Rhus Coriaria L. Extract: Antioxidant Effect and Modulation of Bioenergetic Capacity in Fibroblasts from Parkinson’s Disease Patients and THP-1 Macrophages
Abstract
1. Introduction
2. Results
2.1. Antioxidant Activity of Phenolic Sumac Extract
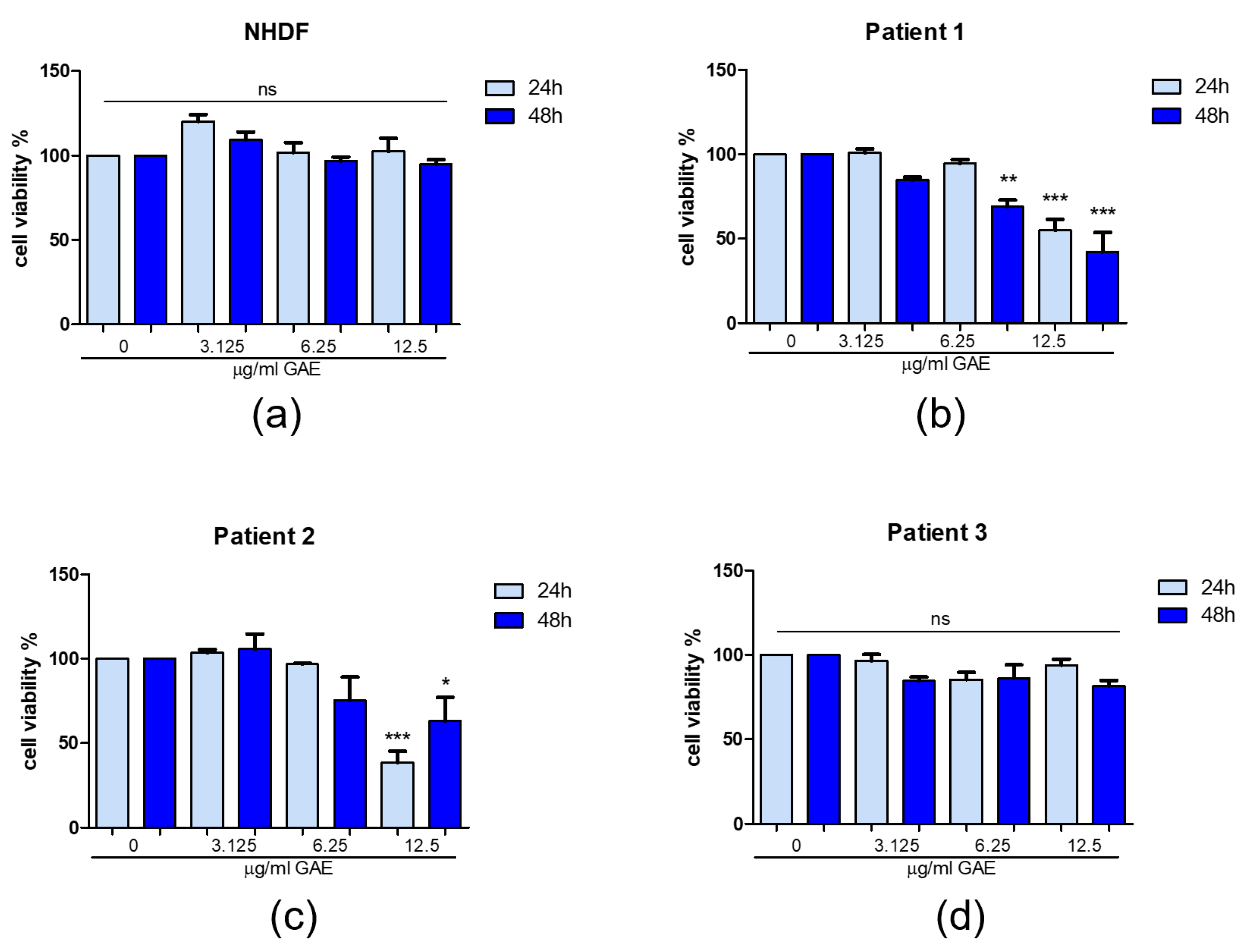
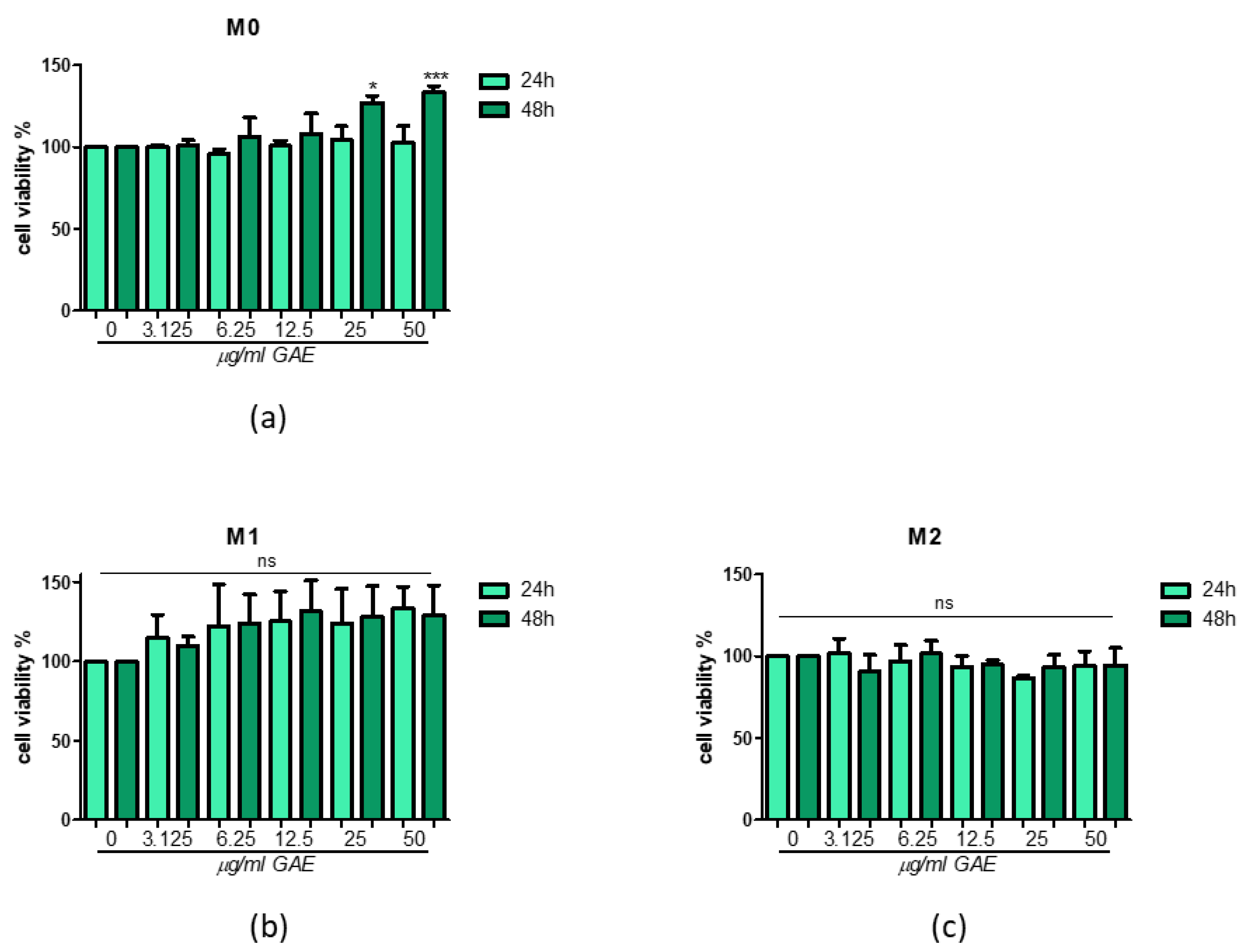
2.2. Effect of Phenolic Sumac Extract on Cell Viability
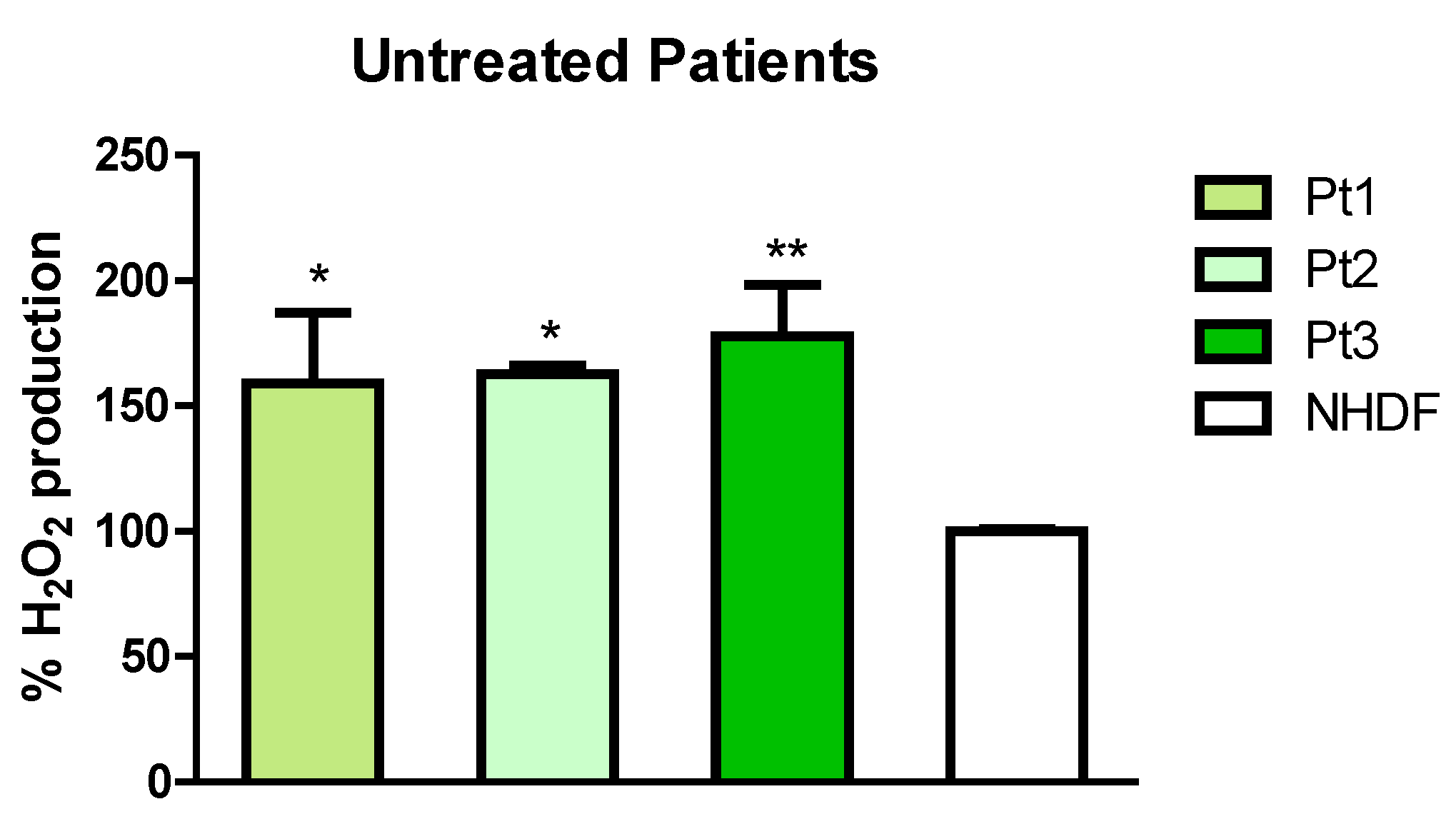
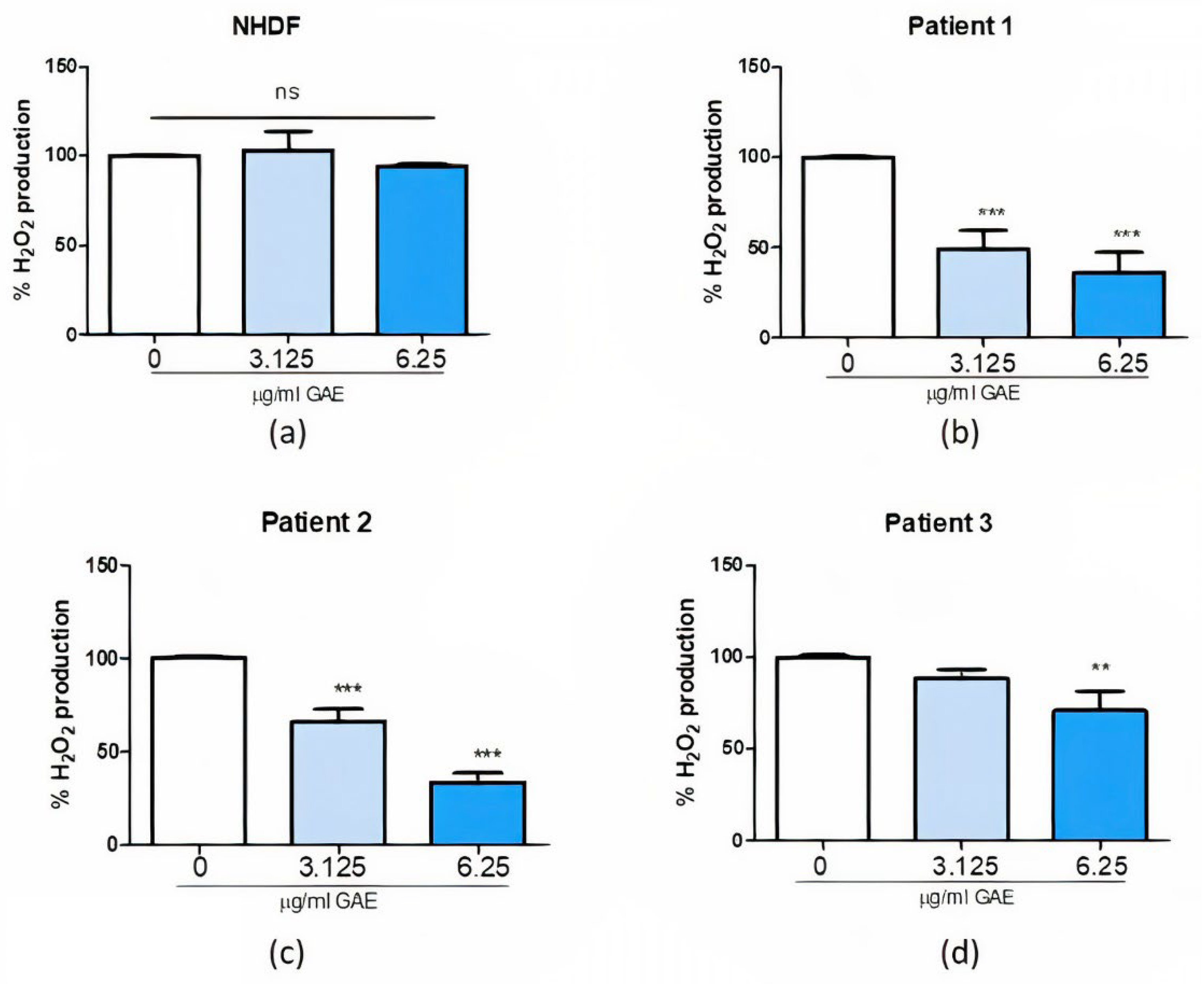
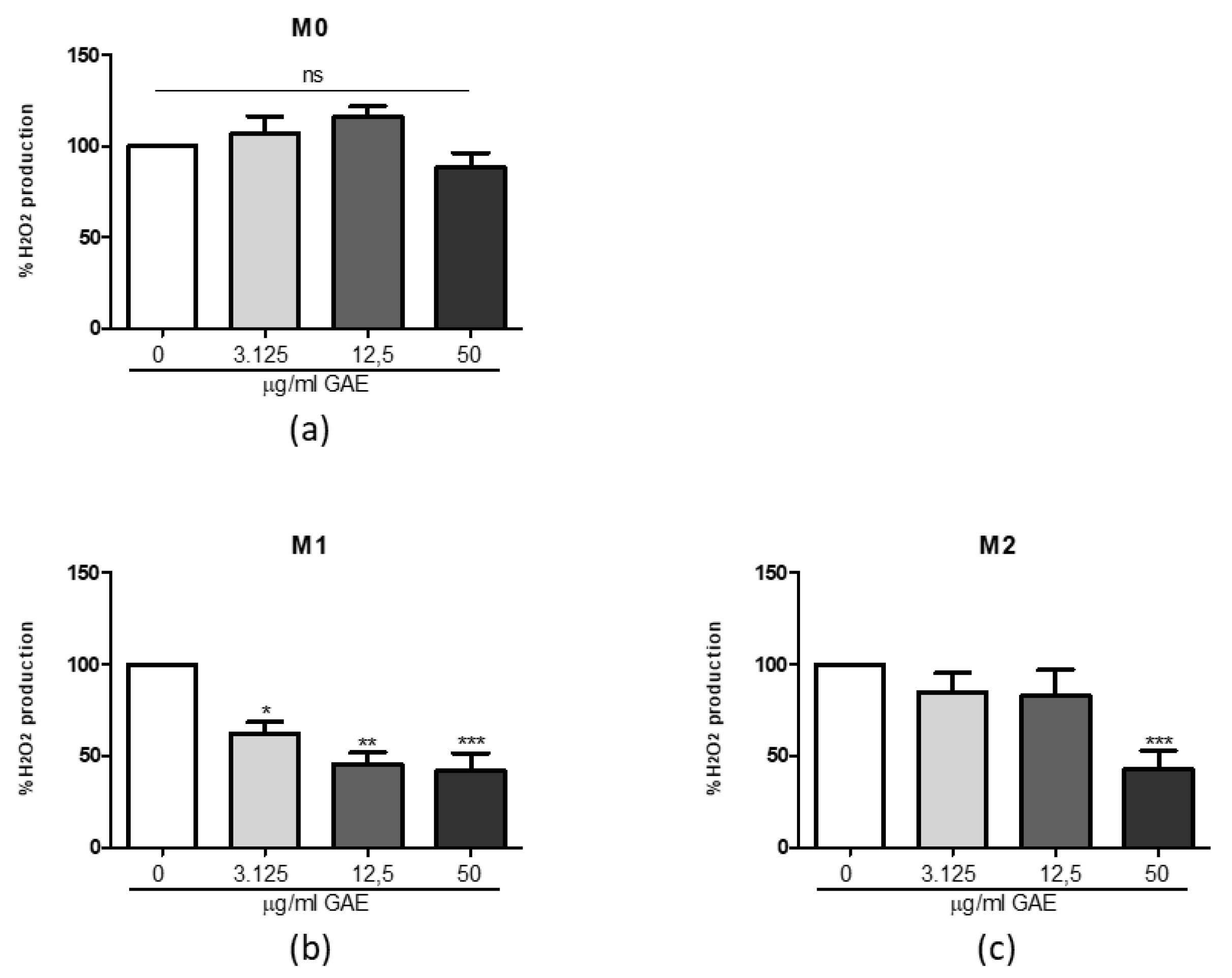
2.3. Effect of Phenolic Sumac Extract Treatment on H2O2 Cellular Production
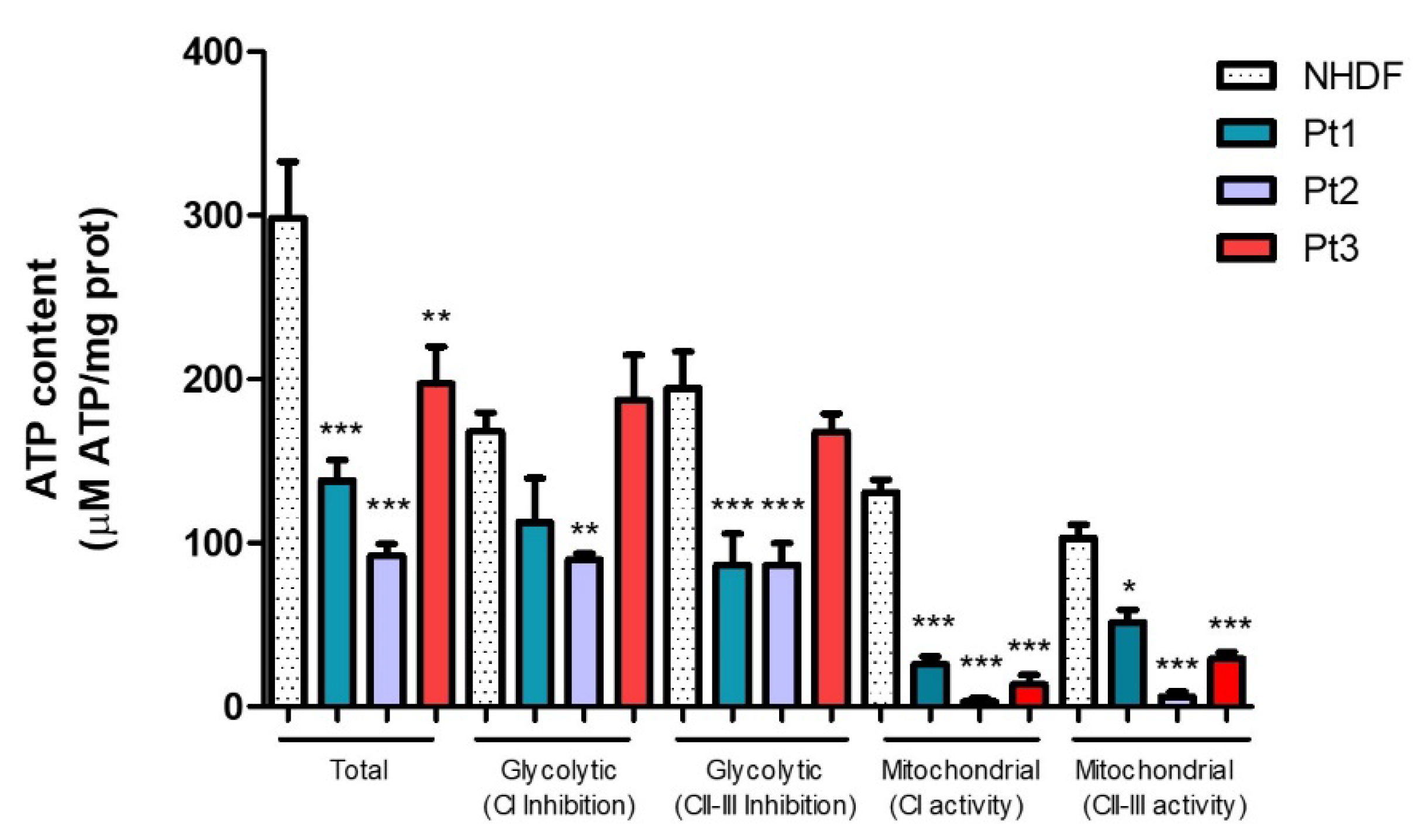
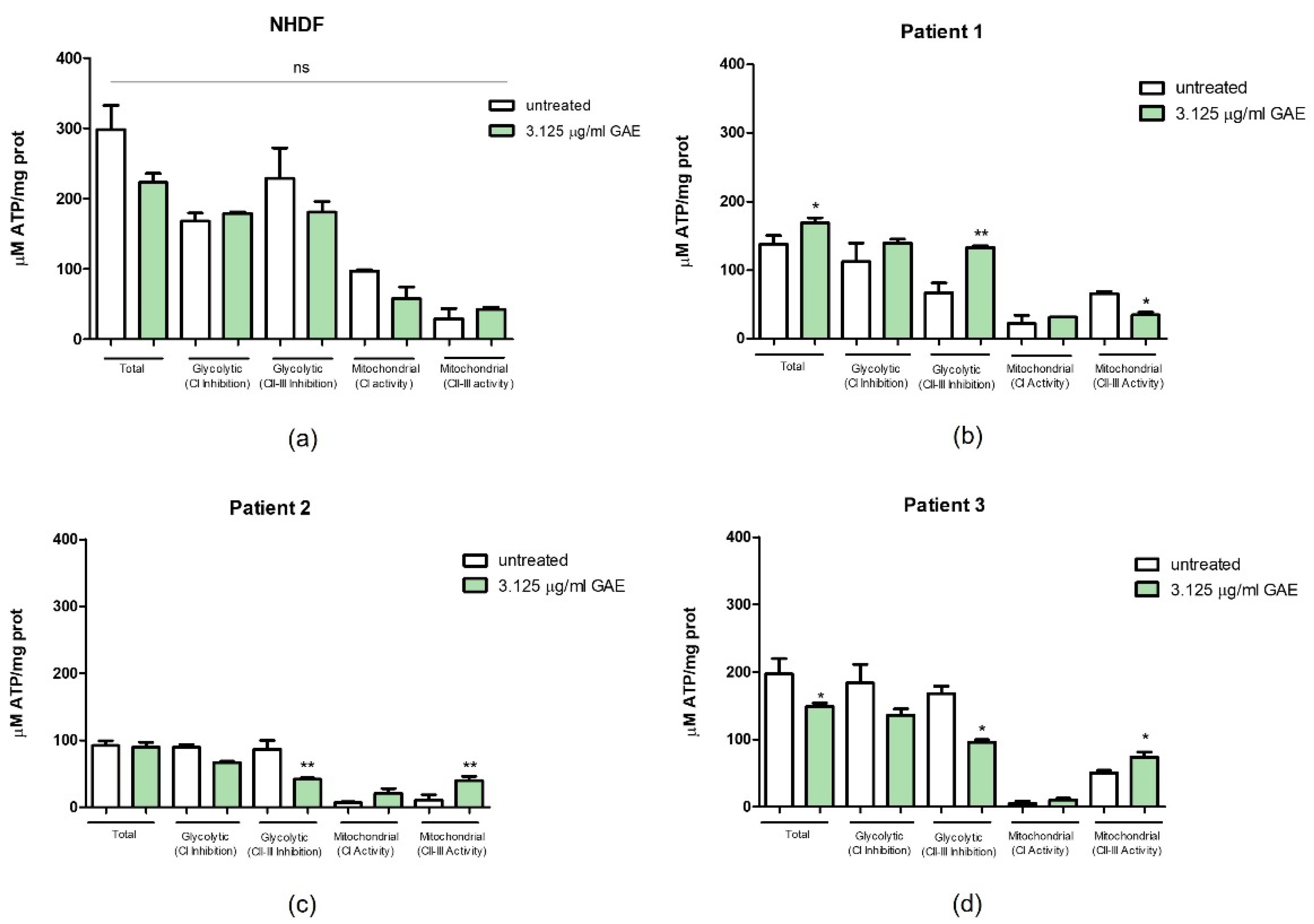
2.4. Effect of Phenolic Sumac Extract on Bioenergetic Capacity
3. Discussion
4. Materials and Methods
4.1. Samples, Cell Growth and Differentiation
4.2. Cytotoxic Effect
4.3. ROS Production
4.4. ATP Assay
4.5. Antioxidant Activity
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kitada, T.; Tomlinson, J.J.; Ao, H.S.; Grimes, D.A.; Schlossmacher, M.G. Considerations Regarding the Etiology and Future Treatment of Autosomal Recessive versus Idiopathic Parkinson Disease. Curr. Treat. Options Neurol. 2012, 14, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Westenberger, A. Genetics of Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a008888. [Google Scholar] [CrossRef] [PubMed]
- Wickremaratchi, M.M.; Knipe, M.D.W.; Sastry, B.D.; Morgan, E.; Jones, A.; Salmon, R.; Weiser, R.; Moran, M.; Davies, D.; Ebenezer, L.; et al. The Motor Phenotype of Parkinson’s Disease in Relation to Age at Onset. Mov. Disord. 2011, 26, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Kilarski, L.L.; Pearson, J.P.; Newsway, V.; Majounie, E.; Knipe, M.D.W.; Misbahuddin, A.; Chinnery, P.F.; Burn, D.J.; Clarke, C.E.; Marion, M.-H.; et al. Systematic Review and UK-Based Study of PARK2 (Parkin), PINK1, PARK7 (DJ-1) and LRRK2 in Early-Onset Parkinson’s Disease. Mov. Disord. 2012, 27, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Zanellati, M.C.; Monti, V.; Barzaghi, C.; Reale, C.; Nardocci, N.; Albanese, A.; Valente, E.M.; Ghezzi, D.; Garavaglia, B. Mitochondrial Dysfunction in Parkinson Disease: Evidence in Mutant PARK2 Fibroblasts. Front. Genet. 2015, 6, 78. [Google Scholar] [CrossRef]
