The Role of IGL-2 Preservation Solution on Rat Livers during SCS and HOPE
Abstract
1. Introduction
2. Results
2.1. Static Cold Storage
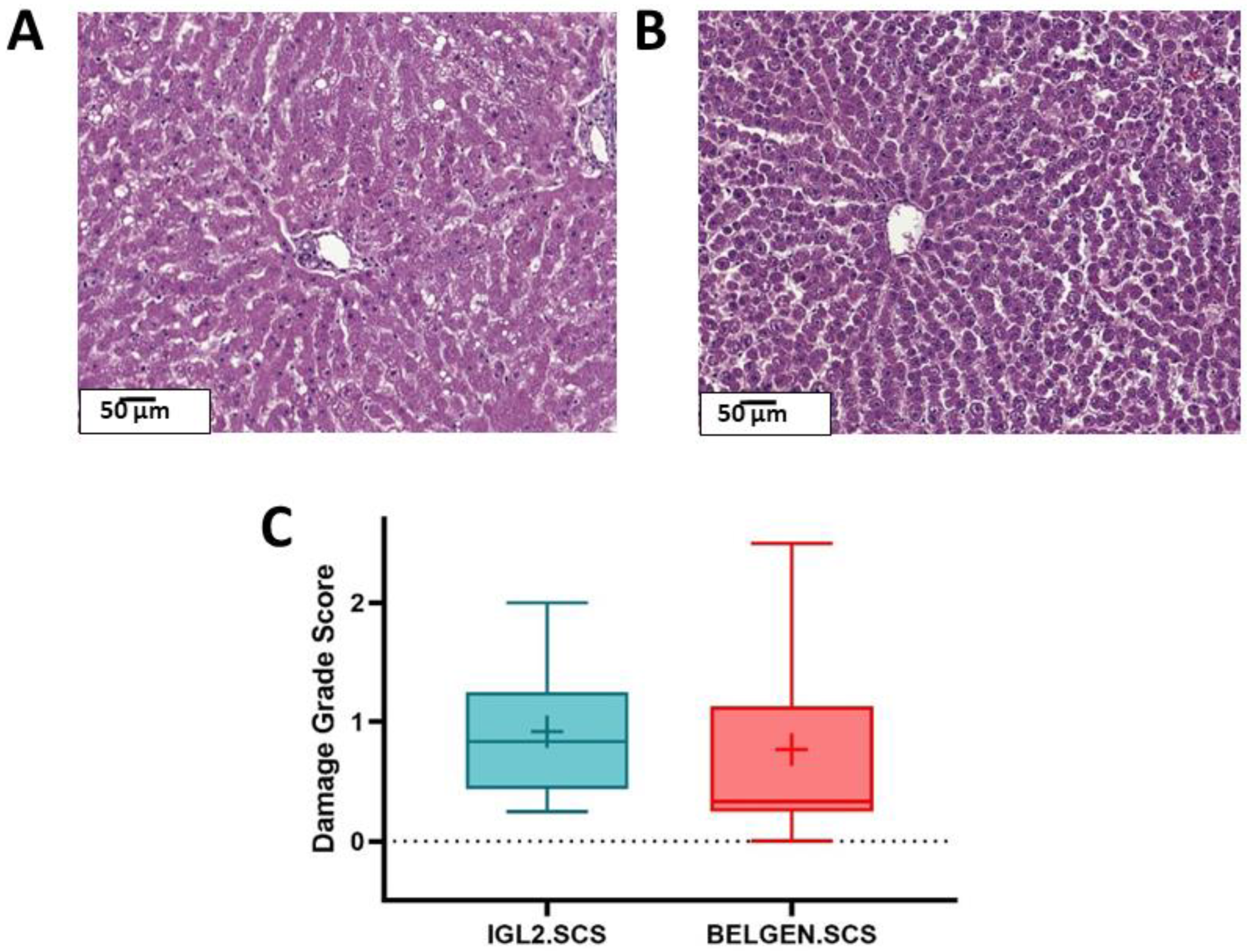
2.1.1. Histology
2.1.2. Liver Function (Transaminases, Lactates)
2.1.3. Glycocalyx
2.1.4. Apoptosis and Inflammation
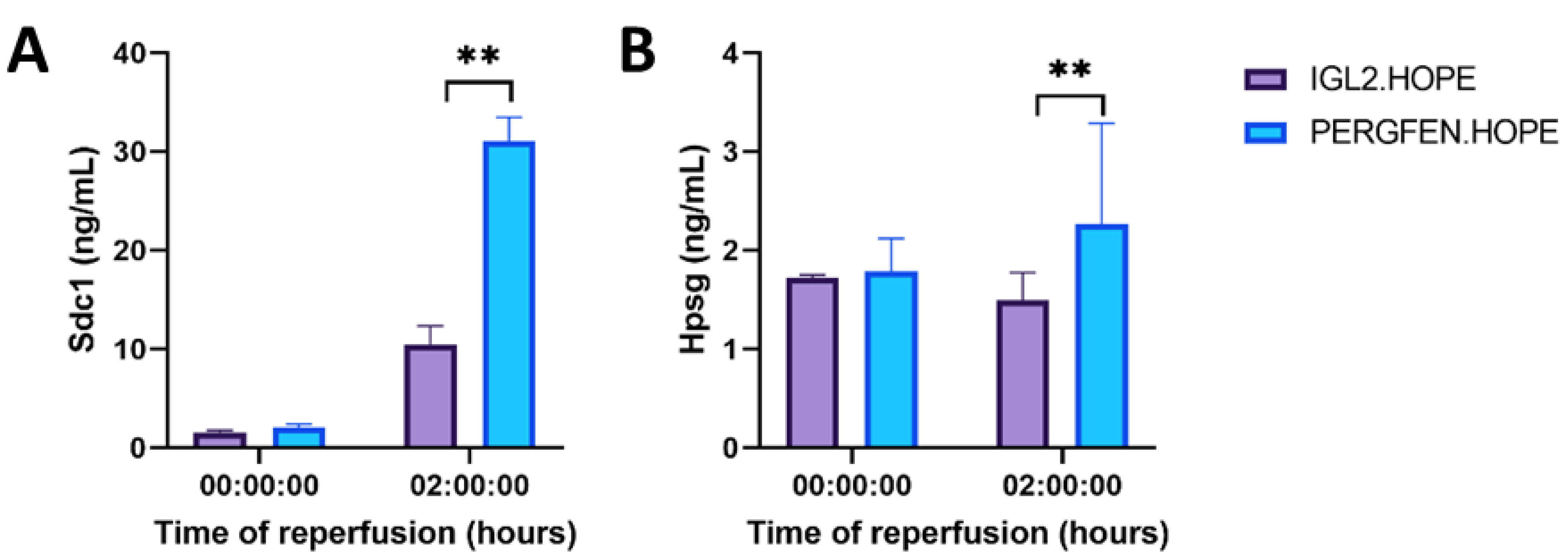
2.2. Hypothermic Oxygenated Perfusion
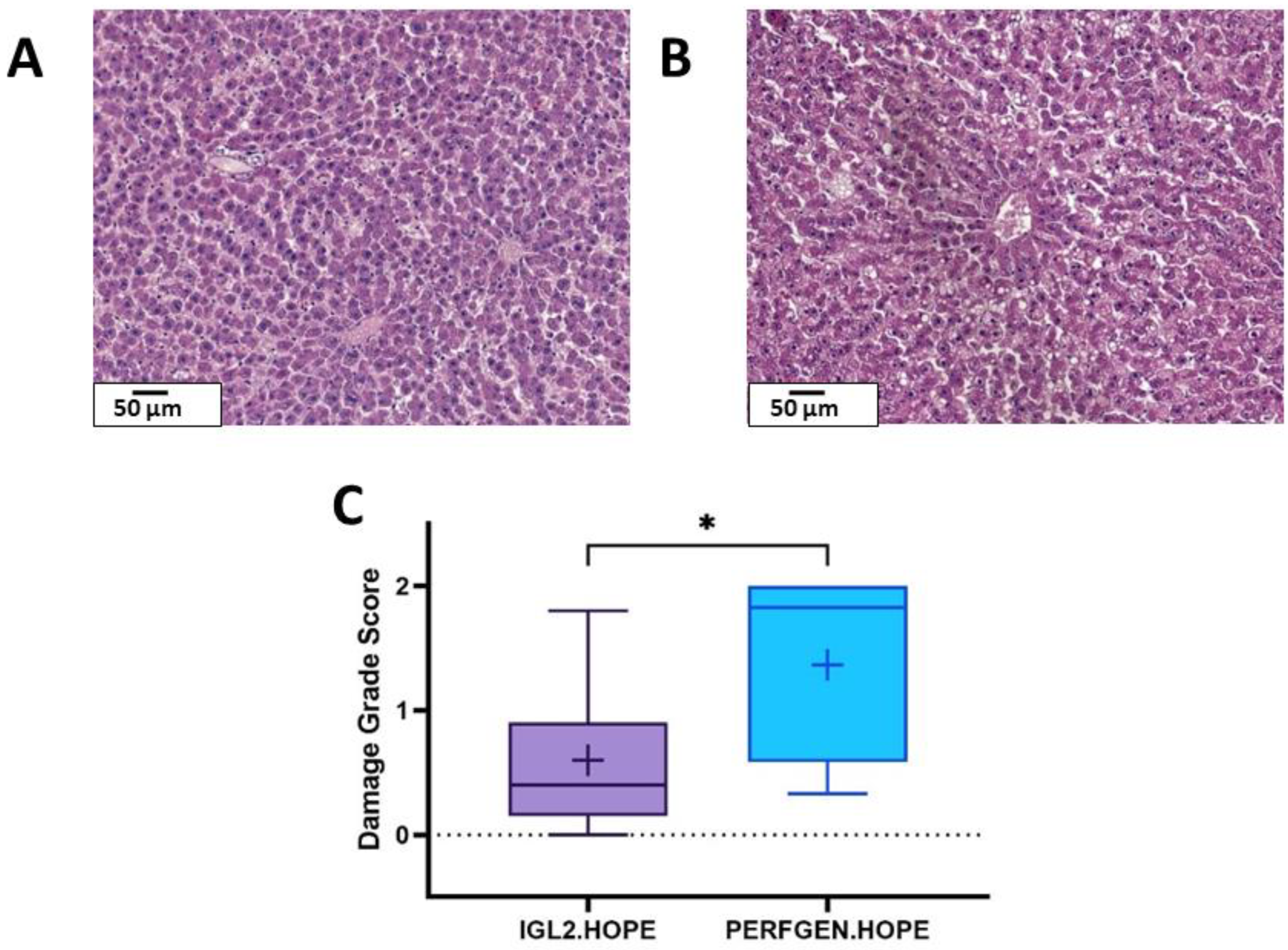
2.2.1. Histology
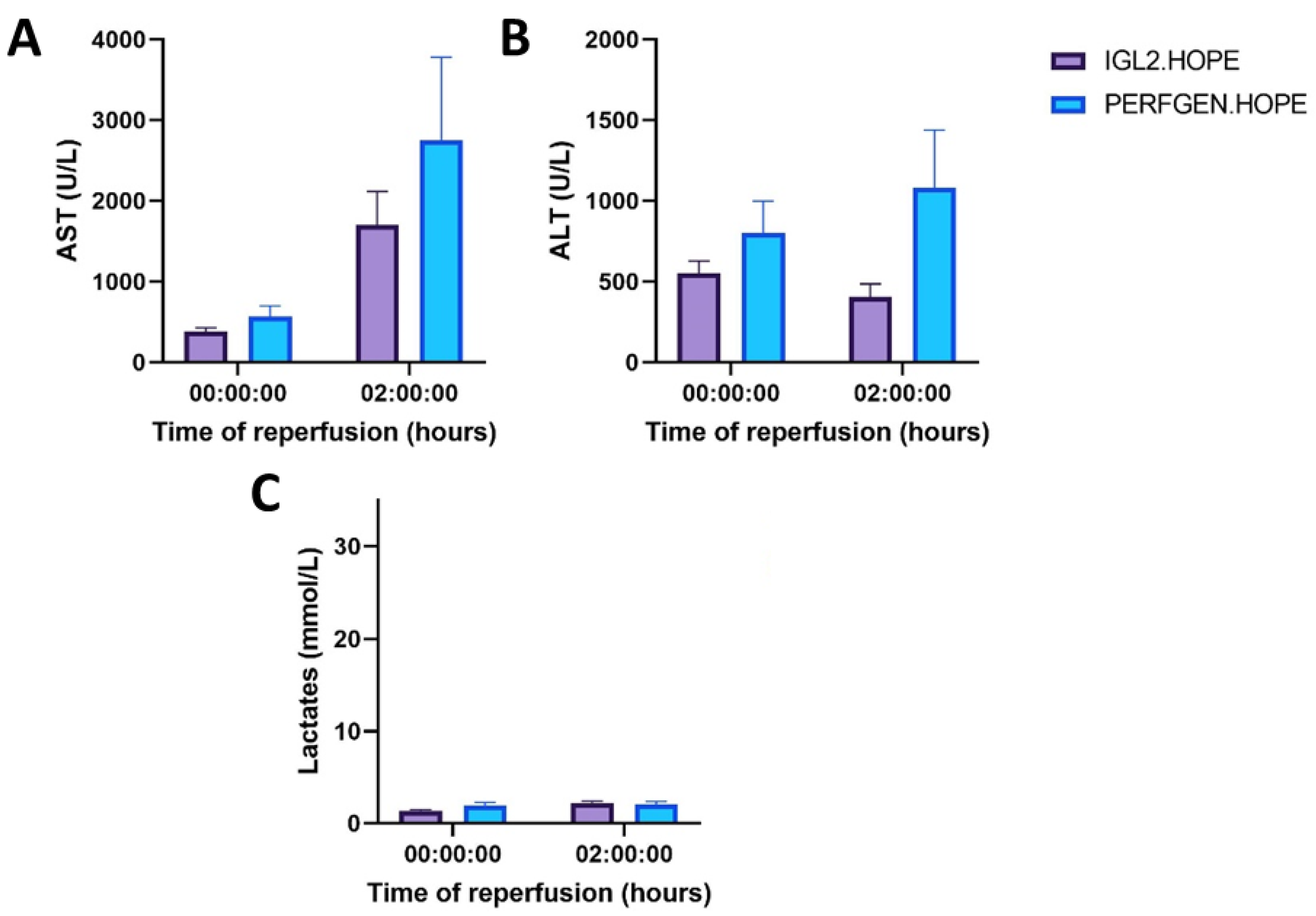
2.2.2. Liver Function (Transaminases, Lactates)
2.2.3. Glycocalyx
2.2.4. Apoptosis and Inflammation
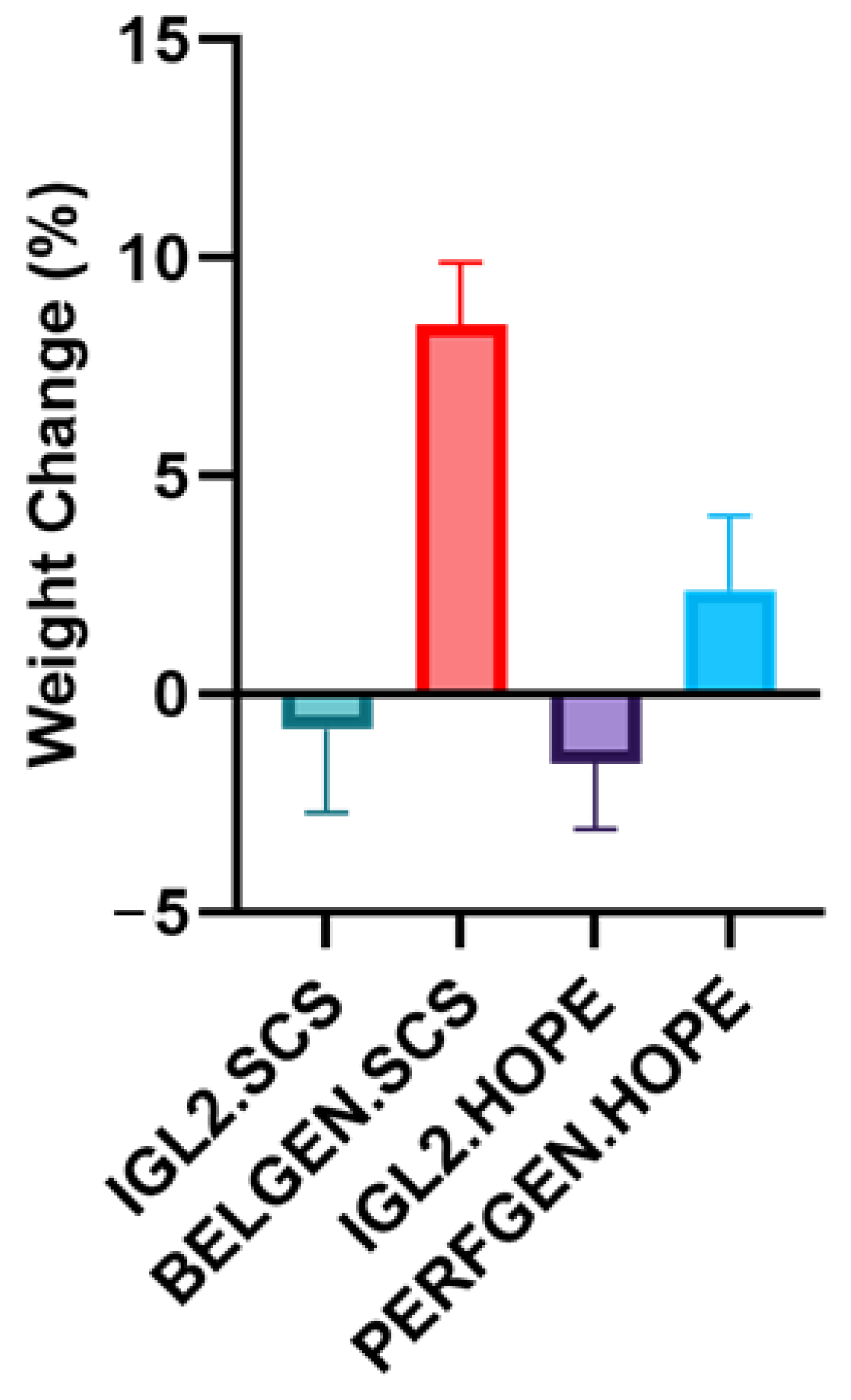
2.3. Edema
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Liver Procurement
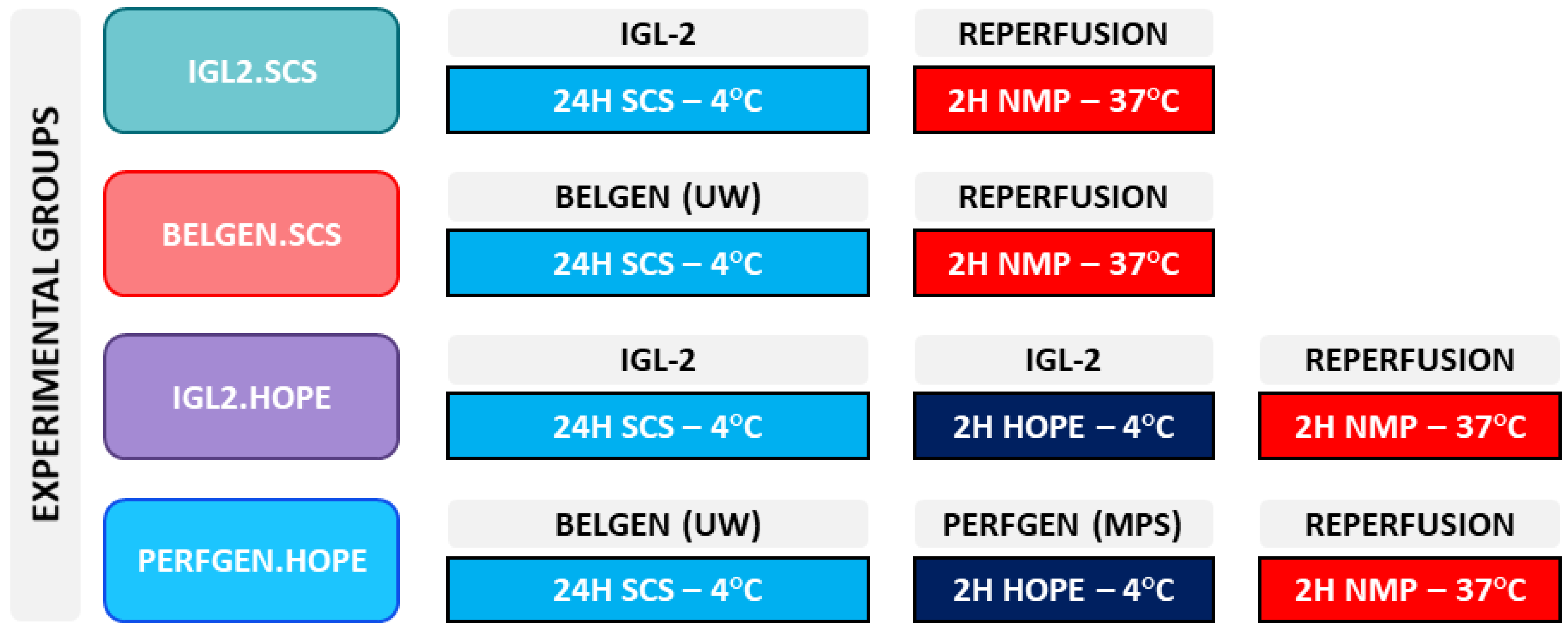
4.3. Experimental Groups
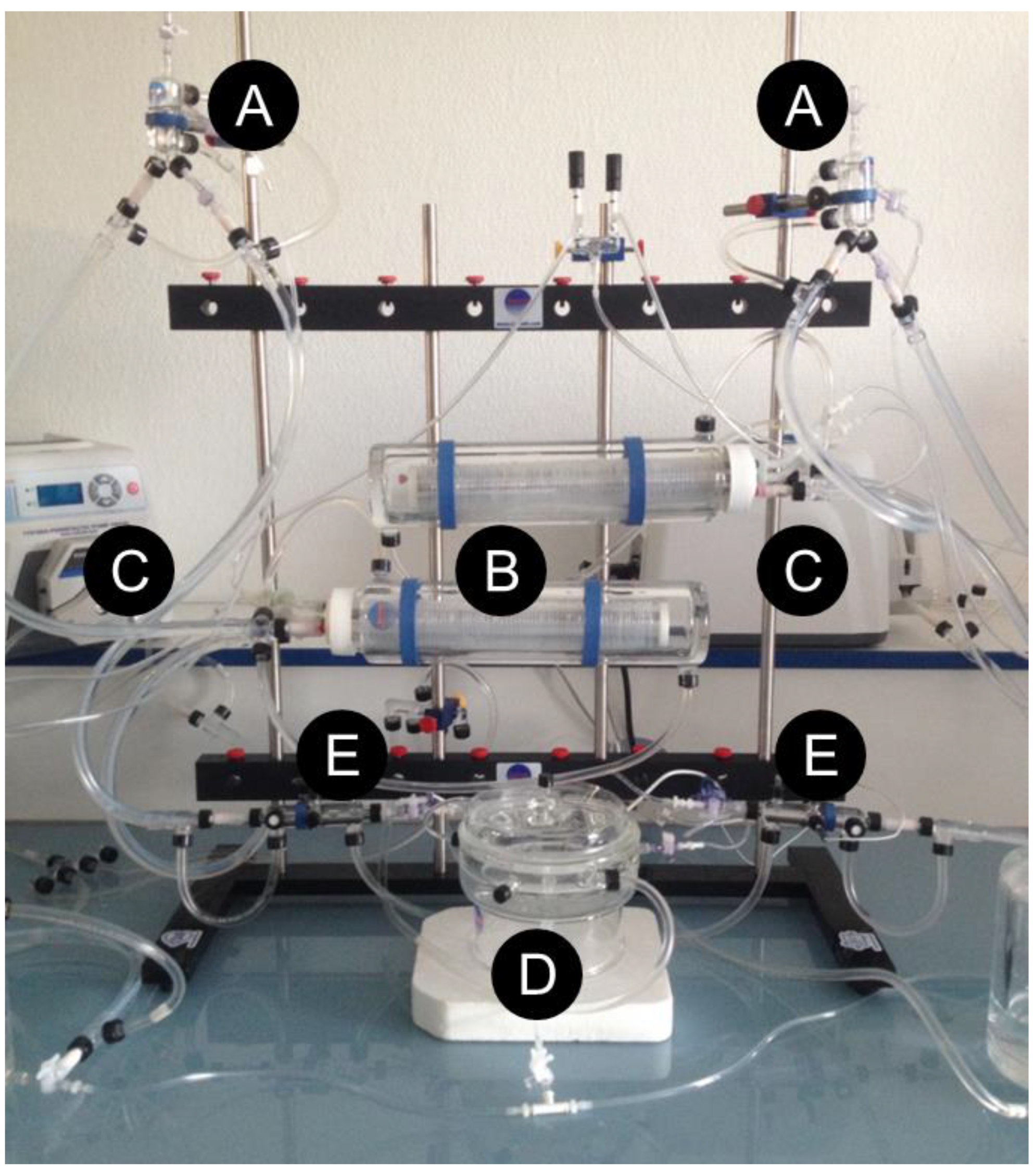
4.4. Hypothermic Oxygenated Perfusion
4.5. Liver Functional Test
4.6. ELISA
4.7. Histology
4.8. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine Amino Transferase |
| AST | Aspartate Amino Transferase |
| BELZER MPS | Belzer Machine Perfusion Solution |
| DGS | Damage Grade Score |
| ECD | Extended Criteria Donors |
| ELTR | European Liver Transplant Registry |
| HES | Haematoxylin-Eosin Saffron (Coloration Agent) |
| HES | Hydroxy Ethyl Starch (Oncotic Agent) |
| HMGB1 | High Mobility Group 1 |
| HOPE | Hypothermic Oxygenation Perfusion |
| HSPG | Heparan Sulfate Proteoglycan |
| IGL | Institut Georges Lopez |
| IRI | Ischemia-Reperfusion Injury |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NMP | Normothermic Machine Perfusion |
| NO | Nitric Oxide |
| PEG | Poly Ethylene Glycol |
| PFA | Paraformaldehyde |
| SCS | Static Cold Storage |
| SD | Standard Deviation |
| SDC1 | Syndecan-1 |
| UNOS | United Network of Organ Sharing |
| UW | University of Wisconsin |
References
- Ahmed, O.; Doyle, M.B.M. Liver transplantation: Expanding the donor and recipient pool. Chin. Clin. Oncol. 2021, 10, 6. [Google Scholar] [CrossRef]
- Vodkin, I.; Kuo, A. Extended Criteria Donors in Liver Transplantation. Clin. Liver Dis. 2017, 21, 289–301. [Google Scholar] [CrossRef]
- Linares, I.; Hamar, M.; Selzner, N.; Selzner, M. Steatosis in Liver Transplantation: Current Limitations and Future Strategies. Transplantation 2019, 103, 78–90. [Google Scholar] [CrossRef]
