Mechanical Stimulation Alters Chronic Ethanol-Induced Changes to VTA GABA Neurons, NAc DA Release and Measures of Withdrawal
Abstract
1. Introduction
2. Results
2.1. Amelioration of Chronic Ethanol-Induced Changes to VTA GABA Neurons by MStim
2.2. Amelioration of Chronic Ethanol-Induced Changes to Dopamine Release in the NAc by MStim
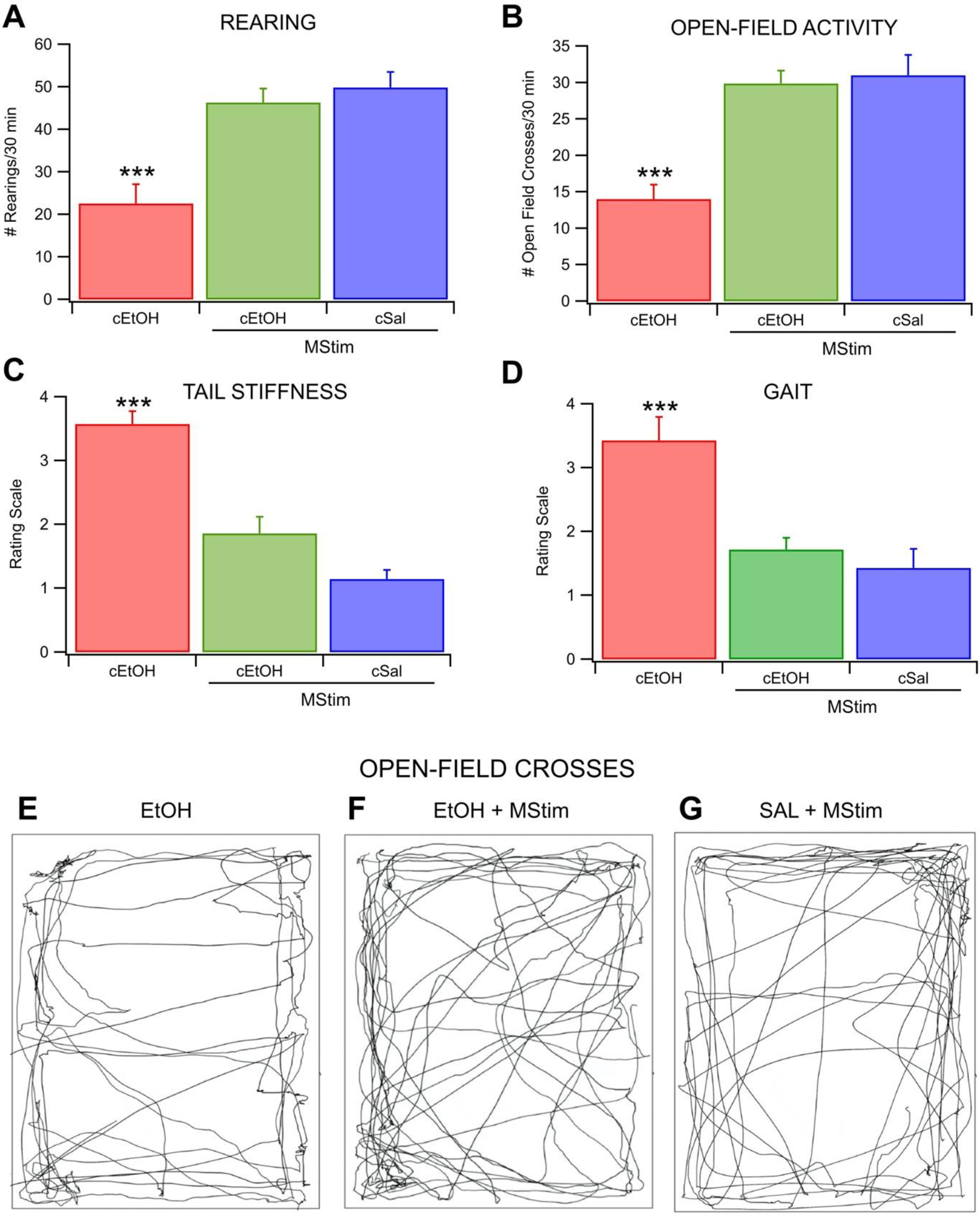
2.3. Amelioration of EtOH Withdrawal Symptoms and Anxiety by MStim
2.4. Effects of MStim on Ultrasonic Vocalizations
3. Discussion
4. Materials and Methods
4.1. Animals, EtOH Administration, and MStim Application
4.2. Single Cell Electrophysiology
4.3. Characterization of VTA GABA Neurons
4.4. Ultrasonic Vocalizations
4.5. Microdialysis and High Performance Liquid Chromatography
4.6. Behavioral Measures of Withdrawal
4.7. Elevated-Plus Maze and Chronic Intermittent Ethanol
4.8. Data Collection and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- SAMHSA. Facing Addiciton in America: The Surgeon Gernal’s Report on Alcohol, Drugs, and Health; US Department of Health and Human Services: Washington, DC, USA, 2016.
