Review: Influence of 25(OH)D Blood Concentration and Supplementation during Pregnancy on Preeclampsia Development and Neonatal Outcomes
Abstract
1. Preeclampsia and 25(OH)D Insufficiency/Deficiency
2. Concentrations of 25(OH)D in the First Trimester of Pregnancy (<14 Weeks) Complicated by Preeclampsia
3. Concentrations of 25(OH)D in the Second Trimester of Pregnancy (14–26 Weeks) Complicated by Preeclampsia
4. Concentrations of 25(OH)Din the Third Trimester of Pregnancy (>26 Weeks) Complicated by Preeclampsia
5. Impact of 25(OH)D Concentrations on the Development of Arterial Hypertension during Pregnancy Complicated by Preeclampsia
6. Impact of Proteinuria on 25(OH)D Concentrations during Pregnancy Complicated by Preeclampsia
7. Relationship between 25(OH)D Concentrations and the Risk of Preeclampsia in Diabetes Mellitus
8. Maternal 25(OH)D Concentrations after Delivery
9. The Influence of Maternal 25(OH)D Concentrations on the Health of Newborns and Young Children
10. Safety of Vitamin D3 Supplements Prescription during Pregnancy
11. Effect of Vitamin D3 Supplementation on Pregnancy Complicated by Preeclampsia
12. Recommendations for the Prevention of Pregnancy Complications with Vitamin D3 Supplements
13. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hypertensive Disorders during Pregnancy, Childbirth and the Postpartum Period. Preeclampsia. Eclampsia. In Russian Clinical Guidelines; Ministry of Health of the Russian Federation: Moscow, Russia, 2016; p. 5.
- Eiland, E.; Nzerue, C.; Faulkner, M. Preeclampsia 2012. J. Pregnancy 2012, 2012, 586578. [Google Scholar] [CrossRef] [PubMed]
- Olson-Chen, C.; Seligman, N.S. Hypertensive emergencies in pregnancy. Crit. Care Clin. 2016, 32, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.; O’Brien, P.; Khalil, A. Current best practice in the management of hypertensive disorders in pregnancy. Integr. Blood Press. Control 2016, 9, 79. [Google Scholar] [PubMed]
- Steegers, E.A.; Von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef]
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia; World Health Organization: Geneva, Switzerland, 2011.
- Mekie, M.; Mekonnen, W.; Assegid, M. Cohabitation duration, obstetric, behavioral and nutritional factors predict preeclampsia among nulliparous women in West Amhara Zones of Ethiopia: Age matched case control study. PLoS ONE 2020, 15, e0228127. [Google Scholar] [CrossRef]
- Kolusari, A.; Kurdoglu, M.; Yildizhan, R.; Adali, E.; Edirne, T.; Cebi, A.; Demir, H.; Yoruk, I.H. Catalase activity, serum trace element and heavy metal concentrations, and vitamin A, D and E levels in pre-eclampsia. J. Int. Med. Res. 2008, 36, 1335–1341. [Google Scholar] [CrossRef]
- Haugen, M.; Brantsæter, A.L.; Trogstad, L.; Alexander, J.; Roth, C.; Magnus, P.; Meltzer, H.M. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology 2009, 20, 720–726. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Catov, J.M.; Simhan, H.N.; Holick, M.F.; Powers, R.W.; Roberts, J.M. Maternal vitamin D deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab. 2007, 92, 3517–3522. [Google Scholar] [CrossRef]
- Tabesh, M.; Salehi-Abargouei, A.; Tabesh, M.; Esmaillzadeh, A. Maternal vitamin D status and risk of pre-eclampsia: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2013, 98, 3165–3173. [Google Scholar] [CrossRef]
- Reeves, I.V.; Bamji, Z.D.; Rosario, G.B.; Lewis, K.M.; Young, M.A.; Washington, K.N. Vitamin D deficiency in pregnant women of ethnic minority: A potential contributor to preeclampsia. J. Perinatol. 2014, 34, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Bakacak, M.; Serin, S.; Ercan, O.; Köstü, B.; Avci, F.; Kılınç, M.; Kıran, H.; Kiran, G. Comparison of Vitamin D levels in cases with preeclampsia, eclampsia and healthy pregnant women. Int. J. Clin. Exp. Med. 2015, 8, 16280–16286. [Google Scholar] [PubMed]
- Pourghassem Gargari, B.; Pourteymour Fard Tabrizi, F.; Sadien, B.; Asghari Jafarabadi, M.; Farzadi, L. Vitamin D Status Is Related to Oxidative Stress But Not High-Sensitive C-Reactive Protein in Women with Pre-Eclampsia. Gynecol. Obstet. Investig. 2016, 81, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Domaracki, P.; Sadlecki, P.; Odrowaz-Sypniewska, G.; Dzikowska, E.; Walentowicz, P.; Siodmiak, J.; Grabiec, M.; Walentowicz-Sadlecka, M. Serum 25(OH) Vitamin D Levels in Polish Women during Pregnancies Complicated by Hypertensive Disorders and Gestational Diabetes. Int. J. Mol. Sci. 2016, 17, 1574. [Google Scholar] [CrossRef]
- Nandi, A.A.; Wadhwani, N.S.; Joshi, S.R. Altered metabolic homeostasis between vitamin D and long chain polyunsaturated fatty acids in preeclampsia. Med. Hypotheses 2017, 100, 31–36. [Google Scholar] [CrossRef]
- Chrisostomo, K.R.; Skare, T.L.; Kulak, J., Jr.; Urbanetz, A.A.; Chrisostomo, E.R.; Nisihara, R. The prevalence and clinical associations of hypovitaminosis D in pregnant women from Brazil. Int. J. Gynaecol. Obstet. 2018, 143, 66–70. [Google Scholar] [CrossRef]
- Pashapour, S.; Golmohammadlou, S.; Behroozi-Lak, T.; Ghasemnejad-Berenji, H.; Sadeghpour, S.; Ghasemnejad-Berenji, M. Relationship between low maternal vitamin D status and the risk of severe preeclampsia: A case control study. Pregnancy Hypertens. 2019, 15, 161–165. [Google Scholar] [CrossRef]
