Abstract
Chilling is a devastating stress that has led to a crisis of production for cucumber (Cucumis sativus L.). To determine the molecular mechanisms underlying chilling responses in cucumber, we investigated physiological changes and transcriptomic responses to chilling stress in the chilling-tolerant inbred line CC and chilling-susceptible inbred line R1461. Physiological analysis showed that CC had a higher survival rate, lower H2O2 accumulation, and ion leakage than R1461 after chilling treatment. RNA-seq analysis identified 938 differentially expressed genes (DEGs) in response to chilling and revealed that chilling stress regulated the transcript levels of genes related to hormones, including auxin, salicylic acid (SA), jasmonic acid (JA), and ethylene. RT-qPCR and pharmacological analysis suggested that cucumber chilling tolerance was associated with variation in the gene expression involved in ethylene biosynthesis and signaling. Exogenously applying 1-aminocyclopropane-1-carboxylic acid (ACC), the precursor of ethylene, improved the chilling tolerance of cucumber, while the exogenous application of the ethylene inhibitor AgNO3 impaired the chilling tolerance of cucumber. After ACC treatment, the difference in chilling tolerance between CC and R1461 disappeared, suggesting that the different chilling tolerance level between CC and R1461 is dependent on the ethylene biosynthesis and signaling pathway. In addition, a comparison of cucumber lines with different chilling tolerances revealed that chilling tolerance is highly associated with the up-regulation of C-repeat binding factor (CBF) genes, while natural variation in the promoter of CsCBF1 is associated with chilling response. This study thus provides information on transcriptomic responses in different varieties of chilling-tolerant cucumber and reveals potential chilling tolerance mechanisms that could be used to improve chilling tolerance in cucumber.
1. Introduction
Cucumber (Cucumis sativus L.), which is one of the most important vegetables, has a tropical origin and is sensitive to chilling temperatures [1,2]. Chilling temperatures often affect cucumber growth and development, limiting its global distribution. Breeding chilling-tolerant cultivars and optimizing cultivation methods are important strategies to avoid chilling injury for cucumber. Dissecting the molecular mechanisms and underlying regulatory network of cucumber responses to chilling stress will provide the necessary theoretical basis for assisting molecular breeding and optimizing cultivation.
Previously studies have indicated that plant hormones and their respective signaling pathways, including auxin (indole-3-acetic acid, IAA) [3], abscisic acid (ABA) [4,5,6], salicylic acid (SA) [7,8], jasmonic acid (JA) [9], and brassinosteroids (BR) [10], play important roles in improving cucumber chilling tolerance. Ethylene is a gaseous plant hormone that also regulates plant chilling stress [11,12,13]. However, this hormone’s role in cucumber chilling tolerance regulation has not been well characterized. Chilling induces ethylene production by inducing the expression of 1-aminocyclopropane-1-carboxylic acid (ACC) synthesis genes in cucumber [10,14,15]. CsEIN2, which is a key regulator in the ethylene signaling pathway, was identified as a candidate gene for chilling stress in cucumber [16]. However, whether ethylene has a positive or negative effect on the chilling tolerance of cucumber is little known. In Arabidopsis thaliana, ethylene production decreased when plants were exposed to chilling temperature, exogenous ethylene biosynthesis inhibitor aminoethoxyvinyl glycine or loss of function of ethylene signaling genes ETR1, EIN2, EIN3, and EIN4 enhance Arabidopsis freezing tolerance, suggesting that ethylene has a negative effect on freezing tolerance in Arabidopsis [13]. In tomato, ethylene production increased upon chilling treatment [12]; exogenous ACC significantly enhanced tomato chilling tolerance; and the SI-miR164a functioned upstream of SINAM3, which directly activated the expression of ACS1A, ACS1B, ACO1, and ACO4, thereby inducing ethylene synthesis upon chilling and thus enhancing tomato chilling tolerance [12]. These results suggested that ethylene plays distinct roles in the regulation of chilling tolerance in different plant species.
Dehydration-responsive element binding factor 1 (DREB1)/C-repeat binding factor (CBF) genes play a hub role in Arabidopsis cold acclimation, where freezing tolerance is enhanced by a prior chilling temperature treatment [17]. In previous studies, CBFs were rapidly induced by chilling [18], and their proteins directly bound to the C-repeat/dehydration-responsive (CRT/DRE) cis-elements (CCGAC) of downstream cold responsive (COR) genes to activate their expressions, thus enhancing the plants’ freezing tolerance [19,20,21,22,23]. CBF/DREB genes were also characterized in the regulation of chilling tolerance in cucumber [24,25]. CsCBF1 and CsCBF2 transcription was rapidly increased by chilling, while CsCBF3 was found to be less sensitive in transcriptional regulation to chilling. Overexpression of CsCBF1-3 in cucumber significantly enhanced the chilling tolerance of cucumber seedlings. In addition, CsCBF1-3 directly activated CsCOR gene expression by binding to the cis-elements of their promoters [25]. These results suggest that the function of CBFs in regulating chilling tolerance in plants is conserved.
Transcriptomic analysis has been identified as an efficient experimental tool to investigate the genome functions and physiological regulation mechanisms of chilling stress [26,27,28]. Recently, physiological and transcriptome analysis has indicated that the interactions between auxin and hydrogen sulfide signaling are involved in cucumber chilling tolerance [3]. In addition, transcriptome profiling uncovered that exogenous nitric oxide (NO) improves low-temperature tolerance in cucumber by modulating the expression of some key transcription factors and their target genes [27]. These results suggest that transcriptomic analysis plays an important role in dissecting the molecular mechanisms of plant responses to chilling stress.
In this study, we compared the transcriptomics of chilling-tolerant and susceptible inbred lines of cucumber to investigate molecular responses to chilling and the chilling tolerance mechanisms in cucumber. We found that activation of the ethylene signaling pathway and transcriptional regulation of ethylene biosynthesis were critical for chilling tolerance in cucumber. Moreover, the expression variation of CBF genes upon chilling was associated with chilling tolerance in cucumber lines, and three polymorphisms of the CBF1 promoter region conferred an expression variation of CBF1 upon chilling. Thus, our study expands our understanding of chilling responses and facilitates the genetic improvement of chilling tolerance in cucumber.
2. Results
2.1. Chilling Tolerance Analysis of Cucumber Inbred Lines
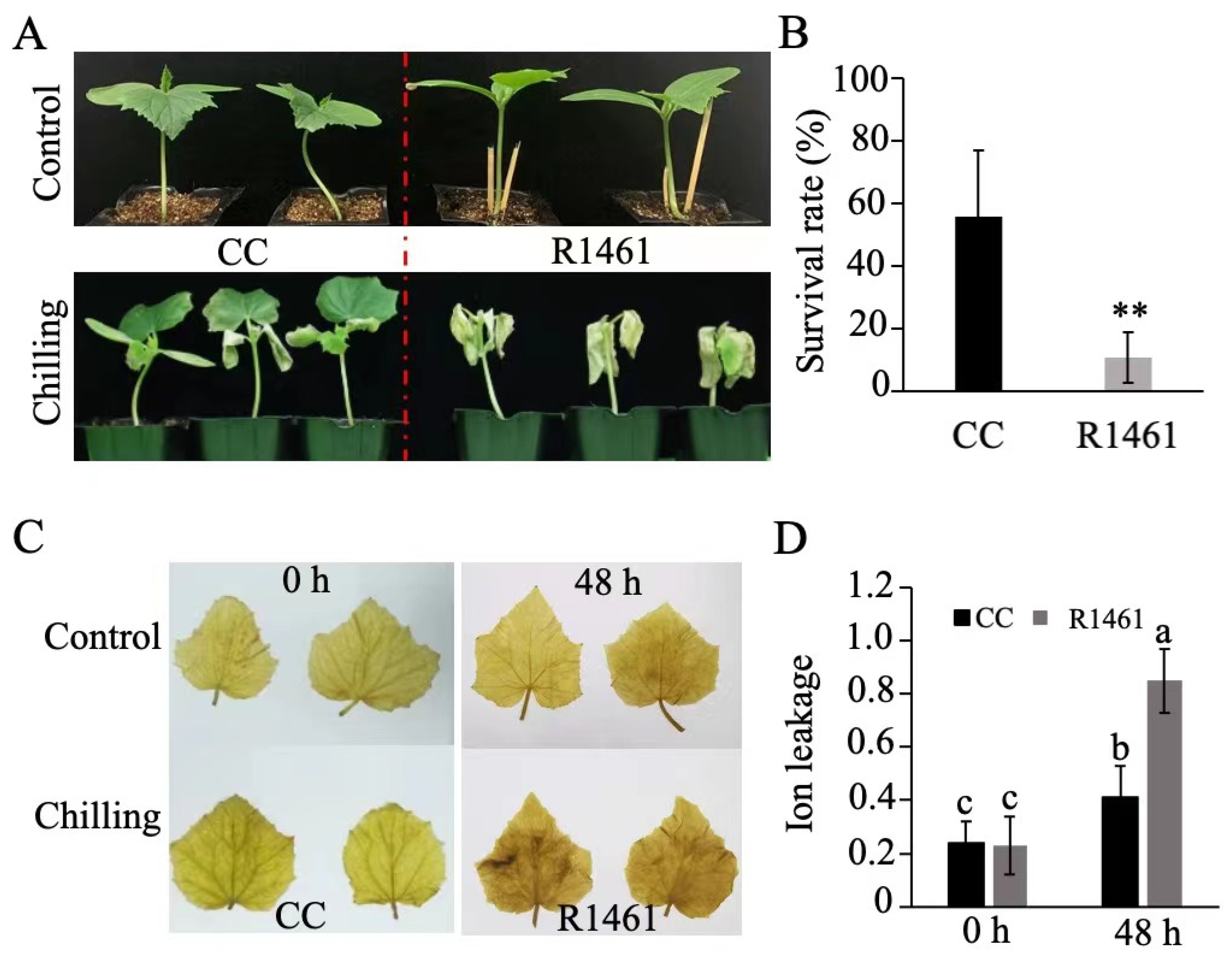
To understand the chilling tolerance of cucumber, we assessed the survival rates of 10 cucumber inbred lines after chilling treatment. Thirty seedlings for each line at the three-leaf stage were exposed to chilling treatment in a 6 °C chamber for 4 days and then transferred to normal conditions for recovery. After 7 days of recovery, CC had the highest survival rate in these inbred lines, whereas R1461 had a medium-to-low survival rate (Figure S1).
