Insulin-like Peptide Receptor (ILPR) in the Cuttlefish Sepiella japonica: Characterization, Expression, and Regulation of Reproduction
Abstract
1. Introduction
2. Results
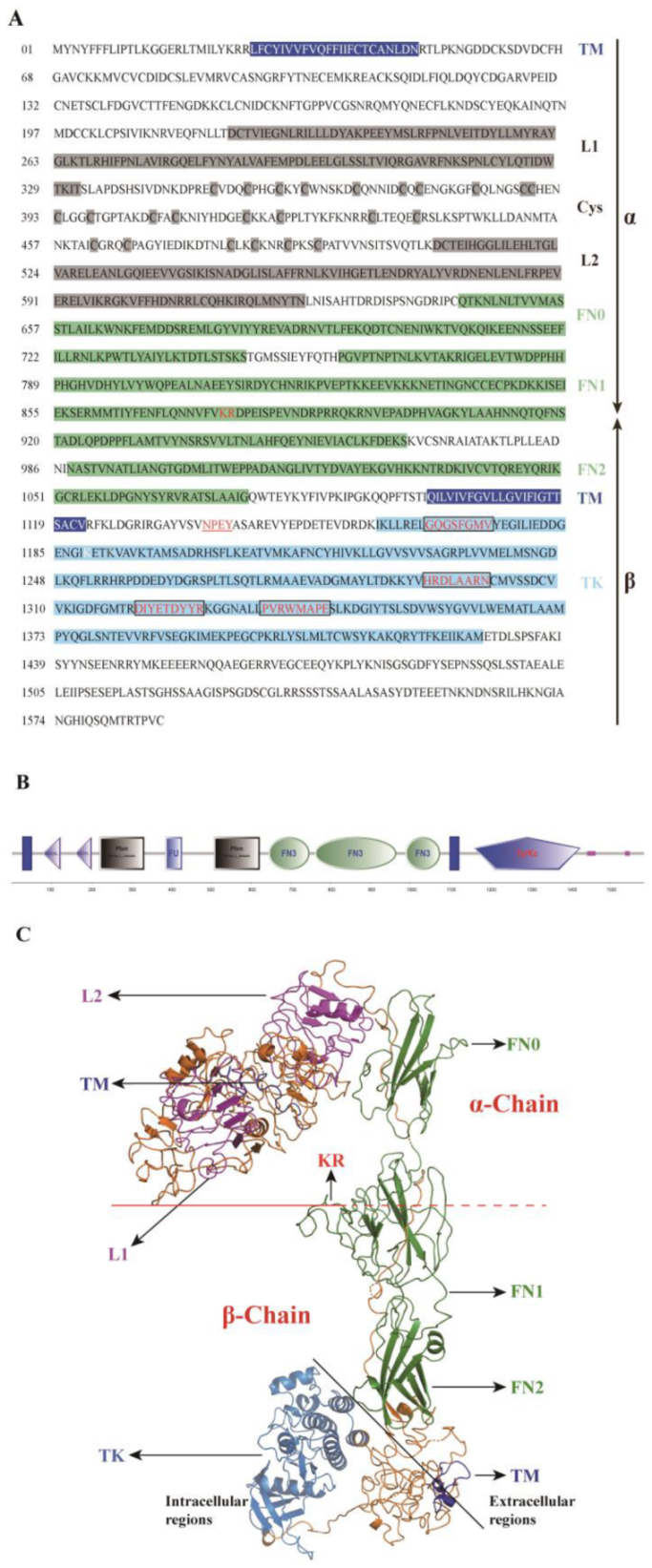
2.1. Cloning and Sequence Analysis of SjILPR
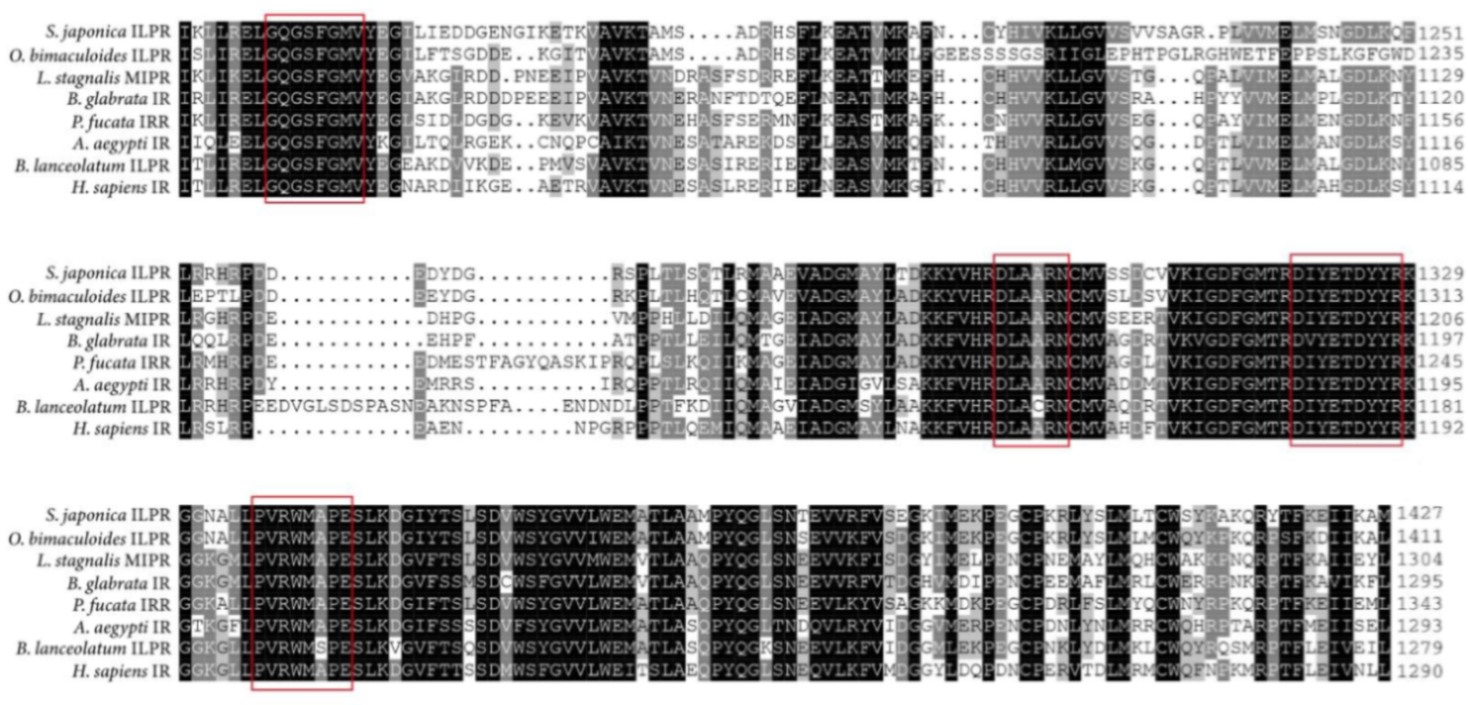
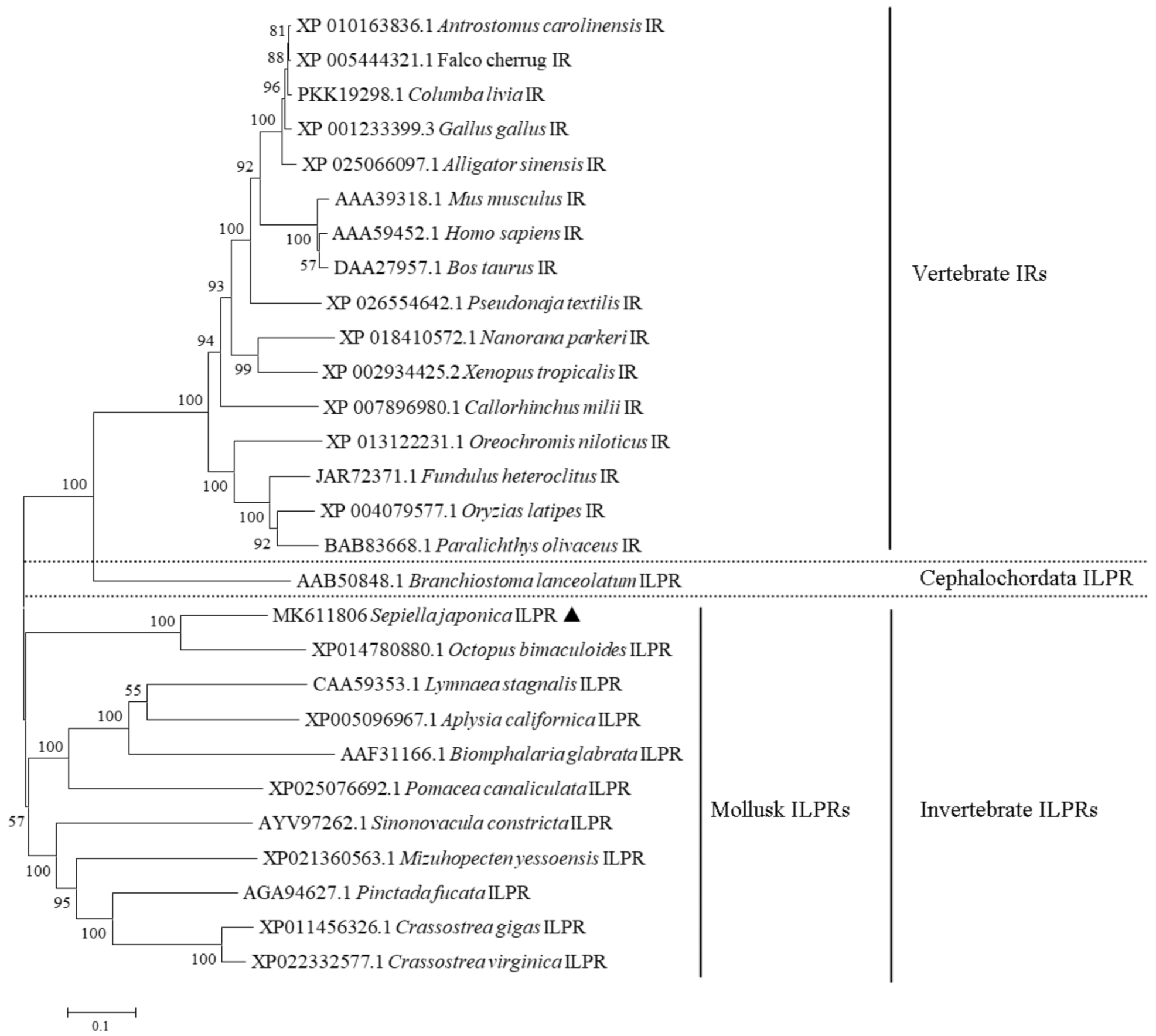
2.2. Phylogenetic Analysis of SjILPR
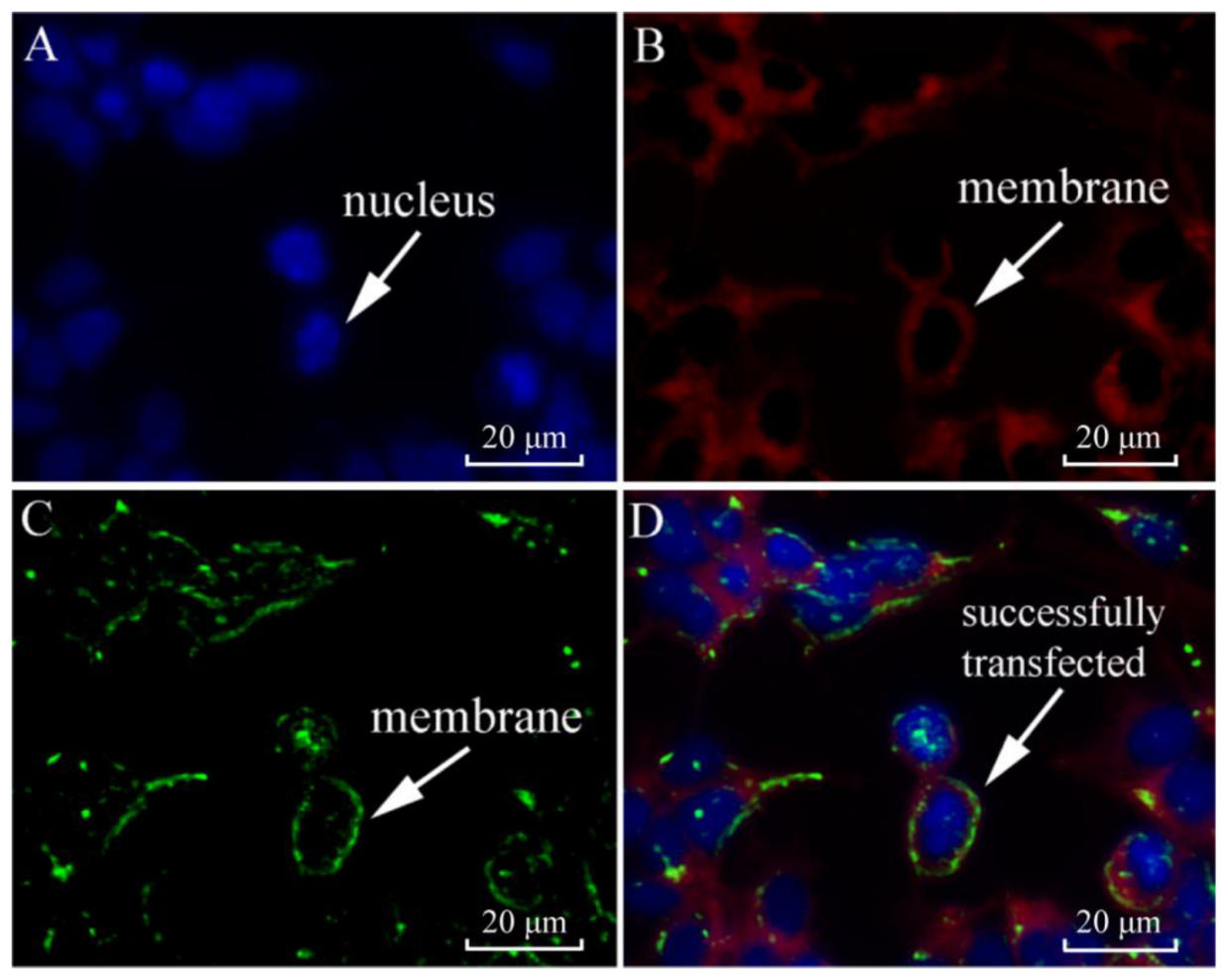
2.3. Subcellular Localization of SjILPR
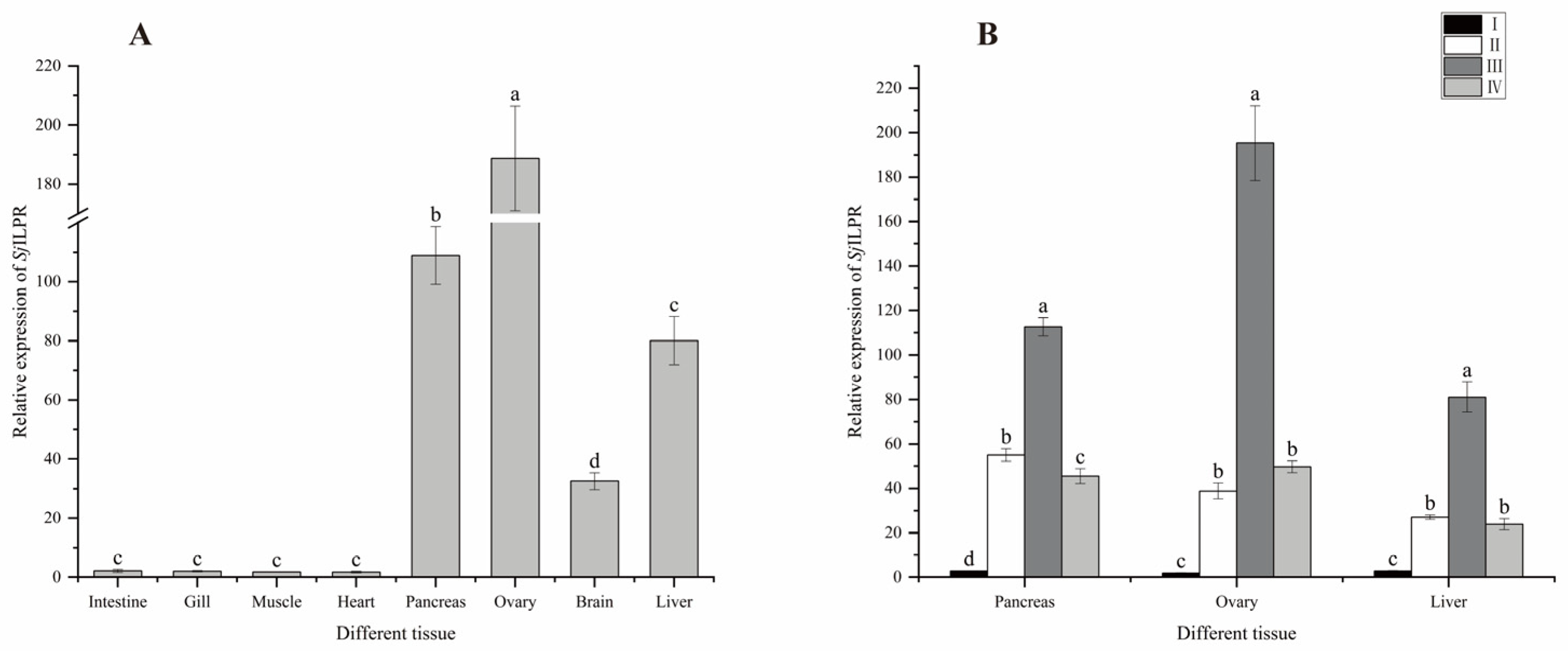
2.4. Expression of SjILPR in Different Tissue
2.5. Expression of SjILPR during the Different Ovarian Development Stages of Female S. japonica
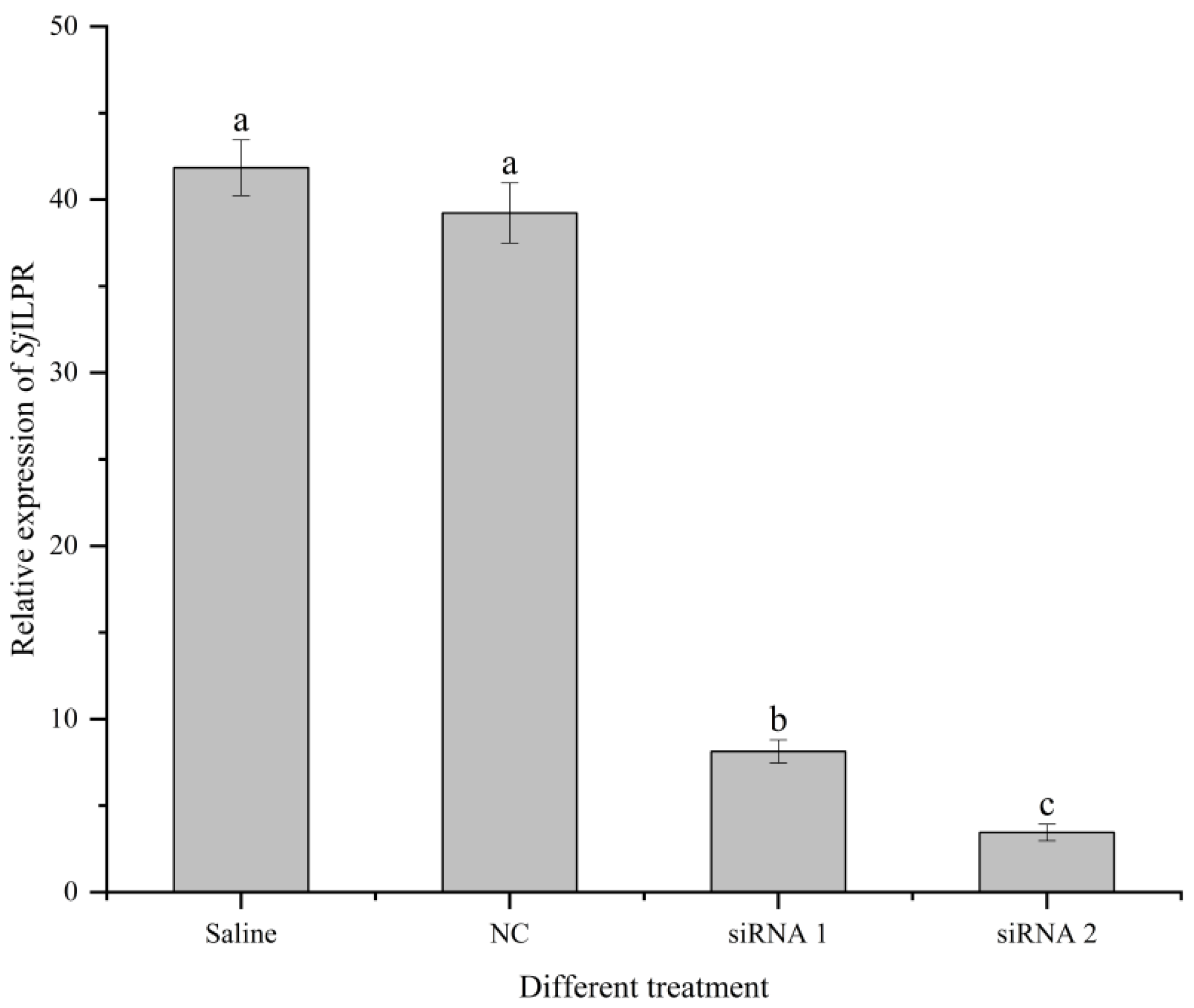
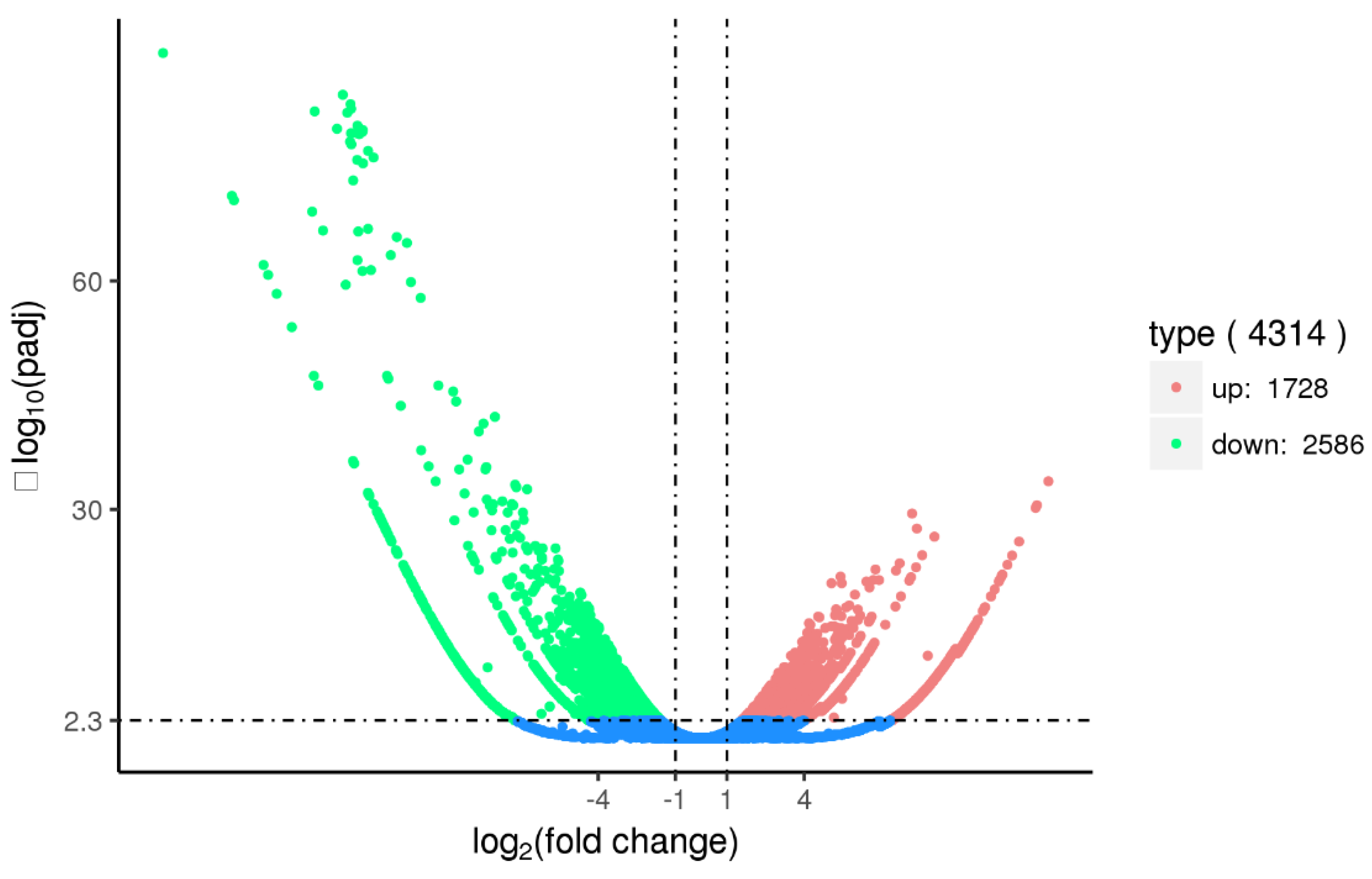
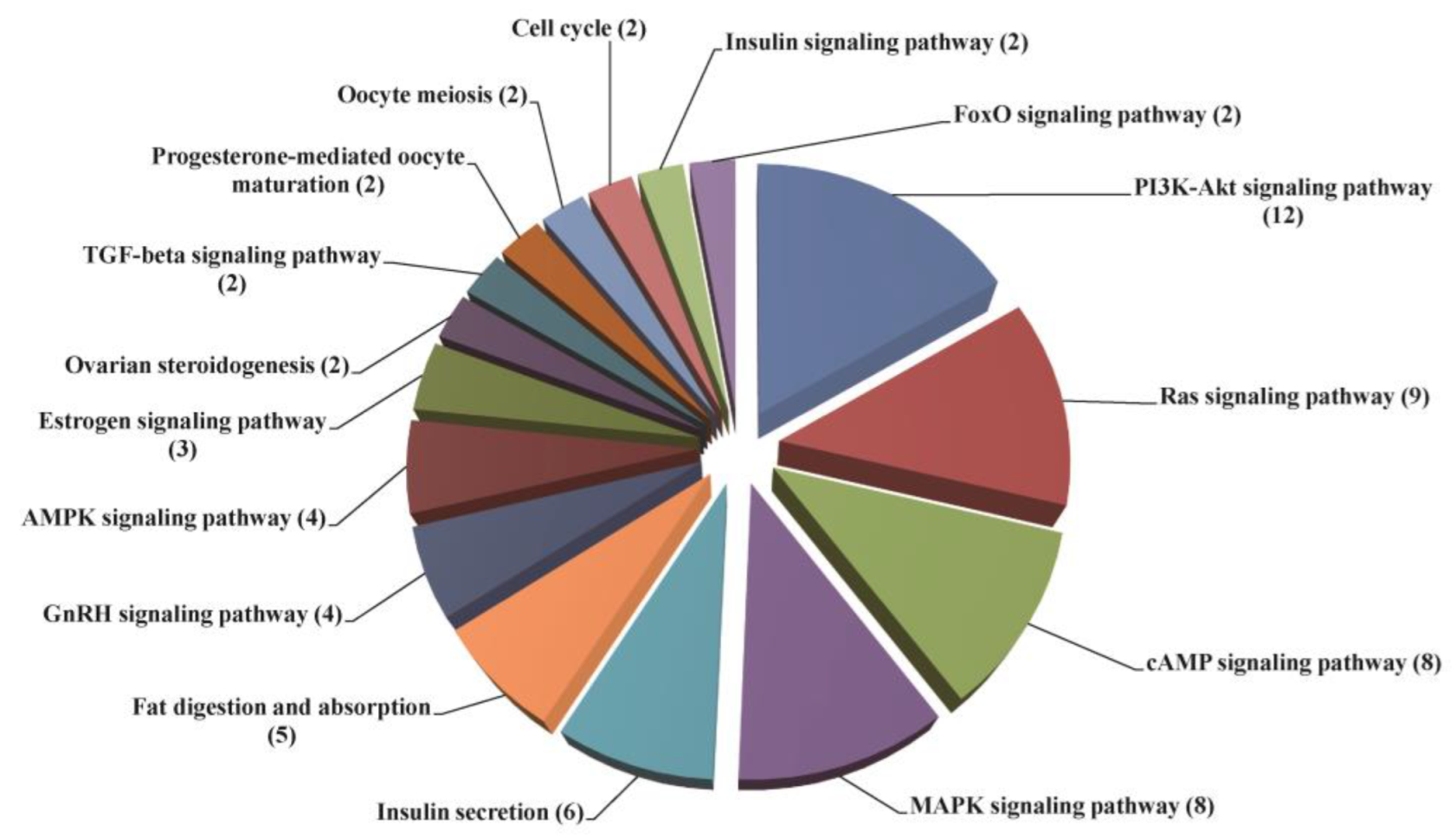
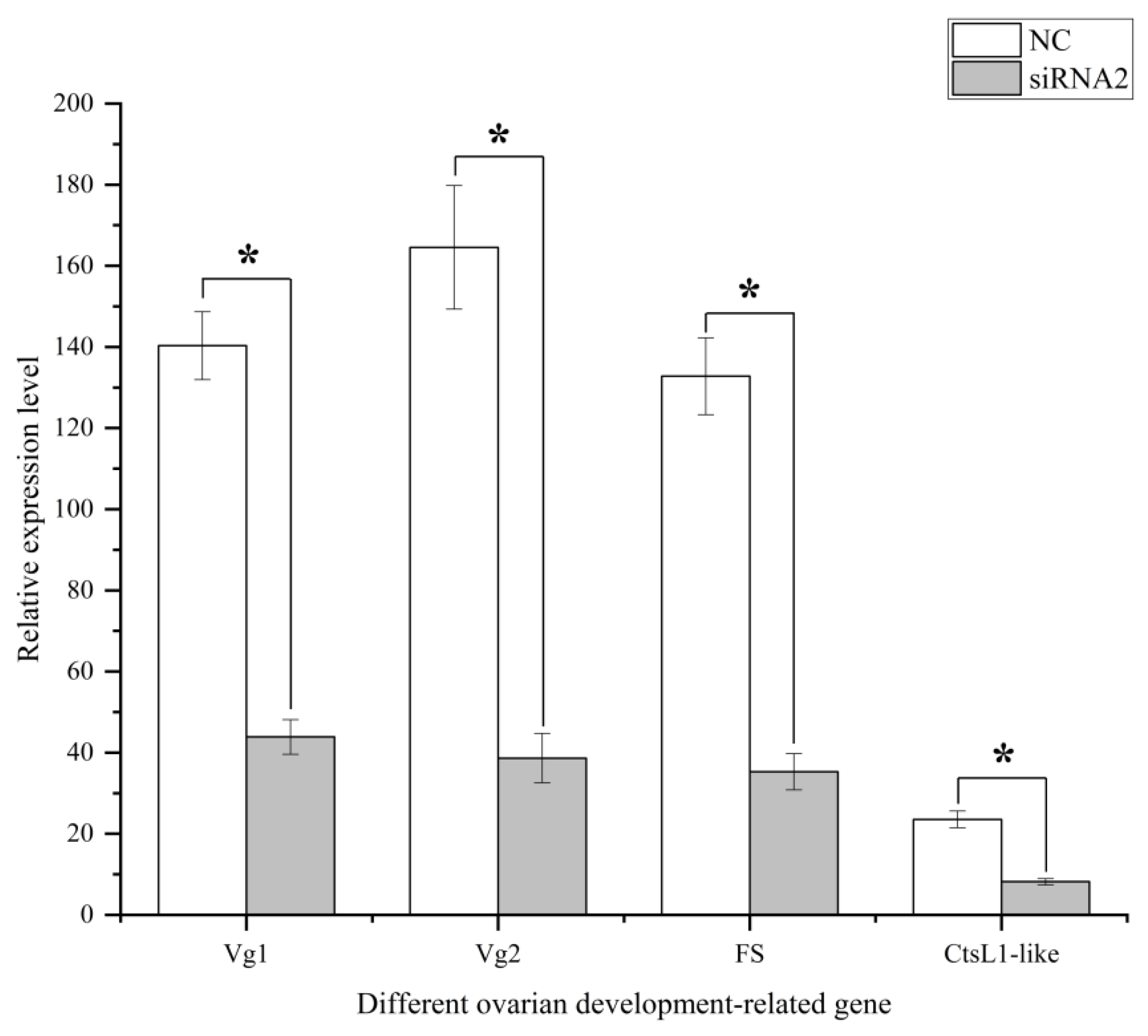
2.6. Transcriptome Analysis of the Ovary after the Silencing of SjILPR
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cloning and Sequence Analysis of SjILPR
4.3. Phylogenetic Analysis of SjILPR
4.4. Subcellular Localization of SjILPR
4.5. Expression of SjILPR in Different Tissue
4.6. Expression of SjILPR during the Different Ovarian Development Stages of Female S. japonica
4.7. RNA Interference (RNAi) In Vivo
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lü, Z.; Liu, W.; Liu, L.; Shi, H.; Ping, H.; Wang, T.; Chi, C.; Wu, C.; Chen, C.-H.; Shen, K.-N.; et al. De novo assembly and comparison of the ovarian transcriptomes of the common Chinese cuttlefish (Sepiella japonica) with different gonadal development. Genom. Data 2016, 7, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Lü, Z.-M.; Liu, W.; Liu, L.-Q.; Wang, T.-M.; Shi, H.-L.; Ping, H.-L.; Chi, C.-F.; Yang, J.-W.; Wu, C.-W. Cloning, characterization, and expression profile of estrogen receptor in common Chinese cuttlefish, Sepiella japonica. J. Exp. Zool. Part A 2016, 325, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Lü, Z.; Zhu, K.; Pang, Z.; Liu, L.; Jiang, L.; Liu, B.; Shi, H.; Ping, H.; Chi, C.; Gong, L. Identification, characterization and mRNA transcript abundance profiles of estrogen related receptor (ERR) in Sepiella japonica imply its possible involvement in female reproduction. Anim. Reprod. Sci. 2019, 211, 106–231. