Abstract
Cardiovascular diseases (CVD) are one of the leading causes of high mortality in patients with systemic lupus erythematosus (SLE). The Framingham risk score and other traditional risk factors do not fully reflect the CVD risk in SLE patients. Therefore, in order to stratify these high-risk patients, additional biomarkers for subclinical CVD are needed. The mechanisms of atherogenesis in SLE are still being investigated. During the past decades, many reports recognized that inflammation plays a crucial role in the development of atherosclerosis. The aim of this report is to present novel proinflammatory and pro-atherosclerotic risk factors that are closely related to SLE inflammation and which determine an increased risk for the occurrence of early cardiovascular events.
1. Introduction
Systemic lupus erythematosus (SLE) is an inflammatory autoimmune disease with the involvement of various organs [1]. It has been demonstrated that cardiovascular diseases (CVD) are currently a leading cause of comorbidity and mortality in SLE patients [2,3,4,5]. Particularly, coronary artery disease (CAD) causes nearly 30% of deaths in SLE [2,6,7]. SLE patients present a great prevalence of asymptomatic CAD [8]. Young women with SLE are prone to higher risks, as they are 50 times more likely than controls to develop a myocardial infarction; nevertheless, males also present the worst outcomes [9,10,11,12]. Regarding the risk of cerebrovascular complications in SLE, it is lower than coronary diseases, but compared to the general population, the risk is twice as high [2,13]. If these occur later in the disease course, the main cause may be attributed to atherosclerosis [14]. Additionally, the risk of peripheral artery occlusive disease has been found to be nine-fold higher in SLE patients than in general population [2,15].
The main traditional risk factors involved in CVD are: older age, arterial hypertension, obesity, and high cholesterol serum levels [2,16,17,18]. Particularly, for almost a century, cholesterol has been regarded as the main factor that promotes the development of atherosclerosis [19]. The burst of cardiovascular events (CVE) in SLE cannot be entirely related to traditional risk factors; it is thought to be driven also by immunologic and inflammatory features found in SLE [9,20,21,22]. Unfortunately, all these traditional cardiovascular models in use underestimate the risk of fatal events in SLE patients. There are currently no lupus-specific screening investigations to classify patients at risk for future major CVE, although traditional Framingham risk factors are insufficient to assess the risk of CVD [9,23,24]. Thus, extensive studies regarding non-traditional SLE-related risk factors that contribute to cardiovascular complications have been conducted [25].
Studies and book chapters relevant to this work were identified using the PubMed, EMBASE, Web of Science and Clinical Key research platforms. We have selected the most important works published in the last 5 years (2017–August 2022). For certain significant data for this review, but for which we found insufficient data in this time period, we extended the search to the last 10 years (2012–2022).
2. Atherosclerosis and Inflammation in SLE
Subclinical atherosclerosis, an early event in SLE individuals, plays an important role related to cardiovascular risk and morbidity. Among the changes it causes, we can list peripheral embolism, arterial hypertension with subsequent left ventricular hypertrophy, and diastolic dysfunction [2,26,27].
Medical conditions characterized by systemic inflammation like SLE have been strongly associated with atherosclerosis. Evidence show that inflammation process is crucial for the development of accelerated atherosclerosis in SLE. Therefore, it is plausible to assume that SLE inflammation may consecutively increase the CVD risk [2,9,28,29]. Inflammation has been linked to the development of atherosclerotic CVD in the general population as well [9,30,31]. Reducing inflammation is one of the essential targets in decreasing the CVD risk in SLE patients; it was highlighted that inflammation is responsible for initiation, formation and destabilization of atherosclerotic plaques [19,25,32,33].
Atherosclerosis Mechanism in SLE
Under a variety of irritant factors, endothelial cells on the vascular wall become activated; this results in a cascade of inflammatory reactions that can lead to the formation of atherosclerotic plaque [34]. There are three stages illustrated in Figure 1, as follows:
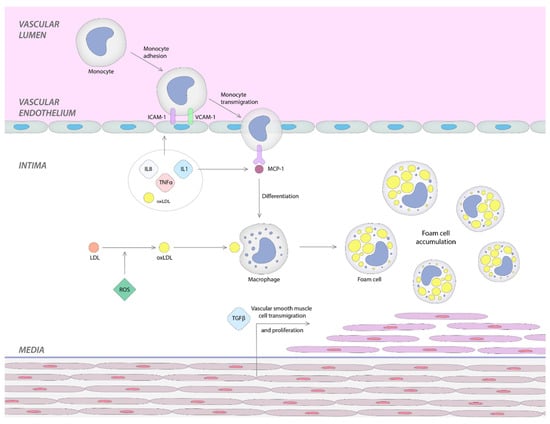
Figure 1.
Stages of atherosclerosis mechanism in SLE patients.
I. Firstly, the inflammatory process leads to the activation of the endothelial cells that determines the expression of surface molecules such as vascular cell and intercellular adhesion molecule-1 (VCAM-1, ICAM-1), selectins, and integrins necessary to leukocyte adhesion, rolling and attachment [2,35,36].
II. Secondly, the adherent leukocytes move through the intima layer and reach the media. This monocyte transmigration into subendothelial layer is promoted by chemokines, such as monocyte chemotactic protein-1 (MCP-1) [2,34,35]. Interestingly, a report showed that a high carotid intima-media thickness (IMT) is associated with elevated circulating levels of MCP-1 in humans [2,31]. The expression of MCP-1 and interleukin-8 (IL-8) can be induced by l-homocysteine, another factor that stimulates leukocyte recruitment [2,37].
At this point, tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and oxidized low-density lipoprotein (OxLDL) play two significant roles: first, they upregulate the adhesion molecules, and second, the adhesion of MCP-1 [2,38].
III. Low-density lipoproteins (LDL) are trapped in the subendothelial region, where they are exposed to reactive oxygen species (ROS) and are transformed into OxLDL. This is the beginning of the third and final stage. OxLDL, in turn, amplifies the inflammatory response by activating endothelial cells that will secrete adhesion molecules and chemokines that will stimulate the recruitment of monocytes. Additionally, OxLDL, found in the subendothelial layer, stimulates monocytes to differentiate into macrophages. The conversion of macrophages into foam cells and the multiplication of smooth muscle cells occur in this last stage, leading to atherosclerotic plaque proliferation [2,34,35,39,40].
On the other hand, the interaction between platelets and endothelial cells generates the production of cytokines such as IL-8 and stimulates ICAM-1, leading to endothelial dysfunction and a high risk of thrombosis. In SLE, these platelet–endothelium interactions are frequently seen and can accelerate the development of CVE [34,41].
3. Non-Traditional Pro-Atherosclerotic Biomarkers in sSLE
Multiple non-Framingham inflammatory biomarkers, such as plasma soluble TNF-like weak inducer of apoptosis (sTWEAK), dysfunctional pro-inflammatory high-density lipoprotein (HDL) (piHDL), leptin, and homocysteine, are independently linked to atherosclerosis in SLE [9,42,43,44,45,46]. Due to the increasing number of individual biomarkers attributed to CVD risk in SLE, a study from the University of California, Los Angeles, has developed a biomarker panel that could help to quantify more accurately the atherosclerosis risk and CVE risk in SLE. Thus, a model was created that is called PREDICTS (the Predictors of Risk for Elevated Flares, Damage Progression, and Increased Cardiovascular Disease in SLE) [45]. It integrates four inflammatory biomarkers (homocysteine, piHDL, TNF-like weak inducer of apoptosis TWEAK, and leptin) and two risk factors (age and diabetes). Among non-traditional risk factors, an increased piHDL function, high leptin levels and plasma soluble TWEAK significantly correlated with plaque formation. Interestingly, this complete panel showed a better predictive capacity for plaque formation in SLE compared with individual markers [45].
3.1. piHDL
Although HDL cholesterol is typically regarded as atheroprotective, in systemic inflammation it can change its properties. This occurs when it shifts from the typical anti-inflammatory form to the proinflammatory HDL (piHDL) form, phenomena that tend to occur during the onset of chronic inflammatory conditions, also seen in SLE [2,47]. A cause may be the ongoing oxidative damage in the chronic inflammatory state that generates piHDL and high levels of ox-LDL [48]. piHDL is found in SLE patients more frequently compared to rheumatoid arthritis (RA) individuals and the general population (Table 1). In this situation, the favorable effects of HDL, the decrease of LDL oxidation, are lost. Instead, this dysfunctional HDL enhances accelerated atherogenesis. McMahon et al. showed that piHDL is strongly linked to the development of carotid plaque and intima-media thickness (IMT) in SLE patients [34,45,48,49,50]. Interestingly, dysfunctional HDL may also be generated by atypical HDL oxidation. This is driven by the release of neutrophil extracellular traps (NETs) and low-density granulocytes that are specific for SLE [9,31,51]. Moreover, piHDL has proteomic and lipidomic abnormalities that are specific for HDL particles derived from SLE individuals [9,52,53,54].

Table 1.
Pro-inflammatory HDL in SLE patients.