- Barodia, S.K.; Creed, R.B.; Goldberg, M.S. Parkin and PINK1 Functions in Oxidative Stress and Neurodegeneration. Brain Res. Bull. 2017, 133, 51–59. [Google Scholar] [CrossRef]
- Ge, P.; Dawson, V.L.; Dawson, T.M. PINK1 and Parkin Mitochondrial Quality Control: A Source of Regional Vulnerability in Parkinson’s Disease. Mol. Neurodegener. 2020, 15, 1–18. [Google Scholar] [CrossRef]
- Grünewald, A.; Kumar, K.R.; Sue, C.M. New Insights into the Complex Role of Mitochondria in Parkinson’s Disease. Prog. Neurobiol. 2019, 177, 73–93. [Google Scholar] [CrossRef]
- Papa, S.; Sardanelli, A.M.; Capitanio, N.; Piccoli, C. Mitochondrial Respiratory Dysfunction and Mutations in Mitochondrial DNA in PINK1 Familial Parkinsonism. J. Bioenerg. Biomembr. 2009, 41, 509–516. [Google Scholar] [CrossRef]
- Pacelli, C.; Rasmo, D.D.; Signorile, A.; Grattagliano, I.; di Tullio, G.; D’Orazio, A.; Nico, B.; Comi, G.P.; Ronchi, D.; Ferranini, E.; et al. Mitochondrial Defect and PGC-1α Dysfunction in Parkin-Associated Familial Parkinson’s Disease. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2011, 1812, 1041–1053. [Google Scholar] [CrossRef]
- Buhlman, L.; Damiano, M.; Bertolin, G.; Ferrando-Miguel, R.; Lombès, A.; Brice, A.; Corti, O. Functional Interplay between Parkin and Drp1 in Mitochondrial Fission and Clearance. Biochim. Et Biophys. Acta-Mol. Cell Res. 2014, 1843, 2012–2026. [Google Scholar] [CrossRef]
- Dorszewska, J.; Kowalska, M.; Prendecki, M.; Piekut, T.; Kozłowska, J.; Kozubski, W. Oxidative Stress Factors in Parkinson’s Disease. Neural Regen. Res. 2021, 16, 1383–1391. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Chang, K.-H.; Lee-Chen, G.-J.; Wu, Y.-R.; Chen, Y.-J.; Lin, J.-L.; Li, M.; Chen, I.-C.; Lo, Y.-S.; Wu, H.-C.; Chen, C.-M. Impairment of Proteasome and Anti-Oxidative Pathways in the Induced Pluripotent Stem Cell Model for Sporadic Parkinson’s Disease. Park. Relat. Disord. 2016, 24, 81–88. [Google Scholar] [CrossRef]