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Lü, Z.M.; Wang, M.T.; Gong, L.; Liu, B.J.; Liu, L.Q. Characterization, relative abundances of mRNA transcripts, and subcellular localization of two forms of membrane progestin receptors (MPRs) in the common chinese cuttlefish, sepiella japonica. Anim. Reprod. Sci. 2019, 208, 106–107. [Google Scholar] [CrossRef]
- Gricourt, L.; Mathieu, M.; Kellner, K. An insulin-like system involved in the control of Pacific oyster Crassostrea gigas reproduction: hrIGF-1 effect on germinal cell proliferation and maturation associated with expression of an homologous insulin receptor-related receptor. Aquaculture 2006, 251, 85–98. [Google Scholar] [CrossRef]
- Riehle, M.A.; Brown, M.R. Insulin receptor expression during development and a reproductive cycle in the ovary of the mosquito Aedes aegypti. Cell Tissue. Res. 2002, 308, 409–420. [Google Scholar] [CrossRef]
- Brown, M.R.; Clark, K.D.; Gulia, M.; Zhao, Z.; Garczynski, S.F.; Crim, J.W.; Suderman, R.J.; Strand, M.R. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2008, 105, 5716–5721. [Google Scholar] [CrossRef]
- Badisco, L.; Marchal, E.; Wielendaele, P.V.; Verlinden, H.; Vleugels, R.; Broeck, J.V. RNA interference of insulin-related peptide and neuroparsins affects vitellogenesis in the desert locust schistocerca gregaria. Peptides 2011, 32, 573–580. [Google Scholar] [CrossRef]
- Badisco, L.; Claeys, I.; Van Hiel, M.B.; Clynen, E.; Huybrechts, J.; Vandersmissen, T.; Van Soest, S.; Bosch, L.V.; Simonet, G.; Broeck, J.V. Purification and characterization of an insulin-related peptide in the desert locust, Schistocerca gregaria: Immunolocalization, cDNA cloning, transcript profiling and interaction with neuroparsin. J. Mol. Endocrinol. 2008, 40, 137–150. [Google Scholar] [CrossRef]
