In Silico and In Vitro Studies of Benzothiazole-Isothioureas Derivatives as a Multitarget Compound for Alzheimer’s Disease
Abstract
1. Introduction
2. Results
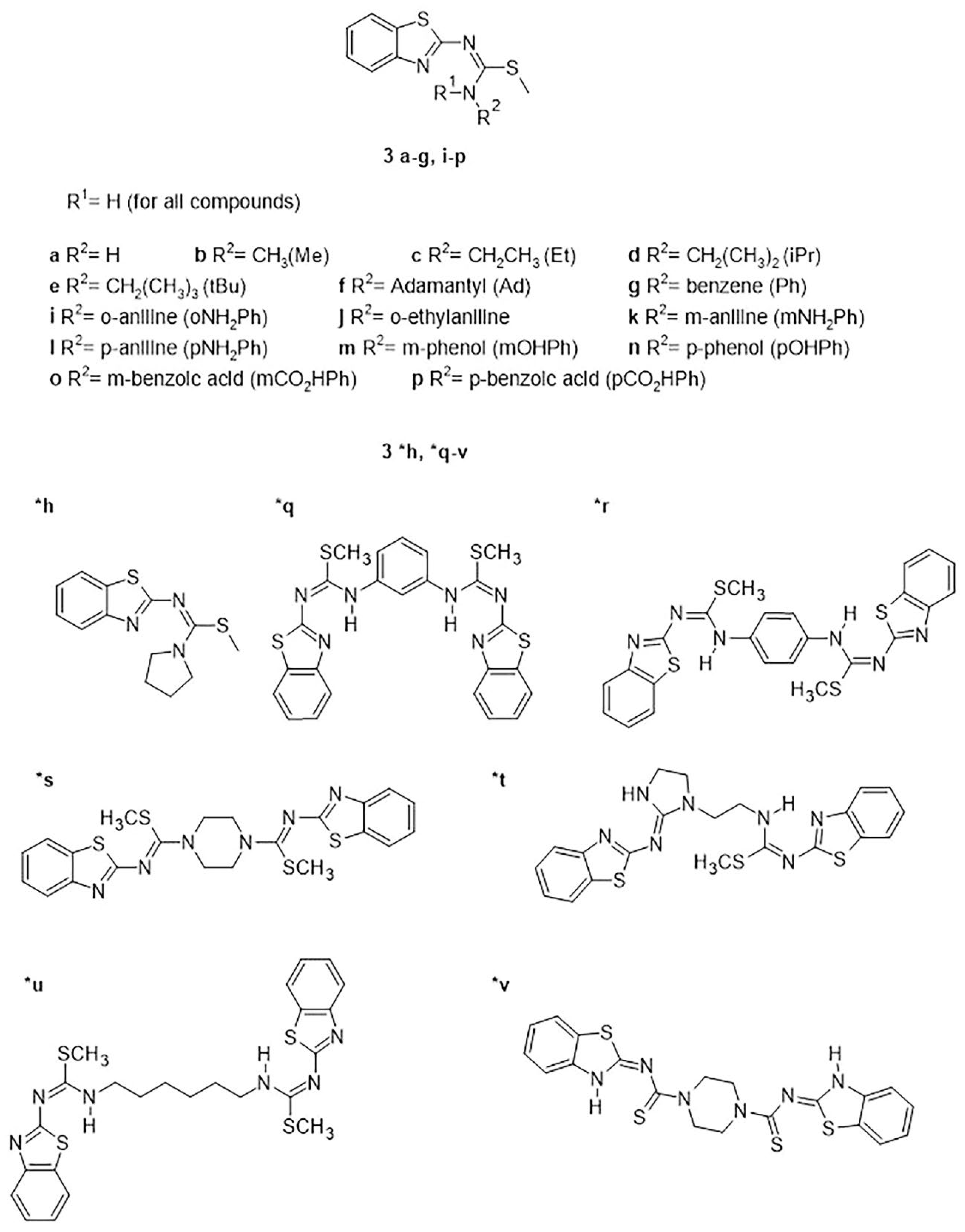
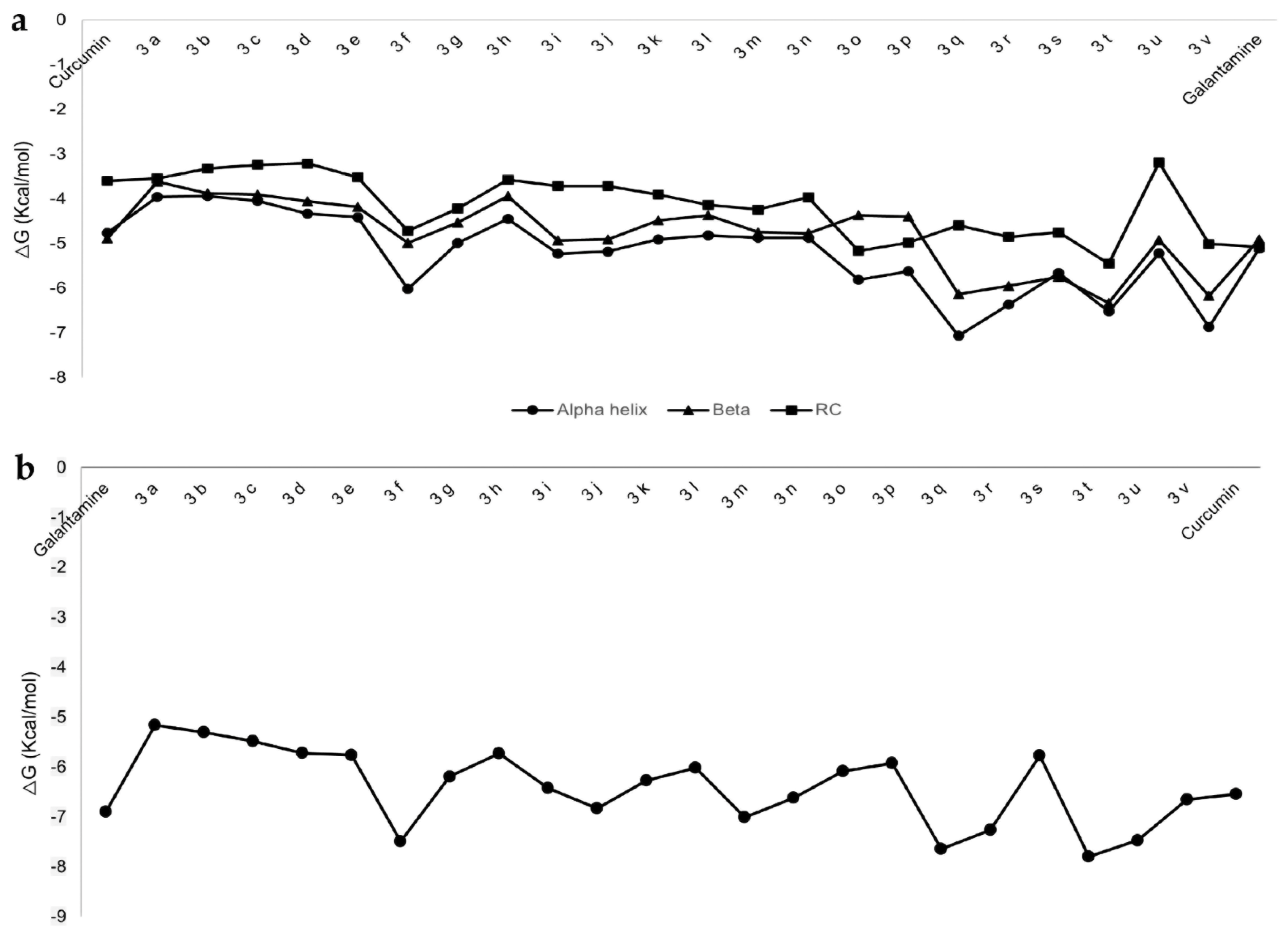
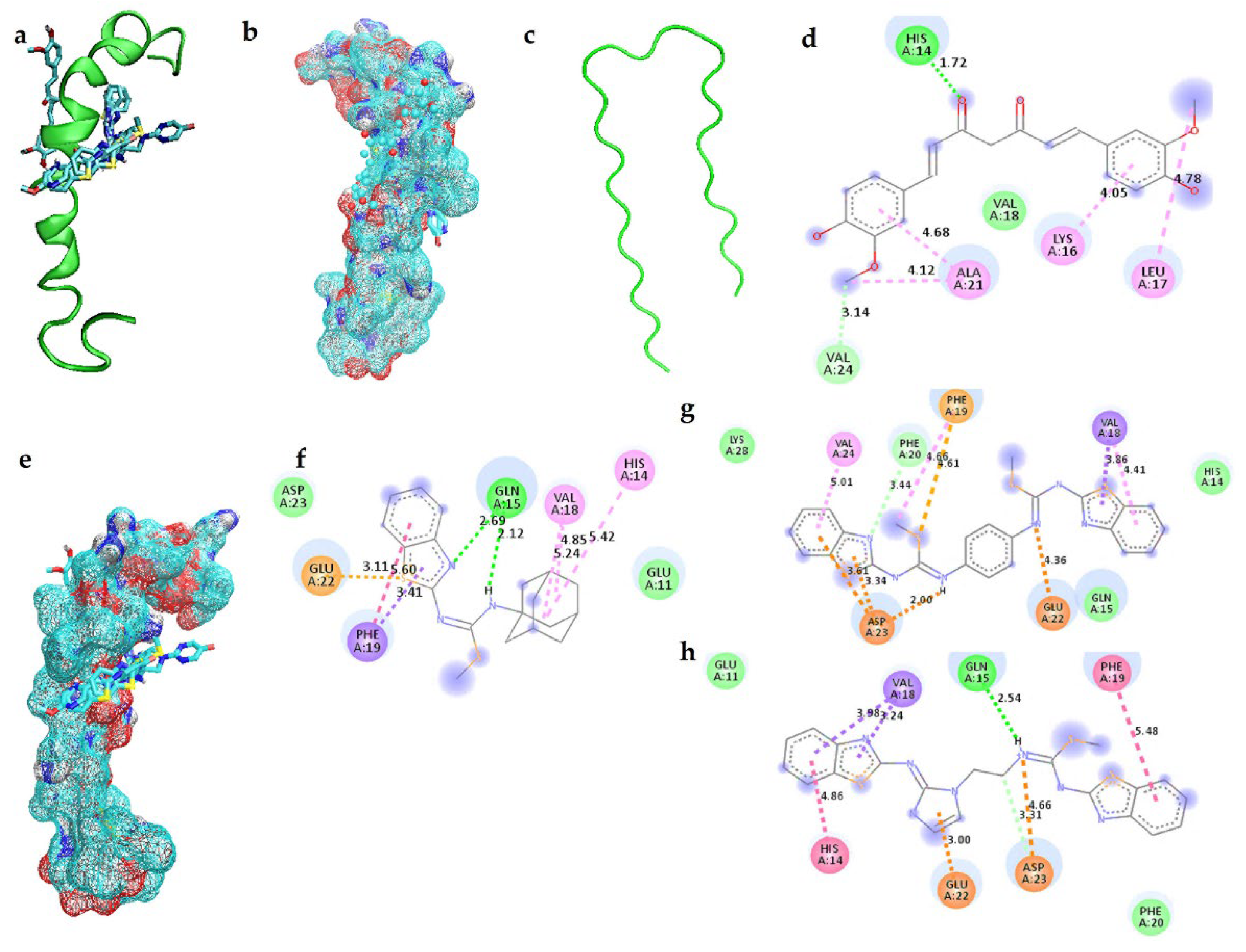
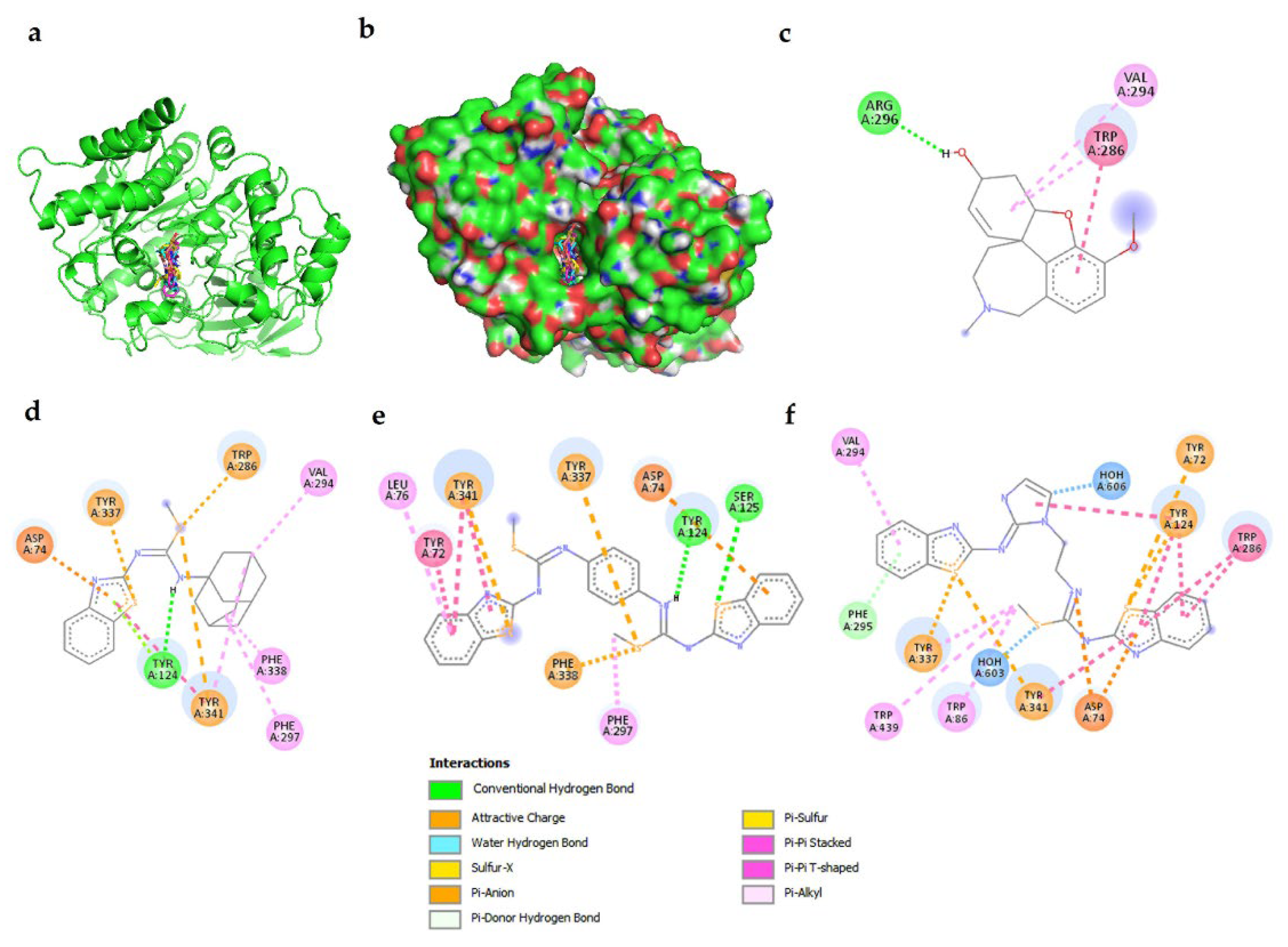
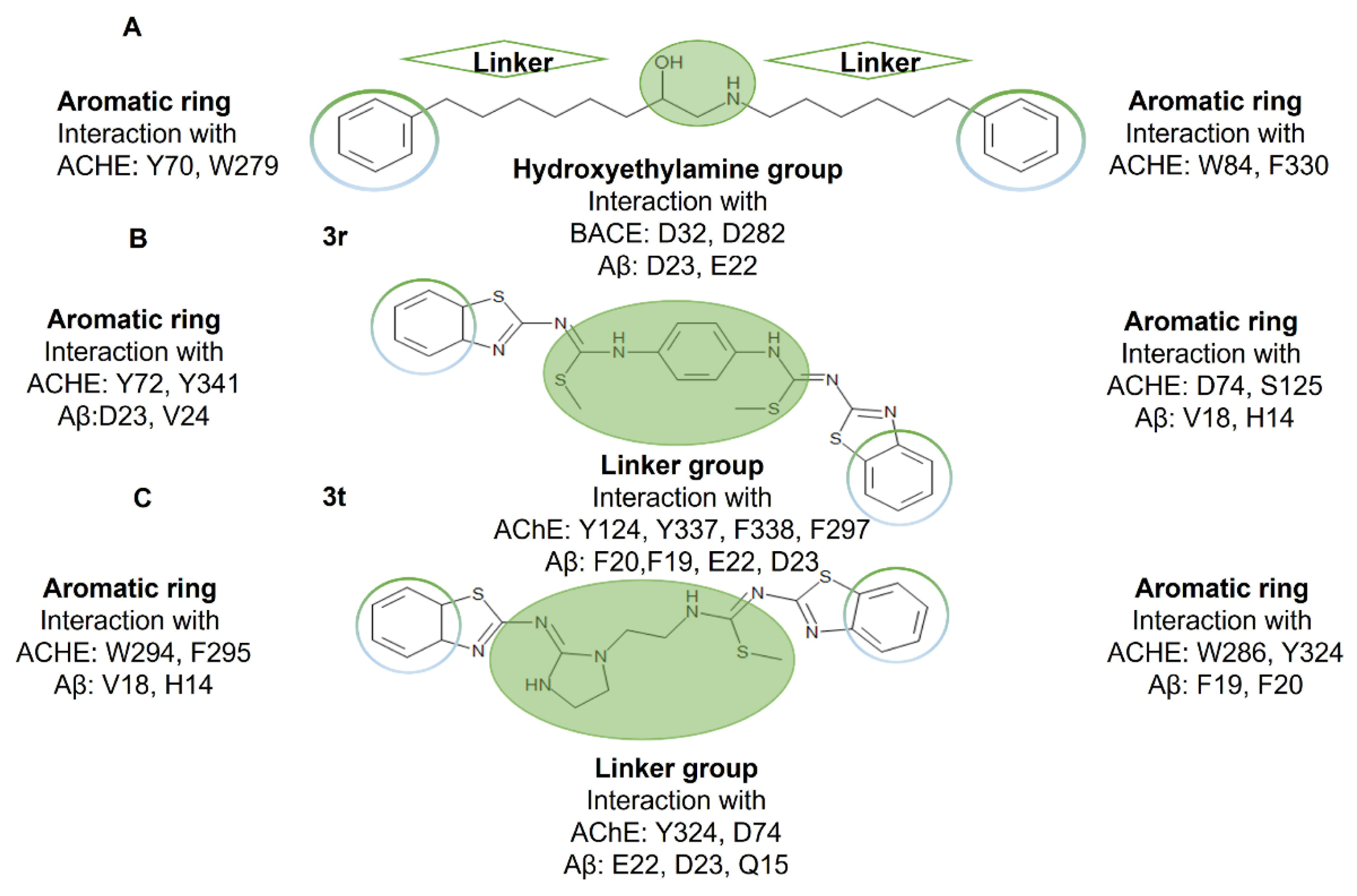
2.1. Interactions of Benzothiazole-Isothiourea Derivatives with AChE and Aβ1-42 by Docking Studies
2.2. ADME, Toxicological and BBB Permeability Prediction
2.3. Activity Assay of 3f, 3r and 3t on AChE
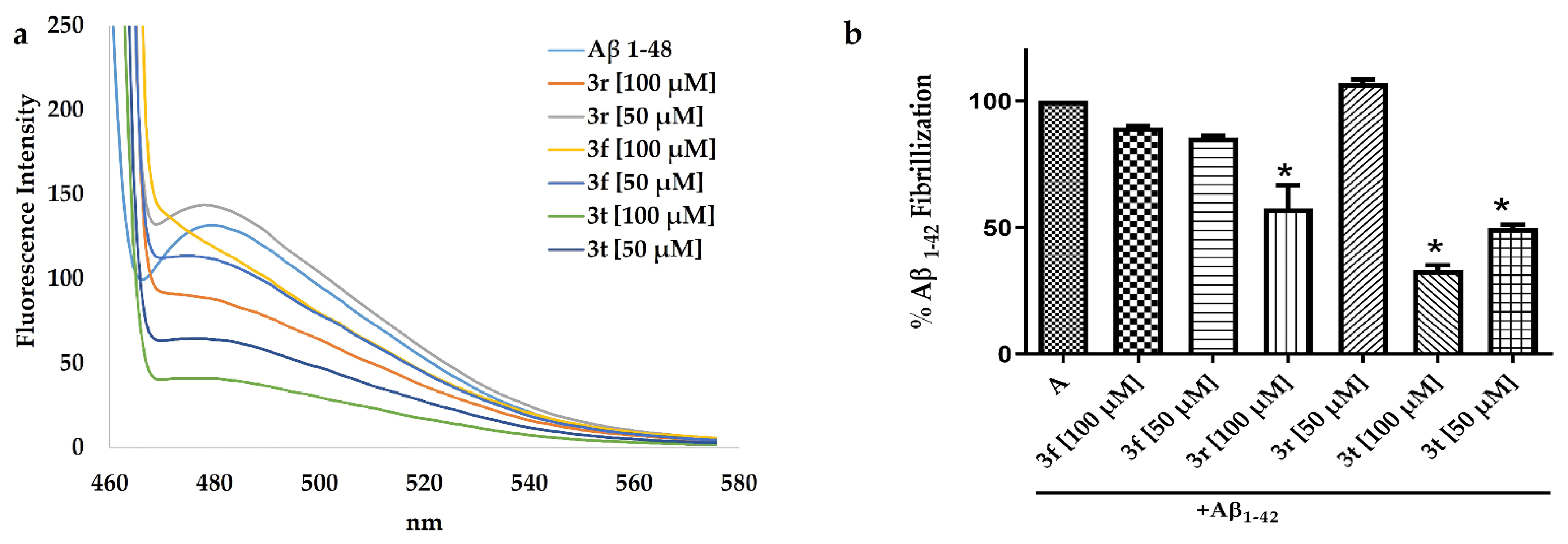
2.4. ThT Assay to Evaluated Aβ1-42 Aggregation with 3t, 3f and 3r Compounds
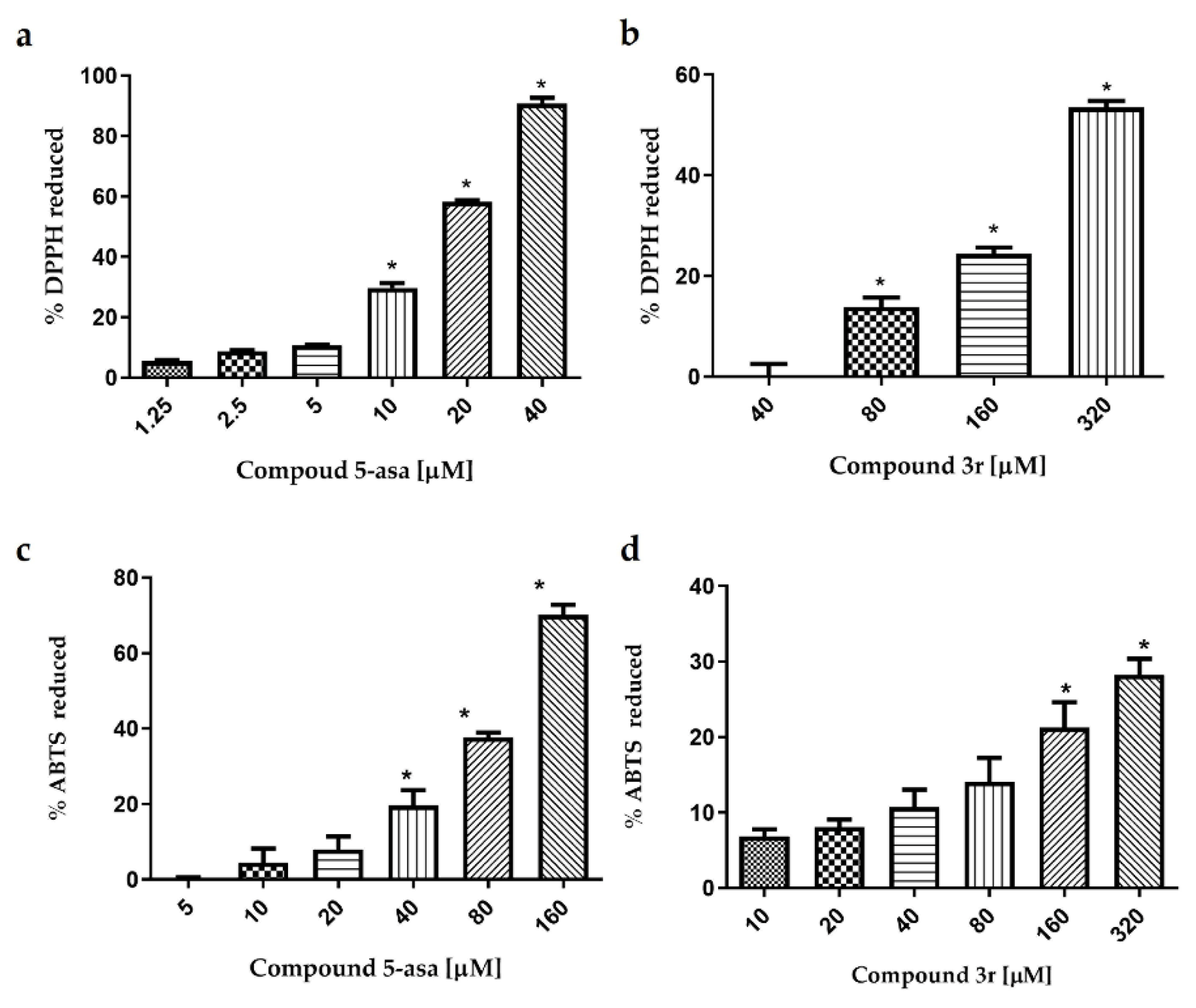
2.5. Antioxidant Activity of 3f, 3r and 3t by DPPH and ABTS
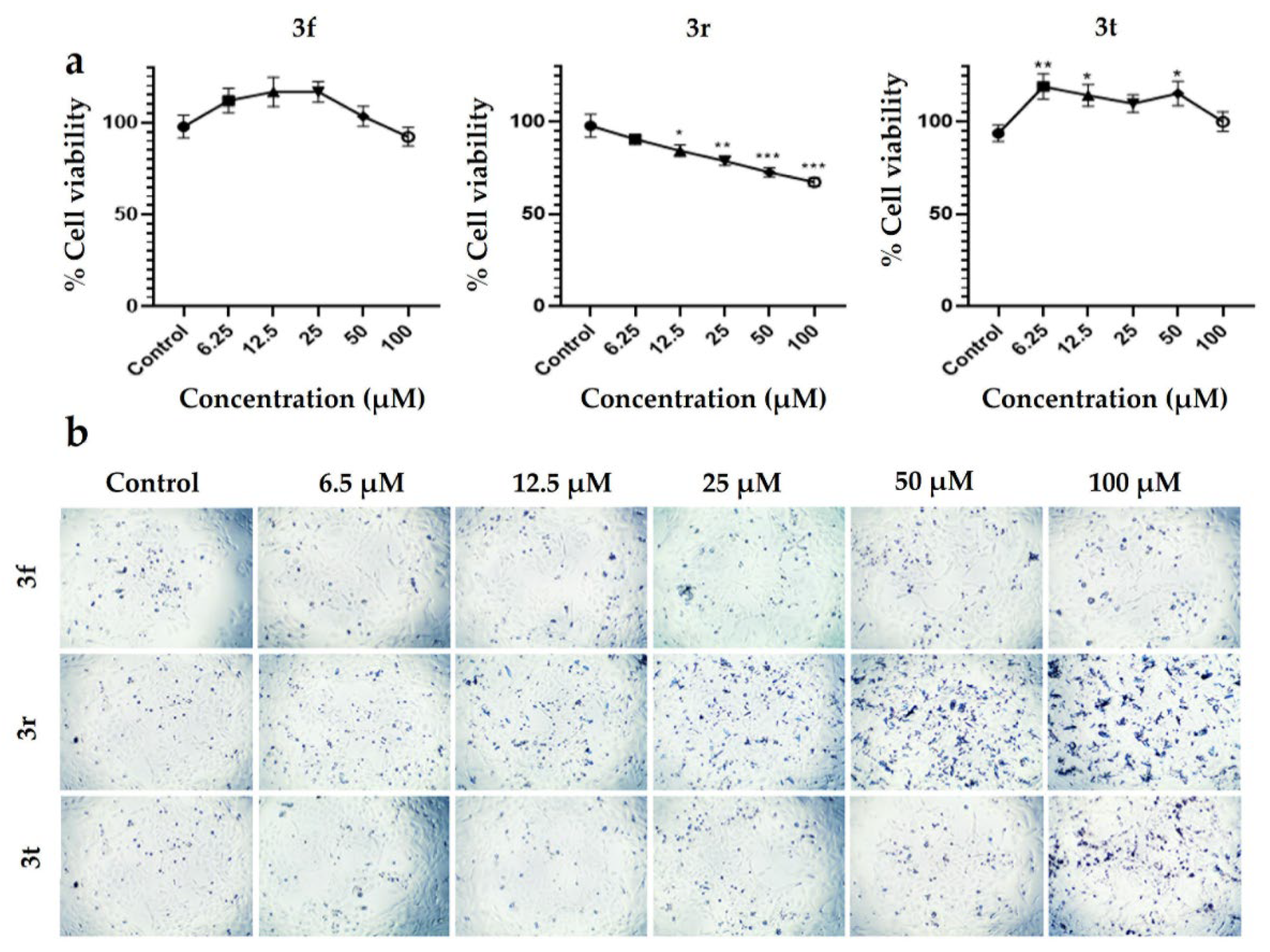
2.6. Cytotoxic Activity of 3f, 3r and 3t Compounds on PC12 Cell Line by MTT Assay
3. Discussion
4. Materials and Methods
4.1. In silico Evaluation
4.1.1. Preparations and Optimization Ligand for Docking Studies
4.1.2. Protein Pre-Optimization for Docking Studies
4.1.3. Docking Studies
4.1.4. Visualization of Protein-Ligand Interactions
4.1.5. ADME, Toxicological and BBB Permeability Prediction
4.2. Reagents for Synthesis and In Vitro Evaluations
4.2.1. Synthesis of Benzothiazolilisothioureas Derivatives
1-Adamantan-1-yl-3-benzothiazol-2-yl-2-methyl-isothiourea (3f)
1-Benzothiazol-2-yl-3-[4-(3-benzothiazol-2-yl-2-methyl-isothioureido)-phenyl]-2-methyl-isothiourea (3r)
1-Benzothiazol-2-yl-3-{2-[2-(benzothiazol-2-ylimino)-imidazolidin-1-yl]-ethyl}-2-methyl-isothiourea (3t)
4.3. In Vitro Assays
4.3.1. AChE Activity In Vitro Evaluation
4.3.2. Evaluation of Aβ1-42 Aggregation In Vitro by Thioflavin T (ThT) Assay
4.3.3. Antioxidant Evaluation
