Shuterin Enhances the Cytotoxicity of the Natural Killer Leukemia Cell Line KHYG-1 by Increasing the Expression Levels of Granzyme B and IFN-γ through the MAPK and Ras/Raf Signaling Pathways
Abstract
1. Introduction
2. Results
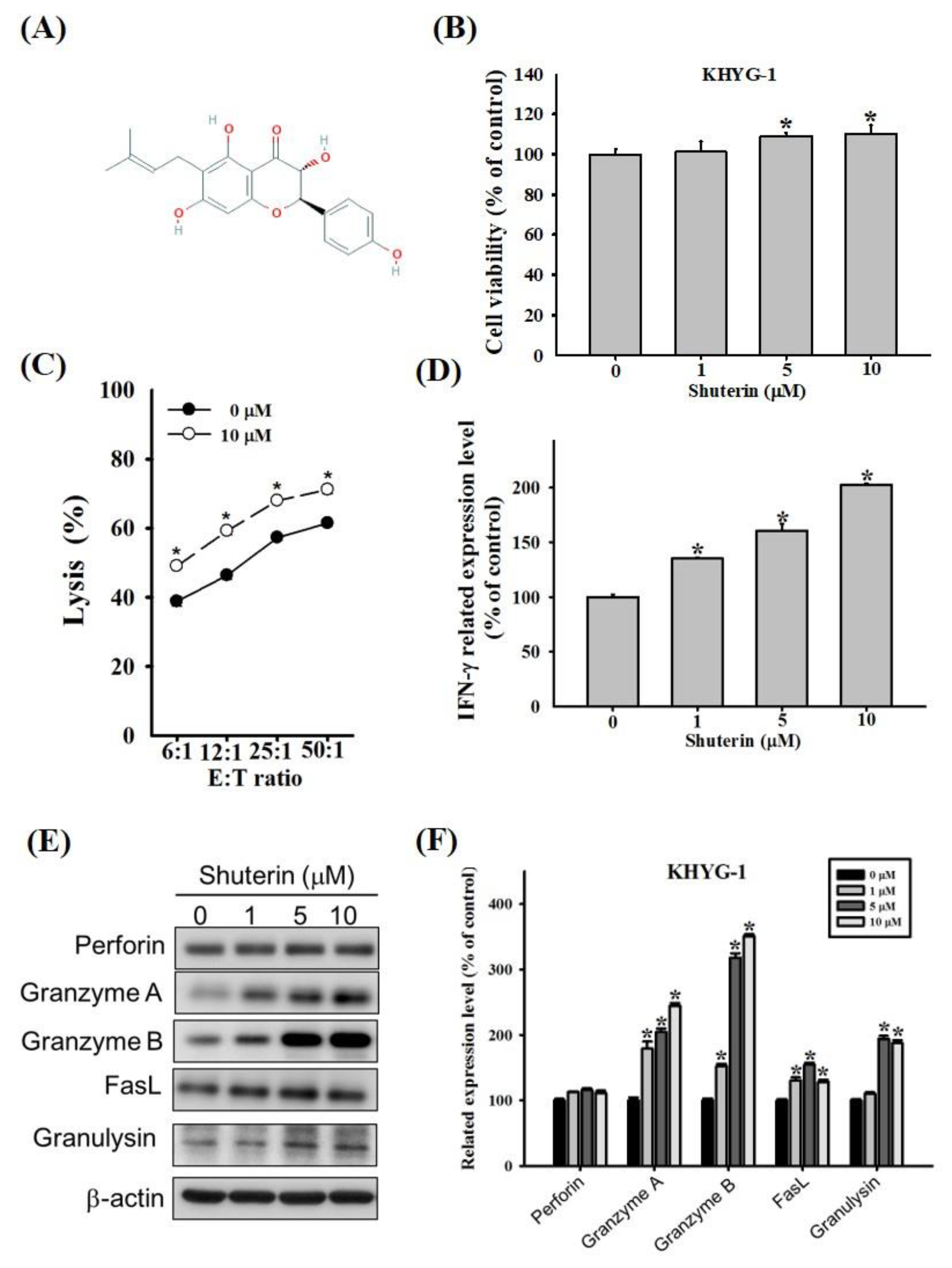
2.1. Effects of Shuterin on the Proliferation and Cytotoxicity of KHYG-1 Cells
2.2. Effects of Shuterin on the Expression Levels of Granzyme B and IFN-γ
2.3. Effects of Shuterin on CREB Phosphorylation and Histone Deacetylation
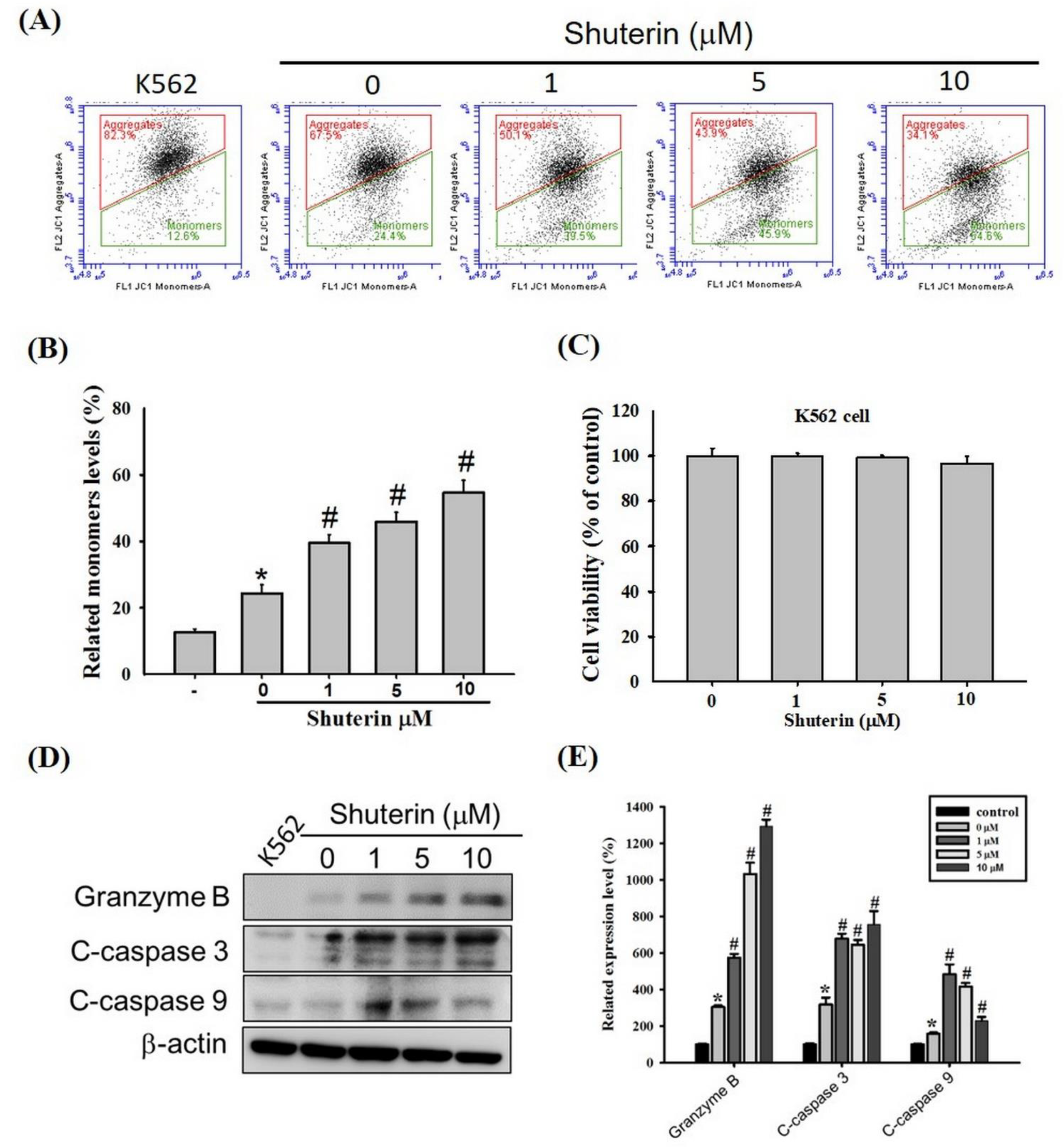
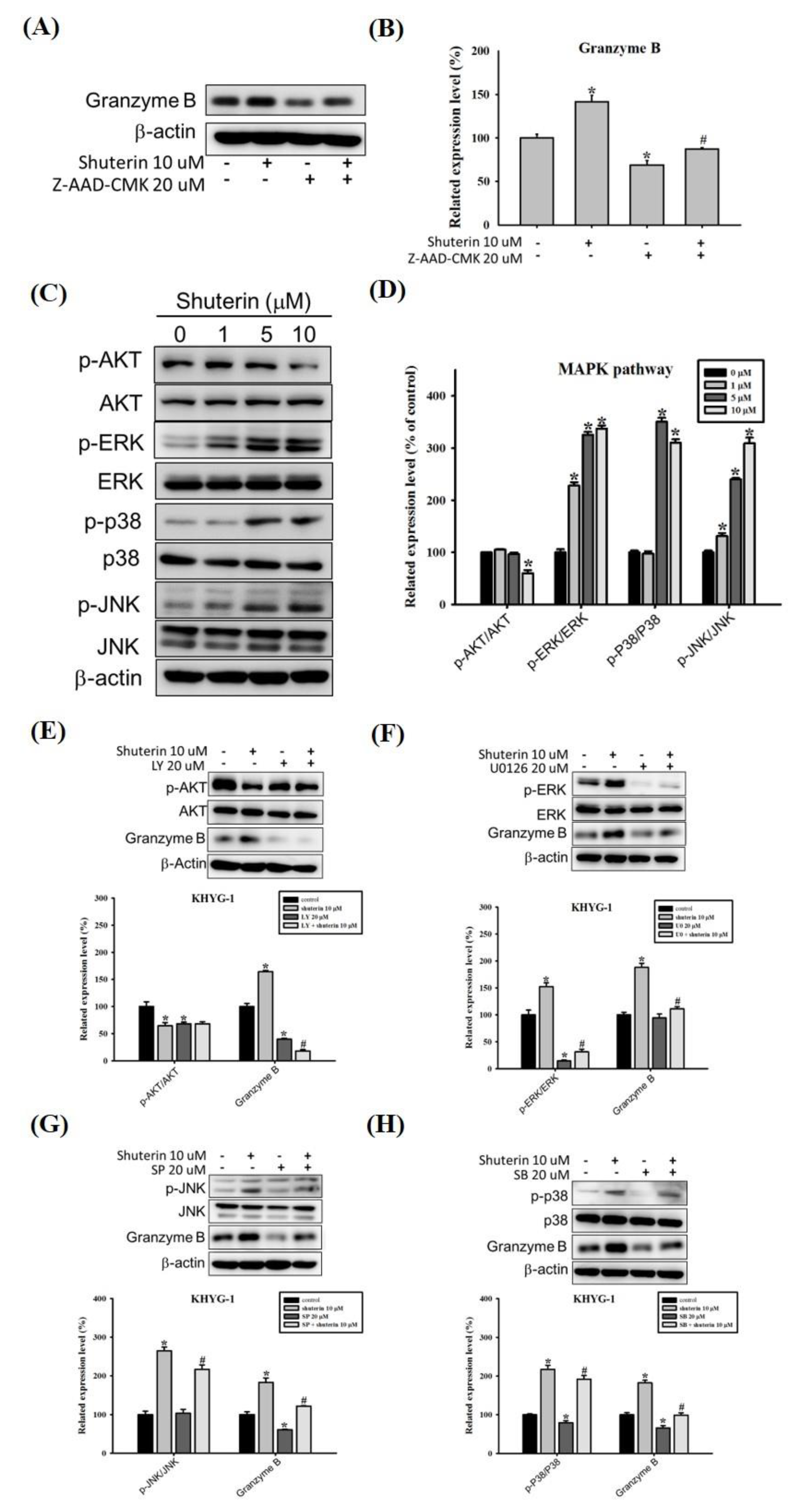
2.4. Effects of Shuterin on the Activation of Caspase-Mediated Apoptosis
2.5. Shuterin Increases Granzyme B Expression via the MAPK Pathway
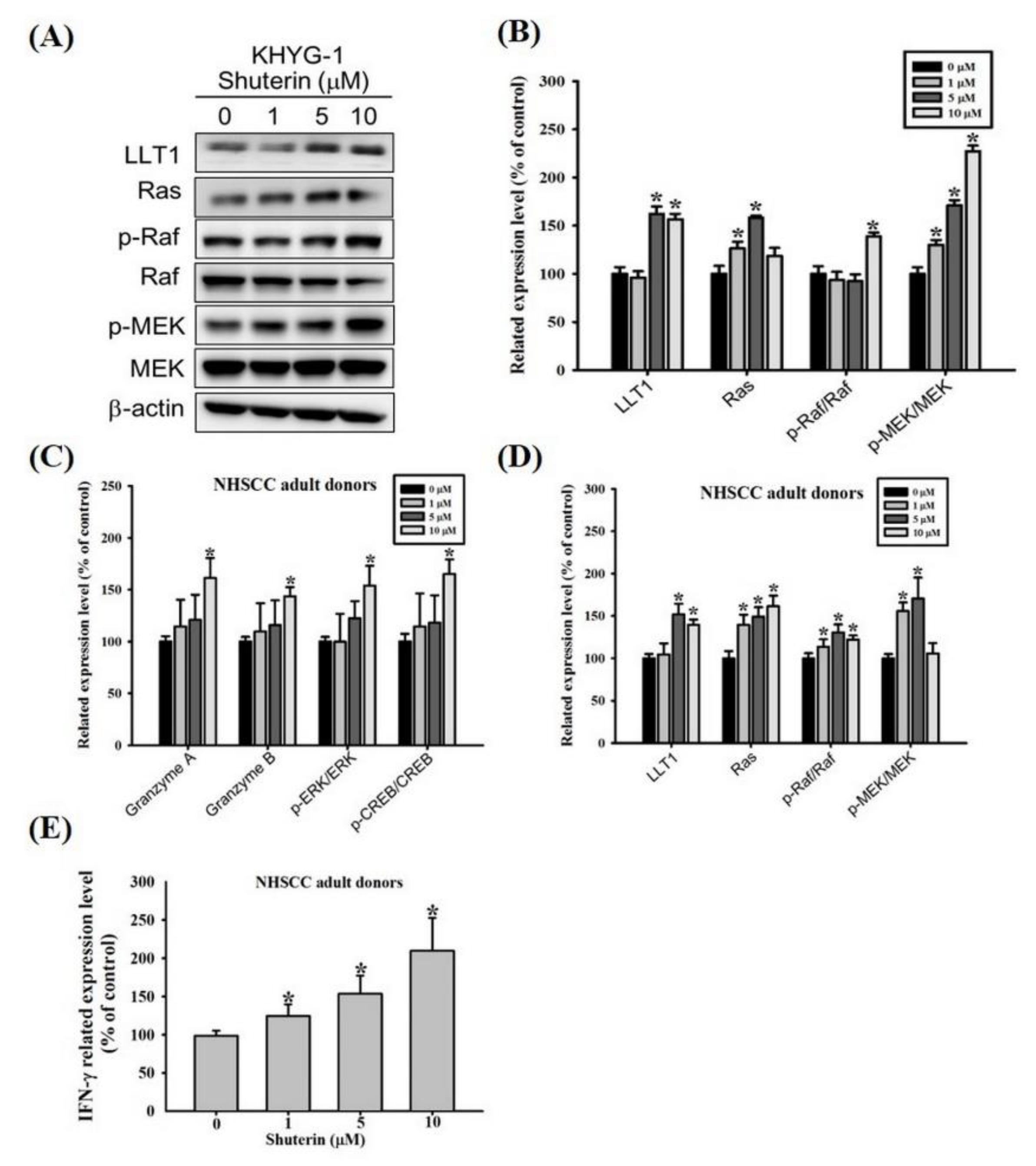
2.6. Shuterin Increase IFN-γ Expression Is Associated with a Higher LLT1 Receptor and Ras/Raf Pathway
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Proliferation Assay
4.4. Cell Cytotoxicity Assay
4.5. Cytokine Secretion
4.6. Western Blotting
4.7. Mitochondrial Membrane Potential Measurement
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nhung, H.T.M.; Anh, B.V.; Huyen, T.L.; Hiep, D.T.; Thao, C.T.; Lam, P.N.; Liem, N.T. Ex vivo expansion of human peripheral blood natural killer cells and cytotoxic T lymphocytes from lung cancer patients. Oncol. Lett. 2018, 15, 5730–5738. [Google Scholar] [CrossRef] [PubMed]
- Terunuma, H. Autologous Immune Enhancement Therapy for Cancer—Our experience since 2004. J. Stem Cells Regen. Med. 2012, 8, 205–206. [Google Scholar] [PubMed]
- Kalos, M.; June, C.H. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity 2013, 39, 49–60. [Google Scholar] [CrossRef]
- Kalinski, P.; Mailliard, R.B.; Giermasz, A.; Zeh, H.J.; Basse, P.; Bartlett, D.L.; Kirkwood, J.M.; Lotze, M.T.; Herberman, R.B. Natural killer-dendritic cell cross-talk in cancer immunotherapy. Expert Opin. Biol. Ther. 2005, 5, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Colucci, F.; Caligiuri, M.A.; Di Santo, J.P. What does it take to make a natural killer? Nat. Rev. Immunol. 2003, 3, 413–425. [Google Scholar] [CrossRef]
