Could Targeted Pharmacotherapies Exert a “Disease Modification Effect” in Patients with Chronic Plaque Psoriasis?
Abstract
1. Introduction
2. Immunopathogenesis of Psoriasis
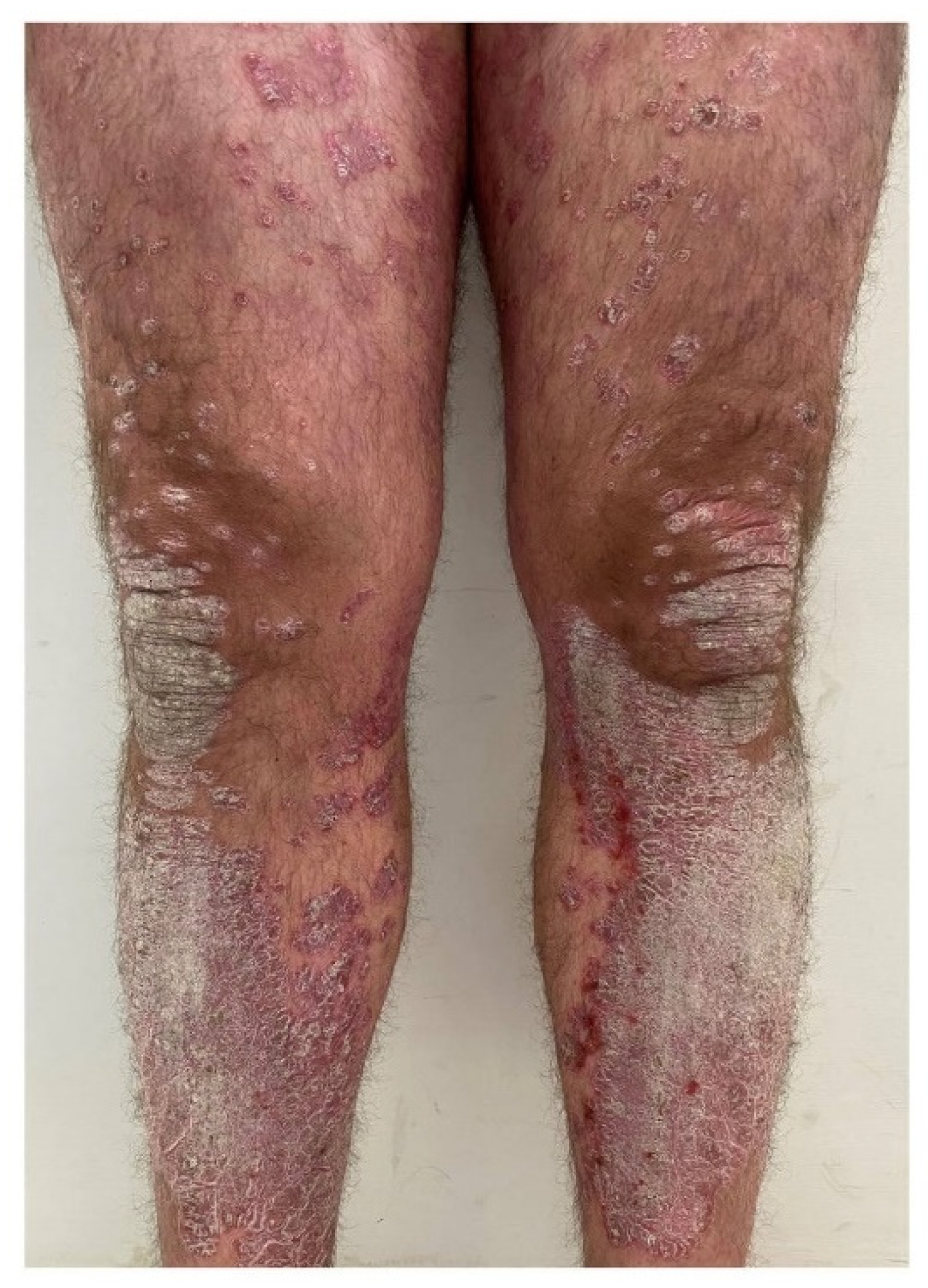
2.1. Immune Memory Characterizing Psoriatic Skin
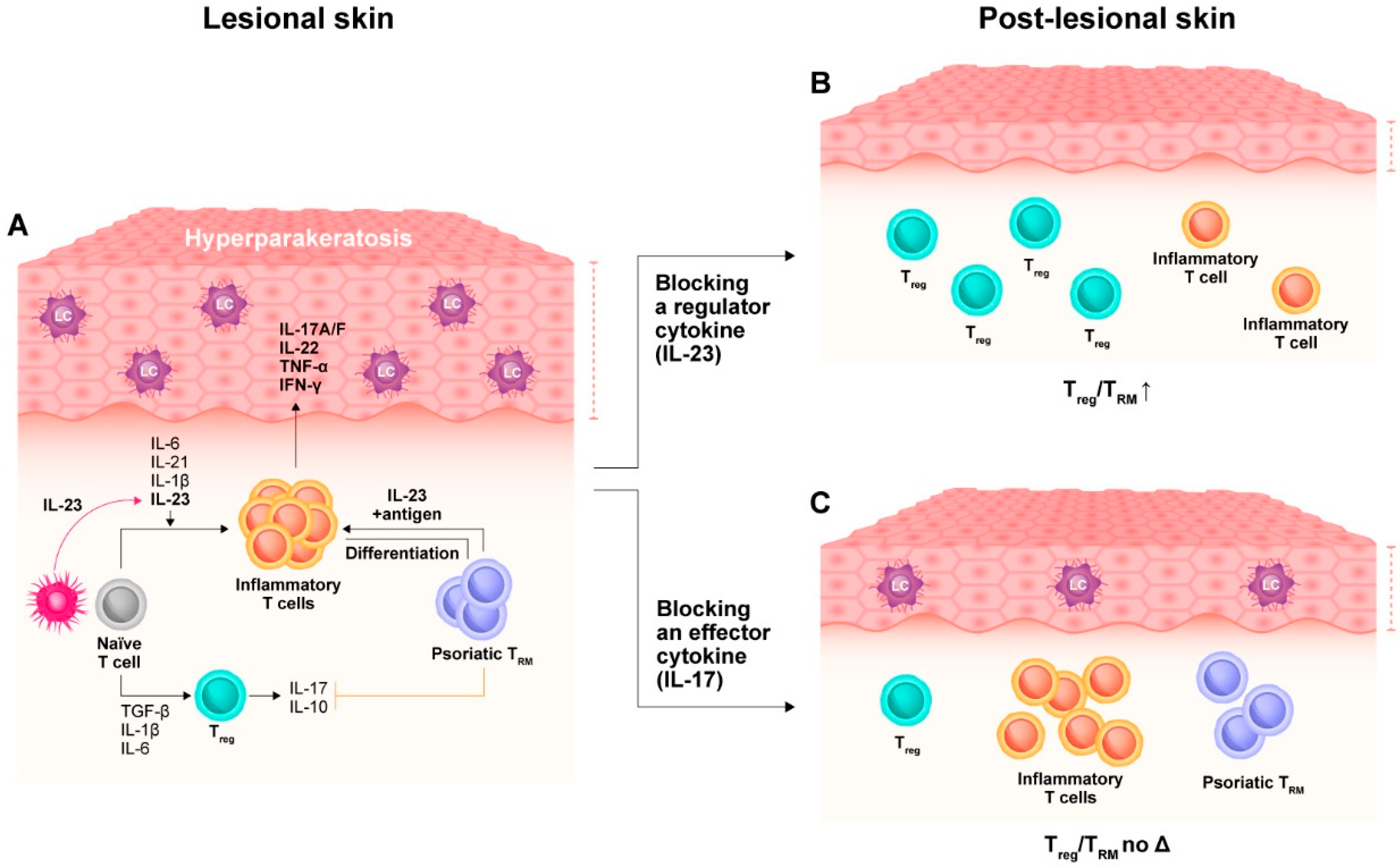
2.2. Molecular Scarring in Post-Lesional Psoriatic Skin
3. Impact of Targeted Pharmacotherapies on the Tissue-Resident Memory Compartment
4. TRM Compartment in the Pathophysiology of Psoriatic Arthritis
5. Targeted Pharmacotherapies May Prevent the Transition from Psoriasis to PsA
5.1. Transition from Psoriasis to PsA
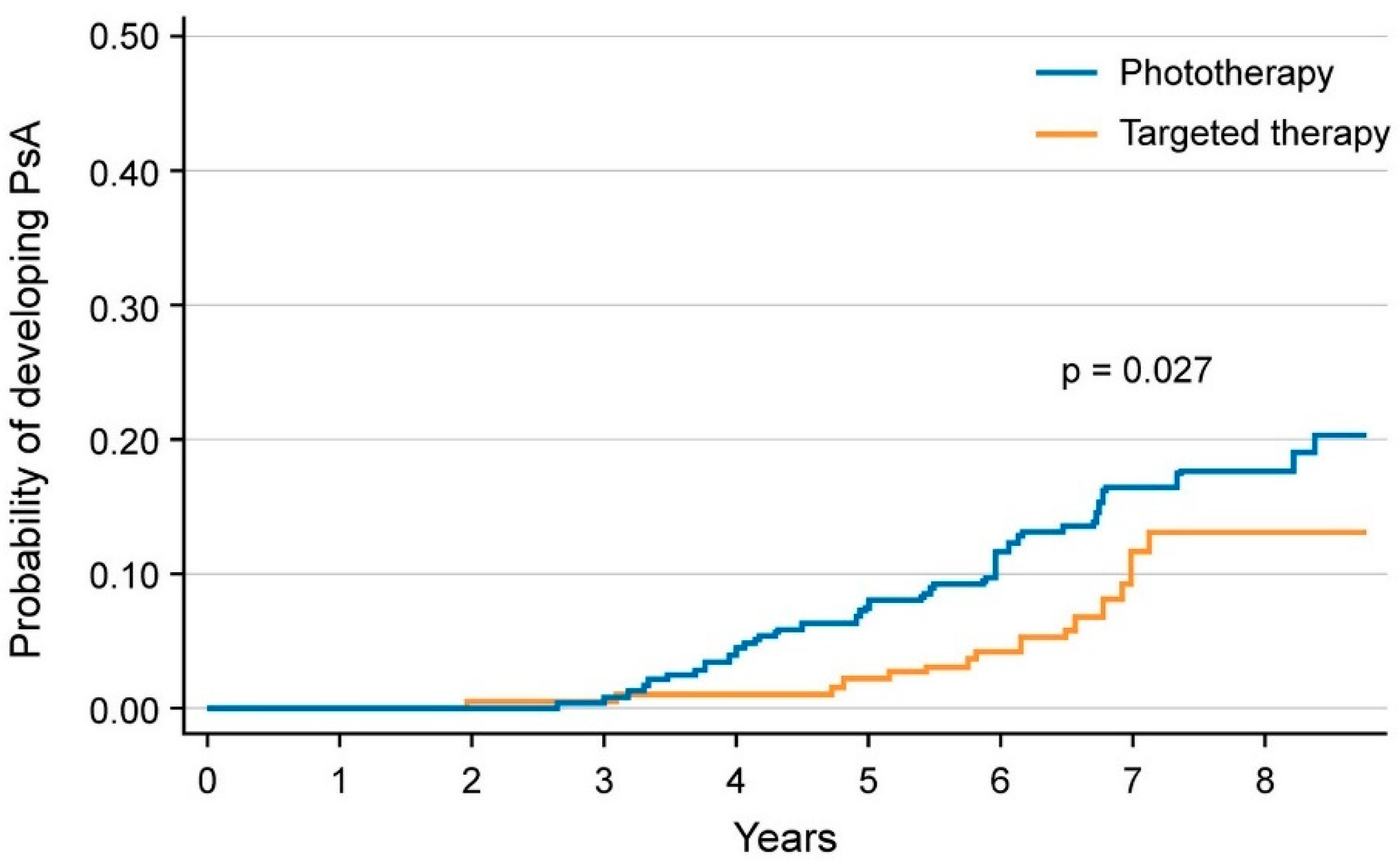
5.2. Reducing the Risk of Developing PsA in Patients with Psoriasis
6. Targeted Pharmacotherapies May Reduce the Burden of the Cumulative Life Course Impairment in Psoriatic Patients
6.1. Social Stigma
6.2. Socio-Economic Status
6.3. Young Patients
6.4. The Potential Role of Targeted Pharmacotherapies
7. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davidovici, B.B.; Sattar, N.; Jörg, P.C.; Puig, L.; Emery, P.; Barker, J.N.; van de Kerkhof, P.; Ståhle, M.; Nestle, F.O.; Girolomoni, G.; et al. Psoriasis and Systemic Inflammatory Diseases: Potential Mechanistic Links between Skin Disease and Co-Morbid Conditions. J. Investig. Dermatol. 2010, 130, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Suárez-Fariñas, M.; Fuentes-Duculan, J.; Lowes, M.A.; Krueger, J.G. Resolved Psoriasis Lesions Retain Expression of a Subset of Disease-Related Genes. J. Investig. Dermatol. 2011, 131, 391–400. [Google Scholar] [CrossRef]
- Tomalin, L.E.; Russell, C.B.; Garcet, S.; Ewald, D.A.; Klekotka, P.; Nirula, A.; Norsgaard, H.; Suàrez-Fariñas, M.; Krueger, J.G. Short-term transcriptional response to IL-17 receptor-A antagonism in the treatment of psoriasis. J. Allergy Clin. Immunol. 2019, 145, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Matos, T.R.; O’Malley, J.T.; Lowry, E.L.; Hamm, D.; Kirsch, I.R.; Robins, H.S.; Kupper, T.S.; Krueger, J.G.; Clark, R.A. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17–producing αβ T cell clones. J. Clin. Investig. 2017, 127, 4031–4041. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, S.; Wikén, M.; Blomqvist, L.; Nylén, S.; Talme, T.; Ståhle, M.; Eidsmo, L. Epidermal Th22 and Tc17 Cells Form a Localized Disease Memory in Clinically Healed Psoriasis. J. Immunol. 2014, 192, 3111–3120. [Google Scholar] [CrossRef]
- Mashiko, S.; Edelmayer, R.M.; Bi, Y.; Olson, L.M.; Wetter, J.B.; Wang, J.; Maari, C.; Proulx, E.S.-C.; Kaimal, V.; Li, X.; et al. Persistence of Inflammatory Phenotype in Residual Psoriatic Plaques in Patients on Effective Biologic Therapy. J. Investig. Dermatol. 2020, 140, 1015–1025.e4. [Google Scholar] [CrossRef]
- Kim, B.R.; Kim, M.; Yang, S.; Choi, C.W.; Lee, K.S.; Youn, S.W. Persistent expression of interleukin-17 and downstream effector cytokines in recalcitrant psoriatic lesions after ustekinumab treatment. J. Dermatol. 2021, 48, 876–882. [Google Scholar] [CrossRef]
- Brodmerkel, C.; Li, K.; Garcet, S.; Hayden, K.; Chiricozzi, A.; Novitskaya, I.; Fuentes-Duculan, J.; Suarez-Farinas, M.; Campbell, K.; Krueger, J.G. Modulation of inflammatory gene transcripts in psoriasis vulgaris: Differences between ustekinumab and etanercept. J. Allergy Clin. Immunol. 2019, 143, 1965–1969. [Google Scholar] [CrossRef]
- Sérézal, I.G.; Classon, C.; Cheuk, S.; Barrientos-Somarribas, M.; Wadman, E.; Martini, E.; Chang, D.; Landén, N.X.; Ehrström, M.; Nylén, S.; et al. Resident T Cells in Resolved Psoriasis Steer Tissue Responses that Stratify Clinical Outcome. J. Investig. Dermatol. 2018, 138, 1754–1763. [Google Scholar] [CrossRef]