2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2,2-Azino-bis(3-ethylbenzothiazolin)-6-sulfonic Acid (ABTS) Assay
4.4. Cytotoxic Evaluation of Compounds on PC12 Cells
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sultana, R.; Butterfield, D.A. Role of Oxidative Stress in the Progression of Alzheimer’s Disease. J. Alzheimers Dis. 2010, 19, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Disease Facts and Figures. Alzheimers Dement. J. Alzheimers Assoc. 2021, 17, 327–406. [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. Risk Factors for Alzheimer’s Disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Wu, Y.-J.; Tee, B.L.; Lo, R.Y. Medical Comorbidity in Alzheimer’s Disease: A Nested Case-Control Study. J. Alzheimers Dis. 2018, 63, 773–781. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s Disease: Pathogenesis, Diagnostics, and Therapeutics. Int. J. Nanomedicine 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- Uddin, M.S.; Al Mamun, A.; Kabir, M.T.; Ashraf, G.M.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Multi-Target Drug Candidates for Multifactorial Alzheimer’s Disease: AChE and NMDAR as Molecular Targets. Mol. Neurobiol. 2021, 58, 281–303. [Google Scholar] [CrossRef]
- Gong, C.-X.; Dai, C.-L.; Liu, F.; Iqbal, K. Multi-Targets: An Unconventional Drug Development Strategy for Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 837649. [Google Scholar] [CrossRef]
- Sampietro, A.; Pérez-Areales, F.J.; Martínez, P.; Arce, E.M.; Galdeano, C.; Muñoz-Torrero, D. Unveiling the Multitarget Anti-Alzheimer Drug Discovery Landscape: A Bibliometric Analysis. Pharmaceuticals 2022, 15, 545. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, S.; Zhu, Z.; Xu, J. Multi-Target Design Strategies for the Improved Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2019, 176, 228–247. [Google Scholar] [CrossRef]