Summarizing, it seems that piHDL is commonly found in SLE patients compared to general population. There is a strong link between piHDL and carotid plaque progression. In SLE cases, piHDL has atherogenic properties due to oxidative damage and also due to proteomic and lipidomic abnormalities.
3.2. Endothelial Progenitor Cells
Endothelial dysfunction, seen in the early stages of atherosclerosis development, has been studied for its contribution to CVD risk in SLE [25,56,57,58]. Data suggested that endothelial dysfunction is already present in SLE patients before CVD development [25,59,60,61,62]. Patients with SLE have functionally and quantitatively decreased levels of endothelial progenitor cells (EPCs) [25,63]. EPCs are a subpopulation of circulating stem cells involved in the repair of blood vessels [34]. They are essential for maintaining the endothelial function, neovascularization, and vascular repair [25]. EPCs are thought to be able to replace damaged endothelial cells and restore endothelial integrity and, therefore, to improve the endothelial function [25,29,64]. They might be used as possible biomarkers to identify patients at risk for developing a CVD [34,65]. Castejon et al. showed that SLE patients with lower levels of progenitor cells had an increased arterial stiffness and a greater preponderance of cardiovascular risk factors, such as smoking and metabolic syndrome [34,66].
In multiple reports evaluating patients with SLE versus healthy controls, the levels of circulating EPCs were significantly low (Table 2). Thus, a reduced level of circulating EPC may be a marker for an early vascular disease [34,66]. The therapeutic and prognostic role of EPCs as an early CVD biomarker in SLE patients remain to be elucidated. New methods for measuring endothelial function and quantifying EPC as prognostic indicators of CVD are needed in SLE patients [25].

Table 2.
Endothelial progenitor cells and the relation with SLE manifestations.
Encouraging in vitro results show the reversibility of the decrease in EPC number after treatment with anti-type 1 INFα and anti B cell activating factor (BAFF) [34].
In summary, it is known that SLE patients present endothelial dysfunction more frequently. It seems that an important biomarker can be considered the low level of EPCs. Moreover, these cells have an altered function. The decrease in the number and alteration of the functionality of EPCs is associated with an increase in arterial stiffness and with the occurrence of CVD in SLE cases.
3.3. Endocan
Endocan is another marker of angiogenesis and endothelial cell activation. It contributes to the recruitment, adhesion and migration of leukocytes across the endothelium [2,74,75].
Icli et al. investigated the impact of endocan on atherosclerotic process in SLE. They found that endocan serum levels were higher in SLE patients versus controls; also, endocan levels were strongly associated with carotid intima-media thickness (cIMT) [2,76]. Its potential role as a useful biomarker in the SLE pathogenesis needs to be further investigated (Table 3). In the literature, only two studies focus on endocan in SLE and on the link between it and atherosclerosis. Furthermore, no animal model studies were identified.

Table 3.
Endocan in SLE cases.
In conclusion, although there are only few studies focused on endocan role in atherosclerosis, it has been found an increased endocan titer in SLE individuals versus controls. Moreover, it seems that there is an association between the endocan level and carotid intima-media thickness. These studies raise the hypothesis that endocan may become a reliable predictor of early atherosclerosis in SLE patients.
3.4. Leptin
Leptin, an adipokine that controls satiety and fat deposits, is considered to be an atherosclerotic risk factor [34,78]. According to the first study that examined the relationship between adipokines and subclinical atherosclerosis in SLE individuals, McMahon et al., showed that higher leptin levels were independently associated with carotid plaques formation and positively correlated with piHDL and oxidized phospholipids levels (Table 4) [34,44,79].
Many immune cell subsets are influenced by leptin [9,80]. For instance, leptin may have particularly pro-inflammatory actions on macrophages in SLE, such as the activation of phagocytosis and an increased presentation of apoptosis-derived self-antigen to T lymphocytes [9,81]. In addition, leptin may induce an increased production of pro-inflammatory cytokines and oxidative stress in endothelial cells and cardiomyocytes [9,82,83].
However, some contradictory studies reported a reduced leptin serum in SLE patients [84,85], while others reported no statistically significant differences between SLE patients and controls [86,87].

Table 4.
Leptin and SLE patients.
Table 4.
Leptin and SLE patients.
| Biomarker | Study | Results |
|---|---|---|
| Leptin | Garcia-Gonzalez et al., 2002 [88] |
|
| Sada et al., 2006 [89] |
| |
| Chung et al., 2009 [90] |
| |
| Al et al., 2009 [91] |
| |
| Kim et al., 2010 [92] |
| |
| McMahon et al., 2011 [44] |
| |
| Vadacca et al., 2013 [93] |
| |
| Wang et al., 2017 [94] |
| |
| Diaz-Rizo et al., 2017 [95] |
| |
| Demir et al., 2018 [96] |
|
piHDL = proinflammatory HDL; Lp(a) = Lipoprotein a; oxPL = oxidised phospholipids; MetS = metabolic syndrome; Tregs = regulatory T cells; pSLE = paediatric SLE; HC = healthy control; LN = lupus nephritis.
Studies confirm the presence of a high level of leptin in SLE patients. In SLE, leptin has particular actions on macrophages, favoring the secretion of inflammatory cytokines and increasing oxidative stress. Moreover, it seems to be positively associated with other pro-atherosclerotic and proinflammatory markers. This evidence suggests that leptin may be a promising marker in SLE.
3.5. Resistin
Resistin is another adipose tissue-specific secretory marker. It contributes to the inflammatory response by upregulating adhesion molecules, expanding the secretion of proinflammatory cytokines such as IL-1, IL-6, and TNF-α. SLE patients with more severe coronary artery calcification (CAC) showed increased levels of resistin [34,71]. Serum resistin may be an encouraging biomarker for renal involvement in SLE patients, but there are insufficient reports (Table 5).

Table 5.
Resistin in SLE patients.
Studies have shown that resistin is present in an increased titer in lupus patients versus controls. The data support that resistin is involved in the inflammatory process and favors arterial calcification Furthermore, it seems that there is a relationship between resistin and kidney damage in SLE cases.
3.6. S100A8 and S100A9
S100A8 and S100A9, two Ca2+ binding proteins, are members of the S100 family. The heterodimeric complex S100 A8/A9 is involved in cytoskeleton rearrangement and arachidonic acid metabolism. It is mainly secreted by phagocytic cells such as neutrophils, monocytes and dendritic cells. S100A8/A9 could be released from activated polymorphonuclear neutrophils (PMNs), as part of NETs [101]. During the inflammatory process, it promotes leukocyte recruitment and induces cytokine secretion [102,103].
Lood et al. evaluated two large cohorts of SLE patients and showed that this proinflammatory and prothrombotic protein complex was found in naïve platelets and has elevated levels in SLE patients. Interestingly, S100A8/A9 is expressed around the thrombus and atherosclerotic plaque and it is synthesized by megakaryocytes, platelet progenitor cells. Wang et al. previously found that this thrombotic effect is mediated through CD36 interaction. This protein complex is associated with CVD, particularly being related to a four-fold increased risk of myocardial infarction [34,103,104].
The serum levels of S100A8/A9 secreted by polymorphonuclear cells are increased in SLE patients, specifically in individuals with positive anti-dsDNA antibodies and glomerulonephritis. Since higher levels of S100A8/A9 have been associated with both inactive and active SLE, serum S100A8/A9 levels may be needed to monitor the disease’s course [101,104,105].
New data consider that S100A8/A9 may also play an interesting role in neuropsychiatric SLE (NPSLE) due to its action on endothelial cells, contributing to vasculopathy and atherosclerosis development at the central nervous system level [106].
Furthermore, S100A8/A9 may be used as a therapeutic biomarker in CVD due to its contribution to atherogenesis, plaque vulnerability, ischemia-associated myocardial inflammation, and heart failure [107] (Table 6). It can generate a proinflammatory response and an increased secretion of IL-6, IL-1β and TNF-α [101]. A promising clinical target may be type-1 interferon (IFN) as it induces the upregulation of platelet-derived S100A8/A9 complex protein [34,103]. S100A8/A9 is becoming a more sensitive biomarker for inflammation activity and for therapeutic response by comparison with traditional inflammation markers, such as C-reactive protein [102,108,109].

Table 6.
Protein complex S100 A8/A9 in SLE patients.
This proinflammatory and prothrombotic protein complex S100 A8/A9, especially the one secreted by platelets, is secreted in excess in SLE patients. It is associated with various clinical manifestations of SLE, such as renal or neuropsychiatric damage. It actively participates in the occurrence of CVD by increasing atherogenesis and the vulnerability of the atherosclerotic plaque. Moreover, it favors the secretion of pro-inflammatory cytokines.
3.7. NETs and Microparticles (MPs)
Neutrophil extracellular traps (NETs) are chromatin fibers released from dying neutrophils. The death of neutrophils with subsequent NETs production is called “NETosis”. During their activation, neutrophils secrete ROS. The major function of NETs consists of trapping and killing pathogens. NETs exert their antimicrobial mechanism through antimicrobial enzymes such as myeloperoxidase (MPO), neutrophil elastase, but also through other molecules such ascathelicidin, histones and DNA [25,110,111].