- Kahn, J.; Schemmer, P. Control of Ischemia-Reperfusion Injury in Liver Transplantation: Potentials for Increasing the Donor Pool. Visc. Med. 2018, 34, 444–448. [Google Scholar] [CrossRef]
- Jadhav, P.V.; Kothakota, S.R.; Sasidharan, M.; Kareem, H.; Nair, A.K. Effect of Donor Hepatic Steatosis on Ischemia Reperfusion Injury in Liver Transplant Recipient. J. Clin. Exp. Hepatol. 2020, 10, 236–244. [Google Scholar] [CrossRef]
- Bodzin, A.S.; Baker, T.B. Liver Transplantation Today: Where We Are Now and Where We Are Going. Liver Transpl. 2018, 24, 1470–1475. [Google Scholar] [CrossRef]
- Burra, P.; Zanetto, A.; Russo, F.P.; Germani, G. Organ Preservation in Liver Transplantation. Semin. Liver Dis. 2018, 38, 260–269. [Google Scholar] [CrossRef]
- Adam, R.; Karam, V.; Cailliez, V.; Grady, J.G.O.; Mirza, D.; Cherqui, D.; Klempnauer, J.; Salizzoni, M.; Pratschke, J.; Jamieson, N.; et al. 2018 Annual Report of the European Liver Transplant Registry (ELTR)-50-year evolution of liver transplantation. Transpl. Int. 2018, 31, 1293–1317. [Google Scholar] [CrossRef]
- Southard, J.H.; Belzer, F.O. Organ preservation. Annu. Rev. Med. 1995, 46, 235–247. [Google Scholar] [CrossRef]
- Southard, J.H. New Solution for Organ Preservation. In Cryopreservation and Low Temperature Biology in Blood Transfusion, Proceedings of the Fourteenth International Symposium on Blood Transfusion, Groningen, The Netherlands, 1989, Organised by the Red Cross Blood Bank Groningen-Drenthe; Developments in Hematology and Immunology; Smit Sibinga, C.T.H., Das, P.C., Meryman, H.T., Eds.; Springer: New York, NY, USA, 1990; pp. 145–153. [Google Scholar] [CrossRef]
- Fujii, Y.; Tanabe, T.; Yamashiro, T.; Shirai, M.; Takewa, Y.; Tatsumi, E. Effect of Hydroxyethyl Starch Priming on the Systemic Inflammatory Response and Lung Edema after Cardiopulmonary Bypass in a Rat Model. ASAIO J. 2017, 63, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Taeger, C.D.; Friedrich, O.; Drechsler, C.; Weigand, A.; Hobe, F.; Geppert, C.I.; Münch, F.; Birkholz, T.; Buchholz, R.; Horch, R.E.; et al. Hydroxyethyl starch solution for extracorporeal tissue perfusion. Clin. Hemorheol. Microcirc. 2016, 64, 91–103. [Google Scholar] [CrossRef]