- Riehle, M.A.; Brown, M.R. Molecular analysis of the serine/threonine kinase Akt and its expression in the mosquito Aedes aegypti. Insect Mol. Biol. 2003, 12, 225–232. [Google Scholar] [CrossRef]
- Smit, A.B.; Vreugdenhil, E.; Ebberink, R.H.; Geraerts, W.P.; Klootwijk, J.; Joosse, J. Growth-controlling molluscan neurons produce the precursor of an insulin-related peptide. Nature 1988, 331, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Floyd, P.D.; Li, L.; Rubakhin, S.S.; Sweedler, J.V.; Horn, C.C.; Kupfermann, I.; Alexeeva, V.Y.; Ellis, T.A.; Dembrow, N.C.; Weiss, K.R.; et al. Insulin prohormone processing, distribution, and relation to metabolism in Aplysia californica. J. Neurosci. 1999, 19, 7732–7741. [Google Scholar] [CrossRef] [PubMed]
- Hamano, K.; Awaji, M.; Usuki, H. cDNA structure of an insulin-related peptide in the Pacific oyster and seasonal changes in the gene expression. J. Endocrinol. 2005, 187, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Cherif-Feildel, M.; Berthelin, C.H.; Adeline, B.; Rivière, G.; Favrel, P.; Kellner, K. Molecular evolution and functional characterization of insulin related peptides in molluscs: Contributions of Crassostrea gigas genomic and transcriptomic-wide screening. Gen. Comp. Endocrinol. 2019, 271, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Leevers, S.J. Growth control: Invertebrate insulin surprises! Curr. Biol. 2001, 11, 209–212. [Google Scholar] [CrossRef]
- Roovers, E.; Vincent, M.E.; van Kesteren, E.; Geraerts, W.P.; Planta, R.J.; Vreugdenhil, E.; van Heerikhuizen, H. Characterization of a putative molluscan insulin-related peptide receptor. Gene 1995, 162, 181–188. [Google Scholar] [CrossRef]
- Jonas, E.A.; Knox, R.J.; Kaczmarek, L.K.; Schwartz, J.H.; Solomon, D.H. Insulin receptor in aplysia neurons: Characterization, molecular cloning, and modulation of ion currents. J. Neurosc. 1996, 16, 1645–1658. [Google Scholar] [CrossRef]
- Lardans, V.; Coppin, J.F.; Jérme Vicogne, A.E.; Delcroix, M.; Dissous, C. Characterization of an insulin receptor-related receptor in biomphalaria glabrata embryonic cells. Biochim. Biophys. Acta (BBA)-Biomembr. 2001, 1510, 321–329. [Google Scholar] [CrossRef]