- Mehta, H.; Mashiko, S.; Angsana, J.; Rubio, M.; Hsieh, Y.-C.M.; Maari, C.; Reich, K.; Blauvelt, A.; Bissonnette, R.; Muñoz-Elías, E.J.; et al. Differential Changes in Inflammatory Mononuclear Phagocyte and T-Cell Profiles within Psoriatic Skin during Treatment with Guselkumab vs. Secukinumab. J. Investig. Dermatol. 2021, 141, 1707–1718.e9. [Google Scholar] [CrossRef] [PubMed]
- Savage, L.; Goodfield, M.; Horton, L.; Watad, A.; Hensor, E.; Emery, P.; Wakefield, R.; Wittmann, M.; McGonagle, D. Regression of Peripheral Subclinical Enthesopathy in Therapy-Naive Patients Treated With Ustekinumab for Moderate-to-Severe Chronic Plaque Psoriasis: A Fifty-Two–Week, Prospective, Open-Label Feasibility Study. Arthritis Rheumatol. 2019, 71, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Kampylafka, E.; Simon, D.; D’Oliveira, I.; Linz, C.; Lerchen, V.; Englbrecht, M.; Rech, J.; Kleyer, A.; Sticherling, M.; Schett, G.; et al. Disease interception with interleukin-17 inhibition in high-risk psoriasis patients with subclinical joint inflammation—Data from the prospective IVEPSA study. Arthritis Res. Ther. 2019, 21, 178. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Bellinato, F.; Targher, G.; Idolazzi, L.; Girolomoni, G. Biological disease-modifying antirheumatic drugs may mitigate the risk of psoriatic arthritis in patients with chronic plaque psoriasis. Ann. Rheum. Dis. 2022, 81, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Felquer, M.L.A.; LoGiudice, L.; Galimberti, M.L.; Rosa, J.; Mazzuoccolo, L.; Soriano, E.R. Treating the skin with biologics in patients with psoriasis decreases the incidence of psoriatic arthritis. Ann. Rheum. Dis. 2022, 81, 74–79. [Google Scholar] [CrossRef]
- Solmaz, D.; Ehlebracht, A.; Karsh, J.; Bakirci, S.; McGonagle, D.; Aydin, S.Z. Evidence that systemic therapies for psoriasis may reduce psoriatic arthritis occurrence. Clin. Exp. Rheumatol. 2020, 38, 257–261. [Google Scholar] [CrossRef]
- Rosenthal, Y.S.; Schwartz, N.; Sagy, I.; Pavlovsky, L. Incidence of Psoriatic Arthritis Among Patients Receiving Biologic Treatments for Psoriasis: A Nested Case–Control Study. Arthritis Rheumatol. 2022, 74, 237–243. [Google Scholar] [CrossRef]
- Karpińska-Mirecka, A.; Bartosińska, J.; Krasowska, D. The effects of selected biologics and a small molecule on Health-Related Quality of Life in adult plaque psoriasis patients: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241604. [Google Scholar] [CrossRef]
- Ayan, G.; Farisogulları, B.; Bilgin, E.; Bolek, E.C.; KübraYardımcı, G.; Duran, E.; Ozsoy, Z.; Uzun, G.S.; Kilic, L.; Akdoğan, A.; et al. Anxiety levels before biologic initiation and changes with treatment in patients with psoriatic arthritis: HUR-BIO biologic registry results. Clin. Rheumatol. 2022, 41, 1439–1446. [Google Scholar] [CrossRef]
- Strober, B.; Gooderham, M.; de Jong, E.M.; Kimball, A.; Langley, R.G.; Lakdawala, N.; Goyal, K.; Lawson, F.; Langholff, W.; Hopkins, L.; et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J. Am. Acad. Dermatol. 2018, 78, 70–80. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Romanelli, P.; Volpe, E.; Borsellino, G.; Romanelli, M. Scanning the Immunopathogenesis of Psoriasis. Int. J. Mol. Sci. 2018, 19, 179. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, A.; Saraceno, R.; Chimenti, M.S.; Guttman-Yassky, E.; Krueger, J.G. Role of IL-23 in the pathogenesis of psoriasis: A novel potential therapeutic target? Expert Opin. Ther. Targets 2014, 18, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, H.L.; Kagami, S.; Phillips, K.G.; Kurtz, S.E.; Jacques, S.L.; Blauvelt, A. IL-23–Mediated Psoriasis-Like Epidermal Hyperplasia Is Dependent on IL-17A. J. Immunol. 2011, 186, 1495–1502. [Google Scholar] [CrossRef]
- Gottlieb, S.L.; Gilleaudeau, P.; Johnson, R.; Estes, L.; Woodworth, T.G.; Gottlieb, A.B.; Krueger, J.G. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat. Med. 1995, 1, 442–447. [Google Scholar] [CrossRef]