- Hagenah, M.; Halberstadt, M.; Wehming, S.; Brewitt, H.; Winter, R. Hydroxyethyl starch for reversing edema in short-term culture media for donor corneas. Klin. Monbl. Augenheilkd. 1996, 208, 107–111. [Google Scholar] [CrossRef]
- Morariu, A.M.; Vd Plaats, A.; VOeveren, W.; Hart, N.; Leuvenink, H.; Graaff, R.; Ploeg, R.; Rakhorst, G. Hyperaggregating effect of hydroxyethyl starch components and University of Wisconsin solution on human red blood cells: A risk of impaired graft perfusion in organ procurement? Transplantation 2003, 76, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.R. Control of water content of non-metabolizing kidney slices by sodium chloride and polyethylene glycol (PEG 6000). J. Physiol. 1971, 213, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.M.; Wicomb, W.N. New organ preservation solutions. Kidney Int. Suppl. 1992, 38, S197–S202. [Google Scholar] [PubMed]
- Wicomb, W.N.; Hill, J.D.; Avery, J.; Collins, G.M. Optimal cardioplegia and 24-hour heart storage with simplified UW solution containing polyethylene glycol. Transplantation 1990, 49, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Panisello-Rosello, A.; Castro-Benitez, C.; Adam, R. Glycocalyx Preservation and NO Production in Fatty Livers-The Protective Role of High Molecular Polyethylene Glycol in Cold Ischemia Injury. Int. J. Mol. Sci. 2018, 19, 2375. [Google Scholar] [CrossRef]
- Rogers, H.; Zibari, G.B.; Roberts, J.; Turnage, R.; Lefer, D.J. Nitric oxide attenuates ischaemia-reperfusion (I/R) injury in the diabetic liver. Clin. Transplant. 2004, 18 (Suppl. 12), 7–11. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Liu, M.; Chen, Y.; Wu, Y.; Li, Q.; Ma, T.; Gao, J.; Xia, Y.; Fan, M.; et al. Protective effect of HINT2 on mitochondrial function via repressing MCU complex activation attenuates cardiac microvascular ischemia-reperfusion injury. Basic Res. Cardiol. 2021, 116, 65. [Google Scholar] [CrossRef]
- Goumard, C.; Turco, C.; Sakka, M.; Aoudjehane, L.; Lesnik, P.; Savier, E.; Conti, F.; Scatton, O. Ex-Vivo Pharmacological Defatting of the Liver: A Review. J. Clin. Med. 2021, 10, 1253. [Google Scholar] [CrossRef]