We then chose CC and R1461 to investigate the cucumber physiological responses to chilling temperature. After 4 days of chilling treatment (6 °C) and 7 days of recovery, CC displayed a slighter leaf curl and higher survival rate than R1461 (Figure 1A,B). Furthermore, when seedlings of CC and R1461 were exposed to 6 °C for 48 h, CC displayed lower H2O2 accumulation (Figure 1C) and significantly lower ion leakage than R1461 (Figure 1D). These results strongly suggest that CC is a chilling-tolerant inbred line, while R1461 is a chilling-susceptible inbred line.
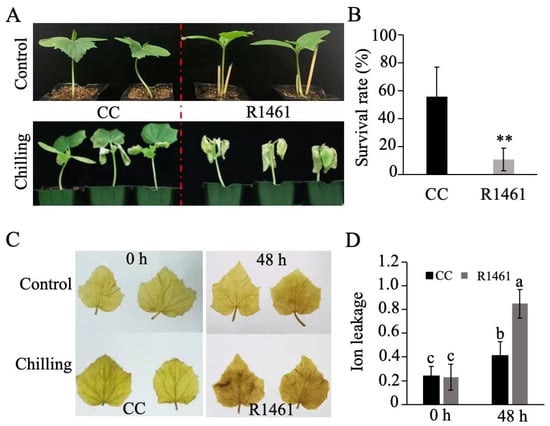
Figure 1.
Effects of chilling treatment on CC and R1461. (A) Morphological phenotypes of CC and R1461 inbred lines before and after 4 days of chilling treatment. (B) Survival rate (%) of CC and R1461 inbred lines after 4 days of chilling treatment. Data are the means ± SD of three biological replicates consisting of 30 seedlings each. ** means p < 0.01, Student’s t-test. (C) DAB staining. (D) ion leakage before and after 48 h chilling treatment. Data are the means ± SD of three biological replicates consisting of 30 seedlings each. Different letters above the bars indicate significant differences among samples as determined by a one-way ANOVA/Duncan (p < 0.05).
2.2. Transcriptomic Analysis of CC and R1461 to Chilling Treatment
To determine the molecular responses to chilling and obtain a better understanding of cucumber chilling tolerance, we performed transcriptome profiling on CC and R1461 seedlings under normal conditions and chilling treatment for 3 h. The total reads from R1461 (between 46,000,000 and 50,000,000 per sample) and CC (47,000,000 and 51,000,000 per sample) were obtained by RNA-seq (Table S2). About 95% of the reads from each sample were uniquely mapped to the cucumber (Chinese Long) v2 Genome (Table S2). Principal component analysis (PCA) analysis showed that CC and R1461 were similar to each other after both 0 and 3 h chilling treatment in the first two principal components, and chilling treatment was the most important factor in differentiating between samples (Figure S2). In addition, the three biological repeats were always clustered together among the samples except for the spreading of the samples of CC after 3 h chilling treatment (Figure S2). These PCA results indicate the high reproducibility of this dataset. Additionally, the chilling treatment induced a similar global transcriptional change in CC and R1461.
2.3. Transcriptomic Response of CC and R1461 to Chilling Temperature
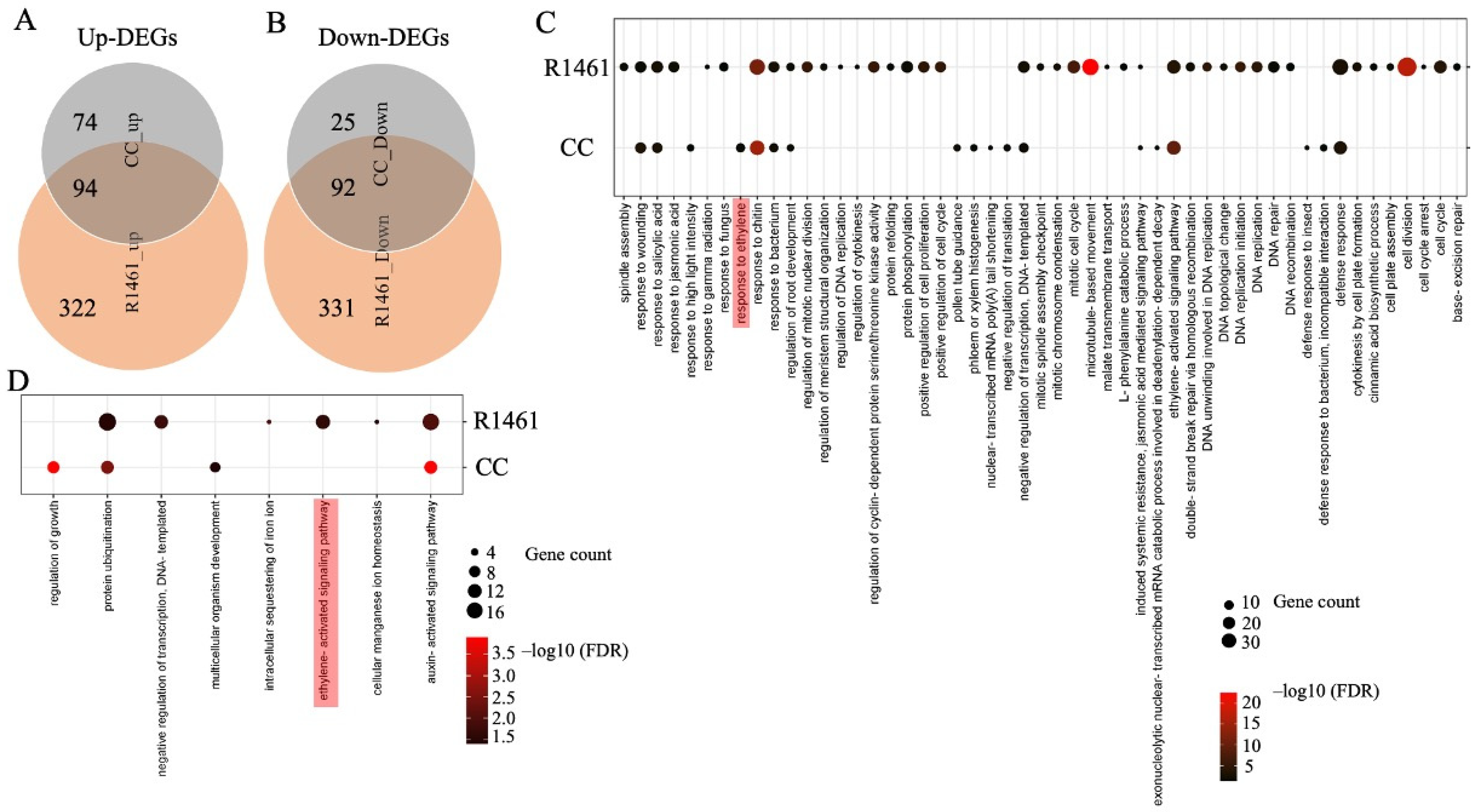
To investigate the responses to chilling at the molecular level, we analyzed the differentially expressed genes (DEGs) and their enriched GO terms for the biological processes (BPs) in CC and R1461 under chilling treatment. More up- and down-regulated DEGs under chilling versus non-chilling (normal) treatment were detected in R1461 than in CC (Figure 2A,B). In total, the Venn diagram analysis revealed that 99 chilling-induced DEGs (74 up- and 25 down-regulated) were unique to CC, while 653 (332 up- and 331 down-regulated) DEGs were unique to R1461 (Figure 2A,B), indicating the specificity of the CC and R1461 to chilling responses and suggesting that these DEGs may be related to the different chilling tolerance levels in CC and R1461. A total of 59 BP terms (51 for up-regulated DEGs, 8 for down-regulated DEGs) were enriched for DEGs in CC or R1461 (Figure 2C,D). CC and R1461 exhibited a differential enrichment of BP terms; 11 BP terms (9 for up-regulated DEGs, 2 for down-regulated DEGs) were unique to CC, while 54 BP terms (50 for up-regulated DEGs, 4 for down-regulated DEGs) were unique to R1461. For up-regulated DEGs, the most significant BP terms for CC and R1461 were “response to chitin” and “cell division”, respectively. For down-regulated DEGs, the most significant BP terms for CC and R1461 were “regulation of growth” and “auxin-activated signaling pathway”, respectively.