- Shi, Y.; Guan, Y.Y.; He, M.X. Molecular identification of insulin-related peptide receptor and its potential role in regulating development in Pinctada fucata. Aquaculture 2013, 408, 118–127. [Google Scholar] [CrossRef][Green Version]
- Zhao, Z.H.; Wang, Z.M.; Deng, Y.W.; Yang, C.Y.; Li, J.H. Gene Cloning and expression pattern analysis of insulin-related peptide receptor from Pinctada maxima. J. Guangdong Ocean. Univ. 2020, 40, 34–42. [Google Scholar]
- Gricourt, L.; Bonnec, G.; Boujard, D.; Mathieu, M.; Kellner, K. Insulin-like system and growth regulation in the Pacific oyster Crassostrea gigas: hrIGF-1 effect on protein synthesis of mantle edge cells and expression of an homologous insulin receptor-related receptor. Gen. Comp. Endocrinol. 2003, 134, 44–56. [Google Scholar] [CrossRef]
- Jouaux, A.; Franco, A.; Heude-Berthelin, C.; Sourdaine, P.; Blin, J.; Mathieu, M.; Kellner, K. Identification of Ras, Pten and p70S6K homologs in the Pacific oyster Crassostrea gigas and diet control of insulin pathway. Gen. Comp. Endocr. 2012, 176, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Lü, Z.M.; Yao, C.H.; Zhao, S.J.; Zhang, Y.; Gong, L.; Liu, B.J.; Liu, L.Q. Characterization of Insulin-like Peptide (ILP) and its potential role in ovarian development of the cuttlefish Sepiella japonica. Curr. Issues Mol. Biol. 2022, 44, 2490–2504. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.J.; Steiner, D.F. Insulin through the ages: Phylogeny of a growth promoting and metabolic regulatory hormone. Am. Zool. 2000, 40, 213–222. [Google Scholar] [CrossRef]
- Ottensmeyer, F.P.; Beniac, D.R.; Luo, R.Z.T.; Yip, C.C. Mechanism of transmembrane signaling: Insulin binding and the insulin receptor. Biochemistry 2000, 39, 12103–12112. [Google Scholar] [CrossRef]
- Boucher, P.; Ditlecadet, D.; Dube, C.; Dufresne, F. Unusual duplication of the insulin-like receptor in the crustacean Daphnia pulex. BMC Evol. Biol. 2010, 10, 305. [Google Scholar] [CrossRef]