- Parente, A.; Osei-Bordom, D.C.; Ronca, V.; Perera, M.T.P.R.; Mirza, D. Organ Restoration With Normothermic Machine Perfusion and Immune Reaction. Front. Immunol. 2020, 11, 565616. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Kron, P.; Dutkowski, P. Hypothermic Oxygenated Liver Perfusion: Basic Mechanisms and Clinical Application. Curr. Transpl. Rep. 2015, 2, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine 2020, 60, 103014. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Hu, X.; He, W.; Wang, Y.; Li, L.; Xiong, Y.; Ye, Q. Hypothermic machine perfusion ameliorates inflammation during ischemia-reperfusion injury via sirtuin-1-mediated deacetylation of nuclear factor-κB p65 in rat livers donated after circulatory death. Mol. Med. Rep. 2017, 16, 8649–8656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.M.J.; Oude Egbrink, M.G.A. The endothelial glycocalyx: Composition, functions, and visualization. Pflugers Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef]
- Ebong, E.E.; Lopez-Quintero, S.V.; Rizzo, V.; Spray, D.C.; Tarbell, J.M. Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1. Integr. Biol. 2014, 6, 338–347. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Koutlas, V.; Pappas, C.; Mitsis, M.; Dounousi, E. The Endothelial Glycocalyx as a Target of Ischemia and Reperfusion Injury in Kidney Transplantation-Where Have We Gone So Far? Int. J. Mol. Sci. 2021, 22, 2157. [Google Scholar] [CrossRef]
- Schiefer, J.; Lebherz-Eichinger, D.; Erdoes, G.; Berlakovich, G.; Bacher, A.; Krenn, C.G.; Faybik, P. Alterations of Endothelial Glycocalyx During Orthotopic Liver Transplantation in Patients With End-Stage Liver Disease. Transplantation 2015, 99, 2118–2123. [Google Scholar] [CrossRef]
- Schiefer, J.; Faybik, P.; Koch, S.; Tudor, B.C.M.; Kollmann, D.; Kuessel, L.; Krenn, C.; Berlakovich, G.; Baron, D.; Baron-Stefaniak, J. Glycocalyx Damage Within Human Liver Grafts Correlates With Graft Injury and Postoperative Graft Function After Orthotopic Liver Transplantation. Transplantation 2020, 104, 72–78. [Google Scholar] [CrossRef]
- Bai, K.; Wang, W. Shear stress-induced redistribution of the glycocalyx on endothelial cells in vitro. Biomech. Model. Mechanobiol. 2014, 13, 303–311. [Google Scholar] [CrossRef]