- Hamedanian, L.; Badehnoosh, B.; Razavi-Khorasani, N.; Mohammadpour, Z.; Mozaffari-Khosravi, H. Evaluation of vitamin D status, parathyroid hormone, and calcium among Iranian pregnant women with preeclampsia: A case-control study. Int. J. Reprod. BioMedicine 2019, 17, 831. [Google Scholar] [CrossRef]
- Kaminskyi, V.; Zhdanovych, O.; Kolomiichenko, T.; Kornienko, S.; Anoshina, T.; Enmaop, S.; Rector, V.; Full, P.; Honoured, S. Preeclampsia-associated homeostasis changes in pregnant woman after ART. Změny homeostázy těhotných po asistované reprodukční technologii asociované s preeklampsii. Ceska Gynekol. 2020, 85, 396–402. [Google Scholar]
- Osman, O.M.; Gaafar, T.; Eissa, T.S.; Abdella, R.; Ebrashy, A.; Ellithy, A. Prevalence of vitamin D deficiency in Egyptian patients with pregnancy-induced hypertension. J. Perinat. Med. 2020, 48, 583–588. [Google Scholar] [CrossRef]
- Huang, X.M.; Liu, Y.H.; Zhang, H.; Cao, Y.; Dou, W.F.; Duan, D.D.; Chen, H.N.; Bo, Y.; Amoah, A.N.; Fu, W.J.; et al. Dietary and serum vitamin D and preeclampsia risk in Chinese pregnant women: A matched case-control study. Br. J. Nutr. 2022; 128, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahiem, S.K.; Ahmed, A.; Sharif, M.E.; Adam, I. Association between maternal serum 25-hydroxyvitamin D concentrations and the risk of pre-eclampsia in central Sudan: A case-control study. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Wahyuningsih, D.; Usman, A.N. Analysis of serum levels L-arginine and 25-hydroxyvitamin D as a predictor of survival of severe preeclampsia mothers. Gac. Sanit. 2021, 35, S224–S226. [Google Scholar] [CrossRef] [PubMed]
- Theobald, G. Effect of Calcium and Vitamins A and D on Incidence of Pregnancy Toxemia. Lancet 1937, 229, 1397–1399. [Google Scholar] [CrossRef]
- Sadin, B.; Pourghassem Gargari, B.; Pourteymour Fard Tabrizi, F. Vitamin D Status in Preeclamptic and Non-preeclamptic Pregnant Women: A Case-Control Study in the North West of Iran. Health Promot. Perspect. 2015, 5, 183–190. [Google Scholar] [CrossRef]
- Das, B.; Singhal, S.R.; Ghalaut, V.S. Evaluating the association between maternal vitamin D deficiency and preeclampsia among Indian gravidas. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 261, 103–109. [Google Scholar] [CrossRef]
- Zeng, S.; Cheng, X.; Chen, R.; Wu, J.; Zhou, J. Low Level of Vitamin D is a Risk Factor for the Occurrence of Early and Late Onset Pre-Eclampsia in Pregnant Women. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Robinson, C.J.; Alanis, M.C.; Wagner, C.L.; Hollis, B.W.; Johnson, D.D. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am. J. Obstet. Gynecol. 2010, 203, 366.e1–366.e3666. [Google Scholar] [CrossRef]
- Baker, A.M.; Haeri, S.; Camargo Jr, C.A.; Espinola, J.A.; Stuebe, A.M. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J. Clin. Endocrinol. Metab. 2010, 95, 5105–5109. [Google Scholar] [CrossRef]
- Robinson, C.J.; Wagner, C.L.; Hollis, B.W.; Baatz, J.E.; Johnson, D.D. Maternal vitamin D and fetal growth in early-onset severe preeclampsia. Am. J. Obstet. Gynecol. 2011, 204, 556-e1. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Simhan, H.N.; Catov, J.M.; Roberts, J.M.; Platt, R.W.; Diesel, J.C.; Klebanoff, M.A. Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology 2014, 25, 207. [Google Scholar] [CrossRef] [PubMed]
- Seely, E.W. Calciotropic hormones in preeclampsia: A renewal of interest. J. Clin. Endocrinol. Metab. 2007, 92, 3402–3403. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.I.; Koch, C.A.; Tamanna, S.; Rouf, S.; Shamsuddin, L. Vitamin D deficiency and the risk of preeclampsia and eclampsia in Bangladesh. Horm. Metab. Res. 2013, 45, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Halhali, A.; Díaz, L.; Avila, E.; Ariza, A.C.; Garabédian, M.; Larrea, F. Decreased fractional urinary calcium excretion and serum 1, 25-dihydroxyvitamin D and IGF-I levels in preeclampsia. J. Steroid Biochem. Mol. Biol. 2007, 103, 803–806. [Google Scholar] [CrossRef]
- Hyppönen, E.; Hartikainen, A.L.; Sovio, U.; Järvelin, M.R.; Pouta, A. Does vitamin D supplementation in infancy reduce the risk of pre-eclampsia? Eur. J. Clin. Nutr. 2007, 61, 1136–1139. [Google Scholar] [CrossRef]
- Bener, A.; Al-Hamaq, A.O.; Saleh, N.M. Association between vitamin D insufficiency and adverse pregnancy outcome: Global comparisons. Int. J. Women’s Health 2013, 5, 523. [Google Scholar] [CrossRef]
- Scholl, T.O.; Chen, X.; Stein, T.P. Maternal calcium metabolic stress and fetal growth. Am. J. Clin. Nutr. 2014, 99, 918–925. [Google Scholar] [CrossRef]
- Chu, J.; Gallos, I.; Tobias, A.; Tan, B.; Eapen, A.; Coomarasamy, A. Vitamin D and assisted reproductive treatment outcome: A systematic review and meta-analysis. Hum. Reprod. 2018, 33, 65–80. [Google Scholar] [CrossRef]
- Abbasalizadeh, S.; Abam, F.; Mirghafourvand, M.; Abbasalizadeh, F.; Taghavi, S.; Hajizadeh, K. Comparing levels of vitamin D, calcium and phosphorus in normotensive pregnant women and pregnant women with preeclampsia. J. Obstet. Gynaecol. 2020, 40, 1069–1073. [Google Scholar] [CrossRef]
- Fischer, D.; Schroer, A.; Lüdders, D.; Cordes, T.; Bücker, B.; Reichrath, J.; Friedrich, M. Metabolism of vitamin D-3 in the placental tissue of normal and preeclampsia complicated pregnancies and premature births. Clin. Exp. Obstet. Gynecol. 2007, 34, 80–84. [Google Scholar]
- Schneuer, F.J.; Roberts, C.L.; Guilbert, C.; Simpson, J.M.; Algert, C.S.; Khambalia, A.Z.; Tasevski, V.; Ashton, A.W.; Morris, J.M.; Nassar, N. Effects of maternal serum 25-hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population. Am. J. Clin. Nutr. 2014, 99, 287–295. [Google Scholar] [CrossRef]