- Sharabi, O.; Manor, R.; Weil, S.; Aflalo, E.; Lezer, Y.; Levy, T.; Aizen, J.; Ventura, T.; Mather, P.B.; Khalaila, I.; et al. Identification and characterization of an insulin-like receptor involved in Crustacean Reproduction. Endocrinology 2016, 157, 928–941. [Google Scholar] [CrossRef]
- Keegan, A.D.; Nelms, K.; White, M.F.; Wang, L.M.; Pierce, J.H.; Paul, W.E. An IL-4 receptor region containing an insulin receptor motif is important for IL-4 mediated IRS-1 phosphorylation and cell growth. Cell 1994, 76, 811–820. [Google Scholar] [CrossRef]
- Denley, A.; Cosgrove, L.J.; Booker, G.W.; Wallace, J.C.; Forbes, B.E. Molecular interactions of the IGF system. Cytokine Growth Factor Rev. 2005, 16, 421–439. [Google Scholar] [CrossRef]
- Lee, J.; Miyazaki, M.; Romeo, G.R.; Shoelson, S.E. Insulin receptor activation with transmembrane domain ligands. J. Biol. Chem. 2014, 289, 19769–19777. [Google Scholar] [CrossRef]
- Hanks, S.K.; Quinn, A.M.; Hunter, T. The protein kinase family: Conserved features and deduced phylogeny of the catalytic domains. Science 1988, 241, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y.; Ullrich, A. Growth factor receptor tyrosine kinases. Annu. Rev. Biochem. 1988, 57, 443–478. [Google Scholar] [CrossRef] [PubMed]
- Klover, P.J.; Mooney, R.A. Hepatocytes: Critical for glucose homeostasis. Int. J. Biochem. Cell Biol. 2004, 36, 753–758. [Google Scholar] [CrossRef]
- Cai, W.J.; Liang, X.F.; Yuan, X.C.; Li, A.X.; He, Y.H.; He, S. Genomic organization and expression of insulin receptors in grass carp, Ctenopharyngodon idellus. Comp. Biochem. Phys. B 2016, 194–195, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Berthelin, C.; Kellner, K.; Mathieu, M. Histological characterization and glucose incorporation into glycogen of the Pacific oyster Crassostrea gigas storage cells. Mar. Biotechnol. 2000, 2, 136–145. [Google Scholar] [CrossRef]
- Gomez-Mendikute, A.; Elizondo, M.; Venier, P.; Cajaraville, M.P. Characterization of mussel gill cells in vivo and in vitro. Cell Tissue Res. 2005, 321, 131–140. [Google Scholar] [CrossRef]