- Loy, C.; Schneider, L. Galantamine for Alzheimer’s Disease and Mild Cognitive Impairment. Cochrane Database Syst. Rev. 2006, CD001747. [Google Scholar] [CrossRef]
- Hampel, H.; Vassar, R.; De Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Zhou, J.; Yan, R.; Vanmechelen, E.; De Vos, A.; et al. The β-Secretase BACE1 in Alzheimer’s Disease. Biol. Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef]
- Menting, K.W.; Claassen, J.A.H.R. β-Secretase Inhibitor; a Promising Novel Therapeutic Drug in Alzheimer’s Disease. Front. Aging Neurosci. 2014, 6, 165. [Google Scholar] [CrossRef]
- Nirmalraj, P.N.; List, J.; Battacharya, S.; Howe, G.; Xu, L.; Thompson, D.; Mayer, M. Complete Aggregation Pathway of Amyloid β (1-40) and (1-42) Resolved on an Atomically Clean Interface. Sci. Adv. 2020, 6, eaaz6014. [Google Scholar] [CrossRef]
- Borgstedt, L.; Blobner, M.; Musiol, M.; Bratke, S.; Syryca, F.; Rammes, G.; Jungwirth, B.; Schmid, S. Neurotoxicity of Different Amyloid Beta Subspecies in Mice and Their Interaction with Isoflurane Anaesthesia. PLoS ONE 2020, 15, e0242989. [Google Scholar] [CrossRef]
- Yang, P.; Sun, F. Aducanumab: The First Targeted Alzheimer’s Therapy. Drug Discov. Ther. 2021, 15, 166–168. [Google Scholar] [CrossRef]
- Rabinovici, G.D. Controversy and Progress in Alzheimer’s Disease—FDA Approval of Aducanumab. N. Engl. J. Med. 2021, 385, 771–774. [Google Scholar] [CrossRef]
- Ali, R.; Siddiqui, N. Biological Aspects of Emerging Benzothiazoles: A Short Review. J. Chem. 2013, 2013, e345198. [Google Scholar] [CrossRef]
- Haroun, M.; Tratrat, C.; Kositsi, K.; Tsolaki, E.; Petrou, A.; Aldhubiab, B.; Attimarad, M.; Harsha, S.; Geronikaki, A.; Venugopala, K.N.; et al. New Benzothiazole-Based Thiazolidinones as Potent Antimicrobial Agents. Design, Synthesis and Biological Evaluation. Curr. Top. Med. Chem. 2018, 18, 75–87. [Google Scholar] [CrossRef]