- Gidlöf, S.; Silva, A.T.; Gustafsson, S.; Lindqvist, P.G. Vitamin D and the risk of preeclampsia–a nested case–control study. Acta Obstet. Et Gynecol. Scand. 2015, 94, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Silvares, E.; Vilouta-Romero, M.; Borrajo-Hernández, E.; Morales-Serrano, M.L.; Alves-Pérez, M.T. Concentraciones séricas maternas de 25-hidroxivitamina D en el primer trimestre y resultados adversos gestacionales. Ginecol. Y Obstet. De México 2017, 84, 150–163. [Google Scholar]
- Artunc-Ulkumen, B.; Kirteke, K.; Koyuncu, F.M. The effect of maternal vitamin D levels on placental shear wave elastography findings in the first trimester. J. Obstet. Gynaecol. 2021, 41, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Benachi, A.; Baptiste, A.; Taieb, J.; Tsatsaris, V.; Guibourdenche, J.; Senat, M.V.; Haidar, H.; Jani, J.; Guizani, M.; Jouannic, J.M.; et al. Relationship between vitamin D status in pregnancy and the risk for preeclampsia: A nested case-control study. Clin. Nutr. 2020, 39, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Achkar, M.; Dodds, L.; Giguère, Y.; Forest, J.C.; Armson, B.A.; Woolcott, C.; Agellon, S.; Spencer, A.; Weiler, H.A. Vitamin D status in early pregnancy and risk of preeclampsia. Am. J. Obstet. Gynecol. 2015, 212, 511.e1–511.e5117. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Q.; Audibert, F.; Hidiroglou, N.; Sarafin, K.; Julien, P.; Wu, Y.; Luo, Z.C.; Fraser, W.D. Longitudinal vitamin D status in pregnancy and the risk of pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Gernand, A.D.; Simhan, H.N.; Caritis, S.; Bodnar, L.M. Maternal vitamin D status and small-for-gestational-age offspring in women at high risk for preeclampsia. Obstet. Gynecol. 2014, 123, 40. [Google Scholar] [CrossRef]
- Yue, C.Y.; Gao, J.P.; Zhang, C.Y.; Ying, C.M. Is serum vitamin D deficiency before gestational 20 weeks a risk factor for preeclampsia? Clin. Nutr. 2021, 40, 4430–4435. [Google Scholar] [CrossRef]
- Wang, W.; Du, T.; Jiang, X. Correlation between 25-Hydroxyvitamin D, sFlt-1, PLGF, and Hypertension in Pregnancy. J. Healthc. Eng. 2021, 2021, 9371953. [Google Scholar] [CrossRef]
- Arisoy, R.; Bostancı, E.; Erdogdu, E.; Polat, M.; Kaya, E.; Tugrul, S. Association between maternal serum 25-hydroxyvitamin D level and pre-eclampsia. J. Matern. Fetal Neonatal Med. 2016, 29, 1941–1944. [Google Scholar] [CrossRef]
- Wetta, L.A.; Biggio, J.R.; Cliver, S.; Abramovici, A.; Barnes, S.; Tita, A.T. Is midtrimester vitamin D status associated with spontaneous preterm birth and preeclampsia? Am. J. Perinatol. 2014, 31, 541–546. [Google Scholar] [PubMed]
- Zhou, J.; Su, L.; Liu, M.; Liu, Y.; Cao, X.; Wang, Z.; Xiao, H. Associations between 25-hydroxyvitamin D levels and pregnancy outcomes: A prospective observational study in southern China. Eur. J. Clin. Nutr. 2014, 68, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lee, M.; Jeyabalan, A.; Roberts, J.M. The relationship of hypovitaminosis D and IL-6 in preeclampsia. Am. J. Obstet. Gynecol. 2014, 210, 149-e1. [Google Scholar] [CrossRef]
- Tamblyn, J.A.; Susarla, R.; Jenkinson, C.; Jeffery, L.E.; Ohizua, O.; Chun, R.F.; Chan, S.Y.; Kilby, M.D.; Hewison, M. Dysregulation of maternal and placental vitamin D metabolism in preeclampsia. Placenta 2017, 50, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Samimi, M.; Kashi, M.; Foroozanfard, F.; Karamali, M.; Bahmani, F.; Asemi, Z.; Hamidian, Y.; Talari, H.R.; Esmaillzadeh, A. The effects of vitamin D plus calcium supplementation on metabolic profiles, biomarkers of inflammation, oxidative stress and pregnancy outcomes in pregnant women at risk for pre-eclampsia. J. Hum. Nutr. Diet. 2016, 29, 505–515. [Google Scholar] [CrossRef]
- Umar, N.; Tauseef, A.; Shahzad, F.; Sabir, S.; Kanwal, S.; Akmal, A.; Zulfiqar, S. Serum 25-Hydroxy Vitamin D Level in Preeclamptic and Normotensive Pregnancies. J. Coll. Physicians Surg. Pak. JCPSP 2016, 26, 673–676. [Google Scholar]
- Weinert, L.S.; Reichelt, A.J.; Schmitt, L.R.; Boff, R.; Oppermann, M.L.R.; Camargo, J.L.; Silveiro, S.P. Serum vitamin D insufficiency is related to blood pressure in diabetic pregnancy. Am. J. Hypertens. 2014, 27, 1316–1320. [Google Scholar] [CrossRef]
- Bärebring, L.; O’Connell, M.; Winkvist, A.; Johannsson, G.; Augustin, H. Serum cortisol and vitamin D status are independently associated with blood pressure in pregnancy. J. Steroid Biochem. Mol. Biol. 2019, 189, 259–264. [Google Scholar] [CrossRef]
- Zhao, R.; Zhou, L.; Wang, S.; Xiong, G.; Hao, L. Association between maternal vitamin D levels and risk of adverse pregnancy outcomes: A systematic review and dose–response meta-analysis. Food Funct. 2022, 13, 14–37. [Google Scholar] [CrossRef]
- Shahid, S.; Ladak, A.; Fatima, S.S.; Zaidi, F.A.; Farhat, S. Association of vitamin D levels with preeclampsia. J. Pak. Med. Assoc. 2020, 70, 2390–2393. [Google Scholar] [CrossRef] [PubMed]
- Adela, R.; Borkar, R.M.; Mishra, N.; Bhandi, M.M.; Vishwakarma, G.; Varma, B.A.; Ragampeta, S.; Banerjee, S.K. Lower serum vitamin D metabolite levels in relation to circulating cytokines/chemokines and metabolic hormones in pregnant women with hypertensive disorders. Front. Immunol. 2017, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.H.; Rifas-Shiman, S.L.; Huh, S.Y.; Kleinman, K.; Litonjua, A.A.; Oken, E.; Rich-Edwards, J.W.; Camargo, C.A.; Gillman, M.W., Jr. Vitamin D status and hypertensive disorders in pregnancy. Ann. Epidemiol. 2014, 24, 399–403.e1. [Google Scholar] [CrossRef] [PubMed]