- Chiricozzi, A. Pathogenic role of IL-17 in psoriasis and psoriatic arthritis. Actas Dermosifiliogr. 2014, 105 (Suppl. 1), 9–20. [Google Scholar] [CrossRef]
- Conrad, C.; Boyman, O.; Tonel, G.; Tun-Kyi, A.; Laggner, U.; De Fougerolles, A.; Kotelianski, V.; Gardner, H.; Nestle, F.O. α1β1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat. Med. 2007, 13, 836–842. [Google Scholar] [CrossRef]
- Boyman, O.; Hefti, H.P.; Conrad, C.; Nickoloff, B.J.; Suter, M.; Nestle, F.O. Spontaneous Development of Psoriasis in a New Animal Model Shows an Essential Role for Resident T Cells and Tumor Necrosis Factor-α. J. Exp. Med. 2004, 199, 731–736. [Google Scholar] [CrossRef]
- Di Meglio, P.; Villanova, F.; Navarini, A.A.; Mylonas, A.; Tosi, I.; Nestle, F.O.; Conrad, C. Targeting CD8+ T cells prevents psoriasis development. J. Allergy Clin. Immunol. 2016, 138, 274–276.e6. [Google Scholar] [CrossRef]
- Lande, R.; Botti, E.; Jandus, C.; Dojcinovic, D.; Fanelli, G.; Conrad, C.; Chamilos, G.; Feldmeyer, L.; Marinari, B.; Chon, S.; et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat. Commun. 2014, 5, 5621. [Google Scholar] [CrossRef]
- Arakawa, A.; Siewert, K.; Stöhr, J.; Besgen, P.; Kim, S.-M.; Rühl, G.; Nickel, J.; Vollmer, S.; Thomas, P.; Krebs, S.; et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J. Exp. Med. 2015, 212, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Res, P.C.M.; Piskin, G.; de Boer, O.; Van Der Loos, C.M.; Teeling, P.; Bos, J.D.; Teunissen, M.B.M. Overrepresentation of IL-17A and IL-22 Producing CD8 T Cells in Lesional Skin Suggests Their Involvement in the Pathogenesis of Psoriasis. PLoS ONE 2010, 5, e14108. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Kikuchi, T.; Fuentes-Duculan, J.; Cardinale, I.; Zaba, L.C.; Haider, A.S.; Bowman, E.P.; Krueger, J.G. Psoriasis Vulgaris Lesions Contain Discrete Populations of Th1 and Th17 T Cells. J. Investig. Dermatol. 2008, 128, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Volpe, E.; Servant, N.; Zollinger, R.; I Bogiatzi, S.; Hupe, P.; Barillot, E.; Soumelis, V. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human TH-17 responses. Nat. Immunol. 2008, 9, 650–657. [Google Scholar] [CrossRef]
- Van Der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.; et al. Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice Is Mediated via the IL-23/IL-17 Axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Chan, J.R.; Blumenschein, W.; Murphy, E.; Diveu, C.; Wiekowski, M.; Abbondanzo, S.; Lucian, L.; Geissler, R.; Brodie, S.; Kimball, A.; et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2–dependent mechanisms with implications for psoriasis pathogenesis. J. Exp. Med. 2006, 203, 2577–2587. [Google Scholar] [CrossRef]
- Conrad, C.; Meller, S.; Gilliet, M. Plasmacytoid dendritic cells in the skin: To sense or not to sense nucleic acids. Semin. Immunol. 2009, 21, 101–109. [Google Scholar] [CrossRef]
- Johnson-Huang, L.M.; McNutt, N.S.; Krueger, J.G.; Lowes, M.A. Cytokine-Producing Dendritic Cells in the Pathogenesis of Inflammatory Skin Diseases. J. Clin. Immunol. 2009, 29, 247–256. [Google Scholar] [CrossRef]
- Catapano, M.; Vergnano, M.; Romano, M.; Mahil, S.K.; Choon, S.-E.; Burden, A.D.; Young, H.S.; Carr, I.M.; Lachmann, H.J.; Lombardi, G.; et al. IL-36 Promotes Systemic IFN-I Responses in Severe Forms of Psoriasis. J. Investig. Dermatol. 2020, 140, 816–826.e81. [Google Scholar] [CrossRef]
- Khalil, S.; Bardawil, T.; Kurban, M.; Abbas, O. Tissue-resident memory T cells in the skin. Agents Actions 2020, 69, 245–254. [Google Scholar] [CrossRef]
