Model. Mech. 2018, 11, dmm036632. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fuchimoto, D.; Sudo, M.; Haruta, H.; Lin, Q.F.; Takayama, T.; Morita, S.; Nochi, T.; Suzuki, S.; Sembon, S.; et al. Development of Human-Like Advanced Coronary Plaques in Low-Density Lipoprotein Receptor Knockout Pigs and Justification for Statin Treatment Before Formation of Atherosclerotic Plaques. J. Am. Heart Assoc. 2016, 5, e002779. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, E.; Zhang, Y.Q. Dog models of human atherosclerotic cardiovascular diseases. Mamm. Genome 2022, 1–8. [Google Scholar] [CrossRef]
- Shively, C.A.; Appt, S.E.; Vitolins, M.Z.; Uberseder, B.; Michalson, K.T.; Silverstein-Metzler, M.G.; Register, T.C. Mediterranean versus Western Diet Effects on Caloric Intake, Obesity, Metabolism, and Hepatosteatosis in Nonhuman Primates. Obesity 2019, 27, 777–784. [Google Scholar] [CrossRef]
- Shim, J.; Al-Mashhadi, R.H.; Sørensen, C.B.; Bentzon, J.F. Large animal models of atherosclerosis—new tools for persistent problems in cardiovascular medicine. J. Pathol. 2016, 238, 257–266. [Google Scholar] [CrossRef]
- Rayner, K.J.; Esau, C.C.; Hussain, F.N.; McDaniel, A.L.; Marshall, S.M.; van Gils, J.M.; Ray, T.D.; Sheedy, F.J.; Goedeke, L.; Liu, X.; et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature 2011, 478, 404–407. [Google Scholar] [CrossRef]
- Tadin-Strapps, M.; Robinson, M.; Le Voci, L.; Andrews, L.; Yendluri, S.; Williams, S.; Bartz, S.; Johns, D.G. Development of lipoprotein(a) siRNAs for mechanism of action studies in non-human primate models of atherosclerosis. J. Cardiovasc. Transl. Res. 2015, 8, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Lameijer, M.; Binderup, T.; van Leent, M.M.T.; Senders, M.L.; Fay, F.; Malkus, J.; Sanchez-Gaytan, B.L.; Teunissen, A.J.P.; Karakatsanis, N. Efficacy and safety assessment of a TRAF6-targeted nanoimmunotherapy in atherosclerotic mice and non-human primates. Nat. Biomed. Eng. 2018, 2, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Gao, Y.; Huang, H.; Wang, X.; Cai, Y.; Luan, Q.X. Tanshinone IIA Attenuates Atherosclerosis in Apolipoprotein E Knockout Mice Infected with Porphyromonas gingivalis. Inflammation 2017, 40, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Jandeleit-Dahm, K.; Hannan, K.M.; Farrelly, C.A.; Allen, T.J.; Rumble, J.R.; Gilbert, R.E.; Cooper, M.E.; Little, P.J. Diabetes-induced vascular hypertrophy is accompanied by activation of Na(+)-H(+) exchange and prevented by Na(+)-H(+) exchange inhibition. Circ. Res. 2000, 87, 1133–1140. [Google Scholar] [CrossRef]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2011, 50, 600–613. [Google Scholar]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies? Pharm. Res. 2019, 37, 12. [Google Scholar] [CrossRef]
- Vahdat Lasemi, F.; Mahjoubin Tehran, M.; Aghaee-Bakhtiari, S.H.; Jalili, A.; Jaafari, M.R.; Sahebkar, A. Harnessing nucleic acid-based therapeutics for atherosclerotic cardiovascular disease: State of the art. Drug Discov. Today 2019, 24, 1116–1131. [Google Scholar] [CrossRef]
- Summerton, J.E. Invention and Early History of Morpholinos: From Pipe Dream to Practical Products. Methods Mol. Biol. 2017, 1565, 1–15. [Google Scholar] [CrossRef]