The main source of autoantigens in SLE is considered to be the inefficient clearance of necrotic and apoptotic cells. Furthermore, there is a deficient clearing of NETs [2]. Hakkim et al. reported that this impaired degradation of NETs in SLE is apparently due to two mechanisms: the presence of antibodies against endonuclease DNase1—necessary for NETs disassembly and the appearance of anti-NET autoantibodies that prevent NETs from degradation [2,112]. In SLE, the development of NETs is stimulated by ribonucleoprotein-specific antibodies [25,113]. SLE neutrophils are stimulated by type I IFN and die upon exposure to SLE-derived anti-ribonucleoprotein antibodies, releasing NETs [113].
According to the latest research, NETs formation might contribute to atherosclerosis progression [110,114]. NETs can directly cause endothelial cell apoptosis by many mechanisms such as: endothelial cell death mediated by endothelial MMP2 stimulation; activation of platelets, coagulation cascade and thrombosis by releasing serine proteases that deteriorate tissue factor pathway inhibitor and activate factor XII and vascular leakage [25,115,116]. Indirectly, NETs determine low-density granulocytes and plasmacytoid dendritic cells to produce large amounts of IFN, which increases IFN-induced endothelial toxicity, enhances HDL-c oxidation and reduces cholesterol outflow capacity [25,117,118,119]. NETs also determine inflammasome activation, consequently increasing the IL1β and IL-18 production, which finally creates a positive loop of NETs production [2,120]. NETs promote the production and release of type I IFN which further promotes NETosis [2,113]. That is why it has been proposed that NETosis could serve as a therapeutic target in SLE [110].
The production of circulating plasma microparticles (MPs) and NETs are two significant processes that have been described in the context of endothelial dysfunction in SLE (Table 7). MPs are generated from apoptotic cells and have high levels in SLE patients [25,121,122]. Moreover, circulating plasma MP can directly cause endothelial cell death. In addition, MPs and MP-immune complexes can cause alterations in endothelial cell permeability and death by increasing the disruption of endothelial microstructure in SLE [25,123]. MPs are not exclusively linked to endothelial cells; individuals with SLE were found to have high levels of MP produced by platelets, monocytes, granulocytes, and lymphocytes [25,124].

Table 7.
Microparticles in SLE cases.
In conclusion, in SLE there is a degradation and deficient clearance of NETs. Thus, through excessive accumulation, atherogenic mechanisms are favored both directly through endothelial cell apoptosis and indirectly through increased IFN secretion, HDL-c oxidation and reduced cholesterol outflow capacity. Moreover, the increase in MPs is associated with the occurrence of CVD due to the increase in vascular permeability and the death of endothelial cells.
3.8. Other Potential Biomarkers of Atherosclerosis in SLE
Homocysteine can lead to lipid peroxidation, endothelial dysfunction, and oxidative damage [127,128]. Several studies have associated homocysteine with atherosclerosis in SLE patients [9,45,46,129].
sTWEAK is a potential biomarker for SLE nephritis and CVD in general population. It can increase IFN- expression in peripheral blood mononuclear cells [9,130,131].
Pentraxin-3 (PTX3), a newly identified pro-atherosclerotic marker in SLE, is synthesized by mononuclear phagocytes, myeloid-derived dendritic cells and endothelium cells as a response to local inflammation. It is recognized as a local vascular inflammatory biomarker in SLE [2,132]. It presents high levels in SLE and correlates with disease activity [2,133]. PTX3 is also associated with other indicators of endothelial dysfunction such as soluble VCAM-1 and vWf [2,134]. These findings suggest that PTX3 may be a novel biomarker for early atherosclerosis in SLE.
Interferon
The synthesis of lipid mediators, of platelet-activating factors and eicosanoids, antigen presentation, and the synthesis of TNF-α and IL-1 are a small number of pro-atherogenic processes that are upregulated by type II interferon (IFN). IFN -γ is one of the most important cytokine in SLE [2,135,136].
Type 1 IFN, which is mainly secreted by plasmacytoid dendritic cells and low-density granulocytes, plays a crucial role in SLE pathogenesis. Type I IFNs are associated with endothelial dysfunction and with CVD progression in SLE, even in patients without risk factors for vascular diseases [34]. IFN-α has a high toxicity against endothelium [25,137]. IFN-α expression is increased as a result of the activation of Toll-like receptors 7 and 9 [2,138]. Additionally, IFN is involved in abnormal vascular repair because it modulates plaque instability by suppressing the development of smooth muscle cells, endothelial cells and the synthesis of collagen [2]. Activation of IFN pathways is linked to a fast atherosclerosis progression in SLE [69,139]. It remains to be established whether type I IFNs would be able to influence platelet S100A8/A9 levels [103].
TNF-α and IL-1 levels are significantly elevated in SLE patients with CVD. Their role is to promote adhesion molecule expression. TNF-α also contributes to the final stage of atherogenesis through down-regulation of lipoprotein lipase, an enzyme that hydrolyses triglycerides in VLDL (very low density lipoproteins) [2,34,140,141,142].
Interleukin-18 (IL-18) manifests its pro-atherosclerotic and pro-inflammatory effects on endothelial cells. Serum IL-18 levels are increased in SLE and correlate with EPC/CAC (circulating angiogenic cells) dysfunction. In SLE, the increased circulating IFN-α levels determine a chronic dysregulation of inflammasome activity with subsequent increased IL-18 levels that led to an abnormal vascular repair [143,144].
Osteoprotegerin (OPG) belongs to the TNF receptor family. Higher levels of CAC (circulating angiogenic cells) and cIMT have been seen in SLE individuals with higher levels of OPG. Kiani et al. suggested that OPG may be a marker for subclinical atherosclerosis in SLE patients, but it requires additional validation in larger trials [145].
The presence of antiphospholipid antibodies (aPL), which are known to enhance the risk of thrombosis in SLE through a variety of pathways, may also be associated with an accelerated atherosclerosis [2]. Interestingly, Lood et al. reported that aPL antibodies, anti-beta 2 glycoprotein 1 (β2-GP1), anti-cardiolipin and lupus anticoagulant, are correlated with high levels of S100A8/A9 in platelets [103]. aPL interact with endothelial cells and monocytes, creating a pro-inflammatory and pro-coagulant state [2,146,147]. aPL also activate the complement generating C5a; in turn, C5a activates neutrophils and a subsequent extrinsic coagulation cascade [2,148].
In contrast with native LDL, oxLDL can form complexes with IgG β2-GP, also increased in SLE. Moreover, the presence of IgG anti-β2-GP1 enhances OxLDL uptake by macrophages. SLE patients with CVD present increased levels of circulating OxLDL and antibodies against OxLDL. The atherosclerotic process has been positively linked to elevated levels of antibodies against OxLDL epitopes [2,149,150].
The extracellular matrix protein osteopontin (OPN) is considered to be a mediator of systemic inflammation. The role of OPN in SLE and atherosclerosis is linked to type I IFN response regulation [151]. Some studies reported that higher OPN levels correlate with SLE and LN compared with other diseases [152,153,154]. A positive association between OPN and cIMT in SLE patients was also described [155].
Low-density granulocytes (LDGs), a subclass of neutrophils that are frequently found in SLE, are highly susceptible to predispose NETosis. Due to their negative effects on endothelium, due to the synthesis of high levels of pro-inflammatory cytokines such as IFN-α and to the disruption of the EPC development into mature endothelial cells, LDG NETs are believed to contribute to atherosclerosis acceleration in SLE. LDGs also determine an important mitochondrial ROS production. This ROS hyperproduction and the stimulation of lipoproteins peroxidation by a decreased paraoxonase 1 activity, are two additional mechanisms of atherogenesis [2].
SLE patients also have higher levels of specific soluble mediators such as annexin A5, platelet endothelial cell adhesion molecule (PECAM1) and activated leukocyte cell adhesion molecule (ALCAM). These mediators have been linked to endothelial dysfunction [25,156]. Moreover, Valer et al. showed that serum annexin A5 levels were independently correlated with endothelial dysfunction and with an increased carotid intima-media thickness in SLE patients [25,157].
4. Conclusions
The balance of endothelial integrity is maintained by the homeostasis between endothelial injury and repair. This is crucial for maintaining a normal endothelial function and consequently, the vascular health. Endothelial dysfunction, which is thought to be the first stage in the pathogenesis of atherosclerosis, develops when endothelial damage exceeds the repair process. It is well known that the atherogenesis process includes both traditional and non-traditional factors, the latter less known, but very important in the mechanism of the disease. Systemic inflammation, also present in SLE, is a main factor in the occurrence of early atherosclerosis, being associated with the presence of a cytokine cascade and multiple cell activations. It is extremely important to find novel biomarkers that can predict the development of atherosclerosis and, why not, to use them as new therapeutic targets.
