1. Introduction
Cardiovascular diseases (CVD) and cancers are the two main causes of death worldwide, representing around 17.9 million and 10 million deaths per year, respectively, according to the World Health Organization (WHO) [
1,
2]. Head and neck cancers (HNC) represent approximatively 900,000 new diagnoses and 400,000 deaths worldwide [
3]. It is well known that CVD and cancers share many comorbidities and risk factors, notably tobacco use, alcohol consumption, unhealthy diets, or physical inactivity.
A pro-inflammatory state with neoangiogenesis and oxidative stress seems to be the key link between them. Atherosclerosis, the major actor in CVD, damages both small and large vessels and involves several interrelated processes, including lipid disturbances, platelet activation, thrombosis, endothelial dysfunction, inflammation, oxidative stress, vascular smooth cell activation, altered matrix metabolism, and extracellular matrix remodeling.
During the first steps of atherosclerosis, low density lipoproteins (LDL) move into the altered subendothelium where they are oxidized by macrophages and smooth muscle cells (SMC). OxLDL transform macrophages into foam cells that release growth factors and cytokines [
4]. In addition, endothelial cells (EC), activated by oxLDL, overexpress some cytokines, such as the Macrophage Colony-Stimulating Factor (M-CSF), to attract additional monocytes and differentiate them into macrophages [
5].
Scavenger receptors, including the cluster of differentiation 36 (CD36), the lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), the scavenger receptor type A (SR-A) and the scavenger receptor class B type 1 (SRB-1), which are strongly expressed on endothelial cells (EC), smooth muscle cells (SMC), and fibroblasts, promote the internalization of oxLDL in macrophages, switching them to foam cells [
6]; macrophages expressing such scavenger receptors on their surface present a decreased migration potential and are then retained within the plaque [
7]. Furthermore, oxLDL fixation on fibroblasts scavenger receptors leads to an increased production of collagen in the plaque, allowing them to catch even more LDL [
8]. Lastly, scavenger receptors induce the formation of reactive oxygen species (ROS) and SMC proliferation that promote some of the previously described alterations [
9].
In cancer, inflammation occurs by the infiltration of immune cells, including macrophages, T cells, or natural killer cells, that secrete large amounts of inflammatory cytokines, pro-angiogenic factors, and ROS, in the tumor microenvironment [
9]. Some studies have already implied oxLDL in cancer; indeed, an increased level of serum oxLDL is observed in patients with colorectal, breast, or ovarian cancer [
10,
11] and animal experiments indicate an increased incidence of prostate, colon, and liver cancers upon fat-enriched diets [
12]. Additionally, a depletion in LOX-1 receptors protects against the apparition of new cancers among several lineages, e.g., breast and cervical cancers and hepatocellular carcinoma [
13], whereas an increased LOX-1 expression has been reported in gastric cancer tissues [
14].
Our team recently studied the migration profile of head and neck cancer cells (HNCC) with Human Papilloma Virus (HPV) negative status, after exposure to commercial oxLDL. We demonstrated an increased expression of LOX-1 and CD36 receptors in HNCC after oxLDL exposure, and a decrease in cell migration via changes in the CD36/b-catenin pathway [
15].
In the present study, we propose to produce well-characterized oxLDL in order to control and standardize their production. These “home-made” oxLDL will be characterized by gel electrophoresis and compared with a commercially available oxLDL batch on one HNCC (93VU-147T) cell line; they will be applied in two additional HPV-positive HNCC to study whether their effects on cell migration involve the CD36 scavenger receptor.