- Nassar, S.Z.; Badae, N.M. Protective effect of vitamin D supplementation in a rat model of preeclampsia: A possible implication of chemerin. Hypertens. Pregnancy 2019, 38, 149–156. [Google Scholar] [CrossRef]
- Albejante, M.C.; Kunz, T.; Ferreira, M.; Júnior, J.; de Almeida, R.J.; Bacigalupo, L.; Matheus, L.; Dalboni, M.A.; Camacho, C.P.; Dellê, H. Proteinuria is Associated with Urinary Loss of Cubilin and Vitamin D-Binding Protein in Patients with Preeclampsia. Sci. Rep. 2020, 10, 3956. [Google Scholar] [CrossRef]
- Poniedziałek-Czajkowska, E.; Mierzyński, R. Could Vitamin D Be Effective in Prevention of Preeclampsia? Nutrients 2021, 13, 3854. [Google Scholar] [CrossRef]
- Jafarzadeh, L.; Motamedi, A.; Behradmanesh, M.; Hashemi, R. A comparison of serum levels of 25-hydroxy vitamin d in pregnant women at risk for gestational diabetes mellitus and women without risk factors. Mater. Socio Med. 2015, 27, 318. [Google Scholar] [CrossRef]
- Ede, G.; Keskin, U.; Cemal Yenen, M.; Samur, G. Lower vitamin D levels during the second trimester are associated with developing gestational diabetes mellitus: An observational cross-sectional study. Gynecol. Endocrinol. 2019, 35, 525–528. [Google Scholar] [CrossRef]
- Weissgerber, T.L.; Mudd, L.M. Preeclampsia and diabetes. Curr. Diabetes Rep. 2015, 15, 1–10. [Google Scholar] [CrossRef]
- Freimane, K.Z.; Kerrigan, L.; Eastwood, K.A.; Watson, C.J. Pre-Eclampsia Biomarkers for Women With Type 1 Diabetes Mellitus: A Comprehensive Review of Recent Literature. Front. Bioeng. Biotechnol. 2022, 10, 809528. [Google Scholar] [CrossRef]
- Azar, M.; Basu, A.; Jenkins, A.J.; Nankervis, A.J.; Hanssen, K.F.; Scholz, H.; Henriksen, T.; Garg, S.K.; Hammad, S.M.; Scardo, J.A.; et al. Serum carotenoids and fat-soluble vitamins in women with type 1 diabetes and preeclampsia: A longitudinal study. Diabetes Care 2011, 34, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Vestgaard, M.; Secher, A.L.; Ringholm, L.; Jensen, J.E.B.; Damm, P.; Mathiesen, E.R. Vitamin D insufficiency, preterm delivery and preeclampsia in women with type 1 diabetes—An observational study. Acta Obstet. Et Gynecol. Scand. 2017, 96, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.B.; Wagner, C.L.; Shary, J.R.; Leyva, M.J.; Yu, J.Y.; Jenkins, A.J.; Nankervis, A.J.; Hanssen, K.F.; Garg, S.K.; Scardo, J.A.; et al. Vitamin D Metabolites and Binding Protein Predict Preeclampsia in Women with Type 1 Diabetes. Nutrients 2020, 12, 2048. [Google Scholar] [CrossRef]
- Tkachuk, A.S.; Vasukova, E.A.; Anopova, A.D.; Karonova, T.L.; Pustozerov, E.A.; Teplova, Y.A.; Eriskovskaya, A.I.; Isakov, A.O.; Vasilieva, E.Y.; Kokina, M.A.; et al. Vitamin D Status and Gestational Diabetes in Russian Pregnant Women in the Period between 2012 and 2021: A Nested Case-Control Study. Nutrients 2022, 14, 2157. [Google Scholar] [CrossRef]
- Bell, R.; Bailey, K.; Cresswell, T.; Hawthorne, G.; Critchley, J.; Lewis-Barned, N.; Northern Diabetic Pregnancy Survey Steering Group. Trends in prevalence and outcomes of pregnancy in women with pre-existing type I and type II diabetes. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L. New insights into the vitamin D requirements during pregnancy. Bone Res. 2017, 5, 1–16. [Google Scholar] [CrossRef]
- Colussi, G.; Catena, C.; Driul, L.; Pezzutto, F.; Fagotto, V.; Darsiè, D.; Badillo-Pazmay, G.V.; Romano, G.; Cogo, P.E.; Sechi, L.A. Secondary hyperparathyroidism is associated with postpartum blood pressure in preeclamptic women and normal pregnancies. J. Hypertens. 2021, 39, 563–572. [Google Scholar] [CrossRef]
- Djekic-Ivankovic, M.; Weiler, H.; Jones, G.; Kaufmann, M.; Kaludjerovic, J.; Aleksic-Velickovic, V.; Mandić, L.M.; Glibetic, M. Vitamin D status in mothers with pre-eclampsia and their infants: A case-control study from Serbia, a country without a vitamin D fortification policy. Public Health Nutr. 2017, 20, 1825–1835. [Google Scholar] [CrossRef]
- Arora, S.; Goel, P.; Chawla, D.; Huria, A.; Arya, A. Vitamin D Status in Mothers and Their Newborns and Its Association with Pregnancy Outcomes: Experience from a Tertiary Care Center in Northern India. J. Obstet. Gynaecol. India 2018, 68, 389–393. [Google Scholar] [CrossRef]
- Thiele, D.K.; Ralph, J.; El-Masri, M.; Anderson, C.M. Vitamin D3 Supplementation During Pregnancy and Lactation Improves Vitamin D Status of the Mother-Infant Dyad. J. Obstet. Gynecol. Neonatal Nurs. JOGNN 2017, 46, 135–147. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. Vitamin D inadequacy in pregnancy: Biology, outcomes, and interventions. Nutr. Rev. 2010, 68, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O’Beirne, M.; Rabi, D.M. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ Clin. Res. Ed. 2013, 346, f1169. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.D.; Fudge, A.N.; Whiting, M.; Coates, P.S. The correlation between third-trimester maternal and newborn-serum 25-hydroxy-vitamin D in a selected South Australian group of newborn samples. BMJ Open 2011, 1, e000236. [Google Scholar] [CrossRef] [PubMed]