- Da Silva, R.T.; Bardallo, R.G.; Folch-Puy, E.; Carbonell, T.; Palmeira, C.M.; Fondevila, C.; Adam, R.; Roselló-Catafau, J.; Panisello-Roselló, A. IGL-2 as a Unique Solution for Cold Static Preservation and Machine Perfusion in Liver and Mitochondrial Protection. Transplant. Proc. 2022, 54, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Bardallo, R.G.; Da Silva, R.T.; Carbonell, T.; Palmeira, C.; Folch-Puy, E.; Roselló-Catafau, J.; Adam, R.; Panisello-Rosello, A. Liver Graft Hypothermic Static and Oxygenated Perfusion (HOPE) Strategies: A Mitochondrial Crossroads. Int. J. Mol. Sci. 2022, 23, 5742. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, G.; Muller, X.; Hervieu, V.; Collardeau-Frachon, S.; Breton, A.; Boulanger, N.; Lesurtel, M.; Dubois, R.; Mohkam, K.; Mabrut, J. Liver transplantation of partial grafts after ex situ splitting during hypothermic oxygenated perfusion-The HOPE-Split pilot study. Liver Transpl. 2022, 28, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Muller, X.; Marcon, F.; Sapisochin, G.; Marquez, M.; Dondero, F.; Rayar, M.; Doyle, M.; Callans, L.; Li, J.; Nowak, G.; et al. Defining Benchmarks in Liver Transplantation: A Multicenter Outcome Analysis Determining Best Achievable Results. Ann. Surg. 2018, 267, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Jedlicka, J.; Becker, B.F.; Chappell, D. Endothelial Glycocalyx. Crit. Care Clin. 2020, 36, 217–232. [Google Scholar] [CrossRef]
- Abassi, Z.; Armaly, Z.; Heyman, S.N. Glycocalyx Degradation in Ischemia-Reperfusion Injury. Am. J. Pathol. 2020, 190, 752–767. [Google Scholar] [CrossRef]
- Passov, A.; Schramko, A.; Mäkisalo, H.; Nordin, A.; Andersson, S.; Pesonen, E.; Ilmakunnas, M. Graft glycocalyx degradation in human liver transplantation. PLoS ONE 2019, 14, e0221010. [Google Scholar] [CrossRef]
- Fernández-Sarmiento, J.; Salazar-Peláez, L.M.; Carcillo, J.A. The Endothelial Glycocalyx: A Fundamental Determinant of Vascular Permeability in Sepsis. Pediatr. Crit. Care Med. 2020, 21, e291–e300. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, Z.; Gao, W.; Duan, Y.; Fan, G.; Geng, X.; Wu, B.; Li, K.; Liu, K.; Peng, C. Aucubin Attenuates Liver Ischemia-Reperfusion Injury by Inhibiting the HMGB1/TLR-4/NF-κB Signaling Pathway, Oxidative Stress, and Apoptosis. Front. Pharmacol. 2020, 11, 544124. [Google Scholar] [CrossRef]
- Hua, S.; Ma, M.; Fei, X.; Zhang, Y.; Gong, F.; Fang, M. Glycyrrhizin attenuates hepatic ischemia-reperfusion injury by suppressing HMGB1-dependent GSDMD-mediated kupffer cells pyroptosis. Int. Immunopharmacol. 2019, 68, 145–155. [Google Scholar] [CrossRef]