- Wei, P.-C.; Lee-Chen, G.-J.; Chen, C.-M.; Wu, Y.-R.; Chen, Y.-J.; Lin, J.-L.; Lo, Y.-S.; Yao, C.-F.; Chang, K.-H. Neuroprotection of Indole-Derivative Compound NC001-8 by the Regulation of the NRF2 Pathway in Parkinson’s Disease Cell Models. Oxidative Med. Cell. Longev. 2019, 2019, 5074367. [Google Scholar] [CrossRef]
- LaVoie, M.J.; Ostaszewski, B.L.; Weihofen, A.; Schlossmacher, M.G.; Selkoe, D.J. Dopamine Covalently Modifies and Functionally Inactivates Parkin. Nat. Med. 2005, 11, 1214–1221. [Google Scholar] [CrossRef]
- Chang, K.-H.; Chen, C.-M. The Role of Oxidative Stress in Parkinson’s Disease. Antioxidants 2020, 9, 597. [Google Scholar] [CrossRef]
- Auburger, G.; Klinkenberg, M.; Drost, J.; Marcus, K.; Morales-Gordo, B.; Kunz, W.S.; Brandt, U.; Broccoli, V.; Reich-mann, H.; Gispert, S.; et al. Primary Skin Fibroblasts as a Model of Parkinson’s Disease. Mol. Neurobiol. 2012, 46, 20–27. [Google Scholar] [CrossRef]
- Burbulla, L.F.; Krüger, R. The Use of Primary Human Fibroblasts for Monitoring Mitochondrial Phenotypes in the Field of Parkinson’s Disease. JoVE 2012, 68, e4228. [Google Scholar] [CrossRef]
- Treutlein, B.; Lee, Q.Y.; Camp, J.G.; Mall, M.; Koh, W.; Shariati, S.A.M.; Sim, S.; Neff, N.F.; Skotheim, J.M.; Wernig, M.; et al. Dissecting Direct Reprogramming from Fibroblast to Neuron Using Single-Cell RNA-Seq. Nature 2016, 534, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.-S.; Chuang, C.-Y.; Yeh, C.-H.; Chiang, W.; Liu, H.-J.; Lin, T.-N.; Kuo, H.-C. Direct Conversion of Human Fibroblasts into Neural Progenitors Using Transcription Factors Enriched in Human ESC-Derived Neural Progenitors. Stem Cell Rep. 2017, 8, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Mortiboys, H.; Thomas, K.J.; Koopman, W.J.H.; Klaffke, S.; Abou-Sleiman, P.; Olpin, S.; Wood, N.W.; Willems, P.H.G.M.; Smeitink, J.A.M.; Cookson, M.R.; et al. Mitochondrial Function and Morphology Are Impaired in Parkin-Mutant Fibroblasts. Ann. Neurol. 2008, 64, 555–565. [Google Scholar] [CrossRef]