- Sacks, J.J.; Gonzales, K.R.; Bouchery, E.E.; Tomedi, L.E.; Brewer, R.D. 2010 National and State Costs of Excessive Alcohol Consumption. Am. J. Prev. Med. 2015, 49, e73–e79. [Google Scholar] [CrossRef] [PubMed]
- Moos, R.H.; Moos, B.S. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction 2006, 101, 212–222. [Google Scholar] [CrossRef] [PubMed]
- White, W.L. Recovery/Remission from Substance Use Disorders; Philadelphia Department of Behavioral Health and Intellectual disAbility Services: Philadelphia, PA, USA, 2012.
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Akbar, M.; Egli, M.; Cho, Y.E.; Song, B.J.; Noronha, A. Medications for alcohol use disorders: An overview. Pharmacol. Ther. 2018, 185, 64–85. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Kim, M.S.; Jang, E.Y.; Lee, J.Y.; Lee, J.G.; Kim, H.Y.; Yoon, S.S.; Lee, B.H.; Chang, S.; Kim, J.H.; et al. Acupuncture reduces relapse to cocaine-seeking behavior via activation of GABA neurons in the ventral tegmental area. Addict. Biol. 2018, 23, 165–181. [Google Scholar] [CrossRef]
- Yoon, S.S.; Yang, E.J.; Lee, B.H.; Jang, E.Y.; Kim, H.Y.; Choi, S.M.; Steffensen, S.C.; Yang, C.H. Effects of acupuncture on stress-induced relapse to cocaine-seeking in rats. Psychopharmacology 2012, 222, 303–311. [Google Scholar] [CrossRef][Green Version]
- Kim, N.J.; Ryu, Y.; Lee, B.H.; Chang, S.; Fan, Y.; Gwak, Y.S.; Yang, C.H.; Bills, K.B.; Steffensen, S.C.; Koo, J.S.; et al. Acupuncture inhibition of methamphetamine-induced behaviors, dopamine release and hyperthermia in the nucleus accumbens: Mediation of group II mGluR. Addict. Biol. 2019, 24, 206–217. [Google Scholar] [CrossRef]
- Yang, C.H.; Yoon, S.S.; Hansen, D.M.; Wilcox, J.D.; Blumell, B.R.; Park, J.J.; Steffensen, S.C. Acupuncture inhibits GABA neuron activity in the ventral tegmental area and reduces ethanol self-administration. Alcohol Clin. Exp. Res. 2010, 34, 2137–2146. [Google Scholar] [CrossRef]
- Kim, S.A.; Lee, B.H.; Bae, J.H.; Kim, K.J.; Steffensen, S.C.; Ryu, Y.H.; Leem, J.W.; Yang, C.H.; Kim, H.Y. Peripheral afferent mechanisms underlying acupuncture inhibition of cocaine behavioral effects in rats. PLoS ONE 2013, 8, e81018. [Google Scholar] [CrossRef]
- Chang, S.; Ryu, Y.; Gwak, Y.S.; Kim, N.J.; Kim, J.M.; Lee, J.Y.; Kim, S.A.; Lee, B.H.; Steffensen, S.C.; Jang, E.Y.; et al. Spinal pathways involved in somatosensory inhibition of the psychomotor actions of cocaine. Sci. Rep. 2017, 7, 5359. [Google Scholar] [CrossRef]
- McLain, R.F.; Raiszadeh, K. Mechanoreceptor endings of the cervical, thoracic, and lumbar spine. Iowa Orthop. J. 1995, 15, 147–155. [Google Scholar] [PubMed]
- Bills, K.; Clarke, T.; Major, G.; Jacobson, C.; Blotter, J.; Feland, J.; Steffensen, S. Targeted Subcutaneous Vibration with Single-Neuron Electrophysiology as a Novel Method for Understanding the Central Effects of Peripheral Vibrational Therapy in a rodent model. Dose Response 2019, 17, 1559325818825172. [Google Scholar] [CrossRef]
- Bills, K.B.; Obray, J.D.; Clarke, T.; Parsons, M.; Brundage, J.; Yang, C.H.; Kim, H.Y.; Yorgason, J.T.; Blotter, J.D.; Steffensen, S.C. Mechanical stimulation of cervical vertebrae modulates the discharge activity of ventral tegmental area neurons and dopamine release in the nucleus accumbens. Brain Stimul. 2020, 13, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Gentry, R.N.; Schuweiler, D.R.; Roesch, M.R. Dopamine signals related to appetitive and aversive events in paradigms that manipulate reward and avoidability. Brain Res. 2019, 1713, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Ranaldi, R. Dopamine and reward seeking: The role of ventral tegmental area. Rev. Neurosci. 2014, 25, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Dalle Grave, R.; Calugi, S.; Marchesini, G. Compulsive exercise to control shape or weight in eating disorders: Prevalence, associated features, and treatment outcome. Compr. Psychiatry 2008, 49, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A. Dopamine and reward: The anhedonia hypothesis 30 years on. Neurotox. Res. 2008, 14, 169–183. [Google Scholar] [CrossRef]