- Smyth, M.J.; Hayakawa, Y.; Takeda, K.; Yagita, H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat. Rev. Cancer 2002, 2, 850–861. [Google Scholar] [CrossRef]
- Lieberman, J. The ABCs of granule-mediated cytotoxicity: New weapons in the arsenal. Nat. Rev. Immunol. 2003, 3, 361–370. [Google Scholar] [CrossRef]
- Zamai, L.; Ahmad, M.; Bennett, I.M.; Azzoni, L.; Alnemri, E.S.; Perussia, B. Natural killer (NK) cell-mediated cytotoxicity: Differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J. Exp. Med. 1998, 188, 2375–2380. [Google Scholar] [CrossRef]
- Metkar, S.S.; Wang, B.; Ebbs, M.L.; Kim, J.H.; Lee, Y.J.; Raja, S.M.; Froelich, C.J. Granzyme B activates procaspase-3 which signals a mitochondrial amplification loop for maximal apoptosis. J. Cell Biol. 2003, 160, 875–885. [Google Scholar] [CrossRef]
- Hanson, R.D.; Grisolano, J.L.; Ley, T.J. Consensus AP-1 and CRE motifs upstream from the human cytotoxic serine protease B (CSP-B/CGL-1) gene synergize to activate transcription. Blood 1993, 82, 2749–2757. [Google Scholar] [CrossRef] [PubMed]
- Wargnier, A.; Legros-Maida, S.; Bosselut, R.; Bourge, J.F.; Lafaurie, C.; Ghysdael, C.J.; Sasportes, M.; Paul, P. Identification of human granzyme B promoter regulatory elements interacting with activated T-cell-specific proteins: Implication of Ikaros and CBF binding sites in promoter activation. Proc. Natl. Acad. Sci. USA 1995, 92, 6930–6934. [Google Scholar] [CrossRef] [PubMed]
- Carotta, S. Targeting NK Cells for Anticancer Immunotherapy: Clinical and Preclinical Approaches. Front. Immunol. 2016, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Hajto, T.; Hostanska, K.; Weber, K.; Zinke, H.; Fischer, J.; Mengs, U.; Lentzen, H.; Saller, R. Effect of a recombinant lectin, Viscum album agglutinin on the secretion of interleukin-12 in cultured human peripheral blood mononuclear cells and on NK-cell-mediated cytotoxicity of rat splenocytes in vitro and in vivo. Nat. Immun. 