Author Contributions
Conceptualization, P.R. and A.C.; methodology, P.R.; software, A.M.B. and A.C.; validation, E.R., A.C. and A.M.B.; formal analysis, C.R.; investigation, P.R.; resources, P.R., A.M.B. and C.R.; data curation, P.R.; writing—original draft preparation, P.R.; writing—review and editing, A.C. and C.R.; visualization, A.M.B. and E.R.; supervision, E.R. and C.R.; project administration, E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hammad, S.M.; Harden, O.C.; Wilson, D.A.; Twal, W.O.; Nietert, P.J.; Oates, J.C. Plasma Sphingolipid Profile Associated With Subclinical Atherosclerosis and Clinical Disease Markers of Systemic Lupus Erythematosus: Potential Predictive Value. Front. Immunol. 2021, 12, 694318. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, V.; Tam, L.S. Novel Insights in Systemic Lupus Erythematosus and Atherosclerosis. Front. Med. 2018, 4, 262. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Cicco, S.; Prete, M.; Solimando, A.G.; Susca, N.; Crudele, L.; Buonavoglia, A.; Colonna, P.; Dammacco, F.; Vacca, A.; et al. Early echocardiographic detection of left ventricular diastolic dysfunction in patients with systemic lupus erythematosus asymptomatic for cardiovascular disease. Clin. Exp. Med. 2020, 20, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Vizcaya, A.; Isenberg, D. Analysis of trends and causes of death in SLE patients over a 40-years period in a cohort of patients in the United Kingdom. Lupus 2021, 30, 702–706. [Google Scholar] [CrossRef]
- Ståhl-Hallengren, C.; Jönsen, A.; Nived, O.; Sturfelt, G. Incidence studies of systemic lupus erythematosus in Southern Sweden: Increasing age, decreasing frequency of renal manifestations and good prognosis. J. Rheumatol. 2000, 27, 685–691. [Google Scholar]
- Aranow, C.; Ginzler, E.M. Epidemiology of cardiovascular disease in systemic lupus erythematosus. Lupus 2000, 9, 166–169. [Google Scholar] [CrossRef]
- Lai, E.C.; Huang, Y.C.; Liao, T.C.; Weng, M.Y. Premature coronary artery disease in patients with immune-mediated inflammatory disease: A population-based study. RMD Open 2022, 8, e001993. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Pinto, C.; Munguía-Realpzo, P.; García-Carrasco, M.; Godinez-Bolaños, K.; Rojas-Villarraga, A.; Morales-Etchegaray, I.; Ayón-Aguilar, J.; Méndez-Martínez, S.; Cervera, R. Asymptomatic coronary artery disease assessed by coronary computed tomography in patients with systemic lupus erythematosus: A systematic review and meta-analysis. Eur. J. Intern. Med. 2022, 100, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Skaggs, B.J.; Grossman, J.; Sahakian, L.; Perry, L.; FitzGerald, J.; Charles-Schoeman, C.; Gorn, A.; Taylor, M.; Moriarty, J.; Ragavendra, N.; et al. A Panel of Biomarkers Associates With Increased Risk for Cardiovascular Events in Women with Systemic Lupus Erythematosus. ACR Open Rheumatol. 2021, 3, 209–220. [Google Scholar] [CrossRef]
- Manzi, S.; Meilahn, E.N.; Rairie, J.E.; Conte, C.G.; Medsger, T.A., Jr.; Jansen-McWilliams, L.; D’Agostino, R.B.; Kuller, L.H. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: Comparison with the Framingham Study. Am. J. Epidemiol. 1997, 145, 408–415. [Google Scholar] [CrossRef]
- Vavlukis, M.; Pop-Gjorcevab, D.; Poposka, L.; Sandevska, E.; Kedev, S. Myocardial Infarction in Systemic Lupus Erythematosus—The Sex-Specific Risk Profile. Curr. Pharm. Des. 2021, 27, 3221–3228. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Hahn, B.H. Atherosclerosis and systemic lupus erythematosus: Mechanistic basis of the association. Curr. Opin. Immunol. 2007, 19, 633–639. [Google Scholar] [CrossRef]
- Arkema, E.V.; Svenungsson, E.; Von Euler, M.; Sjöwall, C.; Simard, J.F. Stroke in systemic lupus erythematosus: A Swedish population-based cohort study. Ann. Rheum. Dis. 2017, 76, 1544–1549. [Google Scholar] [CrossRef]
- Hanly, J.G.; Li, Q.; Su, L.; Urowitz, M.B.; Gordon, C.; Bae, S.C.; Romero-Diaz, J.; Sanchez-Guerrero, J.; Bernatsky, S.; Clarke, A.E.; et al. Cerebrovascular Events in Systemic Lupus Erythematosus: Results From an International Inception Cohort Study. Arthritis Care Res. 2018, 70, 1478–1487. [Google Scholar] [CrossRef]
- Chuang, Y.W.; Yu, M.C.; Lin, C.L.; Yu, T.M.; Shu, K.H.; Kao, C.H. Risk of Peripheral Arterial Occlusive Disease in Patients With Systemic Lupus Erythematosus: A Nationwide Population-Based Cohort Study. Medicine 2015, 94, e2121. [Google Scholar] [CrossRef] [PubMed]
- Manzi, S.; Selzer, F.; Sutton-Tyrrell, K.; Fitzgerald, S.G.; Rairie, J.E.; Tracy, R.P.; Kuller, L.H. Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Rheum. 1999, 42, 51–60. [Google Scholar] [CrossRef]
- Voutilainen, A.; Brester, C.; Kolehmainen, M.; Tuomainen, T.P. Epidemiological analysis of coronary heart disease and its main risk factors: Are their associations multiplicative, additive, or interactive? Ann. Med. 2022, 54, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.A.; Șalaru, D.L.; Prisacariu, C.; Marcu, D.T.M.; Sascău, R.A.; Stătescu, C. Novel Biomarkers of Atherosclerotic Vascular Disease-Latest Insights in the Research Field. Int. J. Mol. Sci. 2022, 23, 4998. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Stojan, G.; Petri, M. Atherosclerosis in systemic lupus erythematosus. J. Cardiovasc. Pharmacol. 2013, 62, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Yazdany, J.; Tonner, C.; Trupin, L.; Panopalis, P.; Gillis, J.Z.; Hersh, A.O.; Julian, L.J.; Katz, P.P.; Criswell, L.A.; Yelin, E.H. Provision of preventive health care in systemic lupus erythematosus: Data from a large observational cohort study. Arthritis Res. Ther. 2010, 12, R84. [Google Scholar] [CrossRef]
- Oliveira, C.B.; Kaplan, M.J. Cardiovascular disease risk and pathogenesis in systemic lupus erythematosus. Semin. Immunopathol. 2022, 44, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Colaco, K.; Ocampo, V.; Ayala, A.P.; Harvey, P.; Gladman, D.D.; Piguet, V.; Eder, L. Predictive Utility of Cardiovascular Risk Prediction Algorithms in Inflammatory Rheumatic Diseases: A Systematic Review. J. Rheumatol. 2020, 47, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Mosca, M.; Tani, C.; Aringer, M.; Bombardieri, S.; Boumpas, D.; Cervera, R.; Doria, A.; Jayne, D.; Khamashta, M.A.; Kuhn, A.; et al. Development of quality indicators to evaluate the monitoring of SLE patients in routine clinical practice. Autoimmun. Rev. 2011, 10, 383–388. [Google Scholar] [CrossRef]
- Mak, A.; Chan, J.K.Y. Endothelial function and endothelial progenitor cells in systemic lupus erythematosus. Nat. Rev. Rheumatol. 2022, 18, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J. The pathophysiology of hypertension in systemic lupus erythematosus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1258–R1267. [Google Scholar] [CrossRef] [PubMed]
- Roldan, C.A.; Alomari, I.B.; Awad, K.; Boyer, N.M.; Qualls, C.R.; Greene, E.R.; Sibbitt, W.L., Jr. Aortic stiffness is associated with left ventricular diastolic dysfunction in systemic lupus erythematosus: A controlled transesophageal echocardiographic study. Clin. Cardiol. 2014, 37, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kaplan, M.J. Cardiovascular disease in systemic lupus erythematosus: An update. Curr. Opin. Rheumatol. 2018, 30, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Tumurkhuu, G.; Montano, E.; Jefferies, C. Innate immune dysregulation in the development of cardiovascular disease in lupus. Curr. Rheumatol. Rep. 2019, 21, 46. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. High-sensitivity C-reactive protein: Potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001, 103, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.T.; Hallerstam, S.; Rosfors, S.; Wallén, N.H. Circulating markers of inflammation are related to carotid artery atherosclerosis. Int. Angiol. 2005, 24, 43–51. [Google Scholar]