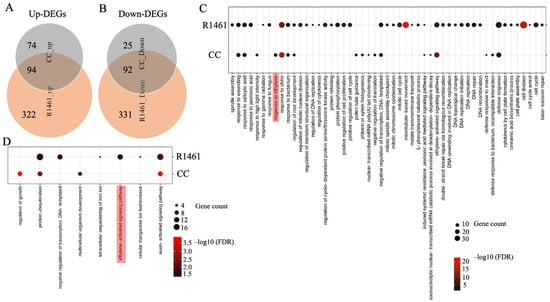
Figure 2.
Analysis of chilling-induced differentially expressed genes (DEGs) and their enriched Biological Process (BP) GO terms in CC and R1461. (A) Venn diagram analysis of the up-regulated DEGs and down-regulated DEGs (B) in CC and R1461 under chilling treatment. The DEGs were identified from chilling versus non-chilling with |log2FC| ≥ 1, FDR ≤ 0.05. A dot bubble diagram is presented for enriched BP terms in up-regulated DEGs (C) and down-regulated DEGs (D) in CC and R1461 under chilling treatment. Significant enrichment of BP terms was established with FDR ≤ 0.05. The x-axis represents the enriched BP terms, and the y-axis indicates the CC and R1461.
It is well known that hormones play critical roles in chilling tolerance in plants [3,5,6,7,8,12,29]. In our data, 7 of the 59 BP terms were related to hormones and their signaling pathways, including “response to salicylic acid”, “response to jasmonic acid”, “response to ethylene”, “induced systemic resistance”, “jasmonic acid mediated signaling pathway”, “ethylene-activated signaling pathway”, and “auxin-activated signaling pathway” (Figure 2C,D), suggesting a large change in hormone responses upon chilling in cucumber. For up-regulated DEGs, the BP term “ethylene-activated signaling pathway” was enriched in both CC and R1461 (Figure 2C). Interestingly, the BP term for up-regulated DEGs “response to ethylene” was uniquely enriched in CC (Figure 2C), and 11 genes belonging to the “ethylene-activated signaling pathway” term were down-regulated in R1461 (Figure 2D). These results suggest that ethylene plays an important role in cucumber chilling responses and that the distinct gene regulation of chilling response in these two BP terms may be the one reason for the different chilling tolerance levels observed in CC and R1461. In addition to ethylene, the BP terms (for up-regulated DEGs) “response to salicylic acid”, “induced systemic resistance”, and jasmonic acid mediated signaling pathway” were enriched in CC and R1461. The BP term (for up-regulated DEGs) “response to jasmonic acid” was uniquely enriched in R1461. The BP term (for down-regulated DEGs) “auxin-activated signaling pathway” was enriched in CC and R1461.
2.4. Expression Analysis of DEGs Involved in the Ethylene Biosynthesis and Signaling Pathway
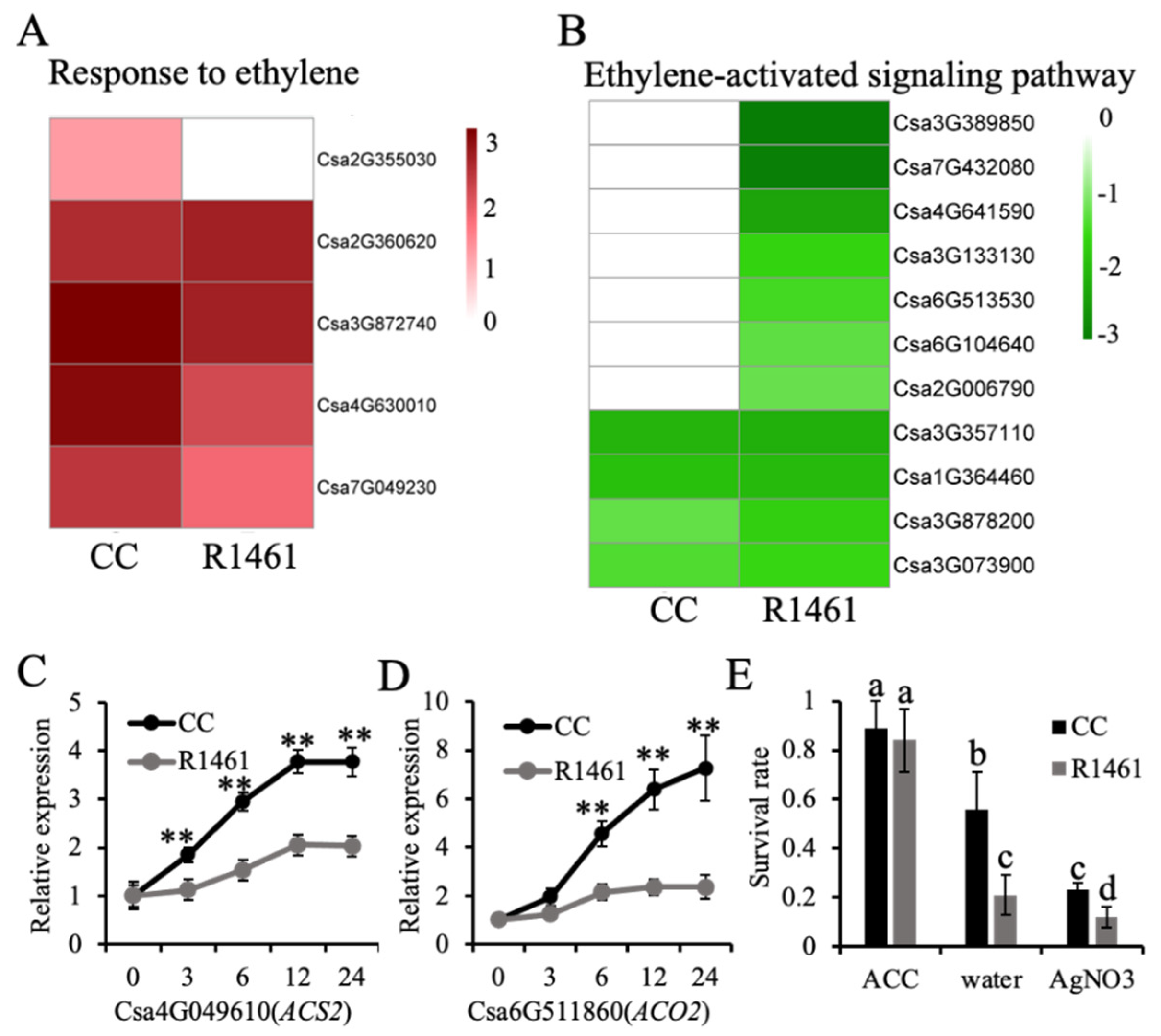
As ethylene signaling is involved in the regulation of plant responses to several stresses, including chilling stress [12,30,31,32,33]. Variation in gene expression in response to chilling in nature may confer chilling tolerance in plants [34]. CC and R1461 featured uniquely enriched terms in ethylene signaling, suggesting that the expression variation of genes involved in ethylene signaling may confer the observed variation in the chilling tolerance of CC and R1461. Therefore, to further investigate the role of ethylene biosynthesis and signaling in response to chilling, we compared the expression levels of DEGs under the CC-unique BP term “response to ethylene” and the R1461-unique BP term “ethylene-activated signaling pathway”. The BP term “response to ethylene” included five genes, all of which were significantly up-regulated in CC. Csa2G355030, encoding an MYB transcription factor, was significantly up-regulated in CC under chilling (Figure 3A). In contrast, Csa2G355030 presented no altered expression in R1461. Although the other genes were also up-regulated in R1461, the fold changes in three of these four genes were less than those in CC (Figure 3A), suggesting that the variation of chilling-induced expression among these genes may confer cucumber chilling tolerance in CC. However, the expression of these four genes was also up-regulated in R1461, and thus the influence of these four genes on chilling tolerance may be less than Csa2G355030. The second examined BP term was “ethylene-activated signaling pathway” (Figure 3B). Four of the genes herein were down-regulated in both CC and R1461. However, the fold change of the down-regulation among these two genes in CC was less than that in R1461. In addition, seven genes in this BP term presented no alteration in CC but were significantly down-regulated in R1461, suggesting that these genes may confer chilling susceptibility in R1461.