- Ni, J.; Zeng, Z.; Han, G.; Huang, H.; Ke, C. Cloning and characterization of the follistatin gene from crassostrea angulata and its expression during the reproductive cycle. Comp. Biochem. Phys. B 2012, 163, 246–253. [Google Scholar] [CrossRef]
- Chen, C.; Li, H.-W.; Ku, W.-L.; Lin, C.-J.; Chang, C.-F.; Wu, G.-C. Two distinct vitellogenin genes are similar in function and expression in the bigfin reef squid Sepioteuthis lessoniana. Biol. Reprod. 2018, 5, 5. [Google Scholar] [CrossRef]
- Gao, J.; Wang, X.W.; Zou, Z.H.; Jia, X.W.; Wang, Y.L.; Zhang, Z.P. Transcriptome analysis of the differences in gene expression between testis and ovary in green mud crab (scylla paramamosain). BMC Genom. 2014, 15, 585. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Han, T.; Liu, T.; Wang, C.; Xiao, J.; Mu, C.; Li, R.; Yu, F.; Shi, H. Ovarian transcriptome analysis of Portunus trituberculatus provides insights into genes expressed during phase III and IV development. PLoS ONE 2015, 10, e0138862. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Q.; Wu, K.; Wei, Z.; Zhou, Z.; Liu, X. De novo transcriptome sequencing and comparative analysis to discover genes involved in ovarian maturity in Strongylocentrotus nudus. Comp. Biochem. Phys. D 2017, 23, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, L.; Jiang, W.; Abasubong, K.P.; Zhang, C.; Zhang, D.; Li, X.; Jiang, G.; Chi, C.; Liu, W. Effects of dietary icariin supplementation on the ovary development-related transcriptome of chinese mitten crab (Eriocheir sinensis). Comp. Biochem. Physiol. Part D 2021, 4, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.G.; Cheng, Y.X.; Sui, L.Y.; Zeng, C.S.; Southgate, P.C.; Yang, X.Z. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of the Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture 2007, 273, 602–613. [Google Scholar] [CrossRef]