- Fares, S.; Sethom, M.M.; Khouaja-Mokrani, C.; Jabnoun, S.; Feki, M.; Kaabachi, N. Vitamin A, E, and D deficiencies in tunisian very low birth weight neonates: Prevalence and risk factors. Pediatr. Neonatol. 2014, 55, 196–201. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, G.M.; Moon, J.E.; Kim, H.M. Severe vitamin D deficiency in preterm infants: Maternal and neonatal clinical features. Korean J. Pediatr. 2015, 58, 427. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.Q.; Zhao, X.; Chen, D.Z.; Liao, X.P.; Zhou, Q. Vitamin D level at birth and influencing factors in preterm infants. Zhongguo Dang Dai Er Ke Za Zhi Chin. J. Contemp. Pediatr. 2017, 19, 800–805. [Google Scholar]
- Gupta, T.; Wahi, S.; Gupta, N.; Arora, S.; Gupta, S.; Bhatia, P. Correlation of vitamin D levels in term normotensive and pre-eclamptic patients in labor. J. Obstet. Gynecol. India 2016, 66, 154–159. [Google Scholar] [CrossRef][Green Version]
- Hajianfar, H.; Esmailzadeh, A.; Feizi, A.; Shahshahan, Z.; Azadbakht, L. Association of maternal serum vitamin D level with risk of pregnancy-related complications and neonatal anthropometric measures: A prospective observational study. Int. J. Prev. Med. 2019, 10. [Google Scholar]
- Nandi, A.; Wadhwani, N.; Randhir, K.; Wagh, G.; Joshi, S.R. Association of vitamin D with fatty acids in pregnancy. Prostaglandins Leukot. Essent. Fat. Acids 2020, 157, 102030. [Google Scholar] [CrossRef]
- Shrestha, D.; Saha, R.; Karki, C.; Mahato, S. Study of Vitamin-D Deficiency among Pregnant Women in their First Trimester Visiting a Tertiary Care Hospital: A Descriptive Cross-sectional Study. JNMA J. Nepal Med. Assoc. 2021, 59, 626. [Google Scholar] [CrossRef]
- Miliku, K.; Vinkhuyzen, A.; Blanken, L.M.; McGrath, J.J.; Eyles, D.W.; Burne, T.H.; Hofman, A.; Tiemeier, H.; Steegers, E.A.; Gaillard, R.; et al. Maternal vitamin D concentrations during pregnancy, fetal growth patterns, and risks of adverse birth outcomes. Am. J. Clin. Nutr. 2016, 103, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Verisokina, N.E.; Kuryaninova, V.A.; Petrosyan, M.A.; Zakharova, I.N.; Zaplatnikov, A.L.; Zubkov, V.V.; Klimov, L.Y.; Dmitrieva, D.V.; Beketova, N.Y.; Momotova, A.A. Analysis of vitamin D availability of premature infants in the south of Russia. Meditsinskiy Sov. 2022, 16, 10–19. (In Russian) [Google Scholar] [CrossRef]
- Vasilyeva, E.N.; Maltseva, L.I.; Denisova, T.G.; Gerasimova, L.I. Health features of newborns depending on vitamin D level of their mothers during pregnancy. Kazan. Meditsinskiy Zhurnal 2017, 98, 691–696. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Aguilar-Cordero, M.J.; Lasserrot-Cuadrado, A.; Mur-Villar, N.; León-Ríos, X.A.; Rivero-Blanco, T.; Pérez-Castillo, I.M. Vitamin D, preeclampsia and prematurity: A systematic review and meta-analysis of observational and interventional studies. Midwifery 2020, 87, 102707. [Google Scholar] [CrossRef] [PubMed]
- Hossain, N.; Kanani, F.H.; Ramzan, S.; Kausar, R.; Ayaz, S.; Khanani, R.; Pal, L. Obstetric and neonatal outcomes of maternal vitamin D supplementation: Results of an open-label, randomized controlled trial of antenatal vitamin D supplementation in Pakistani women. J. Clin. Endocrinol. Metab. 2014, 99, 2448–2455. [Google Scholar] [CrossRef]
- Belderbos, M.E.; Houben, M.L.; Wilbrink, B.; Lentjes, E.; Bloemen, E.M.; Kimpen, J.L.; Rovers, M.; Bont, L. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics 2011, 127, e1513–e1520. [Google Scholar] [CrossRef]
- Onwuneme, C.; Martin, F.; McCarthy, R.; Carroll, A.; Segurado, R.; Murphy, J.; Twomey, A.; Murphy, N.; Kilbane, M.; McKenna, M.; et al. The Association of Vitamin D Status with Acute Respiratory Morbidity in Preterm Infants. J. Pediatr. 2015, 166, 1175–1180.e1. [Google Scholar] [CrossRef]
- Byun, S.Y.; Bae, M.H.; Lee, N.R.; Han, Y.M.; Park, K.H. Association between vitamin D deficiency at one month of age and bronchopulmonary dysplasia. Medicine 2021, 100, e27966. [Google Scholar] [CrossRef]
- Allan, K.M.; Prabhu, N.; Craig, L.C.; McNeill, G.; Kirby, B.; McLay, J.; Helms, P.J.; Ayres, J.G.; Seaton, A.; Turner, S.W.; et al. Maternal vitamin D and E intakes during pregnancy are associated with asthma in children. Eur. Respir. J. 2015, 45, 1027–1036. [Google Scholar] [CrossRef]
- Devereux, G.; Litonjua, A.A.; Turner, S.W.; Craig, L.C.; McNeill, G.; Martindale, S.; Helms, P.J.; Seaton, A.; Weiss, S.T. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am. J. Clin. Nutr. 2007, 85, 853–859. [Google Scholar] [CrossRef]
- Lee, C.L.; Ng, B.K.; Wu, L.L.; Cheah, F.C.; Othman, H.; Ismail, N. Vitamin D deficiency in pregnancy at term: Risk factors and pregnancy outcomes. Horm. Mol. Biol. Clin. Investig. 2017, 31. [Google Scholar] [CrossRef]
- Martínez-Domínguez, S.J.; Tajada, M.; Chedraui, P.; Pérez-López, F.R. Systematic review and meta-analysis of Spanish studies regarding the association between maternal 25-hydroxyvitamin D levels and perinatal outcomes. Gynecol. Endocrinol. 2018, 34, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Velkavrh, M.; Paro-Panjan, D.; Benedik, E.; Mis, N.F.; Godnov, U.; Salamon, A.S. The Influence of Maternal Levels of Vitamin D and Adiponectin on Anthropometrical Measures and Bone Health in Offspring. Prilozi 2019, 40, 91–98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wierzejska, R.; Jarosz, M.; Klemińska-Nowak, M.; Tomaszewska, M.; Sawicki, W.; Bachanek, M.; Siuba-Strzelińska, M. Maternal and Cord Blood Vitamin D Status and Anthropometric Measurements in Term Newborns at Birth. Front. Endocrinol. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Levkovitz, O.; Lagerev, E.; Bauer-Rusak, S.; Litmanovitz, I.; Grinblatt, E.; Sirota, G.L.; Shalit, S.; Arnon, S. Vitamin D Levels in Pregnant Women Do Not Affect Neonatal Bone Strength. Children 2022, 9, 883. [Google Scholar] [CrossRef]