1998, 16, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Cho, H. Improved Anti-Cancer Effect of Curcumin on Breast Cancer Cells by Increasing the Activity of Natural Killer Cells. J. Microbiol. Biotechnol. 2018, 28, 874–882. [Google Scholar] [CrossRef]
- Lu, C.C.; Chen, J.K. Resveratrol enhances perforin expression and NK cell cytotoxicity through NKG2D-dependent pathways. J. Cell Physiol. 2010, 223, 343–351. [Google Scholar] [CrossRef]
- Li, Q.; Huyan, T.; Ye, L.J.; Li, J.; Shi, J.L.; Huang, Q.S. Concentration-dependent biphasic effects of resveratrol on human natural killer cells in vitro. J. Agric. Food Chem. 2014, 62, 10928–10935. [Google Scholar] [CrossRef]
- Ango, P.Y.; Kapche, D.W.; Fotso, G.W.; Fozing, C.D.; Yeboah, E.M.; Mapitse, R.; Demirtas, I.; Ngadjui, B.T.; Yeboah, S.O. Thonningiiflavanonol A and thonningiiflavanonol B, two novel flavonoids, and other constituents of Ficus thonningii Blume (Moraceae). Z. Naturforsch. C J. Biosci. 2016, 71, 65–71. [Google Scholar] [CrossRef]
- Ingham, J.L.; Tahara, S.; Dziedzic, S.Z. New 3-hydroxyflavanone (dihydroflavonol) phytoalexins from the papilionate legume Shuteria vestita. J. Nat. Prod. 1986, 49, 631–638. [Google Scholar] [CrossRef]
- Suck, G.; Branch, D.R.; Smyth, M.J.; Miller, R.G.; Vergidis, J.; Fahim, S.; Keating, A. KHYG-1, a model for the study of enhanced natural killer cell cytotoxicity. Exp. Hematol. 2005, 33, 1160–1171. [Google Scholar] [CrossRef]
- Yagita, M.; Huang, C.L.; Umehara, H.; Matsuo, Y.; Tabata, R.; Miyake, M.; Konaka, Y.; Takatsuki, K. A novel natural killer cell line (KHYG-1) from a patient with aggressive natural killer cell leukemia carrying a p53 point mutation. Leukemia 2000, 14, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Street, S.E.; Cretney, E.; Smyth, M.J. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood 2001, 97, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Abe, D.; Nogata, Y. Polymethoxylated flavones potentiate the cytolytic activity of NK leukemia cell line KHYG-1 via enhanced expression of granzyme B. Biochem. Biophys. Res. Commun. 2015, 456, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Ropero, S.; Esteller, M. The role of histone deacetylases (HDACs) in human cancer. Mol. Oncol. 2007, 1, 19–25. [Google Scholar] [CrossRef]
- Thomas, L.D.; Shah, H.; Green, S.A.; Bankhurst, A.D.; Whalen, M.M. Tributyltin exposure causes decreased granzyme B and perforin levels in human natural killer cells. Toxicology 2004, 200, 221–233. [Google Scholar] [CrossRef]
- Dokmanovic, M.; Clarke, C.; Marks, P.A. Histone deacetylase inhibitors: Overview and perspectives. Mol. Cancer Res. 2007, 5, 981–989. [Google Scholar] [CrossRef]
- Darmon, A.J.; Nicholson, D.W.; Bleackley, R.C. Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature 1995, 377, 446–448. [Google Scholar] [CrossRef]
- Bao, C.X.; Chen, H.X.; Mou, X.J.; Zhu, X.K.; Zhao, Q.; Wang, X.G. GZMB gene silencing confers protection against synovial tissue hyperplasia and articular cartilage tissue injury in rheumatoid arthritis through the MAPK signaling pathway. Biomed. Pharmacother. 2018, 103, 346–354. [Google Scholar] [CrossRef]
- Huang, C.; Bi, E.; Hu, Y.; Deng, W.; Tian, Z.; Dong, C.; Hu, Y.; Sun, B. A novel NF-kappaB binding site controls human granzyme B gene transcription. J. Immunol. 2006, 176, 4173–4181. [Google Scholar] [CrossRef]
- Bambard, N.D.; Mathew, S.O.; Mathew, P.A. LLT1-mediated activation of IFN-gamma production in human natural killer cells involves ERK signalling pathway. Scand. J. Immunol. 2010, 71, 210–219. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Tonn, T.; Becker, S.; Esser, R.; Schwabe, D.; Seifried, E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J. Hematother. Stem Cell Res. 2001, 10, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.S.; Yusa, S.; Kikuchi-Maki, A.; Catina, T.L. NKp44 triggers NK cell activation through DAP12 association that is not influenced by a putative cytoplasmic inhibitory sequence. J. Immunol. 2004, 172, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Raulet, D.H. Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol. 2003, 3, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Suck, G.; Branch, D.R.; Keating, A. Irradiated KHYG-1 retains cytotoxicity: Potential for adoptive immunotherapy with a natural killer cell line. Int. J. Radiat. Biol. 2006, 82, 355–361. [Google Scholar] [CrossRef]
- Smyth, M.J.; Cretney, E.; Kelly, J.M.; Westwood, J.A.; Street, S.E.; Yagita, H.; Takeda, K.; van Dommelen, S.L.; Degli-Esposti, M.A.; Hayakawa, Y. Activation of NK cell cytotoxicity. Mol. Immunol. 2005, 42, 501–510. [Google Scholar] [CrossRef]
- Geiger, T.L.; Sun, J.C. Development and maturation of natural killer cells. Curr. Opin. Immunol. 2016, 39, 82–89. [Google Scholar] [CrossRef]
- Metkar, S.S.; Wang, B.; Aguilar-Santelises, M.; Raja, S.M.; Uhlin-Hansen, L.; Podack, E.; Trapani, J.A.; Froelich, C.J. Cytotoxic cell granule-mediated apoptosis: Perforin delivers granzyme B-serglycin complexes into target cells without plasma membrane pore formation. Immunity 2002, 16, 417–428. [Google Scholar] [CrossRef]
- Motyka, B.; Korbutt, G.; Pinkoski, M.J.; Heibein, J.A.; Caputo, A.; Hobman, M.; Barry, M.; Shostak, I.; Sawchuk, T.; Holmes, C.F.; et al. Mannose 6-phosphate/insulin-like growth factor II receptor is a death receptor for granzyme B during cytotoxic T cell-induced apoptosis. Cell 2000, 103, 491–500. [Google Scholar] [CrossRef]
- Trapani, J.A.; Browne, K.A.; Smyth, M.J.; Jans, D.A. Localization of granzyme B in the nucleus. A putative role in the mechanism of cytotoxic lymphocyte-mediated apoptosis. J. Biol. Chem. 1996, 271, 4127–4133. [Google Scholar] [CrossRef]