- Morén, C.; deSouza, R.M.; Giraldo, D.M.; Uff, C. Antioxidant Therapeutic Strategies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 9328. [Google Scholar] [CrossRef] [PubMed]
- Pogačnik, L.; Ota, A.; Poklar Ulrih, N. An Overview of Crucial Dietary Substances and Their Modes of Action for Prevention of Neurodegenerative Diseases. Cells 2020, 9, 576. [Google Scholar] [CrossRef]
- Aryal, S.; Skinner, T.; Bridges, B.; Weber, J.T. The Pathology of Parkinson’s Disease and Potential Benefit of Dietary Polyphenols. Molecules 2020, 25, 4382. [Google Scholar] [CrossRef]
- Granzotto, A.; Zatta, P. Resveratrol Acts Not through Anti-Aggregative Pathways but Mainly via Its Scavenging Properties against Aβ and Aβ-Metal Complexes Toxicity. PLoS ONE 2011, 6, e21565. [Google Scholar] [CrossRef]
- Ferretta, A.; Gaballo, A.; Tanzarella, P.; Piccoli, C.; Capitanio, N.; Nico, B.; Annese, T.; Paola, M.D.; Dell’Aquila, C.; Mari, M.D.; et al. Effect of Resveratrol on Mitochondrial Function: Implications in Parkin-Associated Familiar Parkinson’s Disease. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 902–915. [Google Scholar] [CrossRef]
- Yan, W.-J.; Liu, R.-B.; Wang, L.-K.; Ma, Y.-B.; Ding, S.-L.; Deng, F.; Hu, Z.-Y.; Wang, D.-B. Sirt3-Mediated Autophagy Contributes to Resveratrol-Induced Protection against ER Stress in HT22 Cells. Front. Neurosci. 2018, 12, 116. [Google Scholar] [CrossRef]
- Khatri, D.K.; Juvekar, A.R. Neuroprotective Effect of Curcumin as Evinced by Abrogation of Rotenone-Induced Motor Deficits, Oxidative and Mitochondrial Dysfunctions in Mouse Model of Parkinson’s Disease. Pharmacol. Biochem. Behav. 2016, 150–151, 39–47. [Google Scholar] [CrossRef]
- Pérez-Hernández, J.; Zaldívar-Machorro, V.J.; Villanueva-Porras, D.; Vega-Ávila, E.; Chavarría, A. A Potential Alternative against Neurodegenerative Diseases: Phytodrugs. Oxidative Med. Cell. Longev. 2016, 2016, 8378613. [Google Scholar] [CrossRef]