- Montarello, N.J.; Nguyen, M.T.; Wong, D.T.L.; Nicholls, S.J.; Psaltis, P.J. Inflammation in Coronary Atherosclerosis and Its Therapeutic Implications. Cardiovasc. Drugs Ther. 2022, 36, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.; Leopoulou, M.; Theofilis, P.; Antonopoulos, A.S.; Siasos, G.; Latsios, G.; Mystakidi, V.C.; Antoniades, C.; Tousoulis, D. A link between inflammation and thrombosis in atherosclerotic cardiovascular diseases: Clinical and therapeutic implications. Atherosclerosis 2020, 309, 16–26. [Google Scholar] [CrossRef]
- Saeli, S.; Bichile, T.; Thakkar, P.; Manzi, S. Cardiovascular disease in systemic lupus erythematosus: An update. In Systemic Lupus Erythematosus. Basic, Applied and Clinical Aspects, 2nd ed.; Tsokos, G.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 44; pp. 415–426. [Google Scholar]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Hansson, G.K. Immune mechanisms in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1876–1890. [Google Scholar] [CrossRef] [PubMed]
- Poddar, R.; Sivasubramanian, N.; DiBello, P.M.; Robinson, K.; Jacobsen, D.W. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: Implications for vascular disease. Circulation 2001, 103, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Khismatullin, D.B. Oxidized low-density lipoprotein contributes to atherogenesis via co-activation of macrophages and mast cells. PLoS ONE 2015, 10, e0123088. [Google Scholar] [CrossRef]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef]
- Nhek, S.; Clancy, R.; Lee, K.A.; Allen, N.M.; Barrett, T.J.; Marcantoni, E.; Nwaukoni, J.; Rasmussen, S.; Rubin, M.; Newman, J.D.; et al. Activated Platelets Induce Endothelial Cell Activation via an Interleukin-1β Pathway in Systemic Lupus Erythematosus. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 707–716. [Google Scholar] [CrossRef]
- Sánchez-Pérez, H.; Quevedo-Abeledo, J.C.; de Armas-Rillo, L.; Rua-Figueroa, Í.; Tejera-Segura, B.; Armas-González, E.; Machado, J.D.; García-Dopico, J.A.; Jimenez-Sosa, A.; Rodríguez-Lozano, C.; et al. Impaired HDL cholesterol efflux capacity in systemic lupus erythematosus patients is related to subclinical carotid atherosclerosis. Rheumatology 2020, 59, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, P.M.; Purmalek, M.M.; Dey, A.K.; Temesgen-Oyelakin, Y.; Sakhardande, S.; Joshi, A.A.; Lerman, J.B.; Fike, A.; Davis, M.; Chung, J.H.; et al. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight 2018, 3, e99276. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Skaggs, B.J.; Sahakian, L.; Grossman, J.; FitzGerald, J.; Ragavendra, N.; Charles-Schoeman, C.; Chernishof, M.; Gorn, A.; Witztum, J.L.; et al. High plasma leptin levels confer increased risk of atherosclerosis in women with systemic lupus erythematosus, and are associated with inflammatory oxidised lipids. Ann. Rheum. Dis. 2011, 70, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Skaggs, B.J.; Grossman, J.M.; Sahakian, L.; Fitzgerald, J.; Wong, W.K.; Lourenco, E.V.; Ragavendra, N.; Charles-Schoeman, C.; Gorn, A.; et al. A panel of biomarkers is associated with increased risk of the presence and progression of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheumatol. 2014, 66, 130–139. [Google Scholar] [CrossRef]
- Roman, M.J.; Crow, M.K.; Lockshin, M.D.; Devereux, R.B.; Paget, S.A.; Sammaritano, L.; Levine, D.M.; Davis, A.; Salmon, J.E. Rate and determinants of progression of atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2007, 56, 3412–3419. [Google Scholar] [CrossRef]
- Pownall, H.J.; Rosales, C.; Gillard, B.K.; Gotto, A.M., Jr. High-density lipoproteins, reverse cholesterol transport and atherogenesis. Nat. Rev. Cardiol. 2021, 18, 712–723. [Google Scholar] [CrossRef]
- McMahon, M.; Grossman, J.; FitzGerald, J.; Dahlin-Lee, E.; Wallace, D.J.; Thong, B.Y.; Badsha, H.; Kalunian, K.; Charles, C.; Navab, M.; et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006, 54, 2541–2549. [Google Scholar] [CrossRef]
- McMahon, M.; Grossman, J.; Skaggs, B.; Fitzgerald, J.; Sahakian, L.; Ragavendra, N.; Charles-Schoeman, C.; Watson, K.; Wong, W.K.; Volkmann, E.; et al. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum. 2009, 60, 2428–2437. [Google Scholar] [CrossRef]
- Reiss, A.B.; Jacob, B.; Ahmed, S.; Carsons, S.E.; DeLeon, J. Understanding Accelerated Atherosclerosis in Systemic Lupus Erythematosus: Toward Better Treatment and Prevention. Inflammation 2021, 44, 1663–1682. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yu, M.; Morin, E.E.; Kang, J.; Kaplan, M.J.; Schwendeman, A. High-Density Lipoprotein in Lupus: Disease Biomarkers and Potential Therapeutic Strategy. Arthritis Rheumatol. 2020, 72, 20–30. [Google Scholar] [CrossRef]
- Han, C.Y.; Tang, C.; Guevara, M.E.; Wei, H.; Wietecha, T.; Shao, B.; Subramanian, S.; Omer, M.; Wang, S.; O’Brien, K.D.; et al. Serum amyloid A impairs the antiinflammatory properties of HDL. J. Clin. Investig. 2016, 126, 266–281. [Google Scholar] [CrossRef]
- Gaál, K.; Tarr, T.; Lőrincz, H.; Borbás, V.; Seres, I.; Harangi, M.; Fülöp, P.; Paragh, G. High-density lipopoprotein antioxidant capacity, subpopulation distribution and paraoxonase-1 activity in patients with systemic lupus erythematosus. Lipids Health Dis. 2016, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Marsillach, J.; Becker, J.O.; Vaisar, T.; Hahn, B.H.; Brunzell, J.D.; Furlong, C.E.; de Boer, I.H.; McMahon, M.A.; Hoofnagle, A.N.; DCCT/EDIC Research Group. Paraoxonase-3 is depleted from the high-density lipoproteins of autoimmune disease patients with subclinical atherosclerosis. J. Proteome Res. 2015, 14, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Skaggs, B.J.; Hahn, B.H.; Sahakian, L.; Grossman, J.; McMahon, M. Dysfunctional, pro-inflammatory HDL directly upregulates monocyte PDGFRβ, chemotaxis and TNFα production. Clin. Immunol. 2010, 137, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Steyers, C.M., III; Miller, F.J., Jr. Endothelial dysfunction in chronic inflammatory diseases. Int. J. Mol. Sci. 2014, 15, 11324–11349. [Google Scholar] [CrossRef] [PubMed]
- Sitia, S.; Tomasoni, L.; Atzeni, F.; Ambrosio, G.; Cordiano, C.; Catapano, A.; Tramontana, S.; Perticone, F.; Naccarato, P.; Camici, P.; et al. From endothelial dysfunction to atherosclerosis. Autoimmun. Rev. 2010, 9, 830–834. [Google Scholar] [CrossRef]
- Mauro, D.; Nerviani, A. Endothelial Dysfunction in Systemic Lupus Erythematosus: Pathogenesis, Assessment and Therapeutic Opportunities. Rev. Recent Clin. Trials 2018, 13, 192–198. [Google Scholar] [CrossRef]
- Stalc, M.; Tomsic, M.; Jezovnik, M.K.; Poredos, P. Endothelium-dependent and independent dilation capability of peripheral arteries in patients with systemic lupus erythematosus and antiphospholipid syndrome. Clin. Exp. Rheumatol. 2011, 29, 616–623. [Google Scholar]
- Johnson, S.R.; Harvey, P.J.; Floras, J.S.; Iwanochko, M.; Ibanez, D.; Gladman, D.D.; Urowitz, M. Impaired brachial artery endothelium dependent flow mediated dilation in systemic lupus erythematosus: Preliminary observations. Lupus 2004, 13, 590–593. [Google Scholar] [CrossRef]
- Kiss, E.; Soltesz, P.; Der, H.; Kocsis, Z.; Tarr, T.; Bhattoa, H.; Shoenfeld, Y.; Szegedi, G. Reduced flow-mediated vasodilation as a marker for cardiovascular complications in lupus patients. J. Autoimmun. 2006, 27, 211–217. [Google Scholar] [CrossRef]