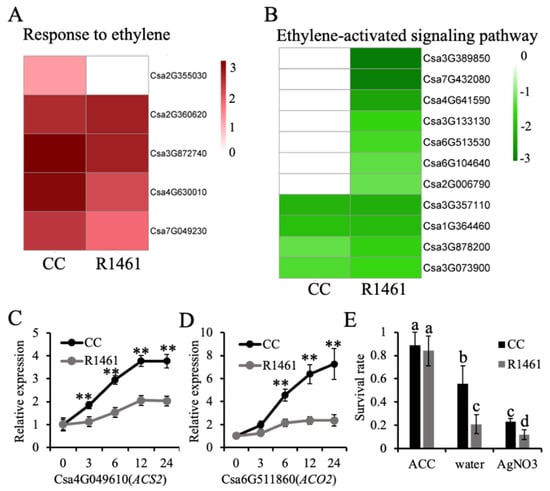
Figure 3.
Ethylene confers chilling tolerance in cucumber. (A,B) Heat maps of the expression levels of genes belonging to “response to ethylene” (A) and “ethylene-activated signaling pathway” (B). Chilling-induced expression changes are shown, expressed as log2FC based on RNA-seq data. (C,D) Relative expression levels of ACS2 (C) and ACO2 (D) in 16 days old seedlings of CC and R1461 under chilling stress. Expression at 0 h was set to a value of 1. Data are the means ± SD of three biological replicates consisting of 3–6 individual seedlings each. ** means p < 0.01, Student’s t-test. (E) Survival rates of CC and R1461 under 4 days of chilling treatment and 7 days of recovery with 100 µM ACC, water, and 100 µM AgNO3 treatment. Data are the means ± SD of three biological replicates consisting of 30 seedlings each. Different letters above the bars indicate significant differences among samples, as determined by one-way ANOVA/Duncan (p < 0.05).
We further performed RT-qPCR to assess the expression levels of genes involved in ethylene synthesis under chilling treatment in CC and R1461. The transcript levels of ACS2 (Csa4G049610) and ACO2 (Csa6G511860) were significantly increased after exposing cucumber plants to chilling temperatures (Figure 3C,D). However, the chilling-induced expression level changes of both ACS2 and ACO2 in CC were larger than those in R1461, suggesting that the variation in ethylene synthesis may confer cucumber chilling tolerance. The CC and R1461 plants were treated with 100 µM exogenous ACC, water, and AgNO3 and then exposed to chilling at 6 °C for 4 days to further investigate the role of ethylene in chilling tolerance. As expected, after 7 days of recovery, 100 µM ACC significantly increased the chilling tolerance of CC and R1461 compared to the water treatment, which was used as a control, while 100 µM AgNO3 decreased the chilling tolerance of CC and R1461 (Figure 3E). Intriguingly, after 100 µM ACC treatment, R1461 showed similar chilling tolerance to CC. These findings suggest that the different chilling tolerance levels between CC and R1461 are dependent on ethylene level.
2.5. Expression Patterns of CsCBFs in CC and R1461 under Chilling Treatments
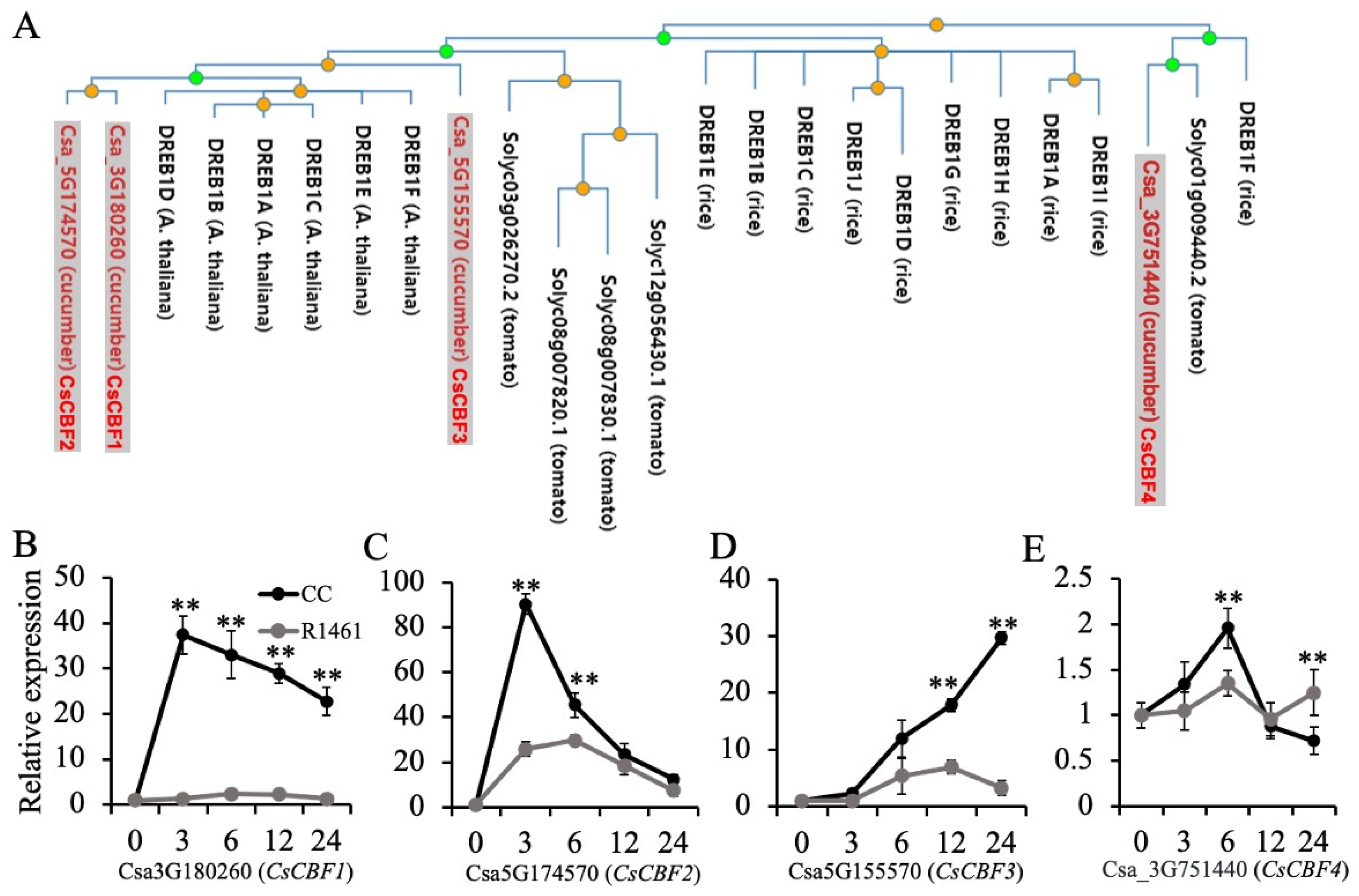
Studies have shown that DREB1/CBFs regulate cold acclimation in Arabidopsis [17] and chilling tolerance in rice [35]. Overexpression of CBF1-3 in cucumber was found to significantly increase cucumber chilling tolerance [25]. To gain insights into the chilling responses of CBF genes in cucumber, we performed a phylogenetic tree analysis using DREB1B in Arabidopsis as a query. As shown in Figure 4A, Csa_3G180260 (CsCBF1), Csa_5G174570 (CsCBF2), and Csa_5G55570 (CsCBF3) were close to DREB1A-F in Arabidopsis. Csa_3G751440 (CsCBF4) was close to the DREB1F in rice and Solyc01g009440 in tomato. Our RNA-seq data showed that the extent of the up-regulation of CsCBF1 differed between CC (416 folds, Table S3) and R1461 (108 folds, Table S4). CsCBF2 was significantly up-regulated (22 folds, Table S3) in CC but showed no altered expression in R1461, suggesting that different extents of up-regulation of the CBF genes contributed to distinct chilling tolerance levels in CC and R1461. Further RT-qPCR analysis revealed that all four CBF genes were significantly up-regulated after chilling in CC (Figure 4B–E). CsCBF1 and CsCBF2 rapidly increased and then decreased after reaching their maximum values at 3 h (Figure 4B,C). However, the expression level of CsCBF3 showed a continual increase over 24 h (Figure 4D). The expression level of CsCBF4 showed a lesser change than the others (Figure 4E), suggesting that this gene was less sensitive to chilling temperatures. In R1461, only the expression level of CsCBF2 and CsCBF3 changed drastically after chilling (Figure 4C,D). These results suggest that the up-regulation of CsCBF genes confers chilling tolerance and that the different expression patterns of these genes in CC and R1461 contribute to their distinct chilling tolerance levels.