- Vo, S.; Watanabe, R.; Koguchi-Yoshioka, H.; Matsumura, Y.; Ishitsuka, Y.; Nakamura, Y.; Okiyama, N.; Fujisawa, Y.; Fujimoto, M. CD 8 resident memory T cells with interleukin 17A-producing potential are accumulated in disease-naïve nonlesional sites of psoriasis possibly in correlation with disease duration. Br. J. Dermatol. 2019, 181, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, K.; Fujiyama, T.; Phadungsaksawasdi, P.; Ito, T.; Honda, T.; Tokura, Y. Epidermal CD8+CD103+ skin resident memory T cells in psoriasis plaques are reduced in number but remain in the basement membrane zone after topical application of corticosteroid and vitamin D3. J. Dermatol. Sci. 2022, 105, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Hijnen, D.; Knol, E.F.; Gent, Y.Y.; Giovannone, B.; Beijn, S.J.; Kupper, T.S.; Bruijnzeel-Koomen, C.A.; Clark, R.A. CD8+ T Cells in the Lesional Skin of Atopic Dermatitis and Psoriasis Patients Are an Important Source of IFN-γ, IL-13, IL-17, and IL-22. J. Investig. Dermatol. 2013, 133, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Gehad, A.; Yang, C.; Scott, L.L.; Teague, J.E.; Schlapbach, C.; Elco, C.P.; Huang, V.; Matos, T.R.; Kupper, T.S.; et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med. 2015, 7, 279ra239. [Google Scholar] [CrossRef]
- Mackay, L.K.; Rahimpour, A.; Ma, J.Z.; Collins, N.; Stock, A.T.; Hafon, M.-L.; Vega-Ramos, J.; Lauzurica, P.; Mueller, S.N.; Stefanovic, T.; et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013, 14, 1294–1301. [Google Scholar] [CrossRef]
- Cheuk, S.; Schlums, H.; Sérézal, I.G.; Martini, E.; Chiang, S.C.; Marquardt, N.; Gibbs, A.; Detlofsson, E.; Introini, A.; Forkel, M.; et al. CD49a Expression Defines Tissue-Resident CD8+ T Cells Poised for Cytotoxic Function in Human Skin. Immunity 2017, 46, 287–300. [Google Scholar] [CrossRef]
- Krueger, J.G.; Fretzin, S.; Suárez-Fariñas, M.; Haslett, P.A.; Phipps, K.M.; Cameron, G.S.; McColm, J.; Katcherian, A.; Cueto, I.; White, T.; et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J. Allergy Clin. Immunol. 2012, 130, 145–154.e9. [Google Scholar] [CrossRef]
- Puig, L.; Costanzo, A.; Muñoz-Elías, E.J.; Jazra, M.; Wegner, S.; Paul, C.F.; Conrad, C. The biological basis of disease recurrence in psoriasis: A historical perspective and current models. Br. J. Dermatol. 2022, 186, 773–781. [Google Scholar] [CrossRef]
- López-Sánchez, C.; Puig, L. Guselkumab in the treatment of moderate-to-severe plaque psoriasis. Immunotherapy 2020, 12, 355–371. [Google Scholar] [CrossRef]
- Blauvelt, A.; Leonardi, C.L.; Gooderham, M.; Papp, K.A.; Philipp, S.; Wu, J.J.; Igarashi, A.; Flack, M.; Geng, Z.; Wu, T.; et al. Efficacy and Safety of Continuous Risankizumab Therapy vs Treatment Withdrawal in Patients With Moderate to Severe Plaque Psoriasis. JAMA Dermatol. 2021, 156, 649–658. [Google Scholar] [CrossRef]
- Gordon, K.B.; Armstrong, A.W.; Foley, P.; Song, M.; Shen, Y.-K.; Li, S.; Muñoz-Elías, E.J.; Branigan, P.; Liu, X.; Reich, K. Guselkumab Efficacy after Withdrawal Is Associated with Suppression of Serum IL-23-Regulated IL-17 and IL-22 in Psoriasis: VOYAGE 2 Study. J. Investig. Dermatol. 2019, 139, 2437–2446.e1. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Armstrong, A.W.; Foley, P.; Song, M.; Wasfi, Y.; Randazzo, B.; Li, S.; Shen, Y.-K.; Gordon, K.B. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J. Am. Acad. Dermatol. 2017, 76, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.; Carrascosa, J.; Fumero, E.; Schoenenberger, A.; Lebwohl, M.; Szepietowski, J.; Reich, K. Time to relapse after tildrakizumab withdrawal in patients with moderate-to-severe psoriasis who were responders at week 28: Post hoc analysis through 64 weeks from reSURFACE 1 trial. J. Eur. Acad. Dermatol. Venereol. 2020, 35, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, K.; Weisenseel, P.; Pinter, A.; Schäkel, K.; Asadullah, K.; Wegner, S.; Muñoz-Elias, E.J.; Bartz, H.; Taut, F.J.H.; Reich, K. IL-23 blockade with guselkumab potentially modifies psoriasis pathogenesis: Rationale and study protocol of a phase 3b, randomised, double-blind, multicentre study in participants with moderate-to-severe plaque-type psoriasis (GUIDE). BMJ Open 2021, 11, e049822. [Google Scholar] [CrossRef]