- Gallegos, R.A.; Criado, J.R.; Lee, R.S.; Henriksen, S.J.; Steffensen, S.C. Adaptive responses of GABAergic neurons in the ventral tegmental area to chronic ethanol. J. Pharmacol. Exp. Ther. 1999, 291, 1045–1053. [Google Scholar]
- Ludlow, K.H.; Bradley, K.D.; Allison, D.W.; Taylor, S.R.; Yorgason, J.T.; Hansen, D.M.; Walton, C.H.; Sudweeks, S.N.; Steffensen, S.C. Acute and chronic ethanol modulate dopamine D2-subtype receptor responses in ventral tegmental area GABA neurons. Alcohol Clin. Exp. Res. 2009, 33, 804–811. [Google Scholar] [CrossRef]
- Stobbs, S.H.; Ohran, A.J.; Lassen, M.B.; Allison, D.W.; Brown, J.E.; Steffensen, S.C. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves NMDA receptors. J. Pharmacol. Exp. Ther. 2004, 311, 282–289. [Google Scholar] [CrossRef]
- Steffensen, S.C.; Taylor, S.R.; Horton, M.L.; Barber, E.N.; Lyle, L.T.; Stobbs, S.H.; Allison, D.W. Cocaine disinhibits dopamine neurons in the ventral tegmental area via use-dependent blockade of GABA neuron voltage-sensitive sodium channels. Eur. J. Neurosci. 2008, 28, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.T.; Tan, K.R.; O’Connor, E.C.; Nikonenko, I.; Muller, D.; Luscher, C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature 2012, 492, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.R.; Yvon, C.; Turiault, M.; Mirzabekov, J.J.; Doehner, J.; Labouebe, G.; Deisseroth, K.; Tye, K.M.; Luscher, C. GABA neurons of the VTA drive conditioned place aversion. Neuron 2012, 73, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, S.C.; Stobbs, S.H.; Colago, E.E.; Lee, R.S.; Koob, G.F.; Gallegos, R.A.; Henriksen, S.J. Contingent and non-contingent effects of heroin on mu-opioid receptor-containing ventral tegmental area GABA neurons. Exp. Neurol. 2006, 202, 139–151. [Google Scholar] [CrossRef]
- Carboni, E.; Imperato, A.; Perezzani, L.; Di Chiara, G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience 1989, 28, 653–661. [Google Scholar] [CrossRef]
- Yoshimoto, K.; McBride, W.J.; Lumeng, L.; Li, T.K. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol 1992, 9, 17–22. [Google Scholar] [CrossRef]
- Bocklisch, C.; Pascoli, V.; Wong, J.C.; House, D.R.; Yvon, C.; de Roo, M.; Tan, K.R.; Luscher, C. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science 2013, 341, 1521–1525. [Google Scholar] [CrossRef]
- Bonci, A.; Williams, J.T. Increased probability of GABA release during withdrawal from morphine. J. Neurosci. 1997, 17, 796–803. [Google Scholar] [CrossRef]
- Koeltzow, T.E.; White, F.J. Behavioral depression during cocaine withdrawal is associated with decreased spontaneous activity of ventral tegmental area dopamine neurons. Behav. Neurosci. 2003, 117, 860–865. [Google Scholar] [CrossRef]
- Karkhanis, A.N.; Huggins, K.N.; Rose, J.H.; Jones, S.R. Switch from excitatory to inhibitory actions of ethanol on dopamine levels after chronic exposure: Role of kappa opioid receptors. Neuropharmacology 2016, 110, 190–197. [Google Scholar] [CrossRef]
- Maisonneuve, I.M.; Ho, A.; Kreek, M.J. Chronic administration of a cocaine “binge” alters basal extracellular levels in male rats: An in vivo microdialysis study. J. Pharmacol. Exp. Ther. 1995, 272, 652–657. [Google Scholar] [PubMed]