- Cooper, C.; Harvey, N.C.; Bishop, N.J.; Kennedy, S.; Papageorghiou, A.T.; Schoenmakers, I.; Fraser, R.; Gandhi, S.V.; Carr, A.; D’Angelo, S.; et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): A multicentre, double-blind, randomised placebo-controlled trial. Lancet. Diabetes Endocrinol. 2016, 4, 393–402. [Google Scholar] [CrossRef]
- Karpen, H.E. Mineral Homeostasis and Effects on Bone Mineralization in the Preterm Neonate. Clin. Perinatol. 2018, 45, 129–141. [Google Scholar] [CrossRef]
- Mahon, P.; Harvey, N.; Crozier, S.; Inskip, H.; Robinson, S.; Arden, N.; Swaminathan, R.; Cooper, C.; Godfrey, K.; SWS Study Group. Low maternal vitamin D status and fetal bone development: Cohort study. J. Bone Miner. Res. 2010, 25, 14–19. [Google Scholar] [CrossRef]
- Viljakainen, H.T.; Korhonen, T.; Hytinantti, T.; Laitinen, E.K.; Andersson, S.; Mäkitie, O.; Lamberg-Allardt, C. Maternal vitamin D status affects bone growth in early childhood—A prospective cohort study. Osteoporos. Int. 2011, 22, 883–891. [Google Scholar] [CrossRef]
- Brooke, O.G.; Brown, I.R.; Bone, C.D.; Carter, N.D.; Cleeve, H.J.; Maxwell, J.D.; Robinson, V.P.; Winder, S.M. Vitamin D supplements in pregnant Asian women: Effects on calcium status and fetal growth. Br. Med. J. 1980, 280, 751–754. [Google Scholar] [CrossRef]
- Karlsson, C.; Obrant, K.J.; Karlsson, M. Pregnancy and lactation confer reversible bone loss in humans. Osteoporos. Int. 2001, 12, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.Q.; Kaplan, A.T.; Lagishetty, V.; Ouyang, Y.B.; Ouyang, Y.; Simmons, C.F.; Equils, O.; Hewison, M. Vitamin D and the regulation of placental inflammation. J. Immunol. 2011, 186, 5968–5974. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, I.N.; Klimov, L.Y.; Kasyanova, A.N. The role of antimicrobial peptides and vitamin D in the formation of anti-infective protection. Pediatrics 2017, 96, 171–179. (In Russian) [Google Scholar] [CrossRef]
- Karatekin, G.; Kaya, A.; Salihoğlu, O.; Balci, H.; Nuhoğlu, A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur. J. Clin. Nutr. 2009, 63, 473–477. [Google Scholar] [CrossRef]
- Melough, M.M.; Murphy, L.E.; Graff, J.C.; Derefinko, K.J.; LeWinn, K.Z.; Bush, N.R.; Enquobahrie, D.A.; Loftus, C.T.; Kocak, M.; Sathyanarayana, S.; et al. Maternal Plasma 25-Hydroxyvitamin D during Gestation Is Positively Associated with Neurocognitive Development in Offspring at Age 4-6 Years. J. Nutr. 2021, 151, 132–139. [Google Scholar] [CrossRef]
- Darling, A.L.; Rayman, M.P.; Steer, C.D.; Golding, J.; Lanham-New, S.A.; Bath, S.C. Association between maternal vitamin D status in pregnancy and neurodevelopmental outcomes in childhood: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Br. J. Nutr. 2017, 117, 1682–1692. [Google Scholar] [CrossRef]
- Strøm, M.; Halldorsson, T.I.; Hansen, S.; Granström, C.; Maslova, E.; Petersen, S.B.; Cohen, A.S.; Olsen, S.F. Vitamin D measured in maternal serum and offspring neurodevelopmental outcomes: A prospective study with long-term follow-up. Ann. Nutr. Metab. 2014, 64, 254–261. [Google Scholar] [CrossRef]
- Miratashi Yazdi, S.A.; Abbasi, M.; Miratashi Yazdi, S.M. Epilepsy and vitamin D: A comprehensive review of current knowledge. Rev. Neurosci. 2017, 28, 185–201. [Google Scholar] [CrossRef]
- Zhu, Y.; Qin, Z.; Gao, J.; Yang, M.; Qin, Y.; Shen, T.; Liu, S. Vitamin D therapy in experimental allergic encephalomyelitis could be limited by opposing effects of sphingosine 1-phosphate and gelsolin dysregulation. Mol. Neurobiol. 2014, 50, 733–743. [Google Scholar] [CrossRef]
- Zayachnikova, T.E.; Belan, E.B.; Krasilnikova, A.S. Vitamin D deficiency in the mother-placenta-fetus system as a risk factor for physical and neurological developmental disorder in premature infants. RMJ Med. Rev. 2019, 5, 20–25. [Google Scholar]
- Tylavsky, F.A.; Kocak, M.; Murphy, L.E.; Graff, J.C.; Palmer, F.B.; Völgyi, E.; Diaz-Thomas, A.M.; Ferry, R.J. Gestational Vitamin 25(OH)D Status as a Risk Factor for Receptive Language Development: A 24-Month, Longitudinal, Observational Study. Nutrients 2015, 7, 9918–9930. [Google Scholar] [CrossRef]
- Pludowski, P.; Grant, W.B.; Bhattoa, H.P.; Bayer, M.; Povoroznyuk, V.; Rudenka, E.; Ramanau, H.; Varbiro, S.; Rudenka, A.; Karczmarewicz, E.; et al. Vitamin D status in central europe. Int. J. Endocrinol. 2014, 2014, 589587. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; De-Regil, L.M.; Lombardo, L.K.; Peña-Rosas, J.P. Vitamin D supplementation during pregnancy: Updated meta-analysis on maternal outcomes. J. Steroid Biochem. Mol. Biol. 2016, 164, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.E. Vitamin D supplementation during pregnancy: Safety considerations in the design and interpretation of clinical trials. J. Perinatol. 2011, 31, 449–459. [Google Scholar] [CrossRef]
- Magielda-Stola, J.; Drews, K.; Wolski, H.; Seremak-Mrozikiewicz, A. Vitamin D3 and its receptor in selected obstetrical complications. Ginekol. Pol. 2021; 92, 460–465. [Google Scholar] [CrossRef]