- Chen, L.; Deshpande, M.; Grisotto, M.; Smaldini, P.; Garcia, R.; He, Z.; Gulko, P.S.; Lira, S.A.; Furtado, G.C. Skin expression of IL-23 drives the development of psoriasis and psoriatic arthritis in mice. Sci. Rep. 2020, 10, 8259. [Google Scholar] [CrossRef]
- Casciano, F.; Diani, M.; Altomare, A.; Granucci, F.; Secchiero, P.; Banfi, G.; Reali, E. CCR4+ Skin-Tropic Phenotype as a Feature of Central Memory CD8+ T Cells in Healthy Subjects and Psoriasis Patients. Front. Immunol. 2020, 11, 529. [Google Scholar] [CrossRef]
- Leijten, E.F.; van Kempen, T.S.; Nordkamp, M.A.O.; Pouw, J.N.; Kleinrensink, N.J.; Vincken, N.L.; Mertens, J.; Balak, D.M.W.; Verhagen, F.H.; Hartgring, S.A.; et al. Tissue-Resident Memory CD8+ T Cells From Skin Differentiate Psoriatic Arthritis From Psoriasis. Arthritis Rheumatol. 2021, 73, 1220–1232. [Google Scholar] [CrossRef]
- Steel, K.J.A.; Srenathan, U.; Ridley, M.; Durham, L.E.; Wu, S.; Ryan, S.; Hughes, C.D.; Chan, E.; Kirkham, B.W.; Taams, L.S. Polyfunctional, Proinflammatory, Tissue-Resident Memory Phenotype and Function of Synovial Interleukin-17A+ CD 8+ T Cells in Psoriatic Arthritis. Arthritis Rheumatol. 2020, 72, 435–447. [Google Scholar] [CrossRef]
- Penkava, F.; Velasco-Herrera, M.D.C.; Young, M.D.; Yager, N.; Nwosu, L.N.; Pratt, A.G.; Lara, A.L.; Guzzo, C.; Maroof, A.; Mamanova, L.; et al. Single-cell sequencing reveals clonal expansions of pro-inflammatory synovial CD8 T cells expressing tissue-homing receptors in psoriatic arthritis. Nat. Commun. 2020, 11, 4767. [Google Scholar] [CrossRef]
- Pennington, S.R.; FitzGerald, O. Early Origins of Psoriatic Arthritis: Clinical, Genetic and Molecular Biomarkers of Progression From Psoriasis to Psoriatic Arthritis. Front. Med. 2021, 8, 723944. [Google Scholar] [CrossRef]
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic Arthritis. N. Engl. J. Med. 2017, 376, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Ogdie, A.; Merola, J.F.; Ritchlin, C. Preventing psoriatic arthritis: Focusing on patients with psoriasis at increased risk of transition. Nat. Rev. Rheumatol. 2019, 15, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Tinazzi, I.; McGONAGLE, D.; Biasi, D.; Confente, S.; Caimmi, C.; Girolomoni, G.; Gisondi, P. Preliminary Evidence That Subclinical Enthesopathy May Predict Psoriatic Arthritis in Patients with Psoriasis. J. Rheumatol. 2011, 38, 2691–2692. [Google Scholar] [CrossRef] [PubMed]
- Naredo, E.; Moller, I.; de Miguel, E.; Batlle-Gualda, E.; Acebes, C.; Brito, E.; Mayordomo, L.; Moragues, C.; Uson, J.; de Agustin, J.J.; et al. High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: A prospective case-control study. Rheumatology 2011, 50, 1838–1848. [Google Scholar] [CrossRef]
- Zabotti, A.; McGonagle, D.G.; Giovannini, I.; Errichetti, E.; Zuliani, F.; Zanetti, A.; Tinazzi, I.; De Lucia, O.; Batticciotto, A.; Idolazzi, L.; et al. Transition phase towards psoriatic arthritis: Clinical and ultrasonographic characterisation of psoriatic arthralgia. RMD Open 2019, 5, e001067. [Google Scholar] [CrossRef]
- Gisondi, P.; Bellinato, F.; Maurelli, M.; Geat, D.; Zabotti, A.; McGonagle, D.; Girolomoni, G. Reducing the Risk of Developing Psoriatic Arthritis in Patients with Psoriasis. Psoriasis Targets Ther. 2022, 12, 213–220. [Google Scholar] [CrossRef]
- Ogdie, A.; Harrison, R.W.; McLean, R.R.; Lin, T.-C.; Lebwohl, M.; Strober, B.E.; Zhuo, J.; Patel, V.; Mease, P.J. Prospective cohort study of psoriatic arthritis risk in patients with psoriasis in a real-world psoriasis registry. J. Am. Acad. Dermatol. 2022. [Google Scholar] [CrossRef]