- Di Meo, F.; Valentino, A.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 2564. [Google Scholar] [CrossRef]
- Arena, K.; Trovato, E.; Cacciola, F.; Spagnuolo, L.; Pannucci, E.; Guarnaccia, P.; Santi, L.; Dugo, P.; Mondello, L.; Dugo, L. Phytochemical Characterization of Rhus Coriaria L. Extracts by Headspace Solid-Phase Micro Extraction Gas Chromatography, Comprehensive Two-Dimensional Liquid Chromatography, and Antioxidant Activity Evaluation. Molecules 2022, 27, 1727. [Google Scholar] [CrossRef]
- Doğan, A.; Çelik, İ. Healing Effects of Sumac (Rhus Coriaria) in Streptozotocin-Induced Diabetic Rats. Pharm. Biol. 2016, 54, 2092–2102. [Google Scholar] [CrossRef]
- Rahideh, S.T.; Shidfar, F.; Khandozi, N.; Rajab, A.; Hosseini, S.P.; Mirtaher, S.M. The Effect of Sumac (Rhus Coriaria L.) Powder on Insulin Resistance, Malondialdehyde, High Sensitive C-Reactive Protein and Paraoxonase 1 Activity in Type 2 Diabetic Patients. J. Res. Med. Sci. 2014, 19, 933–938. [Google Scholar]
- Khalilpour, S.; Behnammanesh, G.; Abdul Majid, A.M.S.; Tamayol, A.; Abdul Majid, A.S. Assessment of Neuroprotective Properties of Rhus Coriaria L. Ethanol Extract in an in Vitro Model of Retinal Degeneration. J. Herb. Med. 2017, 10, 45–52. [Google Scholar] [CrossRef]
- Khalilpour, S.; Latifi, S.; Behnammanesh, G.; Majid, A.M.S.A.; Majid, A.S.A.; Tamayol, A. Ischemic Optic Neuropathy as a Model of Neurodegenerative Disorder: A Review of Pathogenic Mechanism of Axonal Degeneration and the Role of Neuroprotection. J. Neurol. Sci. 2017, 375, 430–441. [Google Scholar] [CrossRef]
- Anand, A.; Modgil, S.; Sharma, V.L.; Shri, R.; Kaushik, S. Preserving Neural Retina through Re-Emerging Herbal Interventions. J. Cell. Biochem. 2014, 115, 1659–1668. [Google Scholar] [CrossRef]