- People’s League of Health. Nutrition of expectant and nursing mothers: Interim report. Lancet 1942, 2, 10–12. [Google Scholar]
- Naghshineh, E.; Sheikhaliyan, S. Effect of vitamin D supplementation in the reduce risk of preeclampsia in nulliparous women. Adv. Biomed. Res. 2016, 5, 7. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Palacios, C.; Lombardo, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Sao Paulo Med. J. Rev. Paul. De Med. 2016, 134, 274–275. [Google Scholar] [CrossRef]
- Baca, K.M.; Simhan, H.N.; Platt, R.W.; Bodnar, L.M. Low maternal 25-hydroxyvitamin D concentration increases the risk of severe and mild preeclampsia. Ann. Epidemiol. 2016, 26, 853–857.e1. [Google Scholar] [CrossRef]
- Fu, Z.M.; Ma, Z.Z.; Liu, G.J.; Wang, L.L.; Guo, Y. Vitamins supplementation affects the onset of preeclampsia. J. Formos. Med. Assoc. Taiwan Yi Zhi 2018, 117, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Stougaard, M.; Damm, P.; Frederiksen, P.; Jacobsen, R.; Heitmann, B.L. Exposure to vitamin D from fortified margarine during fetal life and later risk of pre-eclampsia: The D-tect Study. Public Health Nutr. 2018, 21, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Xiaomang, J.; Yanling, W. Effect of vitamin D3 supplementation during pregnancy on high risk factors—A randomized controlled trial. J. Perinat. Med. 2020, 49, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Kulia, O. Study of the influence of calcium and vitamin d prescription during the pregnancy period on the state of newborns’ health and on the electrolysite balance umbilical cord blood. Georgian Med. News 2018, 284, 19–23. [Google Scholar]
- Asemi, Z.; Esmaillzadeh, A. The Effect of Multi mineral-Vitamin D Supplementation on Pregnancy Outcomes in Pregnant Women at Risk for Pre-eclampsia. Int. J. Prev. Med. 2015, 6, 62. [Google Scholar] [CrossRef]
- Rostami, M.; Tehrani, F.R.; Simbar, M.; Bidhendi Yarandi, R.; Minooee, S.; Hollis, B.W.; Hosseinpanah, F. Effectiveness of Prenatal Vitamin D Deficiency Screening and Treatment Program: A Stratified Randomized Field Trial. J. Clin. Endocrinol. Metab. 2018, 103, 2936–2948. [Google Scholar] [CrossRef]
- Skowrońska-Jóźwiak, E.; Adamczewski, Z.; Tyszkiewicz, A.; Krawczyk-Rusiecka, K.; Lewandowski, K.; Lewiński, A. Assessment of adequacy of vitamin D supplementation during pregnancy. Ann. Agric. Environ. Med. AAEM 2014, 21, 198–200. [Google Scholar]
- Ojo, O.; Weldon, S.M.; Thompson, T.; Vargo, E.J. The Effect of Vitamin D Supplementation on Glycaemic Control in Women with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Int. J. Environ. Res. Public Health 2019, 16, 1716. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Z.; Hu, Y.; Wang, Y.; Wu, Y.; Lian, F.; Li, H.; Yang, J.; Xu, X. The effects of vitamin D supplementation on glycemic control and maternal-neonatal outcomes in women with established gestational diabetes mellitus: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 3148–3157. [Google Scholar] [CrossRef]
- Shahgheibi, S.; Farhadifar, F.; Pouya, B. The effect of vitamin D supplementation on gestational diabetes in high-risk women: Results from a randomized placebo-controlled trial. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2016, 21, 2. [Google Scholar] [CrossRef]
- Saha, S.; Saha, S. A comparison of the risk of cesarean section in gestational diabetes mellitus patients supplemented antenatally with vitamin D containing supplements versus placebo: A systematic review and meta-analysis of double-blinded randomized controlled trials. J. Turk. Ger. Gynecol. Assoc. 2020, 21, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Karamali, M.; Beihaghi, E.; Mohammadi, A.A.; Asemi, Z. Effects of High-Dose Vitamin D Supplementation on Metabolic Status and Pregnancy Outcomes in Pregnant Women at Risk for Pre-Eclampsia. Horm. Metab. Res. 2015, 47, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Mojibian, M.; Soheilykhah, S.; Fallah Zadeh, M.A.; Jannati Moghadam, M. The effects of vitamin D supplementation on maternal and neonatal outcome: A randomized clinical trial. Iran. J. Reprod. Med. 2015, 13, 687–696. [Google Scholar]
- Bi, W.G.; Nuyt, A.M.; Weiler, H.; Leduc, L.; Santamaria, C.; Wei, S.Q. Association Between Vitamin D Supplementation During Pregnancy and Offspring Growth, Morbidity, and Mortality: A Systematic Review and Meta-analysis. JAMA Pediatr. 2018, 172, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, F.R.; Pilz, S.; Chedraui, P. Vitamin D supplementation during pregnancy: An overview. Curr. Opin. Obstet. Gynecol. 2020, 32, 316–321. [Google Scholar] [CrossRef]
- Nausheen, S.; Habib, A.; Bhura, M.; Rizvi, A.; Shaheen, F.; Begum, K.; Iqbal, J.; Ariff, S.; Shaikh, L.; Raza, S.S.; et al. Impact evaluation of the efficacy of different doses of vitamin D supplementation during pregnancy on pregnancy and birth outcomes: A randomised, controlled, dose comparison trial in Pakistan. BMJ Nutr. Prev. Health 2021, 4, 425–434. [Google Scholar] [CrossRef]
- Mirzakhani, H.; Litonjua, A.A.; McElrath, T.F.; O’Connor, G.; Lee-Parritz, A.; Iverson, R.; Macones, G.; Strunk, R.C.; Bacharier, L.B.; Zeiger, R.; et al. Early pregnancy vitamin D status and risk of preeclampsia. J. Clin. Investig. 2016, 126, 4702–4715. [Google Scholar] [CrossRef]
- Behjat Sasan, S.; Zandvakili, F.; Soufizadeh, N.; Baybordi, E. The Effects of Vitamin D Supplement on Prevention of Recurrence of Preeclampsia in Pregnant Women with a History of Preeclampsia. Obstet. Gynecol. Int. 2017, 2017, 8249264. [Google Scholar] [CrossRef]