3. Discussion
As oxLDL are among the main actors in the initiation and progression of atherosclerosis, it is necessary to produce oxLDL for studies involving their biological effects. The method most often applied to isolate lipoproteins producing oxLDL relies on ultracentrifugation, at around 100.000 g during 20–24 h with potassium bromide (KBr), in order to modify the density of the solution at 1.063 g/mL to isolate lipoproteins [
16,
17]. Some older studies even used a higher relative centrifugal force at 250.000–280.000 g during 24 to 48 h [
18,
19]. However, these methods are laborious in daily practice, requiring special and large equipment and long running times [
20]. Therefore, we developed a method easier to implement, that only requires material usually available in most laboratories. All the steps can be performed within a week even for a large production, requiring an initial investment of some 2000€. After selective precipitation from human plasma of a mixture LDL/VLDL, size exclusion liquid chromatography was used to isolate each of these lipoprotein classes, but also to assess the purity of the isolated LDL fraction by verifying the absence of other lipoproteins.
CuSO
4 is commonly used for LDL oxidation because it is easy to use, cheap, and steady. However, the resulting oxidation is “aggressive”, and may not be representative of in vivo oxidations. The intensity of oxidation depends on CuSO
4 concentration, the duration and temperature of incubation (increasing the temperature from 21 °C to 37 °C severely increases oxidation), and LDL concentration [
21]. Thus, it is extremely important that we standardized a process of mild LDL oxidation. It could be interesting to implement an even softer oxidation method to fit with real life situations. Indeed, it has been shown on M0 macrophages that the strength of oxidation influences the polarization to M1 or M2 macrophages [
22]. Additionally, Vlaminck et al. compared two models of LDL oxidation, the classical CuSO
4 oxidation (oxLDL) and a more physiological hydrogen peroxide-myeloperoxidase-based peroxidation that yields MoxLDL. They showed that copper oxidation induces biochemical alterations on both lipids and proteins, while myeloperoxidation induces mainly alterations on proteins; also, on THP-1-derived macrophages, oxLDL and MoxLDL induced different cell death pathways [
23]. Therefore, a majoring effect on HNCC of our LDL Cu
++ oxidation cannot be excluded, and our levels of protein oxidation should be quantitatively assessed, e.g., by an ELISA method. However, we obtained similar biological data by comparing reference and home-made oxLDL, which suggests an acceptable rate of oxidation in our production.
The results obtained in this work on HPV-positive HNCC are in line with our previous results, obtained with reference to oxLDL [
15] on HPV-negative HNCC (Detroit 562, UPCI-SCC-131 and FaDu), that indicated similar scavenger receptors expression and migration profiles.
However, interestingly, recent papers suggest that high-risk HPV may increase the risk of atheromatous plaque. The mechanism remains unclear, but it was proposed that the systemic inflammation induced by HPV could promote atherosclerosis lesions. The pro-inflammatory effects of HPV and the release of extracellular vesicles by HPV-transformed cells are well documented in the scientific literature [
24]. Data from the National Health and Nutrition Examination Survey on U.S. women indicate a strong association between the presence of HPV DNA on vaginal swab and a prior history of myocardial infarction or stroke [
25]. On the other hand, Lawson et al. identified HPV in 55% of atheromatous coronary arteries from deceased donors [
26], while Joo et al. described an elevated long-term cardiovascular risk among healthy young Korean women infected with high-risk [
27]. Thus, it is highly probable that there is a relation between HPV status and atherosclerosis, but further studies need to be implemented to understand the mechanisms involved.
We recognize some limitations of our work, e.g., that different intensities of copper oxidation or myeloperoxidation could be tested on HNCC in order to observe potential variability in scavenger receptors expression or cell migration
Moreover, in vitro results on 2-dimensional (2D) models are not able to mimic the microenvironment of cancers. Some authors have chosen to study HNCC effects on a novel 3-dimensional (3D) model, which comes closest to the in vivo situation. Miserocchi et al. showed that culturing oropharyngeal squamous cell carcinoma (OSCC) on a 3D biomimetic collagen-based scaffold induced different collagen fiber organization, and induced an increased expression of markers related to epithelial–mesenchymal transition (EMT) associated to a different cell migration behavior independently of HPV status. They demonstrated that LOX-1 expression was the most upregulated marker in 3D cultures, approximately ten-fold higher than in monolayer cultures [
28]. In parallel, Engelmann et al. recently worked on primary HNCC from patient’s tumorectomy cultured in a 3D organotypic co-culture model (3D-OTC) that maintain the architecture and cell composition to mimic individual tumors in their entirety, and to test individual long-term tumor responses to standard treatment or targeted therapies [
29].