- Xu, L.; Ge, F.; Hu, Y.; Yu, Y.; Guo, K.; Miao, C. Sevoflurane Postconditioning Attenuates Hepatic Ischemia-Reperfusion Injury by Limiting HMGB1/TLR4/NF-κB Pathway via Modulating microRNA-142 in vivo and in vitro. Front. Pharmacol. 2021, 12, 646307. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.F.; Donovan, M.; Cotter, T.G. Regulation and measurement of oxidative stress in apoptosis. J. Immunol. Methods 2002, 265, 49–72. [Google Scholar] [CrossRef]
- Delgadillo, L.F.; Lomakina, E.B.; Kuebel, J.; Waugh, R.E. Changes in endothelial glycocalyx layer protective ability after inflammatory stimulus. Am. J. Physiol. Cell Physiol. 2021, 320, C216–C224. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.J.; Down, C.J.; Foster, R.R.; Satchell, S.C. The Pathological Relevance of Increased Endothelial Glycocalyx Permeability. Am. J. Pathol. 2020, 190, 742–751. [Google Scholar] [CrossRef]
- Ar’Rajab, A.; Ahrén, B.; Sundberg, R.; Bengmark, S. The function of a colloid in liver cold-storage preservation. Transplantation 1991, 52, 34–38. [Google Scholar] [CrossRef]
- Thuillier, R.; Renard, C.; Rogel-Gaillard, C.; Demars, J.; Milan, D.; Forestier, L.; Ouldmoulene, A.; Goujon, J.M.; Badet, L.; Hauet, T. Effect of polyethylene glycol-based preservation solutions on graft injury in experimental kidney transplantation. Br. J. Surg. 2011, 98, 368–378. [Google Scholar] [CrossRef]
- Bessems, M.; Doorschodt, B.M.; Dinant, S.; de Graaf, W.; van Gulik, T.M. Machine perfusion preservation of the pig liver using a new preservation solution, polysol. Transplant. Proc. 2006, 38, 1238–1242. [Google Scholar] [CrossRef]
- Bessems, M.; Doorschodt, B.M.; Hooijschuur, O.; van Vliet, A.K.; van Gulik, T.M. Optimization of a new preservation solution for machine perfusion of the liver: Which is the preferred colloid? Transplant. Proc. 2005, 37, 329–331. [Google Scholar] [CrossRef]
- Panisello Rosello, A.; Teixeira da Silva, R.; Castro, C.; Bardallo, R.G.; Calvo, M.; Folch-Puy, E.; Carbonell, T.; Palmeira, C.; Catafau, J.R.; Adam, R. Polyethylene Glycol 35 as a Perfusate Additive for Mitochondrial and Glycocalyx Protection in HOPE Liver Preservation. Int. J. Mol. Sci. 2020, 21, 5703. [Google Scholar] [CrossRef]