- Trapani, J.A.; Sutton, V.R. Granzyme B: Pro-apoptotic, antiviral and antitumor functions. Curr. Opin. Immunol. 2003, 15, 533–543. [Google Scholar] [CrossRef]
- Heibein, J.A.; Goping, I.S.; Barry, M.; Pinkoski, M.J.; Shore, G.C.; Green, D.R.; Bleackley, R.C. Granzyme B-mediated cytochrome c release is regulated by the Bcl-2 family members bid and Bax. J. Exp. Med. 2000, 192, 1391–1402. [Google Scholar] [CrossRef]
- Alimonti, J.B.; Shi, L.; Baijal, P.K.; Greenberg, A.H. Granzyme B induces BID-mediated cytochrome c release and mitochondrial permeability transition. J. Biol. Chem. 2001, 276, 6974–6982. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.A.; Du, C.; Xu, M.; Wang, X.; Ley, T.J. DFF45/ICAD can be directly processed by granzyme B during the induction of apoptosis. Immunity 2000, 12, 621–632. [Google Scholar] [CrossRef]
- Martinvalet, D.; Dykxhoorn, D.M.; Ferrini, R.; Lieberman, J. Granzyme A cleaves a mitochondrial complex I protein to initiate caspase-independent cell death. Cell 2008, 133, 681–692. [Google Scholar] [CrossRef]
- Pardo, J.; Pérez-Galán, P.; Gamen, S.; Marzo, I.; Monleón, I.; Kaspar, A.A.; Susín, S.A.; Kroemer, G.; Krensky, A.M.; Naval, J.; et al. A role of the mitochondrial apoptosis-inducing factor in granulysin-induced apoptosis. J. Immunol. 2001, 167, 1222–1229. [Google Scholar] [CrossRef]
- Ali, A.K.; Nandagopal, N.; Lee, S.H. IL-15-PI3K-AKT-mTOR: A Critical Pathway in the Life Journey of Natural Killer Cells. Front. Immunol. 2015, 6, 355. [Google Scholar] [CrossRef]
- Geng, J.; Wang, Y.; Zhang, L.; Wang, R.; Li, C.; Sheng, W.; Li, Z.; Jiang, M. The cajanine derivative LJ101019C regulates the proliferation and enhances the activity of NK cells via Kv1.3 channel-driven activation of the AKT/mTOR pathway. Phytomedicine 2020, 66, 153113. [Google Scholar] [CrossRef]
- Li, C.; Ge, B.; Nicotra, M.; Stern, J.N.; Kopcow, H.D.; Chen, X.; Strominger, J.L. JNK MAP kinase activation is required for MTOC and granule polarization in NKG2D-mediated NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA 2008, 105, 3017–3022. [Google Scholar] [CrossRef]
