Enhanced Flame Retardancy of Rigid Polyurethane Foams by Polyacrylamide/MXene Hydrogel Nanocomposite Coating
Abstract
1. Introduction
2. Results and Discussion
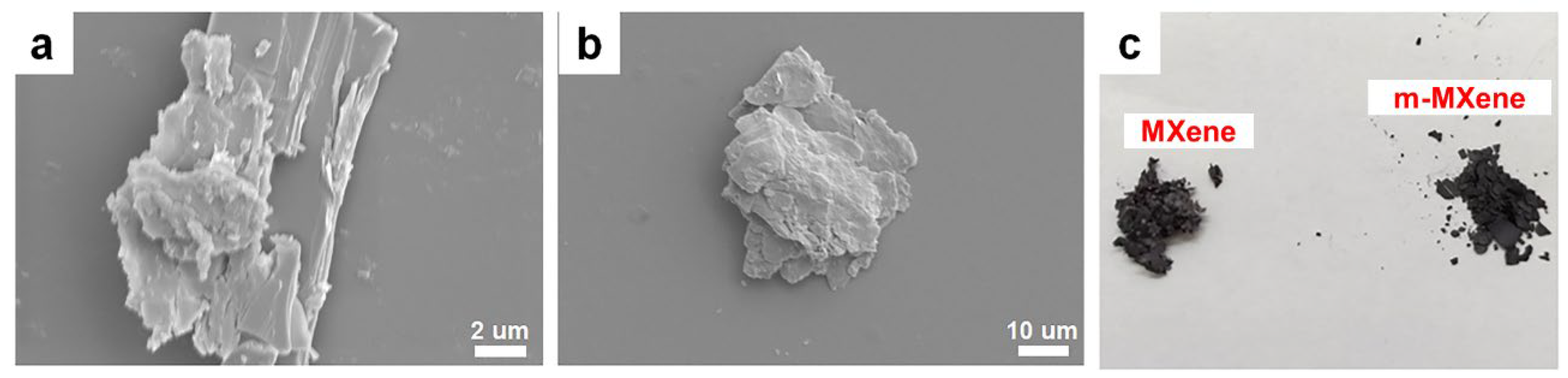
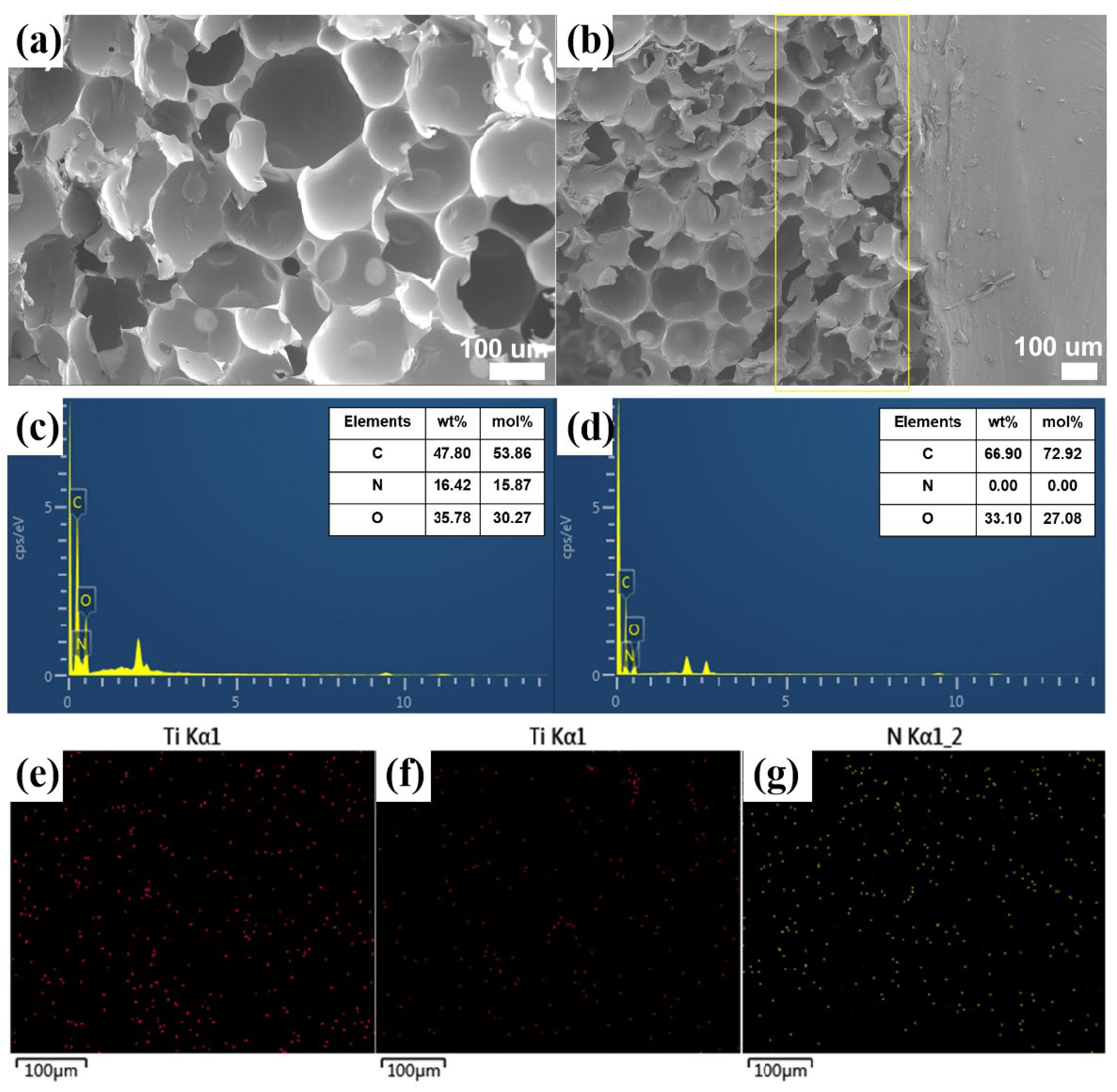
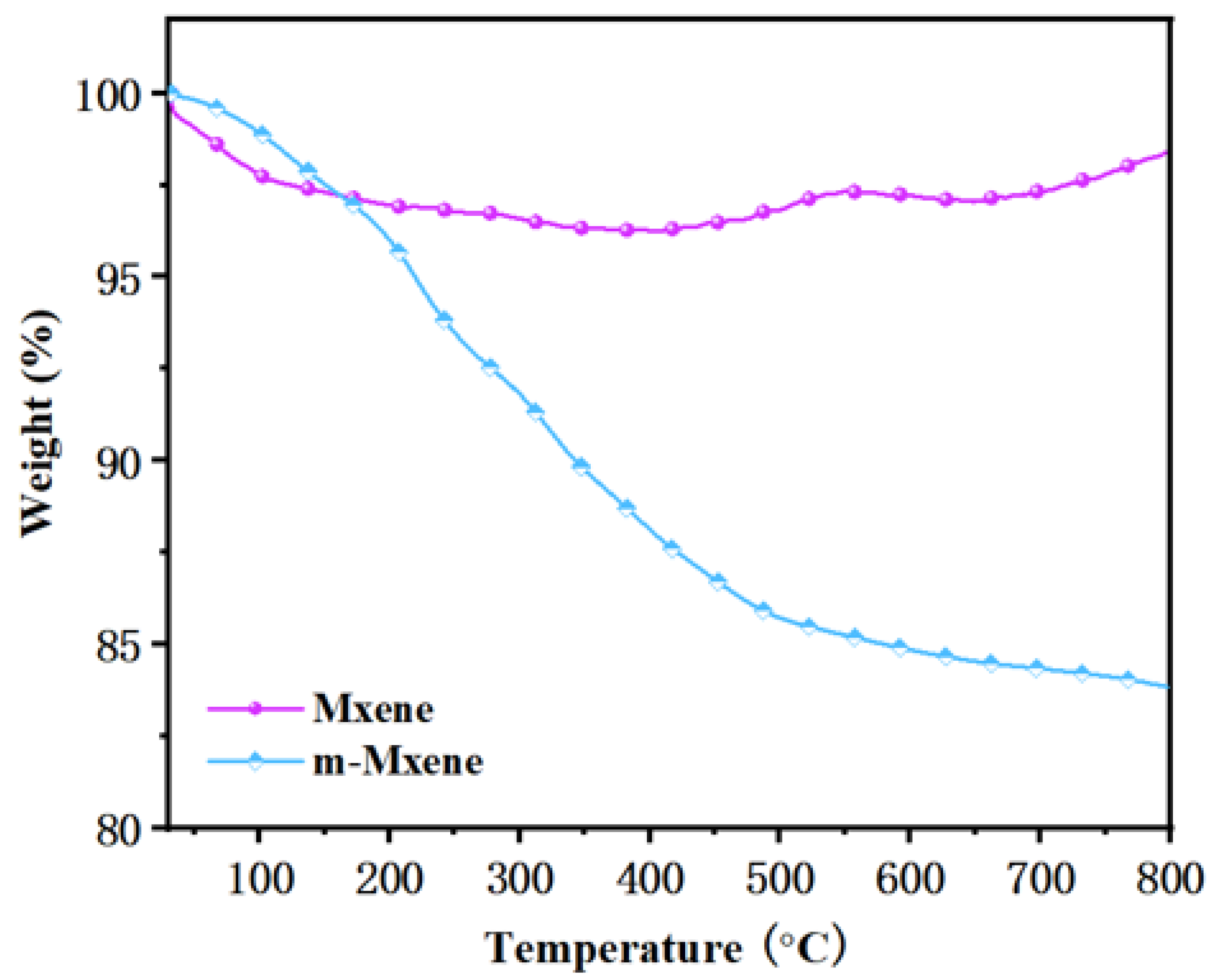
2.1. Characterization of m-MXene
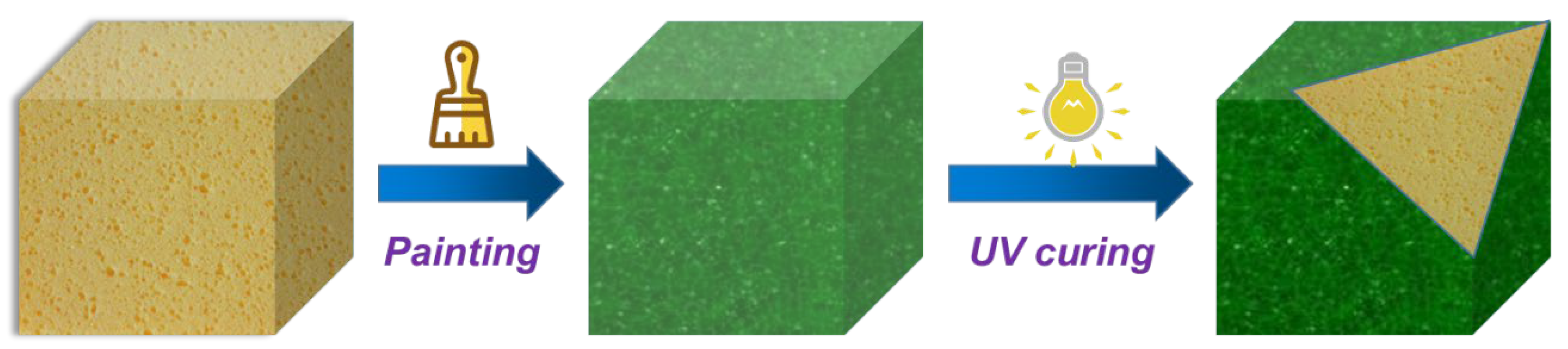
2.2. Morphology and Structure of Coated RPUF
2.3. Thermal Property of Coated RPUF
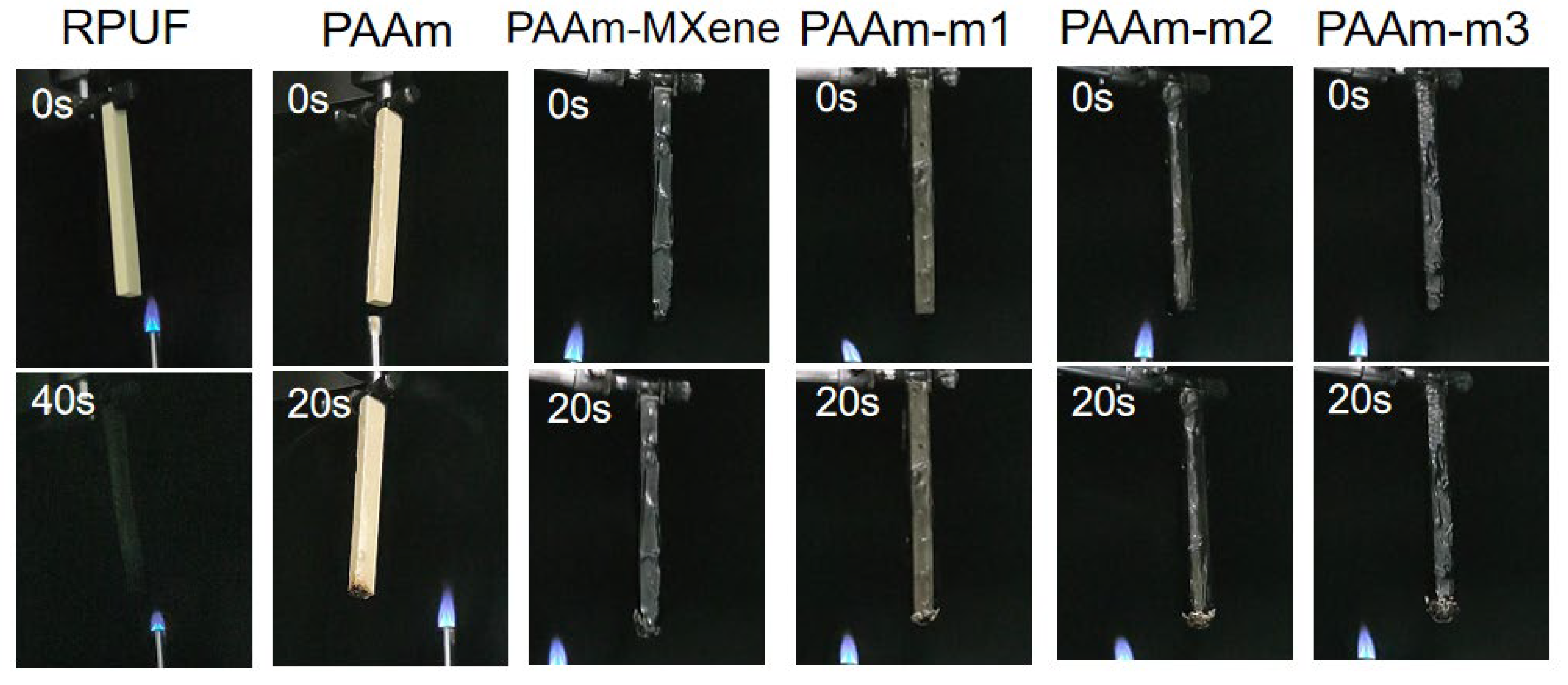
2.4. Flame Retardancy of Coated RPUF
2.5. Tensile Properties of Coatings
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Functionalized MXene (m-MXene)
3.3. Preparation of Coated RPUF
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, S.; Deng, D.; Zhou, L.; Zhang, P.; Tang, G. Flame retardancy and thermal degradation of rigid polyurethane foams composites based on aluminum hypophosphite. Mater. Res. Express 2019, 6, 105365. [Google Scholar] [CrossRef]
- Wu, D.-H.; Zhao, P.-H.; Liu, Y.-Q.; Liu, X.-Y.; Wang, X.-f. Halogen Free flame retardant rigid polyurethane foam with a novel phosphorus—Nitrogen intumescent flame retardant. J. Appl. Polym. Sci. 2014, 131, 39581. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, S.; Liang, R.; Sun, P.; Hai, Y.; Zhang, L. Thermal-triggered insulating fireproof layers: A novel fire-extinguishing MXene composites coating. Chem. Eng. J. 2020, 391, 23621. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, J.; Sun, P.; Zhang, L.; Qian, X.; Jiang, S.; Shi, C. Green, tough and highly efficient flame-retardant rigid polyurethane foam enabled by double network hydrogel coatings. Soft Matter 2021, 17, 10555–10565. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, K.; Shi, Y.; Wang, J.; Ma, S.; Feng, Y.; Lv, Y.; Yang, F.; Liu, M.; Song, P. Fire-safe, mechanically strong and tough thermoplastic Polyurethane/MXene nanocomposites with exceptional smoke suppression. Mater. Today Phys. 2022, 22, 100607. [Google Scholar] [CrossRef]
- Weil, E.D. Fire-protective and flame-retardant coatings—A state-of-the-art review. J. Fire Sci. 2011, 29, 259–296. [Google Scholar] [CrossRef]
- Zhang, D.; Williams, B.L.; Santos, V.H.; Lofink, B.J.; Becher, E.M.; Partyka, A.; Peng, X.; Sun, L. Self-assembled intumescent flame retardant coatings: Influence of pH on the flammability of cotton fabrics. Eng. Sci. 2020, 12, 106–112. [Google Scholar] [CrossRef]
- Gong, K.; Zhou, K.; Qian, X.; Shi, C.; Yu, B. MXene as emerging nanofillers for high-performance polymer composites: A review. Compos. Part B Eng. 2021, 217, 108867. [Google Scholar] [CrossRef]
- Gu, J.-w.; Zhang, G.-c.; Dong, S.-l.; Zhang, Q.-y.; Kong, J. Study on preparation and fire-retardant mechanism analysis of intumescent flame-retardant coatings. Surf. Coat. Technol. 2007, 201, 7835–7841. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, S.; Liang, R.; Liao, Z.; You, G. A green highly-effective surface flame-retardant strategy for rigid polyurethane foam: Transforming UV-cured coating into intumescent self-extinguishing layer. Compos. Part A Appl. Sci. Manuf. 