- Matthews, D.C.; Mao, X.; Dowd, K.; Tsakanikas, D.; Jiang, C.S.; Meuser, C.; Andrews, R.D.; Lukic, A.S.; Lee, J.; Hampilos, N.; et al. Riluzole, a Glutamate Modulator, Slows Cerebral Glucose Metabolism Decline in Patients with Alzheimer’s Disease. Brain J. Neurol. 2021, 144, 3742–3755. [Google Scholar] [CrossRef]
- Pereira, A. Glutamatergic Dysfunction in Cognitive Aging: Riluzole in Mild Alzheimer’s Disease; Clinical Trial Registration NCT01703117; clinicaltrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/results/NCT01703117?view=results. (accessed on 2 September 2022).
- Hascup, K.N.; Findley, C.A.; Britz, J.; Esperant-Hilaire, N.; Broderick, S.O.; Delfino, K.; Tischkau, S.; Bartke, A.; Hascup, E.R. Riluzole Attenuates Glutamatergic Tone and Cognitive Decline in AβPP/PS1 Mice. J. Neurochem. 2021, 156, 513–523. [Google Scholar] [CrossRef]
- da Silva, T.L.; Miolo, L.M.F.; Sousa, F.S.S.; Brod, L.M.P.; Savegnago, L.; Schneider, P.H. New Thioureas Based on Thiazolidines with Antioxidant Potential. Tetrahedron Lett. 2015, 56, 6674–6680. [Google Scholar] [CrossRef]
- Saravanan, K.M.; Zhang, H.; Zhang, H.; Xi, W.; Wei, Y. On the Conformational Dynamics of β-Amyloid Forming Peptides: A Computational Perspective. Front. Bioeng. Biotechnol. 2020, 8, 532. [Google Scholar] [CrossRef]
- Fatafta, H.; Khaled, M.; Sayyed-Ahmad, A.; Strodel, B. Amyloid-β Peptide Dimers Undergo a Random Coil to β-Sheet Transition in the Aqueous Phase but Not at the Neuronal Membrane. bioRxiv 2021. [Google Scholar] [CrossRef]
- Tycko, R.; Ishii, Y. Constraints on Supramolecular Structure in Amyloid Fibrils from Two-Dimensional Solid-State NMR Spectroscopy with Uniform Isotopic Labeling. J. Am. Chem. Soc. 2003, 125, 6606–6607. [Google Scholar] [CrossRef]