- Ghosh, P.; Kumar, A.; Kumar, S.; Aggarwal, A.; Sinha, N.; Misra, R. Subclinical atherosclerosis and endothelial dysfunction in young South-Asian patients with systemic lupus erythematosus. Clin. Rheumatol. 2009, 28, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Xiang, W.; He, X. IFN-I Mediates Dysfunction of Endothelial Progenitor Cells in Atherosclerosis of Systemic Lupus Erythematosus. Front. Immunol. 2020, 11, 581385. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.H.; Chen, P.C.; Yang, Y.H.; Wang, L.C.; Lee, J.H.; Lin, Y.T.; Chiang, B.L. Statin reduces mortality and morbidity in systemic lupus erythematosus patients with hyperlipidemia: A nationwide population-based cohort study. Atherosclerosis 2015, 243, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.A.; Anghel, R.; Marcu, D.T.M.; Mitu, O.; Roca, M.; Mitu, F. Impact of Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors on Arterial Stiffness and Vascular Aging-What Do We Know So Far? (A Narrative Review). Life 2022, 12, 803. [Google Scholar] [CrossRef] [PubMed]
- Castejon, R.; Jimenez-Ortiz, C.; Valero-Gonzalez, S.; Rosado, S.; Mellor, S.; Yebra-Bango, M. Decreased circulating endothelial progenitor cells as an early risk factor of subclinical atherosclerosis in systemic lupus erythematosus. Rheumatology 2014, 53, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Westerweel, P.E.; Luijten, R.K.; Hoefer, I.E.; Koomans, H.A.; Derksen, R.H.; Verhaar, M.C. Haematopoietic and endothelial progenitor cells are deficient in quiescent systemic lupus erythematosus. Ann. Rheum. Dis. 2007, 66, 865–870. [Google Scholar] [CrossRef]
- Moonen, J.R.; de Leeuw, K.; van Seijen, X.J.; Kallenberg, C.G.; van Luyn, M.J.; Bijl, M.; Harmsen, M.C. Reduced number and impaired function of circulating progenitor cells in patients with systemic lupus erythematosus. Arthritis Res. Ther. 2007, 9, R84. [Google Scholar] [CrossRef]
- Denny, M.F.; Thacker, S.; Mehta, H.; Somers, E.C.; Dodick, T.; Barrat, F.J.; McCune, W.J.; Kaplan, M.J. Interferon-alpha promotes abnormal vasculogenesis in lupus: A potential pathway for premature atherosclerosis. Blood 2007, 110, 2907–2915. [Google Scholar] [CrossRef]
- Lee, P.Y.; Li, Y.; Richards, H.B.; Chan, F.S.; Zhuang, H.; Narain, S.; Butfiloski, E.J.; Sobel, E.S.; Reeves, W.H.; Segal, M.S. Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum. 2007, 56, 3759–3769. [Google Scholar] [CrossRef]
- Baker, J.F.; Zhang, L.; Imadojemu, S.; Sharpe, A.; Patil, S.; Moore, J.S.; Mohler, E.R., III; Von Feldt, J. Circulating endothelial progenitor cells are reduced in SLE in the absence of coronary artery calcification. Rheumatol. Int. 2012, 32, 997–1002. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, Y.J.; Kim, K.J.; Choi, J.J.; Kim, W.U.; Cho, C.S. Osteoprotegerin causes apoptosis of endothelial progenitor cells by induction of oxidative stress. Arthritis Rheum. 2013, 65, 2172–2182. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kow, N.Y.; Lee, H.Y.; Fairhurst, A.M.; Mak, A. CD34+CD133+CD309+ circulating angiogenic cell level is reduced but positively related to hydroxychloroquine use in SLE patients-a case-control study and meta-regression analysis. Rheumatology 2021, 60, 3936–3944. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Adam, E.; Lyon, M.; Depontieu, F.; Motte, V.; Landolfi, C.; Lortat-Jacob, H.; Bechard, D.; Lassalle, P.; Delehedde, M. Endocan or endothelial cell specific molecule-1 (ESM-1): A potential novel endothelial cell marker and a new target for cancer therapy. Biochim. Biophys. Acta 2006, 1765, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Choi, H.Y.; Bae, J.S. Endocan as a potential diagnostic or prognostic biomarker for chronic kidney disease. Kidney Int. 2014, 86, 1079–1081. [Google Scholar] [CrossRef]
- Icli, A.; Cure, E.; Cure, M.C.; Uslu, A.U.; Balta, S.; Mikhailidis, D.P.; Ozturk, C.; Arslan, S.; Sakız, D.; Sahin, M.; et al. Endocan Levels and Subclinical Atherosclerosis in Patients with Systemic Lupus Erythematosus. Angiology 2016, 67, 749–755. [Google Scholar] [CrossRef]
- Tokarska, K.; Bogaczewicz, J.; Robak, E.; Woźniacka, A. The role of endocan and selected pro-inflammatory cytokines in systemic lupus erythematosus. Postepy Dermatol. Alergol. 2020, 37, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Tselios, K.; Sheane, B.J.; Gladman, D.D.; Urowitz, M.B. Optimal Monitoring for Coronary Heart Disease Risk in Patients with Systemic Lupus Erythematosus: A Systematic Review. J. Rheumatol. 2016, 43, 54–65. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, G.G. Association between circulating leptin levels and systemic lupus erythematosus: An updated meta-analysis. Lupus 2018, 27, 428–435. [Google Scholar] [CrossRef]
- Yuan, Q.; Chen, H.; Li, X.; Wei, J. Leptin: An unappreciated key player in SLE. Clin. Rheumatol. 2020, 39, 305–317. [Google Scholar] [CrossRef]
- Amarilyo, G.; Iikuni, N.; Liu, A.; Matarese, G.; La Cava, A. Leptin enhances availability of apoptotic cell-derived self-antigen in systemic lupus erythematosus. PLoS ONE 2014, 9, e112826. [Google Scholar] [CrossRef]
- Bouloumie, A.; Marumo, T.; Lafontan, M.; Busse, R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999, 13, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhang, X.; Ren, J. Leptin regulates cardiomyocyte contractile function through endothelin-1 receptor-NADPH oxidase pathway. Hypertension 2006, 47, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Chougule, D.; Nadkar, M.; Venkataraman, K.; Rajadhyaksha, A.; Hase, N.; Jamale, T.; Kini, S.; Khadilkar, P.; Anand, V.; Madkaikar, M.; et al. Adipokine interactions promote the pathogenesis of systemic lupus erythematosus. Cytokine 2018, 111, 20–27. [Google Scholar] [CrossRef]
- De Sanctis, J.B.; Zabaleta, M.; Bianco, N.E.; Garmendia, J.V.; Rivas, L. Serum adipokine levels in patients with systemic lupus erythematosus. Autoimmunity 2009, 42, 272–274. [Google Scholar] [CrossRef]
- Toussirot, E.; Gaugler, B.; Bouhaddi, M.; Nguyen, N.U.; Saas, P.; Dumoulin, G. Elevated adiponectin serum levels in women with systemic autoimmune diseases. Mediat. Inflamm. 2010, 2010, 938408. [Google Scholar] [CrossRef]
- Wisłowska, M.; Rok, M.; Stepień, K.; Kuklo-Kowalska, A. Serum leptin in systemic lupus erythematosus. Rheumatol. Int. 2008, 28, 467–473. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, A.; Gonzalez-Lopez, L.; Valera-Gonzalez, I.C.; Cardona-Muñoz, E.G.; Salazar-Paramo, M.; González-Ortiz, M.; Martínez-Abundis, E.; Gamez-Nava, J.I. Serum leptin levels in women with systemic lupus erythematosus. Rheumatol. Int. 2002, 22, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Sada, K.E.; Yamasaki, Y.; Maruyama, M.; Sugiyama, H.; Yamamura, M.; Maeshima, Y.; Makino, H. Altered levels of adipocytokines in association with insulin resistance in patients with systemic lupus erythematosus. J. Rheumatol. 2006, 33, 1545–1552. [Google Scholar]
- Chung, C.P.; Long, A.G.; Solus, J.F.; Rho, Y.H.; Oeser, A.; Raggi, P.; Stein, C.M. Adipocytokines in systemic lupus erythematosus: Relationship to inflammation, insulin resistance and coronary atherosclerosis. Lupus 2009, 18, 799–806. [Google Scholar] [CrossRef]
- Al, M.; Ng, L.; Tyrrell, P.; Bargman, J.; Bradley, T.; Silverman, E. Adipokines as novel biomarkers in paediatric systemic lupus erythematosus. Rheumatology 2009, 48, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Choi, G.S.; Jeon, J.Y.; Yoon, J.M.; Sung, J.M.; Suh, C.H. Leptin and ghrelin in Korean systemic lupus erythematosus. Lupus 2010, 19, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Vadacca, M.; Zardi, E.M.; Margiotta, D.; Rigon, A.; Cacciapaglia, F.; Arcarese, L.; Buzzulini, F.; Amoroso, A.; Afeltra, A. Leptin, adiponectin and vascular stiffness parameters in women with systemic lupus erythematosus. Intern. Emerg. Med. 2013, 8, 705–712. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, Y.; Yang, L.; Song, S.; Han, Y.; Tian, Y.; Ding, M.; Jin, H.; Shao, F.; Liu, A. Leptin levels in patients with systemic lupus erythematosus inversely correlate with regulatory T cell frequency. Lupus 2017, 26, 1401–1406. [Google Scholar] [CrossRef]
- Diaz-Rizo, V.; Bonilla-Lara, D.; Gonzalez-Lopez, L.; Sanchez-Mosco, D.; Fajardo-Robledo, N.S.; Perez-Guerrero, E.E.; Rodriguez-Jimenez, N.A.; Saldaña-Cruz, A.M.; Vazquez-Villegas, M.L.; Gomez-Bañuelos, E.; et al. Serum levels of adiponectin and leptin as biomarkers of proteinuria in lupus nephritis. PLoS ONE 2017, 12, e0184056. [Google Scholar] [CrossRef]