2019, 125, 105534. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, M.; Shi, Y.; Xu, X.; Ma, Z.; Yu, B.; Fu, S.; Huang, G.; Wang, H.; Song, P. Functionalizing MXene towards highly stretchable, ultratough, fatigue- and fire-resistant polymer nanocomposites. Chem. Eng. J. 2021, 424, 130338. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Wang, Q.; Lin, W. The synergistic effect of layered double hydroxides with other flame retardant additives for polymer nanocomposites: A critical review. Dalton Trans. 2018, 47, 14827–14840. [Google Scholar] [CrossRef] [PubMed]
- Nageswara Rao, T.; Naidu, T.M.; Kim, M.S.; Parvatamma, B.; Prashanthi, Y.; Koo, B.H. Influence of zinc oxide nanoparticles and char forming agent polymer on flame retardancy of intumescent flame retardant coatings. Nanomaterials 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Du, Y.; Zheng, X.; Wang, J.; Wang, Y.; Zhao, S.; Xin, Z.; Li, L. Reduced fire hazards of expandable polystyrene building materials via intumescent flame-retardant coatings. J. Mater. Sci. 2020, 55, 7555–7572. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Fu, L.; Feng, Y.; Lv, Y.; Wang, Z.; Liu, M.; Chen, Z. Highly efficient MXene/Nano-Cu smoke suppressant towards reducing fire hazards of thermoplastic polyurethane. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106600. [Google Scholar] [CrossRef]