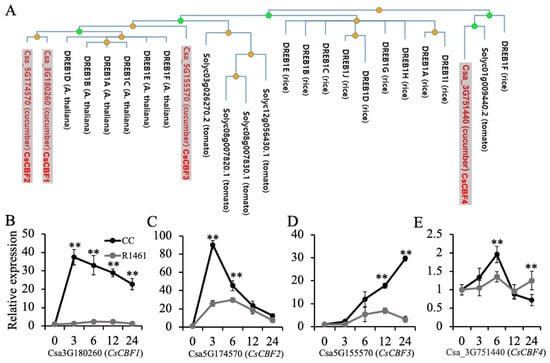
Figure 4.
Expression of DREB1/CBF genes in CC and R1461 under chilling treatment. (A) Phylogenetic tree analysis of DREB1/CBF proteins from cucumber, rice, tomato, and Arabidopsis. The tree is from PhyloGenes (http://www.phylogenes.org) (accessed on 20 August 2022). using the Arabidopsis DREB1B gene as a query on genes from Arabidopsis, tomato, and rice. (B–E) Relative expression levels of CsCBF1-4 genes in CC and R1461 under chilling treatment, analyzed by RT-qPCR. Expression at 0 h was set to a value of 1. Data are the means ± SD of three biological replicates consisting of 3–6 individual seedlings each. ** means p < 0.01, Student’s t-test.
2.6. Natural Variation in CBF1 Promoter Confers a Distinct Chilling Response
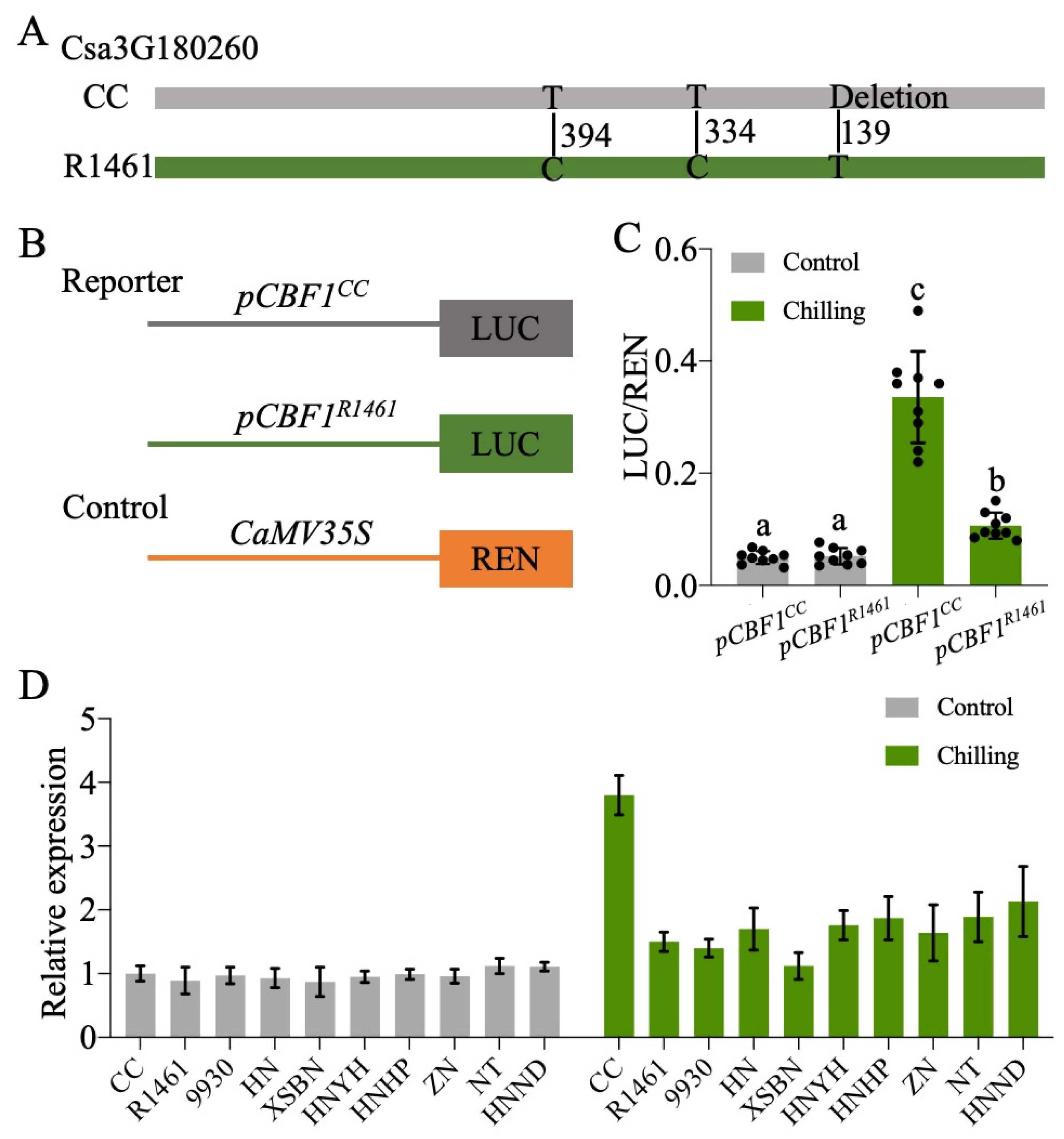
Given the expression variation of CsCBF1-3 in CC and R1461 (Figure 4B–D) and that the overexpression of the CsCBF1-3 enhanced cucumber chilling tolerance [25], we then determined whether there are polymorphisms present in their genomic sequences. We sequenced these genes (about 2.8 kb), including the promoter (about 2 kb) and coding regions (about 0.8 kb), via PCR amplification followed by Sanger sequencing. Compared to the Cucumber (Chinese Long) v2 Genome, no differences in CsCBF2 and CsCBF3 were found between the CC and R1461 genomic sequences, indicating that the different expression patterns under chilling in CC and R1461 could be caused by the other regulators, which were perhaps located in other quantitative trait loci (QTLs). It also indicates the post-translational regulation of CsCBF2 and CsCBF3. For example, stability and activity differences of both transcription factors. Sequence analysis of CsCBF1 identified two single nucleotide polymorphisms (SNPs) and one base deletion in the promoter region between CC and the other lines (Figure 5A, Table S5). Hence, we postulated that these three polymorphisms may contribute to the different expression patterns of CsCBF1 in CC and R1461 under chilling. To confirm our prediction, the 1.5 kb promoters upstream of the transcriptional start site for CsCBF1 from CC and R1461 were cloned, and each was fused with the reporter gene Firefly luciferase (LUC). Another reporter gene, Renilla luciferase (REN), residing in the same vector, was expressed under control of the CaMV 35s promoter to normalize transformation and expression efficiencies (Figure 5B). The reporter constructs pCBF1CC::LUC and pCBF1R1461::LUC were each transformed into protoplasts from the Arabidopsis Col-0 plants. As shown in Figure 5C, there was no difference in luciferase activity at normal temperature. Under chilling temperature, both pCBF1CC::LUC and pCBF1R1461::LUC presented significantly higher luciferase activity compared to that at normal temperature. In addition, at chilling temperatures, pCBF1CC::LUC showed significantly higher LUC activity than pCBF1R1461::LUC. In addition, we examined the CsCBF1 transcript levels in the nine chilling-susceptible cucumber lines at 0 and 3 h after chilling treatment compared to CC. As expected, the CsCBF1 transcript level was strongly induced by chilling in CC (Figure 5D), suggesting that natural variations in the promoter region of CsCBF1 contribute to CsCBF1 expression variation in response to chilling temperatures. However, there were no differences in CsCBF1 transcript levels between the CC and chilling-susceptible lines at normal temperatures, suggesting that the influence on gene expression by the polymorphisms in CsCBF1 was specific to chilling temperatures.