- Demir, S.; Erten, G.; Artım-Esen, B.; Şahinkaya, Y.; Pehlivan, Ö.; Alpay-Kanıtez, N.; Deniz, G.; Inanç, M. Increased serum leptin levels are associated with metabolic syndrome and carotid intima media thickness in premenopausal systemic lupus erythematosus patients without clinical atherosclerotic vascular events. Lupus 2018, 27, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.F.; Morales, M.; Qatanani, M.; Cucchiara, A.; Nackos, E.; Lazar, M.A.; Teff, K.; von Feldt, J.M. Resistin levels in lupus and associations with disease-specific measures, insulin resistance, and coronary calcification. J. Rheumatol. 2011, 38, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.; Ye, Y.; Han, J.; Arriens, C.; Saxena, R.; Li, Q.Z.; Mohan, C.; Wu, T. Resistin as a potential marker of renal disease in lupus nephritis. Clin. Exp. Immunol. 2015, 179, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Gamez-Nava, J.I.; Diaz-Rizo, V.; Perez-Guerrero, E.E.; Muñoz-Valle, J.F.; Saldaña-Cruz, A.M.; Fajardo-Robledo, N.S.; Jacobo-Cuevas, H.; Nava-Valdivia, C.A.; Alcaraz-Lopez, M.F.; Trujillo, X.; et al. Assessment of serum macrophage migration inhibitory factor (MIF), adiponectin, and other adipokines as potential markers of proteinuria and renal dysfunction in lupus nephritis: A cross-sectional study. Biomark. Res. 2020, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A.; Helmy, M.; Barakat, M.; Elneily, D.; Ahmed, O. Serum resistin, insulin resistance and carotid intima-media thickness as an indication of subclinical atherosclerosis in systemic lupus erythematosus patients. Egypt. Rheumatol. 2021, 43, 319–323. [Google Scholar] [CrossRef]
- Tydén, H.; Lood, C.; Gullstrand, B.; Jönsen, A.; Nived, O.; Sturfelt, G.; Truedsson, L.; Ivars, F.; Leanderson, T.; Bengtsson, A.A. Increased serum levels of S100A8/A9 and S100A12 are associated with cardiovascular disease in patients with inactive systemic lupus erythematosus. Rheumatology 2013, 52, 2048–2055. [Google Scholar] [CrossRef]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Lood, C.; Tydén, H.; Gullstrand, B.; Jönsen, A.; Källberg, E.; Mörgelin, M.; Kahn, R.; Gunnarsson, I.; Leanderson, T.; Ivars, F.; et al. Platelet-Derived S100A8/A9 and Cardiovascular Disease in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2016, 68, 1970–1980. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, C.; Gao, H.; Bilodeau, M.L.; Zhang, Z.; Croce, K.; Liu, S.; Morooka, T.; Sakuma, M.; Nakajima, K.; et al. Platelet-derived S100 family member myeloid-related protein-14 regulates thrombosis. J. Clin. Investig. 2014, 124, 2160–2171. [Google Scholar] [CrossRef] [PubMed]
- Tydén, H.; Lood, C.; Gullstrand, B.; Jönsen, A.; Ivars, F.; Leanderson, T.; Bengtsson, A.A. Pro-inflammatory S100 proteins are associated with glomerulonephritis and anti-dsDNA antibodies in systemic lupus erythematosus. Lupus 2017, 26, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Zervides, K.A.; Jern, A.; Nystedt, J.; Gullstrand, B.; Nilsson, P.C.; Sundgren, P.C.; Bengtsson, A.A.; Jönsen, A. Serum S100A8/A9 concentrations are associated with neuropsychiatric involvement in systemic lupus erythematosus: A cross-sectional study. BMC Rheumatol. 2022, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Schiopu, A.; Cotoi, O.S. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediat. Inflamm. 2013, 2013, 828354. [Google Scholar] [CrossRef]
- Soyfoo, M.S.; Roth, J.; Vogl, T.; Pochet, R.; Decaux, G. Phagocyte-specific S100A8/A9 protein levels during disease exacerbations and infections in systemic lupus erythematosus. J. Rheumatol. 2009, 36, 2190–2194. [Google Scholar] [CrossRef]
- Ometto, F.; Friso, L.; Astorri, D.; Botsios, C.; Raffeiner, B.; Punzi, L.; Doria, A. Calprotectin in rheumatic diseases. Exp. Biol. Med. 2017, 242, 859–873. [Google Scholar] [CrossRef]
- Salemme, R.; Peralta, L.N.; Meka, S.H.; Pushpanathan, N.; Alexander, J.J. The Role of NETosis in Systemic Lupus Erythematosus. J. Cell. Immunol. 2019, 1, 33–42. [Google Scholar]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Hakkim, A.; Fürnrohr, B.G.; Amann, K.; Laube, B.; Abed, U.A.; Brinkmann, V.; Herrmann, M.; Voll, R.E.; Zychlinsky, A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. USA 2010, 107, 9813–9818. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Romo, G.S.; Caielli, S.; Vega, B.; Connolly, J.; Allantaz, F.; Xu, Z.; Punaro, M.; Baisch, J.; Guiducci, C.; Coffman, R.L.; et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra20. [Google Scholar] [CrossRef] [PubMed]
- Mozzini, C.; Garbin, U.; Fratta Pasini, A.M.; Cominacini, L. An exploratory look at NETosis in atherosclerosis. Intern. Emerg. Med. 2017, 12, 13–22. [Google Scholar] [CrossRef]
- Pieterse, E.; Rother, N.; Garsen, M.; Hofstra, J.M.; Satchell, S.C.; Hoffmann, M.; Loeven, M.A.; Knaapen, H.K.; van der Heijden, O.W.H.; Berden, J.H.M.; et al. Neutrophil Extracellular Traps Drive Endothelial-to-Mesenchymal Transition. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1371–1379. [Google Scholar] [CrossRef]
- von Brühl, M.L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- Lande, R.; Ganguly, D.; Facchinetti, V.; Frasca, L.; Conrad, C.; Gregorio, J.; Meller, S.; Chamilos, G.; Sebasigari, R.; Riccieri, V.; et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra19. [Google Scholar] [CrossRef]
- Döring, Y.; Manthey, H.D.; Drechsler, M.; Lievens, D.; Megens, R.T.; Soehnlein, O.; Busch, M.; Manca, M.; Koenen, R.R.; Pelisek, J.; et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 2012, 125, 1673–1683. [Google Scholar] [CrossRef]
- Knight, J.S.; Subramanian, V.; O’Dell, A.A.; Yalavarthi, S.; Zhao, W.; Smith, C.K.; Hodgin, J.B.; Thompson, P.R.; Kaplan, M.J. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann. Rheum. Dis. 2015, 74, 2199–2206. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Carmona-Rivera, C.; Smith, C.K.; Kaplan, M.J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 2013, 190, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Mobarrez, F.; Vikerfors, A.; Gustafsson, J.T.; Gunnarsson, I.; Zickert, A.; Larsson, A.; Pisetsky, D.S.; Wallén, H.; Svenungsson, E. Microparticles in the blood of patients with systemic lupus erythematosus (SLE): Phenotypic characterization and clinical associations. Sci. Rep. 2016, 6, 36025. [Google Scholar] [CrossRef] [PubMed]
- Rother, N.; Yanginlar, C.; Pieterse, E.; Hilbrands, L.; van der Vlag, J. Microparticles in Autoimmunity: Cause or Consequence of Disease? Front. Immunol. 2022, 13, 822995. [Google Scholar] [CrossRef]
- Atehortúa, L.; Rojas, M.; Vásquez, G.; Muñoz-Vahos, C.H.; Vanegas-García, A.; Posada-Duque, R.A.; Castaño, D. Endothelial activation and injury by microparticles in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 34. [Google Scholar] [CrossRef]
- López, P.; Rodríguez-Carrio, J.; Martínez-Zapico, A.; Caminal-Montero, L.; Suárez, A. Circulating microparticle subpopulations in systemic lupus erythematosus are affected by disease activity. Int. J. Cardiol. 2017, 236, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Dieker, J.; Tel, J.; Pieterse, E.; Thielen, A.; Rother, N.; Bakker, M.; Fransen, J.; Dijkman, H.B.; Berden, J.H.; de Vries, J.M.; et al. Circulating Apoptotic Microparticles in Systemic Lupus Erythematosus Patients Drive the Activation of Dendritic Cell Subsets and Prime Neutrophils for NETosis. Arthritis Rheumatol. 2016, 68, 462–472. [Google Scholar] [CrossRef]