- Doytchinova, I.; Atanasova, M.; Salamanova, E.; Ivanov, S.; Dimitrov, I. Curcumin Inhibits the Primary Nucleation of Amyloid-Beta Peptide: A Molecular Dynamics Study. Biomolecules 2020, 10, 1323. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin Inhibits Formation of Amyloid Beta Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid in Vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.D.; Malagodi, A.J.; Chi, E.Y.; Evans, D.G. Computational Study of the Driving Forces and Dynamics of Curcumin Binding to Amyloid-β Protofibrils. J. Phys. Chem. B 2019, 123, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Goa, K.L. Galantamine. Drugs 2000, 60, 1095–1122. [Google Scholar] [CrossRef] [PubMed]
- Wallin, A.K.; Wattmo, C.; Minthon, L. Galantamine Treatment in Alzheimer’s Disease: Response and Long-Term Outcome in a Routine Clinical Setting. Neuropsychiatr. Dis. Treat. 2011, 7, 565–576. [Google Scholar] [CrossRef]
- Ali, M.R.; Sadoqi, M.; Møller, S.G.; Boutajangout, A.; Mezei, M. Assessing the Binding of Cholinesterase Inhibitors by Docking and Molecular Dynamics Studies. J. Mol. Graph. Model. 2017, 76, 36–42. [Google Scholar] [CrossRef]
- Dvir, H.; Silman, I.; Harel, M.; Rosenberry, T.L.; Sussman, J.L. Acetylcholinesterase: From 3D Structure to Function. Chem. Biol. Interact. 2010, 187, 10–22. [Google Scholar] [CrossRef]
- Imramovsky, A.; Stepankova, S.; Vanco, J.; Pauk, K.; Monreal-Ferriz, J.; Vinsova, J.; Jampilek, J. Acetylcholinesterase-Inhibiting Activity of Salicylanilide N-Alkylcarbamates and Their Molecular Docking. Molecules 2012, 17, 10142–10158. [Google Scholar] [CrossRef]
- Novales, N.A.; Schwans, J.P. Comparing the Effects of Organic Cosolvents on Acetylcholinesterase and Butyrylcholinesterase Activity. Anal. Biochem. 2022, 654, 114796. [Google Scholar] [CrossRef]