Future experiments on the involvement of LOX-1 and CD36 receptors in migration might be interesting in determining an eventual synergistic effect. It would also be interesting to observe a possible effect of the LDL oxidation level on cancer cell migration and scavenger receptor expression, e.g., by changing the CuSO4 incubation conditions, or by applying myeloperoxidation or a superoxide anion generator. Finally, it would be interesting to reproduce these experiments on a 3D-model in order to mimic the in vivo tumor environment.
In conclusion, we developed an original method to produce oxLDL and demonstrated that oxLDL exposure of HPV-positive HNCC enhanced their lipid uptake and increased the expression of CD36 and LOX-1 scavenger receptors, while decreasing their migration capacity.
4. Material and Methods
4.1. oxLDL Production
Commercial oxLDL (Invitrogen, ThermoFisher Scientific, Waltham, MA, USA) were used as reference comparators for the “home-made” oxLDL. On 10 mL human plasma (Croix-Rouge, Bruxelles, Belgium), we applied a purification kit (Cell Biolabs Inc., San Diego, CA, USA) based on a precipitation and centrifugation step to isolate a 1 mL fraction containing LDL and Very Low Density Lipoproteins (VLDL). This kit is based on dextran sulfate to selectively precipitate LDL and VLDL without the need for ultracentrifugation. LDL were isolated from VLDL by high performance liquid chromatography (system HPLC 626-DAD 2996, Waters™, Framingham, MA, USA), by injecting 150 µL of the purified LDL-VLDL fraction in an aqueous size exclusion chromatography column (Shodex PROTEIN KW-804, 8.0 mm i.d. × 300 mm, couple