- Carmona-Pérez, L.; Rojas, M.; Muñoz-Vahos, C.; Vanegas-García, A.; Vásquez, G. Plasma microparticles from patients with systemic lupus erythematosus modulate the content of miRNAs in U937 cells. Immunology 2021, 164, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Austin, R.C. Contributions of hyperhomocysteinemia to atherosclerosis: Causal relationship and potential mechanisms. Biofactors 2009, 35, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Davì, G.; Falco, A. Oxidant stress, inflammation and atherogenesis. Lupus 2005, 14, 760–764. [Google Scholar] [CrossRef]
- Lertratanakul, A.; Wu, P.; Dyer, A.R.; Kondos, G.; Edmundowicz, D.; Carr, J.; Ramsey-Goldman, R. Risk factors in the progression of subclinical atherosclerosis in women with systemic lupus erythematosus. Arthritis Care Res. 2014, 66, 1177–1185. [Google Scholar] [CrossRef]
- Suttichet, T.B.; Kittanamongkolchai, W.; Phromjeen, C.; Anutrakulchai, S.; Panaput, T.; Ingsathit, A.; Kamanamool, N.; Ophascharoensuk, V.; Sumethakul, V.; Avihingsanon, Y. Urine TWEAK level as a biomarker for early response to treatment in active lupus nephritis: A prospective multicentre study. Lupus Sci. Med. 2019, 6, e000298. [Google Scholar] [CrossRef]
- Xue, L.; Liu, L.; Huang, J.; Wen, J.; Yang, R.; Bo, L.; Tang, M.; Zhang, Y.; Liu, Z. Tumor necrosis factor-like weak inducer of apoptosis activates type I interferon signals in lupus nephritis. BioMed Res. Int. 2017, 2017, 4927376. [Google Scholar] [CrossRef]
- Mantovani, A.; Garlanda, C.; Doni, A.; Bottazzi, B. Pentraxins in innate immunity: From C-reactive protein to the long pentraxin PTX3. J. Clin. Immunol. 2008, 28, 1–13. [Google Scholar] [CrossRef]
- Pang, Y.; Tan, Y.; Li, Y.; Zhang, J.; Guo, Y.; Guo, Z.; Zhang, C.; Yu, F.; Zhao, M.H. Pentraxin 3 is closely associated with tubulointerstitial injury in lupus nephritis: A large multicenter cross-sectional study. Medicine 2016, 95, e2520. [Google Scholar] [CrossRef]
- Cieślik, P.; Hrycek, A. Pentraxin 3 as a biomarker of local inflammatory response to vascular injury in systemic lupus erythematosus. Autoimmunity 2015, 48, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Peilot, H.; Rosengren, B.; Bondjers, G.; Hurt-Camejo, E. Interferon-gamma induces secretory group IIA phospholipase A2 in human arterial smooth muscle cells. Involvement of cell differentiation, STAT-3 activation, and modulation by other cytokines. J. Biol. Chem. 2000, 275, 22895–22904. [Google Scholar] [CrossRef]
- Kuriyama, Y.; Shimizu, A.; Kanai, S.; Oikawa, D.; Motegi, S.I.; Tokunaga, F.; Ishikawa, O. Coordination of retrotransposons and type I interferon with distinct interferon pathways in dermatomyositis, systemic lupus erythematosus and autoimmune blistering disease. Sci. Rep. 2021, 11, 23146. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.; Kow, N.Y. Imbalance between endothelial damage and repair: A gateway to cardiovascular disease in systemic lupus erythematosus. BioMed Res. Int. 2014, 2014, 178721. [Google Scholar] [CrossRef] [PubMed]
- Avalos, A.M.; Busconi, L.; Marshak-Rothstein, A. Regulation of autoreactive B cell responses to endogenous TLR ligands. Autoimmunity 2010, 43, 76–83. [Google Scholar] [CrossRef]
- Kirou, K.A.C.P.; Salmon, J.E.; Roman, M.J.; Crow, M.K. Identification of molecular pathways associated with progression of carotid atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2006, 54, S807. [Google Scholar]
- Agarwal, S.; Elliott, J.R.; Manzi, S. Atherosclerosis risk factors in systemic lupus erythematosus. Curr. Rheumatol. Rep. 2009, 11, 241–247. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Kaplan, M.J. The interplay of inflammation and cardiovascular disease in systemic lupus erythematosus. Arthritis Res. Ther. 2011, 13, 203. [Google Scholar] [CrossRef]
- Arida, A.; Protogerou, A.D.; Kitas, G.D.; Sfikakis, P.P. Systemic Inflammatory Response and Atherosclerosis: The Paradigm of Chronic Inflammatory Rheumatic Diseases. Int. J. Mol. Sci. 2018, 19, 1890. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Thacker, S.G.; Berthier, C.C.; Cohen, C.D.; Kretzler, M.; Kaplan, M.J. Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. J. Immunol. 2011, 187, 6143–6156. [Google Scholar] [CrossRef]
- Rezaieyazdi, Z.; AkbariRad, M.; Saadati, N.; Salari, M.; Orang, R.; Sedighi, S.; Esmaily, H.; Azarpazhooh, M.R.; Firoozi, A.; Akbarpour, E. Serum interleukin-18 and its relationship with subclinical atherosclerosis in systemic lupus erythematosus. ARYA Atheroscler. 2021, 17, 1. [Google Scholar]
- Kiani, A.N.; Aukrust, P.; Ueland, T.; Hollan, I.; Barr, E.; Magder, L.S.; Petri, M. Serum osteoprotegrin (OPG) in subclinical atherosclerosis in systemic lupus erythematosus. Lupus 2017, 26, 865–870. [Google Scholar] [CrossRef]
- Harris, E.N.; Pierangeli, S.S. Primary, secondary, and catastrophic antiphospholipid syndrome: What’s in a name? Semin. Thromb. Hemost. 2008, 34, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Meroni, P.L.; Raschi, E.; Testoni, C.; Borghi, M.O. Endothelial cell activation by antiphospholipid antibodies. Clin. Immunol. 2004, 112, 169–174. [Google Scholar] [CrossRef]
- Ritis, K.; Doumas, M.; Mastellos, D.; Micheli, A.; Giaglis, S.; Magotti, P.; Rafail, S.; Kartalis, G.; Sideras, P.; Lambris, J.D. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J. Immunol. 2006, 177, 4794–4802. [Google Scholar] [CrossRef] [PubMed]
- Doria, A.; Shoenfeld, Y.; Wu, R.; Gambari, P.F.; Puato, M.; Ghirardello, A.; Gilburd, B.; Corbanese, S.; Patnaik, M.; Zampieri, S.; et al. Risk factors for subclinical atherosclerosis in a prospective cohort of patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2003, 62, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Svenungsson, E.; Jensen-Urstad, K.; Heimbürger, M.; Silveira, A.; Hamsten, A.; de Faire, U.; Witztum, J.L.; Frostegård, J. Risk factors for cardiovascular disease in systemic lupus erythematosus. Circulation 2001, 104, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Wirestam, L.; Saleh, M.; Svensson, C.; Compagno, M.; Zachrisson, H.; Wetterö, J.; Sjöwall, C. Plasma osteopontin versus intima media thickness of the common carotid arteries in well-characterised patients with systemic lupus erythematosus. Lupus 2021, 30, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Wirestam, L.; Enocsson, H.; Skogh, T.; Padyukov, L.; Jönsen, A.; Urowitz, M.B.; Gladman, D.D.; Romero-Diaz, J.; Bae, S.C.; Fortin, P.R.; et al. Osteopontin and Disease Activity in Patients with Recent-onset Systemic Lupus Erythematosus: Results from the SLICC Inception Cohort. J. Rheumatol. 2019, 46, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, M.; Chiocchetti, A.; Cena, T.; Musetti, C.; Monti, S.; Clemente, N.; Dianzani, U.; Magnani, C.; Stratta, P. Osteopontin circulating levels correlate with renal involvement in systemic lupus erythematosus and are lower in ACE inhibitor-treated patients. Clin. Rheumatol. 2014, 33, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Wirestam, L.; Frodlund, M.; Enocsson, H.; Skogh, T.; Wetterö, J.; Sjöwall, C. Osteopontin is associated with disease severity and antiphospholipid syndrome in well characterised Swedish cases of SLE. Lupus Sci. Med. 2017, 4, e000225. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Dallegri, F.; Montecucco, F.; Poggi, A.; Nobili, F.M.; Cacciapaglia, F.; Afeltra, A.; Moccetti, T.; Colombo, B.M. Serum osteopontin negatively impacts on intima-media thickness in patients with systemic lupus erythematosus. Eur. J. Clin. Investig. 2019, 49, e13089. [Google Scholar] [CrossRef] [PubMed]
- Mancardi, D.; Arrigo, E.; Cozzi, M.; Cecchi, I.; Radin, M.; Fenoglio, R.; Roccatello, D.; Sciascia, S. Endothelial dysfunction and cardiovascular risk in lupus nephritis: New roles for old players? Eur. J. Clin. Investig. 2021, 51, e13441. [Google Scholar] [CrossRef]
- Valer, P.; Paul, B.; Eugenia, B.; Camelia, B. Annexin A5 as independent predictive biomarker for subclinical atherosclerosis and endothelial dysfunction in systemic lupus erythematosus patients. Clin. Lab. 2013, 59, 359–367. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).