- Chiang, E.T.; Camp, S.M.; Dudek, S.M.; Brown, M.E.; Usatyuk, P.V.; Zaborina, O.; Alverdy, J.C.; Garcia, J.G.N. Protective effects of high-molecular weight polyethylene glycol (PEG) in human lung endothelial cell barrier regulation: Role of actin cytoskeletal rearrangement. Microvasc. Res. 2009, 77, 174–186. [Google Scholar] [CrossRef]
- Zaouali, M.A.; Bejaoui, M.; Calvo, M.; Folch-Puy, E.; Pantazi, E.; Pasut, G.; Rimola, A.; Abdennebi, H.B.; Adam, R.; Roselló-Catafau, J. Polyethylene glycol rinse solution: An effective way to prevent ischemia-reperfusion injury. World J. Gastroenterol. 2014, 20, 16203–16214. [Google Scholar] [CrossRef] [PubMed]
- Ferrero-Andrés, A.; Panisello-Roselló, A.; Serafín, A.; Roselló-Catafau, J.; Folch-Puy, E. Polyethylene Glycol 35 (PEG35) Protects against Inflammation in Experimental Acute Necrotizing Pancreatitis and Associated Lung Injury. Int. J. Mol. Sci. 2020, 21, 917. [Google Scholar] [CrossRef] [PubMed]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Bardallo, R.G.; da Silva, R.T.; Carbonell, T.; Folch-Puy, E.; Palmeira, C.; Roselló-Catafau, J.; Pirenne, J.; Adam, R.; Panisello-Roselló, A. Role of PEG35, Mitochondrial ALDH2, and Glutathione in Cold Fatty Liver Graft Preservation: An IGL-2 Approach. Int. J. Mol. Sci. 2021, 22, 5332. [Google Scholar] [CrossRef] [PubMed]
- Panisello-Roselló, A.; Alva, N.; Flores, M.; Lopez, A.; Benítez, C.C.; Folch-Puy, E.; Rolo, A.; Palmeira, C.; Adam, R.; Carbonell, T. Aldehyde Dehydrogenase 2 (ALDH2) in Rat Fatty Liver Cold Ischemia Injury. Int. J. Mol. Sci. 2018, 19, 2479. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, T.A.; Bruinsma, B.G.; Lee, J.; D’Andrea, V.; Liu, Q.; Izamis, M.; Uygun, K.; Yarmush, M.L. A simplified subnormothermic machine perfusion system restores ischemically damaged liver grafts in a rat model of orthotopic liver transplantation. Transplant. Res. 2012, 1, 6. [Google Scholar] [CrossRef][Green Version]
- Asong-Fontem, N.; Panisello-Rosello, A.; Lopez, A.; Imai, K.; Zal, F.; Delpy, E.; Rosello-Catafau, J.; Adam, R. A Novel Oxygen Carrier (M101) Attenuates Ischemia-Reperfusion Injuries during Static Cold Storage in Steatotic Livers. Int. J. Mol. Sci. 2021, 22, 8542. [Google Scholar] [CrossRef]