- Marín, I.D.G.; López, R.H.C.; Martínez, O.A.; Padilla-Martínez, I.I.; Correa-Basurto, J.; Rosales-Hernández, M.C. New Compounds from Heterocyclic Amines Scaffold with Multitarget Inhibitory Activity on Aβ Aggregation, AChE, and BACE1 in the Alzheimer Disease. PLoS ONE 2022, 17, e0269129. [Google Scholar] [CrossRef]
- Anzini, M.; Chelini, A.; Mancini, A.; Cappelli, A.; Frosini, M.; Ricci, L.; Valoti, M.; Magistretti, J.; Castelli, L.; Giordani, A.; et al. Synthesis and Biological Evaluation of Amidine, Guanidine, and Thiourea Derivatives of 2-Amino(6-Trifluoromethoxy)Benzothiazole as Neuroprotective Agents Potentially Useful in Brain Diseases. J. Med. Chem. 2010, 53, 734–744. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Gabr, M.T. Multitarget Therapeutic Strategies for Alzheimer’s Disease. Neural Regen. Res. 2019, 14, 437–440. [Google Scholar] [CrossRef]
- Demir Özkay, Ü.; Can, Ö.D.; Sağlık, B.N.; Acar Çevik, U.; Levent, S.; Özkay, Y.; Ilgın, S.; Atlı, Ö. Design, Synthesis, and AChE Inhibitory Activity of New Benzothiazole-Piperazines. Bioorg. Med. Chem. Lett. 2016, 26, 5387–5394. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Deng, L.; Haynes, P.A.; Wu, Y.; Amirkhani, A.; Kamath, K.S.; Wu, J.X.; Pushpitha, K.; Gupta, V.; Graham, S.; Gupta, V.K.; et al. Amyloid-Beta Peptide Neurotoxicity in Human Neuronal Cells Is Associated with Modulation of Insulin-like Growth Factor Transport, Lysosomal Machinery and Extracellular Matrix Receptor Interactions. Neural Regen. Res. 2020, 15, 2131–2142. [Google Scholar] [CrossRef]
- LeVine, H. Thioflavine T Interaction with Amyloid β-Sheet Structures. Amyloid 1995, 2, 1–6. [Google Scholar] [CrossRef]