- Asemi, Z.; Tabassi, Z.; Heidarzadeh, Z.; Khorammian, H.; Sabihi, S.S.; Samimi, M. Effect of calcium-vitamin D supplementation on metabolic profiles in pregnant women at risk for pre-eclampsia: A randomized placebo-controlled trial. Pak. J. Biol. Sci. PJBS 2012, 15, 316–324. [Google Scholar] [CrossRef]
- Abedi, P.; Mohaghegh, Z.; Afshary, P.; Latifi, M. The relationship of serum vitamin D with pre-eclampsia in the Iranian women. Matern. Child Nutr. 2014, 10, 206–212. [Google Scholar] [CrossRef]
- Curtis, E.M.; Moon, R.J.; Harvey, N.C.; Cooper, C. Maternal vitamin D supplementation during pregnancy. Br. Med. Bull. 2018, 126, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Christoph, P.; Challande, P.; Raio, L.; Surbek, D. High prevalence of severe vitamin D deficiency during the first trimester in pregnant women in Switzerland and its potential contributions to adverse outcomes in the pregnancy. Swiss Med. Wkly. 2020, 150, w20238. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Low vitamin D may explain the link between preeclampsia and cardiovascular disease. Am. Heart J. 2010, 159, e19. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Dietary Reference Values for Vitamin D; EFSA Panel on Dietetic Products, Nutrition and Allergies: Parma, Italy, 2016. [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Mansur, J.L. Vitamina D en pediatría, embarazo y lactancia. Arch. Argent. De Pediatría 2018, 116, 286–590. [Google Scholar]
- Pigarova, E.A.; Rozhinskaya, L.Y.; Belaya, J.E.; Dzeranova, L.K.; Karonova, T.L.; Ilyin, A.V.; Dedov, I.I. Russian Association of Endocrinologists recommendations for diagnosis, treatment and prevention of vitamin D deficiency in adults. Probl. Endocrinol. 2016, 62, 60–84. [Google Scholar] [CrossRef]
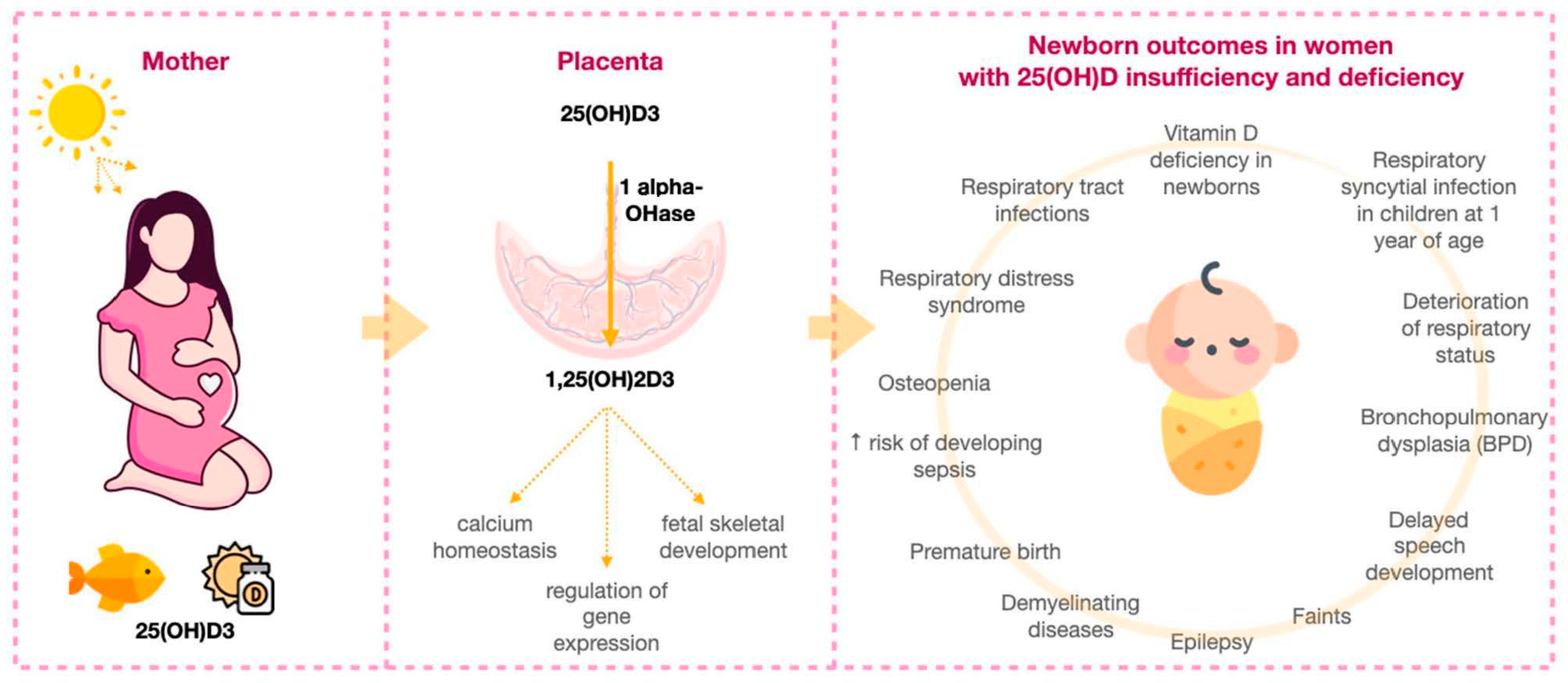
| Week of Gestation | 25(OH)D Concentrations during Normal Pregnancy, mean ± SD (ng/mL) | 25(OH)D Concentrations during Pregnancy Complicated by Preeclampsia, mean ± SD (ng/mL) 2 | References |
|---|---|---|---|
| First trimester of gestation (<14) | |||
| 12 | 19.44 ± 8.2 | 20.88 ± 8.2 | [44] |
| 14 | 18.88 ± 7.08 | 20.92 ± 6.88 | [48] |
| First–second trimester of gestation | |||
| 12–18 | 20.1 ± 9.3 | 22.3 ± 11.1 | [47] |
| 12–18 | 19.33 ± 4.75 | pre-eclampsia 12.29 ± 2.79 severe pre-eclampsia 9.56 ± 2.68 | [52] |
| Second trimester of gestation (15–26) | |||
| 15–20 | 39.2 (27.2–45.2) 1 | 30 (18.8–42.8) 1 | [31] |
| 15–21 | 28.6 ± 12.6 | 27.4 ± 14.4 | [54] |
| 16 | 18.84–23.96 (21.24) 1 | 18.16 (15.44–21.36) 1 | [11] |
| 24–26 | 22.8 ± 7.64 | 19.56 ± 6.72 | [49] |
| Second–third trimester of gestation | |||
| 20–32 | 19.76 ± 9.04 | 16.92 ± 6.92 | [57] |
| 25–35 | 15.73 ± 5.85 | 10.09 ± 6.66 | [27] |
| 25–38 | 22.76 (17.56–28.32) 1 | All preeclampsia 21.84 (18.2–27.84) 1 | [43] |
| Early onset preeclampsia 21.4 (16.96–24.68) 1 | |||
| 25–39 | 14.9 ± 12.0 | Severe pre-eclampsia 5.8 ± 4.5 | [53] |
| Non-severe pre-eclampsia 11.8 ± 7.3 | |||
| Third trimester of gestation (>26) | |||
| 26–31 | 32 (20–44) 1 | 18 (13–31) 1 | [30] |
| 26–37 | 13.41 ± 8.05 | 6.88 ± 9.46 | [20] |
| 30 | 10.09 ± 6.6 | 15.73 ± 5.85 | [15] |
| 30–40 | 23.7 ± 5.93 | 19.3 ± 4.31 | [14] |
| 30–40 | 19.5 ± 6.5 | 14.8 ± 5.4 | [22] |
| 30–40 | 31.4 ± 1.7 | 11.0 ± 7.1 | [28] |
| 30–36 | 22.57 ± 4.33 | pre-eclampsia 18.68 ± 3.50 severe pre-eclampsia 9.48 ± 2.98 | [52] |
| 32–38 | 30.8 ± 11.0 | 27.7 ± 12.2 | [47] |
| 35–40 | 24.86 ± 1.02 | 23.96 ± 1.31 | [35] |
| 35–41 | 23.84 ± 6.93 | 15.27 ± 3.52 | [19] |
| Adverse Neonatal and Postnatal Outcomes | 25(OH)D Threshold Concentrations in ng/mL 2 | Reference |
|---|---|---|
| Neonatal Outcomes | ||
| 25(OH)D Deficiency in Newborns | <12 | [80] |
| Low Body Mass at Birth | <30 | [50] |
| Birth of Premature Babies | <20 | [94] |
| <30 | [96] | |
| Apgar score at 1 and 5 min < 7 points | <30 | [97] |
| Deterioration of Respiratory Status | <12 | [99] |
| BPD in Premature Infants | <20 1 | [100] |
| Postnatal Outcomes | ||
| Respiratory Syncytial Infection in Children at First Year of Life | <20 | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpova, N.; Dmitrenko, O.; Arshinova, E.; Nurbekov, M. Review: Influence of 25(OH)D Blood Concentration and Supplementation during Pregnancy on Preeclampsia Development and Neonatal Outcomes. Int. J. Mol. Sci. 2022, 23, 12935. https://doi.org/10.3390/ijms232112935
Karpova N, Dmitrenko O, Arshinova E, Nurbekov M. Review: Influence of 25(OH)D Blood Concentration and Supplementation during Pregnancy on Preeclampsia Development and Neonatal Outcomes. International Journal of Molecular Sciences. 2022; 23(21):12935. https://doi.org/10.3390/ijms232112935
Chicago/Turabian StyleKarpova, Nataliia, Olga Dmitrenko, Ekaterina Arshinova, and Malik Nurbekov. 2022. "Review: Influence of 25(OH)D Blood Concentration and Supplementation during Pregnancy on Preeclampsia Development and Neonatal Outcomes" International Journal of Molecular Sciences 23, no. 21: 12935. https://doi.org/10.3390/ijms232112935
APA StyleKarpova, N., Dmitrenko, O., Arshinova, E., & Nurbekov, M. (2022). Review: Influence of 25(OH)D Blood Concentration and Supplementation during Pregnancy on Preeclampsia Development and Neonatal Outcomes. International Journal of Molecular Sciences, 23(21), 12935. https://doi.org/10.3390/ijms232112935