- Wang, K.C.; Yeh, Y.T.; Nguyen, P.; Limqueco, E.; Lopez, J.; Thorossian, S.; Guan, K.L.; Li, Y.J.; Chien, S. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc. Natl. Acad. Sci. USA 2016, 113, 11525–11530. [Google Scholar] [CrossRef]
- Hu, Y.X.; Zhu, R.F.; Qin, Y.W.; Zhao, X.X.; Jing, Q. Zfp36l1b protects angiogenesis through Notch1b/Dll4 and Vegfa regulation in zebrafish. Atherosclerosis 2020, 309, 56–64. [Google Scholar] [CrossRef]
- Yu, Z.; Peng, Q.; Li, S.; Hao, H.; Deng, J.; Meng, L.; Shen, Z.; Yu, W.; Nan, D.; Bai, Y.; et al. Myriocin and d-PDMP ameliorate atherosclerosis in ApoE-/- mice via reducing lipid uptake and vascular inflammation. Clin. Sci. 2020, 134, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, D.; Nguyen, M.A.; Geoffrion, M.; Vreeken, D.; Lister, Z.; Cheng, H.S.; Otte, N.; Essebier, P.; Wyatt, H.; Kandiah, J.W.; et al. RIPK1 Expression Associates with Inflammation in Early Atherosclerosis in Humans and Can Be Therapeutically Silenced to Reduce NF-κB Activation and Atherogenesis in Mice. Circulation 2021, 143, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, L.; Xie, Y.; Wang, P.; Deng, S.; Qin, A.; Zhang, J.; Yu, X.; Zheng, W.; Jiang, X. Triple-Targeting Delivery of CRISPR/Cas9 To Reduce the Risk of Cardiovascular Diseases. Angew. Chem. Int. Ed. 2019, 58, 12404–12408. [Google Scholar] [CrossRef] [PubMed]
- Carreras, A.; Pane, L.S.; Nitsch, R.; Madeyski-Bengtson, K.; Porritt, M.; Akcakaya, P.; Taheri-Ghahfarokhi, A.; Ericson, E.; Bjursell, M.; Perez-Alcazar, M.; et al. In Vivo genome and base editing of a human PCSK9 knock-in hypercholesterolemic mouse model. BMC Biol. 2019, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, A.C.; Evitt, N.H.; Lv, W.; Musunuru, K. Reduced Blood Lipid Levels With In Vivo CRISPR-Cas9 Base Editing of ANGPTL3. Circulation 2018, 137, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Y.; He, L.; Pu, W.; Yu, W.; Li, Y.; Wu, Y.T.; Xu, C.; Wei, Y.; Ding, Q.; et al. In Vivo AAV-CRISPR/Cas9-Mediated Gene Editing Ameliorates Atherosclerosis in Familial Hypercholesterolemia. Circulation 2020, 141, 67–79. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamato, D.; Ilyas, I.; Xu, S.; Little, P.J. Non-Mouse Models of Atherosclerosis: Approaches to Exploring the Translational Potential of New Therapies. Int. J. Mol. Sci. 2022, 23, 12964. https://doi.org/10.3390/ijms232112964
Kamato D, Ilyas I, Xu S, Little PJ. Non-Mouse Models of Atherosclerosis: Approaches to Exploring the Translational Potential of New Therapies. International Journal of Molecular Sciences. 2022; 23(21):12964. https://doi.org/10.3390/ijms232112964
Chicago/Turabian StyleKamato, Danielle, Iqra Ilyas, Suowen Xu, and Peter J. Little. 2022. "Non-Mouse Models of Atherosclerosis: Approaches to Exploring the Translational Potential of New Therapies" International Journal of Molecular Sciences 23, no. 21: 12964. https://doi.org/10.3390/ijms232112964
APA StyleKamato, D., Ilyas, I., Xu, S., & Little, P. J. (2022). Non-Mouse Models of Atherosclerosis: Approaches to Exploring the Translational Potential of New Therapies. International Journal of Molecular Sciences, 23(21), 12964. https://doi.org/10.3390/ijms232112964