- Karaca, Ş.; Osmaniye, D.; Sağlık, B.N.; Levent, S.; Ilgın, S.; Özkay, Y.; Karaburun, A.Ç.; Kaplancıklı, Z.A.; Gundogdu-Karaburun, N. Synthesis of Novel Benzothiazole Derivatives and Investigation of Their Enzyme Inhibitory Effects against Alzheimer’s Disease. RSC Adv. 2022, 12, 23626–23636. [Google Scholar] [CrossRef] [PubMed]
- Reinke, A.A.; Gestwicki, J.E. Structure–Activity Relationships of Amyloid Beta-Aggregation Inhibitors Based on Curcumin: Influence of Linker Length and Flexibility. Chem. Biol. Drug Des. 2007, 70, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Monte, F.L.; Kramer, T.; Boländer, A.; Plotkin, B.; Eldar-Finkelman, H.; Fuertes, A.; Dominguez, J.; Schmidt, B. Synthesis and Biological Evaluation of Glycogen Synthase Kinase 3 (GSK-3) Inhibitors: An Fast and Atom Efficient Access to 1-Aryl-3-Benzylureas. Bioorg. Med. Chem. Lett. 2011, 21, 5610–5615. [Google Scholar] [CrossRef] [PubMed]
- De Simone, A.; Tumiatti, V.; Andrisano, V.; Milelli, A. Glycogen Synthase Kinase 3β: A New Gold Rush in Anti-Alzheimer’s Disease Multitarget Drug Discovery? J. Med. Chem. 2021, 64, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, F.; Lucas, J.J.; Avila, J. GSK3 and Tau: Two Convergence Points in Alzheimer’s Disease. J. Alzheimers Dis. 2013, 33 (Suppl. S1), S141–S144. [Google Scholar] [CrossRef]
- Domínguez, J.L.; Fernández-Nieto, F.; Castro, M.; Catto, M.; Paleo, M.R.; Porto, S.; Sardina, F.J.; Brea, J.M.; Carotti, A.; Villaverde, M.C.; et al. Computer-Aided Structure-Based Design of Multitarget Leads for Alzheimer’s Disease. J. Chem. Inf. Model. 2015, 55, 135–148. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Dennington, R.; Millan, J.; Todd, K. GaussView 5; Semichem Inc.: Shawnee, KS, USA, 2009. [Google Scholar]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- RCSB PDB: Homepage—Protein Data Bank. Available online: https://www.rcsb.org/ (accessed on 13 September 2018).