- Gezici, S. Neuroprotective Effect, Antimicrobial and Antioxidant Potentials of Sumac (Rhus Coriaria L.) Fruit Extracts. Hacet. J. Biol. Chem. 2019, 47, 165–170. [Google Scholar] [CrossRef][Green Version]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef]
- Lohmann, E.; Periquet, M.; Bonifati, V. How much phenotypic variation can be attributed to parkin genotype? Ann. Neurol. 2003, 54, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Rawal, N.; Periquet, M.; Lohmann, E.; Lücking, C.; Teive, H.; Ambrosio, G.; Raskin, S.; Lincoln, S.; Hattori, N.; Guimaraes, J.; et al. Brice. New Parkin Mutations and Atypical Phenotypes in Families with Autosomal Recessive Parkinsonism. Neurology 2003, 60, 1378–1381. [Google Scholar] [CrossRef] [PubMed]
- Periquet, M.; Lücking, C.; Vaughan, J.; Bonifati, V.; Dürr, A.; De Michele, G.; Horstink, M.; Farrer, M.; Illarioshkin, S.; Pollak, P.; et al. Origin of the Mutations in the Parkin Gene in Europe: Exon Rearrangements Are Independent Recurrent Events, Whereas Point Mutations May Result from Founder Effects. Am. J. Hum. Genet. 2001, 68, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Prim. 2017, 3, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Morato Torres, C.A.; Wassouf, Z.; Zafar, F.; Sastre, D.; Outeiro, T.F.; Schüle, B. The Role of Alpha-Synuclein and Other Parkinson’s Genes in Neurodevelopmental and Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 5724. [Google Scholar] [CrossRef]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beni-nati, S.; et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. BioMed Res. Int. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Kim, T.Y.; Leem, E.; Lee, J.M.; Kim, S.R. Control of Reactive Oxygen Species for the Prevention of Parkinson’s Disease: The Possible Application of Flavonoids. Antioxidants 2020, 9, 583. [Google Scholar] [CrossRef]
- Sardanelli, A.M.; Amati, F.; Trentadue, R.; Sgaramella, G.; Martino, N.A.; Dell’Aquila, M.E.; Criscuolo, C.; De Michele, G. Mitochondrial respiratory dysfunction in PARK6 and PARK2 familial Parkinsonism. In Proceedings of the 36th FEBS Congress Biochemistry for Tomorrow’s Medicine, Torino, Italy, 25–30 June 2011; p. 275. [Google Scholar] [CrossRef]