- McGonagle, D.G.; Zabotti, A.; Watad, A.; Bridgewood, C.; De Marco, G.; Kerschbaumer, A.; Aletaha, D. Intercepting psoriatic arthritis in patients with psoriasis: Buy one get one free? Ann. Rheum. Dis. 2022, 81, 7–10. [Google Scholar] [CrossRef]
- Bellinato, F.; Gisondi, P.; Girolomoni, G. A dermatologist perspective in the pharmacological treatment of patients with psoriasis and psoriatic arthritis. Expert Rev. Clin. Pharmacol. 2020, 13, 481–491. [Google Scholar] [CrossRef]
- Gerriets, V.; Goyal, A.; Khaddour, K. Tumor Necrosis Factor Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Korman, N. Management of psoriasis as a systemic disease: What is the evidence? Br. J. Dermatol. 2020, 182, 840–848. [Google Scholar] [CrossRef]
- Meer, E.; Merola, J.F.; Fitzsimmons, R.; Love, T.J.; Wang, S.; Shin, D.; Chen, Y.; Xie, S.; Choi, H.; Zhang, Y.; et al. Does biologic therapy impact the development of PsA among patients with psoriasis? Ann. Rheum. Dis. 2021, 81, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.; Hotman, A.; Stricker, B.H. Confounding by Indication: An Example of Variation in the Use of Epidemiologic Terminology. Am. J. Epidemiol. 1999, 149, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.; Kleyn, C.; Gulliver, W. Cumulative life course impairment in psoriasis: Patient perception of disease-related impairment throughout the life course. Br. J. Dermatol. 2011, 164 (Suppl. 1), 1–14. [Google Scholar] [CrossRef]
- Kimball, A.B.; Gieler, U.; Linder, D.; Sampogna, F.; Warren, R.; Augustin, M. Psoriasis: Is the impairment to a patient’s life cumulative? J. Eur. Acad. Dermatol. Venereol. 2010, 24, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.; Piaserico, S.; Augustin, M.; Fortina, A.B.; Cohen, A.; Gieler, U.; Jemec, G.; Kimball, A.; Peserico, A.; Sampogna, F.; et al. Psoriasis—The Life Course Approach. Acta Derm. Venereol. 2016, 96, 102–108. [Google Scholar] [CrossRef][Green Version]
- Vardy, D.; Besser, A.; Amir, M.; Gesthalter, B.; Biton, A.; Buskila, D. Experiences of stigmatization play a role in mediating the impact of disease severity on quality of life in psoriasis patients. Br. J. Dermatol. 2002, 147, 736–742. [Google Scholar] [CrossRef]
- Schmid-Ott, G.; Künsebeck, H.-W.; Jäger, B.; Sittig, U.; Hofste, N.; Ott, R.; Malewski, P.; Lamprecht, F. Significance of the Stigmatization Experience of Psoriasis Patients: A 1-Year Follow-up of the Illness and its Psychosocial Consequences in Men and Women. Acta Derm. Venereol. 2005, 85, 27–32. [Google Scholar] [CrossRef]
- Richards, H.; Fortune, D.; Main, C.; Griffiths, C. Stigmatization and psoriasis. Br. J. Dermatol. 2003, 149, 209–211. [Google Scholar] [CrossRef]
- Armstrong, A.; Jarvis, S.; Boehncke, W.-H.; Rajagopalan, M.; Fernández-Peñas, P.; Romiti, R.; Bewley, A.; Vaid, B.; Huneault, L.; Fox, T.; et al. Patient perceptions of clear/almost clear skin in moderate-to-severe plaque psoriasis: Results of the Clear About Psoriasis worldwide survey. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2200–2207. [Google Scholar] [CrossRef]
- van Beugen, S.; van Middendorp, H.; Ferwerda, M.; Smit, J.; Zeeuwen-Franssen, M.; Kroft, E.; de Jong, E.; Donders, A.; van de Kerkhof, P.; Evers, A. Predictors of perceived stigmatization in patients with psoriasis. Br. J. Dermatol. 2017, 176, 687–694. [Google Scholar] [CrossRef]
- Szepietowski, J.; Hrehorów, E.; Salomon, J.; Reich, A. Patients with Psoriasis Feel Stigmatized. Acta Derm. Venereol. 2012, 92, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, I.H.; Link, B.G. Psychosocial Consequences of Rejection and Stigma Feelings in Psoriasis Patients. Int. J. Dermatol. 1993, 32, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Khoury, L.; Skov, L.; Møller, T. Facing the dilemma of patient-centred psoriasis care: A qualitative study identifying patient needs in dermatological outpatient clinics. Br. J. Dermatol. 2017, 177, 436–444. [Google Scholar] [CrossRef]