- Okuno, A.; Yang, W.J.; Jayasankar, V.; Saido-Sakanaka, H.; Huong, D.T.; Jasmani, S.; Atmomarsono, M.; Subramoniam, T.; Tsutsui, N.; Ohira, T.; et al. Deduced primary structure of vitellogenin in the giant freshwater prawn, Macrobrachium rosenbergii, and yolk processing during ovarian maturation. J. Exp. Zool. 2002, 292, 417–429. [Google Scholar] [CrossRef]
- Chen, L.Q.; Jiang, H.B.; Zhou, Z.L.; Li, K.; Deng, G.Y.; Liu, Z.J. Purification of vitellin from the ovary of Chinese mitten-handed crab (Eriocheir sinensis) and development of an antivitellin ELISA. Comp. Biochem. Physiol. Part B 2004, 138, 305–311. [Google Scholar] [CrossRef]
- Terra, W.R.; Dias, R.O.; Ferreira, C. Recruited lysosomal enzymes as major digestive enzymes in insects. Biochem. Soc. Trans. 2019, 47, 615–623. [Google Scholar] [CrossRef]
- Tingaud-Sequeira, A.; Cerdà, J. Phylogenetic relationships and gene expression pattern of three different cathepsin L. (Ctsl) isoforms in zebrafish: Ctsla is the putative yolk processing enzyme. Gene 2007, 386, 98–106. [Google Scholar] [CrossRef]
- Zhu, J.; Fu, H.; Qiao, H.; Jin, S.; Xiong, Y. Expression and functional analysis of cathepsin L1 in ovarian development of the oriental river prawn, macrobrachium nipponense. Aquacult. Rep. 2021, 20, 100724. [Google Scholar] [CrossRef]
- Jiang, N.; Jin, X.; He, J.Y.; Yin, Z. The roles of follistatin 1 in regulation of zebrafish fecundity and sexual differentiation. Biol. Reprod. 2012, 87, 54. [Google Scholar] [CrossRef]
- Schultz, J.; Milpetz, F.; Bork, P.; Ponting, C.P. SMART, a simple modular architecture research tool: Identification of signaling domains. Proc. Natl. Acad. Sci. USA 1998, 95, 5857–5864. [Google Scholar] [CrossRef]
- Zhou, X.G.; Zheng, W.; Li, Y.; Pearce, R.; Zhang, C.X.; Bell, E.W.; Zhang, G.J.; Zhang, Y. I-TASSER-MTD: A deep-learning based platform for multidomain protein structure and function prediction. Nat. Protoc. 2022, 17, 2326–2353. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dudley, J.; Nei, M.; Tamura, K. MEGA: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 2008, 9, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gore, S.R.; Harms, C.A.; Kukanich, B.; Forsythe, J.; Lewbart, G.A.; Papich, M.G. Enrofloxacin pharmacokinetics in the European cuttlefish, Sepia officinalis, after a single i.v. injection and bath administration. J. Vet. Pharmacol. Ther. 2005, 28, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Z.; Tao, C.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the kegg orthology (ko) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
| Sample | Raw_Reads | Clean_Reads | Clean_Bases (Gb) | Q20 (%) | Q30 (%) | GC_pct (%) | Mapped_Reads |
|---|---|---|---|---|---|---|---|
| siRNA2-injected | 71733238 | 70292362 | 10.54 | 96.77 | 91.78 | 38.48 | 55027712 |
| Control | 68396864 | 67258492 | 10.09 | 96.85 | 91.92 | 38.17 | 51430472 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lü, Z.; Liu, Y.; Yan, J.; Zhang, Y.; Gong, L.; Liu, B.; Liu, J.; Xu, Z.; Liu, L. Insulin-like Peptide Receptor (ILPR) in the Cuttlefish Sepiella japonica: Characterization, Expression, and Regulation of Reproduction. Int. J. Mol. Sci. 2022, 23, 12903. https://doi.org/10.3390/ijms232112903
Lü Z, Liu Y, Yan J, Zhang Y, Gong L, Liu B, Liu J, Xu Z, Liu L. Insulin-like Peptide Receptor (ILPR) in the Cuttlefish Sepiella japonica: Characterization, Expression, and Regulation of Reproduction. International Journal of Molecular Sciences. 2022; 23(21):12903. https://doi.org/10.3390/ijms232112903
Chicago/Turabian StyleLü, Zhenming, Yantao Liu, Jun Yan, Yao Zhang, Li Gong, Bingjian Liu, Jing Liu, Zhijin Xu, and Liqin Liu. 2022. "Insulin-like Peptide Receptor (ILPR) in the Cuttlefish Sepiella japonica: Characterization, Expression, and Regulation of Reproduction" International Journal of Molecular Sciences 23, no. 21: 12903. https://doi.org/10.3390/ijms232112903
APA StyleLü, Z., Liu, Y., Yan, J., Zhang, Y., Gong, L., Liu, B., Liu, J., Xu, Z., & Liu, L. (2022). Insulin-like Peptide Receptor (ILPR) in the Cuttlefish Sepiella japonica: Characterization, Expression, and Regulation of Reproduction. International Journal of Molecular Sciences, 23(21), 12903. https://doi.org/10.3390/ijms232112903