- Hernández-Rodríguez, M.; Correa-Basurto, J.; Benitez-Cardoza, C.G.; Resendiz-Albor, A.A.; Rosales-Hernández, M.C. In Silico and in Vitro Studies to Elucidate the Role of Cu2+ and Galanthamine as the Limiting Step in the Amyloid Beta (1-42) Fibrillation Process. Protein Sci. Publ. Protein Soc. 2013, 22, 1320–1335. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System; DeLano Scientific: San Carlos, CA, USA, 2002. [Google Scholar]
- Systèmes, D. Free Download: BIOVIA Discovery Studio Visualizer. Dassault Systèmes. Available online: https://discover.3ds.com/discovery-studio-visualizer-download (accessed on 30 August 2022).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Cruz, A.; Padilla-Martínez, I.I.; García-Báez, E.V.; Juárez, M.J. S-Methyl-(-N-Aryl and -N-Alkyl)Isothioureas Derived from 2-Aminobenzothiazole. Arkivoc 2007, 2008, 200–209. [Google Scholar] [CrossRef]
- Merchán, F.; Garín, J.; Meléndez, E. A Facile Synthesis of Dimethyl N-(2-Benzothiazolyl)-Dithiocarbonimidates and Methyl N-(2-Benzothiazolyl)-Dithiocarbamates. Synthesis 1982, 1982, 590–591. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.; Correa-Basurto, J.; Nicolás-Vázquez, M.I.; Miranda-Ruvalcaba, R.; Benítez-Cardoza, C.G.; Reséndiz-Albor, A.A.; Méndez-Méndez, J.V.; Rosales-Hernández, M.C. Virtual and In Vitro Screens Reveal a Potential Pharmacophore That Avoids the Fibrillization of Aβ1–42. PLoS ONE 2015, 10, e0130263. [Google Scholar] [CrossRef]
- Rivera-Antonio, A.; Rosales-Hernández, M.C.; Balbuena-Rebolledo, I.; Santiago-Quintana, J.M.; Mendieta-Wejebe, J.E.; Correa-Basurto, J.; García-Vázquez, J.B.; García-Báez, E.V.; Padilla-Martínez, I.I. Myeloperoxidase Inhibitory and Antioxidant Activities of (E)-2-Hydroxy-α-Aminocinnamic Acids Obtained through Microwave-Assisted Synthesis. Pharmaceuticals 2021, 14, 513. [Google Scholar] [CrossRef]
- Prism 5 Updates—GraphPad. Available online: https://www.graphpad.com/support/prism-5-updates/ (accessed on 2 September 2022).

| Ligand | ΔG (kcal/mol) | Amino Acid Residues |
|---|---|---|
| Aβ1-42 in α-helix conformation | ||
| Curcumin | −4.76 | H13, H14, K16, L17, V18, A21, E22, V24, G25, S26 |
| 3f | −6.02 | F20, F19, Q15, V12, H14, E11, D7, V18, E22, D23, N27 |
| 3q | −7.06 | V12, E11, H14, Q15, V18, F19, F20, E22, D23, V24, N27, K28 |
| 3r | −6.37 | N27, K28, D23, V24, A21, F20, E22, F19, Q15, V18, H14, E11, V12, Y10 |
| 3t | −6.52 | Y10, E11, H14, Q15, V18, F19, F20, E22, D23, N27 |
| AChE | ||
| Galantamine | −6.9 | Y341, S293, V294, F295, R296, F297 |
| 3f | −7.49 | W86, D74, R296, F295, V294, Y341, F338 |
| 3q | −7.64 | S293, V294, R296, F295, D74, F338, T83, N87, W86, G122, G121 |
| 3r | −7.26 | G120, G121, R296, F295, V294, S293, Y341, F338, T83, D74, W86, N87 |
| 3t | −7.8 | Y341, D74, V294, F338, T83, F295, G121, G122 |
| Molecule | MW | #Heavy Atoms | #Aromatic Heavy Atoms | Fraction Csp3 | #Rotatable Bonds | #H-bond Acceptors | #H-bond Donors | MR | TPSA | Lipinski #Violations |
| 3f | 357.54 | 24 | 9 | 0.58 | 4 | 2 | 1 | 105.76 | 90.82 | 1 |
| 3r | 520.72 | 34 | 24 | 0.08 | 8 | 4 | 2 | 153.67 | 181.64 | 2 |
| 3t | 467.63 | 31 | 18 | 0.24 | 7 | 4 | 2 | 141.39 | 159.58 | 0 |
| Predicted Toxicity | ||||||||||
| Molecule | Class | LD50: (mg/kg) | Carcinogenicity | Immunotoxicity | Mutagenicity | Cytotoxicity | ||||
| Prediction | Probability | Prediction | Probability | Prediction | Probability | Prediction | Probability | |||
| 3f | 4 | 1000 | Inactive | 0.60 | Inactive | 0.99 | Active | 0.63 | Inactive | 0.77 |
| 3r | 4 | 1000 | Inactive | 0.59 | Inactive | 0.98 | Active | 0.68 | Inactive | 0.77 |
| 3t | 5 | 4000 | Inactive | 0.59 | Inactive | 0.94 | Active | 0.51 | Active | 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales Hernández, M.C.; Fragoso Morales, L.G.; Correa Basurto, J.; Olvera Valdez, M.; García Báez, E.V.; Román Vázquez, D.G.; Anaya García, A.P.; Cruz, A. In Silico and In Vitro Studies of Benzothiazole-Isothioureas Derivatives as a Multitarget Compound for Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12945. https://doi.org/10.3390/ijms232112945
Rosales Hernández MC, Fragoso Morales LG, Correa Basurto J, Olvera Valdez M, García Báez EV, Román Vázquez DG, Anaya García AP, Cruz A. In Silico and In Vitro Studies of Benzothiazole-Isothioureas Derivatives as a Multitarget Compound for Alzheimer’s Disease. International Journal of Molecular Sciences. 2022; 23(21):12945. https://doi.org/10.3390/ijms232112945
Chicago/Turabian StyleRosales Hernández, Martha Cecilia, Leticia Guadalupe Fragoso Morales, José Correa Basurto, Marycruz Olvera Valdez, Efrén Venancio García Báez, Dania Guadalupe Román Vázquez, Ana Paola Anaya García, and Alejandro Cruz. 2022. "In Silico and In Vitro Studies of Benzothiazole-Isothioureas Derivatives as a Multitarget Compound for Alzheimer’s Disease" International Journal of Molecular Sciences 23, no. 21: 12945. https://doi.org/10.3390/ijms232112945
APA StyleRosales Hernández, M. C., Fragoso Morales, L. G., Correa Basurto, J., Olvera Valdez, M., García Báez, E. V., Román Vázquez, D. G., Anaya García, A. P., & Cruz, A. (2022). In Silico and In Vitro Studies of Benzothiazole-Isothioureas Derivatives as a Multitarget Compound for Alzheimer’s Disease. International Journal of Molecular Sciences, 23(21), 12945. https://doi.org/10.3390/ijms232112945