- Schmitt, J.M.; Ford, D.E. Work Limitations and Productivity Loss Are Associated with Health-Related Quality of Life but Not with Clinical Severity in Patients with Psoriasis. Dermatology 2006, 213, 102–110. [Google Scholar] [CrossRef]
- Sampogna, F.; Gisondi, P.; Tabolli, S.; Abeni, D. Impairment of Sexual Life in Patients with Psoriasis. Dermatology 2007, 214, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.A.; Gupta, A. Psychiatric and Psychological Co-Morbidity in Patients with Dermatologic Disorders. Am. J. Clin. Dermatol. 2003, 4, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.A.; Ilchef, R.; Cooper, A.J. Psychiatric morbidity in psoriasis: A review. Australas. J. Dermatol. 2004, 45, 155–161; quiz 160–151. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Saraceno, R.; Giunta, A.; Maccarone, M.; Chimenti, S. An Italian Study on Psoriasis and Depression. Dermatology 2006, 212, 123–127. [Google Scholar] [CrossRef]
- Modalsli, E.H.; Snekvik, I.; Romundstad, P.R.; Naldi, L.; Saunes, M.; Åsvold, B.O. The association between the clinical diversity of psoriasis and depressive symptoms: The HUNT Study, Norway. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 2062–2068. [Google Scholar] [CrossRef]
- Papp, K.; Poulin, Y.; Vieira, A.; Shelton, J.; Poulin-Costello, M. Disease characteristics in patients with and without psoriatic arthritis treated with etanercept. J. Eur. Acad. Dermatol. Venereol. 2013, 28, 581–589. [Google Scholar] [CrossRef]
- Krueger, G.; Koo, J.; Lebwohl, M.; Menter, A.; Stern, R.S.; Rolstad, T. The impact of psoriasis on quality of life: Results of a 1998 National Psoriasis Foundation patient-membership survey. Arch. Dermatol. 2001, 137, 280–284. [Google Scholar] [PubMed]
- Fleming, P.; Bai, J.; Pratt, M.; Sibbald, C.; Lynde, C.; Gulliver, W. The prevalence of anxiety in patients with psoriasis: A systematic review of observational studies and clinical trials. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 798–807. [Google Scholar] [CrossRef] [PubMed]
- McAleer, M.; Mason, D.; Cunningham, S.; O’Shea, S.; McCormick, P.; Stone, C.; Collins, P.; Rogers, S.; Kirby, B. Alcohol misuse in patients with psoriasis: Identification and relationship to disease severity and psychological distress. Br. J. Dermatol. 2011, 164, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Zink, A.; Herrmann, M.; Fischer, T.; Lauffer, F.; Garzorz-Stark, N.; Böhner, A.; Spinner, C.; Biedermann, T.; Eyerich, K. Addiction: An underestimated problem in psoriasis health care. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1308–1315. [Google Scholar] [CrossRef]
- Horn, E.J.; Fox, K.M.; Patel, V.; Chiou, C.-F.; Dann, F.; Lebwohl, M. Association of patient-reported psoriasis severity with income and employment. J. Am. Acad. Dermatol. 2007, 57, 963–971. [Google Scholar] [CrossRef]
- Bronckers, I.M.G.J.; van Geel, M.J.; van de Kerkhof, P.C.M.; de Jong, E.M.G.J.; Seyger, M.M.B. A cross-sectional study in young adults with psoriasis: Potential determining factors in quality of life, life course and work productivity. J. Dermatol. Treat. 2019, 30, 208–215. [Google Scholar] [CrossRef]
- Hung, W.-K.; Tung, T.-H.; Wang, T.-Y.; Liao, S.-C.; Chi, C.-C. Risk for incident suicidality among psoriasis patients: A systematic review and meta-analysis. Arch. Dermatol. Res. 2022, 1–11. [Google Scholar] [CrossRef]
- Piaserico, S.; Marinello, E.; Dessi, A.; Linder, M.; Coccarielli, D.; Peserico, A. Efficacy of Biofeedback and Cognitive-behavioural Therapy in Psoriatic PatientsA Single-blind, Randomized and Controlled Study with Added Narrow-band Ultraviolet B Therapy. Acta Derm. Venereol. 2016, 96, 91–95. [Google Scholar] [CrossRef]