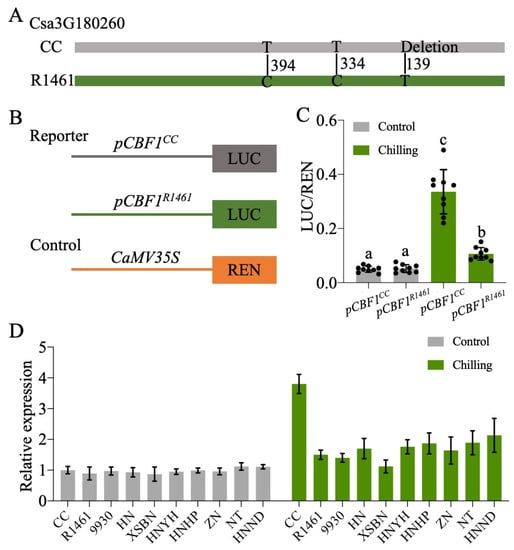
Figure 5.
Association of natural polymorphisms in the promoter region of CBF1 with chilling responses. (A) Sequence variations of CBF1 in CC and R1461. Positions were defined relative to the transcription start site of CsCBF1. (B) Schematics of the reporter and control constructs used in the dual-luciferase expression assay. LUC, Firefly luciferase; REN, Renilla luciferase. (C) Effects of the polymorphisms on the activity of the CBF1 promoter. pCBF1CC::LUC and pCBF1R1461::LUC were expressed in protoplasts and incubated at 6 °C and 22 °C for 6 h, respectively. Relative reporter activity (LUC/REN) was calculated. The points represent the mean values of 9 independent biological repeats. Different plants growing at different times were used for each biological repeat. Different letters above the bars indicate significant differences among samples as determined by a one-way ANOVA/Duncan (p < 0.05). (D) RT-qPCR of CsCBF1 in CC and 9 chilling-susceptible cucumber lines after 0 h (Control) and 3 h (Chilling) of chilling treatment. Expression at 0 h in CC was set to a value of 1. Data are the means ± SD of three biological replicates consisting of 3–6 individual seedlings each.
3. Discussion
Chilling stress severely affects cucumber plant growth and production [36,37,38]. How cucumber plants respond and adapt to chilling temperature is an important biological question, but the corresponding molecular mechanisms are still poorly understood. Here, we identified an extreme chilling-tolerant cucumber inbred line CC with a high survival rate, low H2O2 accumulation, and ion leakage after chilling treatment (Figure 1). Transcriptome analysis in a chilling-tolerant inbred line (CC) and a susceptible inbred line (R1462) revealed a variation in gene expression that could contribute to differences in chilling tolerance in cucumber.
Transcriptome analysis identified 285 and 839 DEGs in chilling-tolerant (CC) and chilling-susceptible (R1461) cucumber cultivars, respectively (Tables S3 and S4), suggesting that these genes may confer chilling tolerance in cucumber. GO analysis was used to analyze the functions of plant genes in response to low-temperature stress [5,27,28,35,39]. Previous studies found that chilling induces ethylene production in cucumber [14,15]. However, it was not tested in these studies whether ethylene signaling plays a role in cucumber chilling tolerance. In the present study, we found that the BP term “response to ethylene” for up-regulated DEGs was enriched in the chilling-tolerant line CC (Figure 2C). Additionally, we observed that “ethylene-activated signaling pathway” for down-regulated DEGs was enriched in the chilling-susceptible R1461 (Figure 2D), indicating that these two processes may confer distinct chilling tolerance in CC and R1461. In addition, the expression levels of ethylene biosynthesis genes in CC were significantly higher than those in R1461 (Figure 3C,D), suggesting that chilling-induced ethylene production is essential for cucumber chilling tolerance. This result is supported by the similar chilling tolerance presented by CC and R1461 when sprayed with ACC (Figure 3E).
Ethylene signaling is known to be very important for low-temperature stress in Arabidopsis and tomato [12,13]. In Arabidopsis thaliana, ethylene was found to rapidly decrease under chilling temperatures and negatively regulate plant responses to freezing stress [13]. Conversely, chilling temperatures rapidly induced the expression of ethylene synthesis genes in tomato and increased ethylene production, thereby enhancing tomato chilling tolerance [12]. These results suggest that ethylene plays distinct roles in the regulation of chilling tolerance among different plant species. Hence, there is a need to investigate the roles of ethylene in chilling tolerance regulation in different plant species. In our work, we identified a chilling-responsiveness of transcripts of the two BP terms “response to ethylene” and “ethylene-activated signaling pathway” and revealed the variation in gene expression among these genes or ethylene synthesis genes in chilling-tolerant and chilling-susceptible cucumber inbred lines, suggesting that there are natural variations in ethylene signaling and biosynthesis between these two inbred lines possibly contributing to chilling tolerance. In the future, it would be interesting to generate populations from these two lines to map the QTLs related to chilling tolerance in cucumber. Moreover, we found that spraying with 100 µM ACC can significantly enhance cucumber chilling tolerance (Figure 3E). This treatment could be directly used for cucumber cultivation to avoid damage from chilling temperatures.
CBF genes are considered to be a hub in cold acclimation [17]; their roles in cucumber chilling tolerance were also previously characterized [25]. Our study indicated that CsCBF1-3 genes were more strongly up-regulated by chilling in the cucumber chilling-tolerant line CC than in the chilling-susceptible R1461 (Figure 4B–D), suggesting that the expression variation in CsCBF1-3 genes upon chilling confers cucumber chilling tolerance. This result is supported by the observation that the overexpression of CsCBF1-3 significantly enhances cucumber chilling tolerance [25]. On the genetic basis of the two SNPs and one deletion in CsCBF1 identified in this study, we suggest that CsCBF1 has great potential for improving cucumber chilling tolerance via molecular breeding techniques. Ethylene-insensitive 3 (EIN3), a nuclear transcription factor, functions downstream in ethylene signaling [40,41]. EIN3 directly binds to CBF1-3 promoter regions to negatively regulate the expression of CBFs and freezing tolerance in Arabidopsis [13]. Our work revealed that ethylene plays a critical role in the distinct chilling tolerance levels of CC and R1461. We speculate that other natural variations in CsCBF regulators involved in ethylene signaling contribute to cucumber chilling tolerance. Therefore, mapping the genes and dissecting the molecular mechanisms underlying how ethylene regulates CsCBF gene expression under chilling stress in cucumber will be an interesting future research direction.
4. Materials and Methods
4.1. Plant Materials and Chilling Treatment
All the plants used in this study were grown in a mixture of soil and vermiculite (1:1) at (22 ± 2 °C) under long-day conditions (16 h light/8 h dark, 130 μmol/m2/s) for 16 days before being sent to the growth chamber (6 °C, 16 h light/8 h dark, 130 μmol/m2/s) for chilling treatment. Survival was defined as the plant showing the emergence of new leaves. Survival rate was calculated as surviving seedlings versus the total number of plants. To determine the cucumber seedling survival rate after chilling, 30 seedlings for each replicate at the three-leaf stage were subjected to chilling treatment for 4 days and then moved to normal conditions for 7 days before plant survival was assessed. To determine the cucumber seedling survival rate after chilling with ACC and AgNO3 treatment, 30 seedlings for each replicate were grown for 16 days in a solution containing 1/2 MS until the three-leaf stage. Before chilling treatment, plants were sprayed with 100 µM ACC, water, and 100 µM AgNO3. After 4 days of chilling treatment, seedlings were moved to normal conditions for 7 days before survival rate was assessed.
4.2. DAB Staining
The accumulation of H2O2 in cucumber seedlings after chilling treatment was detected via DAB staining as described previously, with some modifications [42]. In brief, tissues were collected in 15 mL tubes and incubated in a 1 mg/mL diaminobenzidine (DAB) solution (Sigma, NJ, USA) for 8 h in the dark to determine the accumulation of H2O2.