- Si, J.-Y.; Tawiah, B.; Sun, W.-L.; Lin, B.; Wang, C.; Yuen, A.C.Y.; Yu, B.; Li, A.; Yang, W.; Lu, H.-D.; et al. Functionalization of MXene Nanosheets for Polystyrene towards High Thermal Stability and Flame Retardant Properties. Polymers 2019, 11, 976. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Y.; Li, Y.; Shao, Q.; Yan, X.; Han, C.; Wang, Z.; Liu, Z.; Guo, Z. Flame-retardant rigid polyurethane foam with a phosphorus-nitrogen single intumescent flame retardant. Polym. Adv. Technol. 2018, 29, 668–676. [Google Scholar] [CrossRef]
- Wang, N.-N.; Wang, H.; Wang, Y.-Y.; Wei, Y.-H.; Si, J.-Y.; Yuen, A.C.Y.; Xie, J.-S.; Yu, B.; Zhu, S.-E.; Lu, H.D.; et al. Robust, Lightweight, Hydrophobic, and Fire-Retarded Polyimide/MXene Aerogels for Effective Oil/Water Separation. ACS Appl. Mater. Interfaces 2019, 11, 40512–40523. [Google Scholar] [CrossRef]
- Yang, W.; Liu, J.-J.; Wang, L.-L.; Wang, W.; Yuen, A.C.Y.; Peng, S.; Yu, B.; Lu, H.-D.; Yeoh, G.H.; Wang, C.-H. Multifunctional MXene/natural rubber composite films with exceptional flexibility and durability. Compos. Part B Eng. 2020, 188, 61–71. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Dong, H.; Xu, R.; Jiang, S. Flame-retardant shape memory polyurethane/MXene paper and the application for early fire alarm sensor. Compos. Part B Eng. 2021, 223, 109149. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; El-Demellawi, J.K.; Jiang, Q.; Ge, G.; Liang, H.; Lee, K.; Dong, X.; Alshareef, H.N. MXene hydrogels: Fundamentals and applications. Chem. Soc. Rev. 2020, 49, 7229–7251. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Z.; Huang, Y.; Hai, Y.; Zhong, X.; Xiao, S.; Jiang, S. Influence of phytic acid on flame retardancy and adhesion performance enhancement of poly (vinyl alcohol) hydrogel coating to wood substrate. Prog. Org. Coat. 2021, 161, 106453. [Google Scholar] [CrossRef]