- Talamonti, M.; Malara, G.; Natalini, Y.; Bardazzi, F.; Conti, A.; Chiricozzi, A.; Mugheddu, C.; Gisondi, P.; Piaserico, S.; Pagnanelli, G.; et al. Effect of Secukinumab on Perception of Anxiety and Depression in Patients with Moderate to Severe Psoriasis: A Post hoc Analysis of the SUPREME Study. Acta Derm. Venereol. 2021, 101, adv00422. [Google Scholar] [CrossRef]
- Fleming, P.; Roubille, C.; Richer, V.; Starnino, T.; McCourt, C.; McFarlane, A.; Siu, S.; Kraft, J.; Lynde, C.; Pope, J.; et al. Effect of biologics on depressive symptoms in patients with psoriasis: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1063–1070. [Google Scholar] [CrossRef]
| Area of Influence of Pharmacotherapies | Treatment(s) | Results |
|---|---|---|
| Immunophenotype of post -esional skin | Ixekizumab vs. etanercept vs. placebo | Downmodulation of genes involved in multiple inflammatory pathways to a greater extent after ixekizumab compared to etanercept [4] |
| Brodalumab vs. etanercept vs. ustekinumab | Suppression of genes involved in multiple inflammatory pathways to a greater extent after brodalumab compared to ustekinumab and etanercept [10] | |
| Guselkumab vs. secukinumab | Higher Treg/TRM ratio after IL-23 compared to IL-17 blockade [11] | |
| Transition from psoriasis alone to PsA | Ustekinumab | Reduction of subclinical entheseal inflammation scores [12] |
| Secukinumab | Improvement of arthralgia, PsAMRIS and synovitis subscore [13] | |
| TNF-α, IL-17 and IL-12/23 inhibitors vs. NB-UVB phototherapy | Lower risk of incident PsA in patients on biologics compared to phototherapy [14] | |
| TNF-α, IL-17 and IL-12/23 inhibitors vs. topicals and cDMARDS | Lower risk of incident PsA in patients on biologics compared to topicals but not to cDMARDs [15] | |
| TNF-α inhibitors, secukinumab and ustekinumab and cDMARDS vs. topicals/no treatment | Lower risk of incident PsA in patients on biologics and cDMARDs compared to topicals/no treatment [16] | |
| Biologics § vs. non-biologics | Lower risk of incident PsA in patients on biologics compared to non-biologic treatments [17] | |
| Biologics § vs. cDMARDS and phototherapy | Higher risk of incident PsA in patients on biologics compared to cDMARDS and phototherapy | |
| Quality of life | Biologics vs. conventional treatments | Significant reduction of the DLQI score after biologics compared to other treatments [18] |
| Anxiety | Biologics | Improvement in anxiety symptoms [19] |
| Depression | Biologics | Lower risk of depressive symptoms [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellinato, F.; Chiricozzi, A.; Piaserico, S.; Targher, G.; Gisondi, P. Could Targeted Pharmacotherapies Exert a “Disease Modification Effect” in Patients with Chronic Plaque Psoriasis? Int. J. Mol. Sci. 2022, 23, 12849. https://doi.org/10.3390/ijms232112849
Bellinato F, Chiricozzi A, Piaserico S, Targher G, Gisondi P. Could Targeted Pharmacotherapies Exert a “Disease Modification Effect” in Patients with Chronic Plaque Psoriasis? International Journal of Molecular Sciences. 2022; 23(21):12849. https://doi.org/10.3390/ijms232112849
Chicago/Turabian StyleBellinato, Francesco, Andrea Chiricozzi, Stefano Piaserico, Giovanni Targher, and Paolo Gisondi. 2022. "Could Targeted Pharmacotherapies Exert a “Disease Modification Effect” in Patients with Chronic Plaque Psoriasis?" International Journal of Molecular Sciences 23, no. 21: 12849. https://doi.org/10.3390/ijms232112849
APA StyleBellinato, F., Chiricozzi, A., Piaserico, S., Targher, G., & Gisondi, P. (2022). Could Targeted Pharmacotherapies Exert a “Disease Modification Effect” in Patients with Chronic Plaque Psoriasis? International Journal of Molecular Sciences, 23(21), 12849. https://doi.org/10.3390/ijms232112849