- Chini, C.C.; Boos, M.D.; Dick, C.J.; Schoon, R.A.; Leibson, P.J. Regulation of p38 mitogen-activated protein kinase during NK cell activation. Eur. J. Immunol. 2000, 30, 2791–2798. [Google Scholar] [CrossRef]
- Suck, G.; Branch, D.R.; Aravena, P.; Mathieson, M.; Helke, S.; Keating, A. Constitutively polarized granules prime KHYG-1 NK cells. Int. Immunol. 2006, 18, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Boles, K.S.; Barten, R.; Kumaresan, P.R.; Trowsdale, J.; Mathew, P.A. Cloning of a new lectin-like receptor expressed on human NK cells. Immunogenetics 1999, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mathew, P.A.; Chuang, S.S.; Vaidya, S.V.; Kumaresan, P.R.; Boles, K.S.; Pham, H.T. The LLT1 receptor induces IFN-gamma production by human natural killer cells. Mol. Immunol. 2004, 40, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.P.; Shema, S.; Wu, S.; Gomez, S.L.; Chang, E.T.; Le, Q.T. Head and neck cancer-specific survival based on socioeconomic status in Asians and Pacific Islanders. Cancer 2011, 117, 1935–1945. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, L.L.; Xiang, R.L.; Yu, G.Y. Efficacy and Safety of Intro-Arterial Chemotherapy Combined with Radiotherapy on Head and Neck Cancer: A Systematic Review and Meta-Analysis. J. Cancer 2019, 10, 6233–6243. [Google Scholar] [CrossRef]
- Kumar, V.B.; Hsieh, M.J.; Mahalakshmi, B.; Chuang, Y.C.; Lin, C.C.; Lo, Y.S.; Ho, H.Y.; Lin, J.T. 7-Epitaxol Induces Apoptosis and Autophagy in Head and Neck Squamous Cell Carcinoma through Inhibition of the ERK Pathway. Cells 2021, 10, 2633. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Hsieh, M.C.; Lin, C.C.; Lo, Y.S.; Ho, H.Y.; Hsieh, M.J.; Lin, J.T. Pinosylvin inhibits migration and invasion of nasopharyngeal carcinoma cancer cells via regulation of epithelial-mesenchymal transition and inhibition of MMP-2. Oncol. Rep. 2021, 46, 143. [Google Scholar] [CrossRef]