4.3. Assay of Relative Electrolyte Leakage
Relative ion leakage was previously used as an indicator of membrane damage under chilling [39]. Relative ion leakage in this study was measured as previously described [43], with few modifications. Briefly, 0.1 g leaves were cut into 0.5 cm pieces and incubated in 0.4 M mannitol at 25 °C with gentle shaking for 3 h before the initial conductivity of the solution was measured with a conductivity meter (DDS-307A, Ningbo Hinotek Technology, Ningbo, China). Total conductivity of the solution was measured after incubation at 85 °C for 20 min. An assay was done on 3 biological replicates consisting of 3–6 individual seedlings each, and the ion leakage rate was calculated as the initial conductivity versus total conductivity.
4.4. RNA Sequencing and RT-qPCR
The three-leaf-stage (16 days old) seedlings of CC and R1461 were treated at 6 °C for 0 and 3 h, and leaves were sampled with 3 biological replicates consisting of 3–6 individual seedlings each. Total RNA was extracted using TRIzol reagent. RNA integrity was evaluated using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The samples with an RNA Integrity Number (RIN) ≥ 7 were subjected to subsequent analysis. The libraries were constructed using a TruSeq Stranded mRNA LTSample Prep Kit (Illumina, San Diego, CA, USA). Then, these libraries were sequenced on an Illumina sequencing platform (HiSeqTM 2500 or Illumina HiSeq X Ten), and 125 bp/150 bp paired-end reads were generated. The transcriptome sequencing and analysis were conducted by OE Biotech Co., Ltd. (Shanghai, China).
For RT-qPCR, the three-leaf-stage seedlings of CC and R1461 were treated at 6 °C for 0, 3, 6, 12, and 24 h. Leaves were then sampled with 3 biological replicates consisting of 3–6 individual seedlings each. Total RNA was isolated from seedling tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and treated with DNase (TRANSGEN BIOTECH, http://www.transgen.com.cn) (accessed on 5 March 2022), before being used for cDNA synthesis. Reverse transcription was done using a PrimerScript II 1st Strand cDNA Synthesis Kit (TaKaRa). First-strand cDNA synthesis of the cDNA strand of mRNA was performed using the previously reported method [34]. ACTIN was used as the control for mRNAs. RT-qPCR was performed using diluted cDNA on a Step One Plus (ABI) real-time thermocycler. Three independent biological replicates were performed. Gene-specific primers are shown in Table S1.
4.5. RNA-Seq Data Analysis
The clean data were mapped to the Cucumber (Chinese Long) v2 Genome (http://plants.ensembl.org/index.html) (accessed on 7 November 2021), using HISAT2 [44]. Differential expression analysis was performed using the DESeq (2012) R package. A value of p < 0.05 and fold change >2 or fold change <0.5 were set as the threshold for significantly different expressions. GO enrichment analyses of DEGs were performed using R base with hypergeometric distribution. The GO terms with q < 0.05 were considered significantly enriched. Venn diagrams and principal component analysis (PCA) were performed on the platform OuyiCloud (https://cloud.oebiotech.cn/task/category/pipeline/) (accessed on 11 November 2021). Enrichment dot bubble plots and heat maps were generated by http://www.bioinformatics.com.cn (accessed on 11 November 2021).
4.6. Promoter Activity Assay
The 1.5 kb promoter fragments upstream of CsCBF1 (Csa3G180260) transcription start site were amplified from CC and R1461 and then cloned into the pGREEN II 0800-LUC reporter vector [45] to generate pCBF1CC::LUC and CBF1R1461::LUC plasmids, respectively. Protoplasts were isolated from 14-day-old seedlings of Arabidopsis Col-0 plants grown on 1/2 Murashige and Skoog media under long-day conditions. Plasmid DNA was transformed into protoplasts following methods described previously [46]. Transfected protoplasts were incubated at 22 °C for 8 h under dark and then shifted to 6 and 22 °C for 6 h incubation. Firefly and Renilla luciferase activities were measured using a Dual-Luciferase Reporter Assay System (Promega, https://www.promega.com) (accessed on 20 May 2022).
4.7. Phylogenetic Tree
The phylogenetic tree of DREB1 was generated using PhyloGenes (http://www.phylogenes.org) (accessed on 21 Match 2022) with the Arabidopsis DREB1B gene as a query on genes from Arabidopsis, rice, cucumber, and tomato.
5. Conclusions
In this study, transcriptome analysis in the chilling-tolerant inbred line CC and chilling-susceptible inbred line R1461 revealed that chilling tolerance in cucumber is associated with the ethylene biosynthesis and signaling pathway, as well as the expression variations of CsCBF1-3 genes under chilling. The expression variation of CsCBF1 in CC and R1461 upon chilling was associated with natural variations in its promoter region. This study provides more comprehensive information on cucumber chilling response and underscores the potential significance of CsCBF1 for future molecular breeding chilling tolerance in cucumber.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232112834/s1.
Author Contributions
Data curation, S.M. and H.M.; funding acquisition, X.W.; supervision, X.W.; writing—original draft, X.W.; writing—review and editing, S.M. and H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Scientific Research and Development Foundation of Zhejiang A&F University (2021FR010).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Shengjun Feng for providing seeds for the cucumber inbred lines.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jakobsen, H.C. Reduction of bacterial growth by a vesicular arbuscular mycorrhizal fungus in the rhizosphere of cucumber (Cucumis sativus L.). Biol. Fertil. Soils 1993, 15, 253–258. [Google Scholar]
- Sheng, Y.; Pan, Y.; Li, Y.; Yang, L.; Weng, Y. Quantitative trait loci for fruit size and flowering time-related traits under domestication and diversifying selection in cucumber (Cucumis sativus). Plant Breed. 2019, 139, 176–191. [Google Scholar] [CrossRef]
- Zhang, X.W.; Liu, F.J.; Zhai, J.; Li, F.D.; Bi, H.G.; Ai, X.Z. Auxin acts as a downstream signaling molecule involved in hydrogen sulfide-induced chilling tolerance in cucumber. Planta 2020, 251, 69. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mao, L.; Mi, H.; Lu, W.; Ying, T.; Luo, Z. Involvement of three annexin genes in the ripening of strawberry fruit regulated by phytohormone and calcium signal transduction. Plant Cell Rep. 2016, 35, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yang, S.; Wang, Y.; Lu, L.; Sun, M.; He, C.; Wang, J.; Li, Y.; Yu, X.; Li, Q.; et al. Physiological and molecular mechanisms of ABA and CaCl2 regulating chilling tolerance of cucumber Seedlings. Plants 2021, 10, 2746. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Li, F.; Ai, X.; Bi, H. H2O2 participates in ABA regulation of grafting-induced chilling tolerance in cucumber. Plant Cell Rep. 2022, 41, 1115–1130. [Google Scholar] [CrossRef]
- Dong, C.J.; Li, L.; Shang, Q.M.; Liu, X.Y.; Zhang, Z.G. Endogenous salicylic acid accumulation is required for chilling tolerance in cucumber (Cucumis sativus L.) seedlings. Planta 2014, 240, 687–700. [Google Scholar] [CrossRef]
- Fu, X.; Feng, Y.Q.; Zhang, X.W.; Zhang, Y.Y.; Bi, H.G.; Ai, X.Z. Salicylic acid is involved in rootstock-scion communication in improving the chilling tolerance of grafted cucumber. Front. Plant Sci. 2021, 12, 693344. [Google Scholar] [CrossRef]
- Qi, C.; Dong, D.; Li, Y.; Wang, X.; Guo, L.; Liu, L.; Dong, X.; Li, X.; Yuan, X.; Ren, S.; et al. Heat shock-induced cold acclimation in cucumber through CsHSFA1d-activated JA biosynthesis and signaling. Plant J. 2022, 111, 85–102. [Google Scholar] [CrossRef]