- Zhong, Q.; Li, Y.; Zhang, G. Two-dimensional MXene-based and MXene-derived photocatalysts: Recent developments and perspectives. Chem. Eng. J. 2021, 409, 128099. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, W.; Sun, Q.; Yu, B.; Yin, X.; Cao, X.; Feng, Y.; Li, R.K.Y.; Qu, J. Surface treatment of two dimensional MXene for poly(vinylidene fluoride) nanocomposites with tunable dielectric permittivity. Compos. Commun. 2021, 23, 100562. [Google Scholar] [CrossRef]
- Xue, Y.; Feng, J.; Huo, S.; Song, P.; Yu, B.; Liu, L.; Wang, H. Polyphosphoramide-intercalated MXene for simultaneously enhancing thermal stability, flame retardancy and mechanical properties of polylactide. Chem. Eng. J. 2020, 397, 125336. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, Y.; Liu, H.; Xie, D.; Wan, C.; Pan, H.; Jiang, S. Mechanical, thermal and fire performance of an inorganic-organic insulation material composed of hollow glass microspheres and phenolic resin. J. Colloid Interface Sci. 2018, 530, 163–170. [Google Scholar] [CrossRef]
- Yu, B.; Yuen, A.C.Y.; Xu, X.; Zhang, Z.-C.; Yang, W.; Lu, H.; Fei, B.; Yeoh, G.H.; Song, P.; Wang, H. Engineering MXene surface with POSS for reducing fire hazards of polystyrene with enhanced thermal stability. J. Hazard. Mater. 2021, 401, 123342. [Google Scholar] [CrossRef]