- Ta, W.; Chawla, A.; Pollard, J. Origins and Hallmarks of Macrophages: Development, Homeostasis, and Disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Huang, S.C.-C.; Everts, B.; Ivanova, Y.; O’Sullivan, D.; Nascimento, M.; Smith, A.M.; Beatty, W.; Love-Gregory, L.; Lam, W.Y.; O’Neill, C.M.; et al. Cell-Intrinsic Lysosomal Lipolysis Is Essential for Alternative Activation of Macrophages. Nat. Immunol. 2014, 15, 846–855. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Aharoni, S.; Lati, Y.; Aviram, M.; Fuhrman, B. Pomegranate Juice Polyphenols Induce a Phenotypic Switch in Macrophage Polarization Favoring a M 2 Anti-inflammatory State. BioFactors 2015, 41, 44–51. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Sreedhar, R.; Giridharan, V.V.; Afrin, R.; Harima, M.; Miyashita, S.; Hara, M.; Suzuki, K.; et al. Curcumin Alleviates Renal Dysfunction and Suppresses Inflammation by Shifting from M1 to M2 Macrophage Polarization in Daunorubicin Induced Ne-phrotoxicity in Rats. Cytokine 2016, 84, 1–9. [Google Scholar] [CrossRef]
- Dugo, L.; Belluomo, M.G.; Fanali, C.; Russo, M.; Cacciola, F.; Maccarrone, M.; Sardanelli, A.M. Effect of Cocoa Polyphenolic Extract on Macrophage Polarization from Proinflammatory M1 to Anti-Inflammatory M2 State. Oxidative Med. Cell. Longev. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Sakaki, H.; Tsukimoto, M.; Harada, H.; Moriyama, Y.; Kojima, S. Autocrine Regulation of Macrophage Activation via Exocytosis of ATP and Activation of P2Y11 Receptor. PLoS ONE 2013, 8, e59778. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-Laboratory Validation of Microplate Methods for Total Phenolic Content and Antioxidant Activity on Polyphenolic Extracts, and Comparison with Conventional Spectrophotometric Methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]

| DPPH Assay | TEAC Assay | |||
|---|---|---|---|---|
| TE (mmol TE/g) | IC50 (mg/mL) | TE (mmol TE/g) | IC50 (mg/mL) | |