- Asong-Fontem, N.; Panisello-Rosello, A.; Beghdadi, N.; Lopez, A.; Rosello-Catafau, J.; Adam, R. Pre-Ischemic Hypothermic Oxygenated Perfusion Alleviates Protective Molecular Markers of Ischemia-Reperfusion Injury in Rat Liver. Transplant. Proc. 2022, 1–16. [Google Scholar] [CrossRef]




| IGL-2 | BEL-GEN (UW) | PERF-GEN (Belzer MPS) | |
|---|---|---|---|
| Electrolytes (mmol/L) | |||
| K+ | 25 | 125 | 25 |
| Na+ | 125 | 30 | 120 |
| Mg2+ | 5 | 5 | 5 |
| SO42− | 5 | 5 | 5 |
| Ca+ | 0.5 | x | x |
| Zn2+ | 0.091 | x | x |
| Buffers (mmol/L) | |||
| Phosphate | 25 | 25 | 25 |
| HEPES | 10 | x | x |
| Histidine | 30 | x | x |
| Impermeants (mmol/L) | |||
| Mannitol | 60 | X | 30 |
| Lactobionic acid | 100 | 100 | x |
| Dextrose | x | x | 10 |
| Ribose | x | x | 5 |
| Gluconate | x | x | 85 |
| Colloids (g/L) | |||
| Hydroxyethyl starch (HES) | x | 50 | 50 |
| Polyethylene glycol 35 (PEG-35) | 5 | x | x |
| Antioxidants | |||
| Glutathione (g/L) | 9 | 3 | 3 |
| Metabolic precursors (mmol/L) | |||
| Adenosine | 5 | 5 | x |
| Adenine | x | x | 5 |
| NaNO2 (nmol/L) | 50 | x | x |
| pH | 7.4 | 7.4 | 7.4 |
| Osmolarity (mosmol/L) | 360 | 320 | 320 |
| Viscosity (Cp) | 1.7 | x | 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asong-Fontem, N.; Panisello-Rosello, A.; Sebagh, M.; Gonin, M.; Rosello-Catafau, J.; Adam, R. The Role of IGL-2 Preservation Solution on Rat Livers during SCS and HOPE. Int. J. Mol. Sci. 2022, 23, 12615. https://doi.org/10.3390/ijms232012615
Asong-Fontem N, Panisello-Rosello A, Sebagh M, Gonin M, Rosello-Catafau J, Adam R. The Role of IGL-2 Preservation Solution on Rat Livers during SCS and HOPE. International Journal of Molecular Sciences. 2022; 23(20):12615. https://doi.org/10.3390/ijms232012615
Chicago/Turabian StyleAsong-Fontem, Njikem, Arnau Panisello-Rosello, Mylène Sebagh, Mathilde Gonin, Joan Rosello-Catafau, and René Adam. 2022. "The Role of IGL-2 Preservation Solution on Rat Livers during SCS and HOPE" International Journal of Molecular Sciences 23, no. 20: 12615. https://doi.org/10.3390/ijms232012615
APA StyleAsong-Fontem, N., Panisello-Rosello, A., Sebagh, M., Gonin, M., Rosello-Catafau, J., & Adam, R. (2022). The Role of IGL-2 Preservation Solution on Rat Livers during SCS and HOPE. International Journal of Molecular Sciences, 23(20), 12615. https://doi.org/10.3390/ijms232012615