- Wei, L.J.; Deng, X.G.; Zhu, T.; Zheng, T.; Li, P.X.; Wu, J.Q.; Zhang, D.W.; Lin, H.H. Ethylene is involved in brassinosteroids induced alternative respiratory pathway in cucumber (Cucumis sativus L.) seedlings response to abiotic stress. Front. Plant Sci. 2015, 6, 982. [Google Scholar] [CrossRef]
- Catala, R.; Lopez-Cobollo, R.; Mar Castellano, M.; Angosto, T.; Alonso, J.M.; Ecker, J.R.; Salinas, J. The Arabidopsis 14-3-3 protein RARE COLD INDUCIBLE 1A links low-temperature response and ethylene biosynthesis to regulate freezing tolerance and cold acclimation. Plant Cell 2014, 26, 3326–3342. [Google Scholar] [CrossRef]
- Dong, Y.; Tang, M.; Huang, Z.; Song, J.; Xu, J.; Ahammed, G.J.; Yu, J.; Zhou, Y. The miR164a-NAM3 module confers cold tolerance by inducing ethylene production in tomato. Plant J. 2022, 111, 440–456. [Google Scholar] [CrossRef]
- Shi, Y.; Tian, S.; Hou, L.; Huang, X.; Zhang, X.; Guo, H.; Yang, S. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 2012, 24, 2578–2595. [Google Scholar] [CrossRef]
- Wang, C.Y.; Adams, D.O. Chilling-induced ethylene production in cucumbers (Cucumis sativus L.). Plant Physiol. 1982, 69, 424–427. [Google Scholar] [CrossRef]
- Wang, C.Y.; Adams, D.O. Ethylene Production by Chilled Cucumbers (Cucumis sativus L.). Plant Physiol. 1980, 66, 841–843. [Google Scholar] [CrossRef]
- Dong, S.; Wang, W.; Bo, K.; Miao, H.; Song, Z.; Wei, S.; Zhang, S.; Gu, X. Quantitative trait loci mapping and candidate gene analysis of low temperature tolerance in cucumber seedlings. Front. Plant Sci. 2019, 10, 1620. [Google Scholar] [CrossRef]
- Thomashow, M.F. PLANT COLD ACCLIMATION: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef]
- Gilmour, S.J.; Zarka, D.G.; Stockinger, E.J.; Salazar, M.P.; Houghton, J.M.; Thomashow, M.F. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 1998, 16, 433–442. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef]
- Sakuma, Y.; Liu, Q.; Dubouzet, J.G.; Abe, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 2002, 290, 998–1009. [Google Scholar] [CrossRef]
- Stockinger, E.J.; Gilmour, S.J.; Thomashow, M.F. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA 1997, 94, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ding, Y.; Shi, Y.; Ma, L.; Wang, Y.; Song, C.; Wilkins, K.A.; Davies, J.M.; Knight, H.; Knight, M.R.; et al. The calcium transporter ANNEXIN1 mediates cold-induced calcium signaling and freezing tolerance in plants. EMBO J. 2021, 40, e104559. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yang, H.; Wu, S.; Fu, D.; Li, M.; Gong, Z.; Yang, S. CPK28-NLP7 module integrates cold-induced Ca2+ signal and transcriptional reprogramming in Arabidopsis. Sci. Adv. 2022, 8, eabn7901. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Liu, Y.; Mu, Y.; Anwar, A.; He, C.; Yan, Y.; Li, Y.; Yu, X. Heterotrimeric G-Protein gamma Subunit CsGG3.2 Positively Regulates the Expression of CBF Genes and Chilling Tolerance in Cucumber. Front. Plant Sci. 2018, 9, 488. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H.; Quan, X.; Shan, Q.; Wang, W.; Yin, N.; Wang, S.; Wang, Z.; He, W. Comprehensive analysis of cucumber C-repeat/dehydration-responsive element binding factor family genes and their potential roles in cold tolerance of cucumber. BMC Plant Biol. 2022, 22, 270. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, R.; Xiang, C.; Zhang, R.; Wang, Q.; Wang, T.; Li, X.; Lu, X.; Gao, S.; Liu, Z.; et al. Transcriptomic and physiological analysis reveal that alpha-linolenic acid biosynthesis responds to early chilling tolerance in pumpkin rootstock varieties. Front. Plant Sci. 2021, 12, 669565. [Google Scholar] [CrossRef]
- Wu, P.; Kong, Q.; Bian, J.; Ahammed, G.J.; Cui, H.; Xu, W.; Yang, Z.; Cui, J.; Liu, H. Unveiling molecular mechanisms of nitric oxide-induced low-temperature tolerance in cucumber by transcriptome profiling. Int. J. Mol. Sci. 2022, 23, 5615. [Google Scholar] [CrossRef]
- Xue, C.; Jiang, Y.; Wang, Z.; Shan, X.; Yuan, Y.; Hua, J. Tissue-level transcriptomic responses to local and distal chilling reveal potential chilling survival mechanisms in maize. J. Exp. Bot. 2021, 72, 7610–7625. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Zhang, Z.; Luo, W.; Tang, Y.; Niu, Y.; Chong, K.; Xu, Y. OsGRF6 interacts with SLR1 to regulate OsGA2ox1 expression for coordinating chilling tolerance and growth in rice. J. Plant Physiol. 2021, 260, 153406. [Google Scholar] [CrossRef]
- He, X.J.; Mu, R.L.; Cao, W.H.; Zhang, Z.G.; Zhang, J.S.; Chen, S.Y. AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. Plant J. 2005, 44, 903–916. [Google Scholar] [CrossRef]
- Xu, L.; Xiang, G.; Sun, Q.; Ni, Y.; Jin, Z.; Gao, S.; Yao, Y. Melatonin enhances salt tolerance by promoting MYB108A-mediated ethylene biosynthesis in grapevines. Hortic. Res. 2019, 6, 114. [Google Scholar] [CrossRef]
- Wang, K.L.; Li, H.; Ecker, J.R. Ethylene biosynthesis and signaling networks. Plant Cell 2002, 14 (Suppl. S1), S131–S151. [Google Scholar] [CrossRef]
- Kim, G.; Ryu, H.; Sung, J. Hormonal crosstalk and root suberization for drought stress tolerance in plants. Biomolecules 2022, 12, 811. [Google Scholar] [CrossRef]
- Wang, X.; Zou, B.; Shao, Q.; Cui, Y.; Lu, S.; Zhang, Y.; Huang, Q.; Huang, J.; Hua, J. Natural variation reveals that OsSAP16 controls low-temperature germination in rice. J. Exp. Bot. 2018, 69, 413–421. [Google Scholar] [CrossRef]
- Wang, H.; Lu, S.; Guan, X.; Jiang, Y.; Wang, B.; Hua, J.; Zou, B. Dehydration-responsive element binding protein 1C, 1E, and 1G promote stress tolerance to Chilling, Heat, Drought, and Salt in Rice. Front. Plant Sci. 2022, 13, 851731. [Google Scholar] [CrossRef]
- Liu, X.; Liu, B.; Xue, S.; Cai, Y.; Qi, W.; Jian, C.; Xu, S.; Wang, T.; Ren, H. Cucumber (Cucumis sativus L.) nitric oxide synthase associated Gene 1 (CsNOA1) plays a role in chilling stress. Front. Plant Sci. 2016, 7, 1652. [Google Scholar] [CrossRef]
- Xu, P.L.; Guo, Y.K.; Bai, J.G.; Shang, L.; Wang, X.J. Effects of long-term chilling on ultrastructure and antioxidant activity in leaves of two cucumber cultivars under low light. Physiol. Plant 2008, 132, 467–478. [Google Scholar] [CrossRef]
- Yang, H.; Wu, F.; Cheng, J. Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food Chem. 2011, 127, 1237–1242. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, S.; Li, Z.; Cheng, J.; Hu, P.; Zhu, T.; Wang, X.; Jin, M.; Wang, X.; Li, L.; et al. CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 promote tolerance to heat and chilling in rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef]
- Chao, Q.; Rothenberg, M.; Solano, R.; Roman, G.; Terzaghi, W.; Ecker, J.R. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 1997, 89, 1133–1144. [Google Scholar] [CrossRef]
- Solano, R.; Stepanova, A.; Chao, Q.; Ecker, J.R. Nuclear events in ethylene signaling: A transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 1998, 12, 3703–3714. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhong, X.; Zhao, F.; Wang, Y.; Yan, B.; Li, Q.; Chen, G.; Mao, B.; Wang, J.; Li, Y.; et al. Overexpression of receptor-like kinase ERECTA improves thermotolerance in rice and tomato. Nat. Biotechnol. 2015, 33, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Charng, Y.Y.; Liu, H.C.; Liu, N.Y.; Chi, W.T.; Wang, C.N.; Chang, S.H.; Wang, T.T. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol. 2007, 143, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Hellens, R.P.; Allan, A.C.; Friel, E.N.; Bolitho, K.; Grafton, K.; Templeton, M.D.; Karunairetnam, S.; Gleave, A.P.; Laing, W.A. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 2005, 1, 13. [Google Scholar] [CrossRef]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).