- Hu, C.D.; Kosaka, Y.; Marcus, P.; Rashedi, I.; Keating, A. Differential Immunomodulatory Effects of Human Bone Marrow-Derived Mesenchymal Stromal Cells on Natural Killer Cells. Stem Cells Dev. 2019, 28, 933–943. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-T.; Chuang, Y.-C.; Chen, M.-K.; Lo, Y.-S.; Lin, C.-C.; Ho, H.-Y.; Liu, Y.-T.; Hsieh, M.-J. Shuterin Enhances the Cytotoxicity of the Natural Killer Leukemia Cell Line KHYG-1 by Increasing the Expression Levels of Granzyme B and IFN-γ through the MAPK and Ras/Raf Signaling Pathways. Int. J. Mol. Sci. 2022, 23, 12816. https://doi.org/10.3390/ijms232112816
Lin J-T, Chuang Y-C, Chen M-K, Lo Y-S, Lin C-C, Ho H-Y, Liu Y-T, Hsieh M-J. Shuterin Enhances the Cytotoxicity of the Natural Killer Leukemia Cell Line KHYG-1 by Increasing the Expression Levels of Granzyme B and IFN-γ through the MAPK and Ras/Raf Signaling Pathways. International Journal of Molecular Sciences. 2022; 23(21):12816. https://doi.org/10.3390/ijms232112816
Chicago/Turabian StyleLin, Jen-Tsun, Yi-Ching Chuang, Mu-Kuan Chen, Yu-Sheng Lo, Chia-Chieh Lin, Hsin-Yu Ho, Yen-Tze Liu, and Ming-Ju Hsieh. 2022. "Shuterin Enhances the Cytotoxicity of the Natural Killer Leukemia Cell Line KHYG-1 by Increasing the Expression Levels of Granzyme B and IFN-γ through the MAPK and Ras/Raf Signaling Pathways" International Journal of Molecular Sciences 23, no. 21: 12816. https://doi.org/10.3390/ijms232112816
APA StyleLin, J.-T., Chuang, Y.-C., Chen, M.-K., Lo, Y.-S., Lin, C.-C., Ho, H.-Y., Liu, Y.-T., & Hsieh, M.-J. (2022). Shuterin Enhances the Cytotoxicity of the Natural Killer Leukemia Cell Line KHYG-1 by Increasing the Expression Levels of Granzyme B and IFN-γ through the MAPK and Ras/Raf Signaling Pathways. International Journal of Molecular Sciences, 23(21), 12816. https://doi.org/10.3390/ijms232112816