| SE | 1.02 ± 0.05 | 0.41 ± 0.02 | 1.76 ± 0.10 | 0.21 ± 0.005 |
| GA | 26.68 ± 1.05 | 0.016 ± 0.001 | 29.19 ± 2.55 | 0.012 ± 0.0003 |
| Patient | Code | Sex | Age Onset (yr) | Age at Skin Biopsy (yr) | Disease Duration (yr) | PARK2 Mutation | H-Y Stage | UPDRS |
|---|---|---|---|---|---|---|---|---|
| Pt1 | NP | F | 28 | 36 | 8 | Cys253Tyr/ex5del | 1 | 5 |
| Pt2 | IT-048-NA099 [41,42] | M | 47 | 64 | 17 | ex3-4del/ex3-4del | 3 | 30 |
| Pt3 | IT-031-NA025 [43] | M | 33 | 65 | 32 | ex2del/ex2-4del | 4 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isgrò, C.; Spagnuolo, L.; Pannucci, E.; Mondello, L.; Santi, L.; Dugo, L.; Sardanelli, A.M. Rhus Coriaria L. Extract: Antioxidant Effect and Modulation of Bioenergetic Capacity in Fibroblasts from Parkinson’s Disease Patients and THP-1 Macrophages. Int. J. Mol. Sci. 2022, 23, 12774. https://doi.org/10.3390/ijms232112774
Isgrò C, Spagnuolo L, Pannucci E, Mondello L, Santi L, Dugo L, Sardanelli AM. Rhus Coriaria L. Extract: Antioxidant Effect and Modulation of Bioenergetic Capacity in Fibroblasts from Parkinson’s Disease Patients and THP-1 Macrophages. International Journal of Molecular Sciences. 2022; 23(21):12774. https://doi.org/10.3390/ijms232112774
Chicago/Turabian StyleIsgrò, Camilla, Ludovica Spagnuolo, Elisa Pannucci, Luigi Mondello, Luca Santi, Laura Dugo, and Anna Maria Sardanelli. 2022. "Rhus Coriaria L. Extract: Antioxidant Effect and Modulation of Bioenergetic Capacity in Fibroblasts from Parkinson’s Disease Patients and THP-1 Macrophages" International Journal of Molecular Sciences 23, no. 21: 12774. https://doi.org/10.3390/ijms232112774
APA StyleIsgrò, C., Spagnuolo, L., Pannucci, E., Mondello, L., Santi, L., Dugo, L., & Sardanelli, A. M. (2022). Rhus Coriaria L. Extract: Antioxidant Effect and Modulation of Bioenergetic Capacity in Fibroblasts from Parkinson’s Disease Patients and THP-1 Macrophages. International Journal of Molecular Sciences, 23(21), 12774. https://doi.org/10.3390/ijms232112774