| Samples | TTI (S) | pHRR (kW/m2) | THR (MJ/m2) |
|---|---|---|---|
| RPUF | 7 | 335.7 | 72.4 |
| PAAm-MXene | 172 | 220.4 | 59.3 |
| PAAm-m1 | 101 | 249.0 | 70.8 |
| PAAm-m2 | 154 | 265.0 | 55.9 |
| PAAm-m3 | 113 | 322.5 | 54.6 |
| Sample | AM (g) | BIS (mg) | APS (mg) | MXene (mg) | m-MXene (mg) | Water (mL) |
|---|---|---|---|---|---|---|
| PAAm | 2.5 | 5 | 50 | - | - | 10 |
| PAAm-MXene | 2.5 | 5 | 50 | 10 | - | 10 |
| PAAm-m1 | 2.5 | 5 | 50 | - | 5 | 10 |
| PAAm-m2 | 2.5 | 5 | 50 | - | 10 | 10 |
| PAAm-m3 | 2.5 | 5 | 50 | - | 15 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Yang, L. Enhanced Flame Retardancy of Rigid Polyurethane Foams by Polyacrylamide/MXene Hydrogel Nanocomposite Coating. Int. J. Mol. Sci. 2022, 23, 12632. https://doi.org/10.3390/ijms232012632
Chen B, Yang L. Enhanced Flame Retardancy of Rigid Polyurethane Foams by Polyacrylamide/MXene Hydrogel Nanocomposite Coating. International Journal of Molecular Sciences. 2022; 23(20):12632. https://doi.org/10.3390/ijms232012632
Chicago/Turabian StyleChen, Bin, and Lizhong Yang. 2022. "Enhanced Flame Retardancy of Rigid Polyurethane Foams by Polyacrylamide/MXene Hydrogel Nanocomposite Coating" International Journal of Molecular Sciences 23, no. 20: 12632. https://doi.org/10.3390/ijms232012632
APA StyleChen, B., & Yang, L. (2022). Enhanced Flame Retardancy of Rigid Polyurethane Foams by Polyacrylamide/MXene Hydrogel Nanocomposite Coating. International Journal of Molecular Sciences, 23(20), 12632. https://doi.org/10.3390/ijms232012632