- Rose, J.H.; Karkhanis, A.N.; Chen, R.; Gioia, D.; Lopez, M.F.; Becker, H.C.; McCool, B.A.; Jones, S.R. Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. Int. J. Neuropsychopharmacol. 2016, 19, pyv127. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004, 5, 483–494. [Google Scholar] [CrossRef]
- Lyness, W.H.; Smith, F.L. Influence of dopaminergic and serotonergic neurons on intravenous ethanol self-administration in the rat. Pharmacol. Biochem. Behav. 1992, 42, 187–192. [Google Scholar] [CrossRef]
- Simola, N.; Granon, S. Ultrasonic vocalizations as a tool in studying emotional states in rodent models of social behavior and brain disease. Neuropharmacology 2019, 159, 107420. [Google Scholar] [CrossRef] [PubMed]
- Schwarting, R.; Wöhr, M. On the relationships between ultrasonic calling and anxiety-related behavior in rats. Braz. J. Med. Biol. Res. 2012, 45, 337–348. [Google Scholar] [CrossRef]
- Xia, Y.; Driscoll, J.R.; Wilbrecht, L.; Margolis, E.B.; Fields, H.L.; Hjelmstad, G.O. Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J. Neurosci. 2011, 31, 7811–7816. [Google Scholar] [CrossRef]
- Yim, H.J.; Gonzales, R.A. Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. Alcohol 2000, 22, 107–115. [Google Scholar] [CrossRef]
- Saland, L.C.; Hastings, C.M.; Abeyta, A.; Chavez, J.B. Chronic ethanol modulates delta and mu-opioid receptor expression in rat CNS: Immunohistochemical analysis with quantitiative confocal microscopy. Neurosci. Lett. 2005, 381, 163–168. [Google Scholar] [CrossRef]
- Knutson, B.; Burgdorf, J.; Panksepp, J. Ultrasonic vocalizations as indices of affective states in rats. Psychol. Bull. 2002, 128, 961–977. [Google Scholar] [CrossRef]
- Coffey, K.R.; Marx, R.G.; Neumaier, J.F. DeepSqueak: A deep learning-based system for detection and analysis of ultrasonic vocalizations. Neuropsychopharmacology 2019, 44, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, S.C.; Svingos, A.L.; Pickel, V.M.; Henriksen, S.J. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J. Neurosci. 1998, 18, 8003–8015. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M.; Pniak, A. Social contacts and production of 50-kHz short ultrasonic calls in adult rats. J. Comp. Psychol. 2002, 116, 73–82. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bills, K.B.; Otteson, D.Z.; Jones, G.C.; Brundage, J.N.; Baldwin, E.K.; Small, C.A.; Kim, H.Y.; Yorgason, J.T.; Blotter, J.D.; Steffensen, S.C. Mechanical Stimulation Alters Chronic Ethanol-Induced Changes to VTA GABA Neurons, NAc DA Release and Measures of Withdrawal. Int. J. Mol. Sci. 2022, 23, 12630. https://doi.org/10.3390/ijms232012630
Bills KB, Otteson DZ, Jones GC, Brundage JN, Baldwin EK, Small CA, Kim HY, Yorgason JT, Blotter JD, Steffensen SC. Mechanical Stimulation Alters Chronic Ethanol-Induced Changes to VTA GABA Neurons, NAc DA Release and Measures of Withdrawal. International Journal of Molecular Sciences. 2022; 23(20):12630. https://doi.org/10.3390/ijms232012630
Chicago/Turabian StyleBills, Kyle B., Dallin Z. Otteson, Gavin C. Jones, James N. Brundage, Emily K. Baldwin, Christina A. Small, Hee Young Kim, Jordan T. Yorgason, Jonathan D. Blotter, and Scott C. Steffensen. 2022. "Mechanical Stimulation Alters Chronic Ethanol-Induced Changes to VTA GABA Neurons, NAc DA Release and Measures of Withdrawal" International Journal of Molecular Sciences 23, no. 20: 12630. https://doi.org/10.3390/ijms232012630
APA StyleBills, K. B., Otteson, D. Z., Jones, G. C., Brundage, J. N., Baldwin, E. K., Small, C. A., Kim, H. Y., Yorgason, J. T., Blotter, J. D., & Steffensen, S. C. (2022). Mechanical Stimulation Alters Chronic Ethanol-Induced Changes to VTA GABA Neurons, NAc DA Release and Measures of Withdrawal. International Journal of Molecular Sciences, 23(20), 12630. https://doi.org/10.3390/ijms232012630