to a KW-G 6B column, 6.0 mm i.d. × 50 mm). The chromatographic separation was achieved at 30 °C at a constant flow rate of 1 mL/min using Phosphate-Buffered Saline 1X (PBS, Gibco Life Technologies, Paisley, UK) as the mobile phase. The DAD detection wavelength was set at 280 nm.
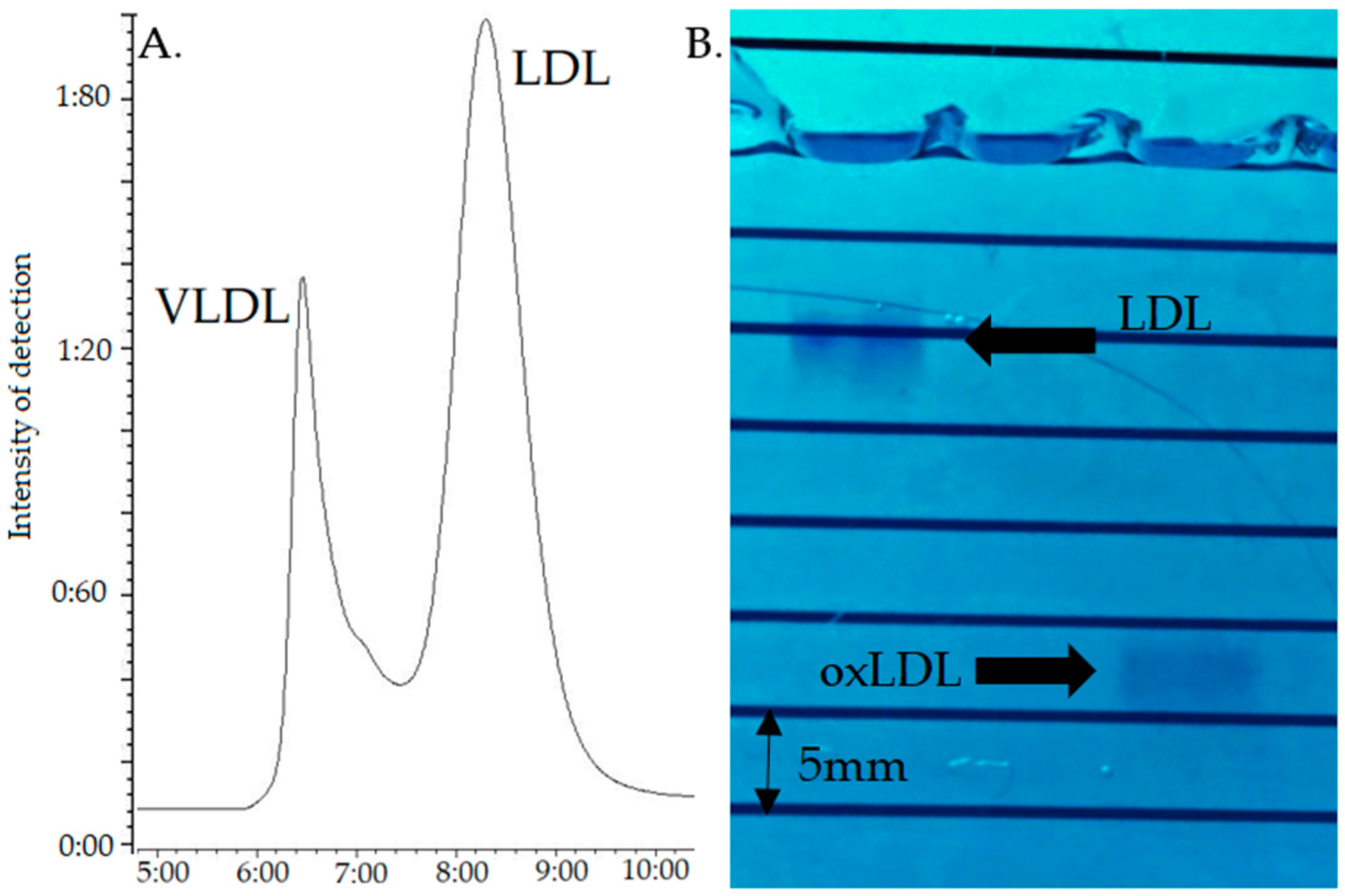
Under these conditions, VLDL and LDL eluted at about 6.6 and 8.6 min (Tmax), respectively (
Figure 1A); given the non-baseline resolution, the LDL peak was recovered after complete elution of the VLDL, i.e., starting at about 8.0 min. This LDL fraction (3 mL) was oxidized by incubation with 5 µM CuSO
4 (VWR international, Avantor, Radnor, PA, USA) for 20 h at 37 °C, the reaction being stopped with 0.2 mM EDTA. The oxLDL was then dialyzed against PBS containing 1 mM EDTA during 24 h at 4 °C, as described by Vrieling et al., using the Slide-A-Lyzer™ MINI dialysis 20K MWCO device (ThermoFischer Scientific, Waltham, MA, USA) [
30], with 2 buffer replacement steps.
The quality of oxidation was assessed by 0.5% agarose gel electrophoresis running for 60 min at 70 Volt [
31], the bands being visualized with Coomassie Blue staining (SimplyBlue™ SafeStain, Invitrogen™, Carlsbad, CA, USA) during 24 h followed by 48 h deionized water washing. As electronegativity increases with oxidation, native LDL migrate under these conditions at approximatively 11 mm against 12 to 30 mm for the oxLDL, depending on their oxidation status [
21].
4.2. Cell Culture
The 93VU-147T cell line was grown in Dulbecco’s Modified Eagle Medium (DMEM, Lonza, Verviers, Belgium) supplemented with 10% fetal bovine serum (FBS), 2% L-Glutamine, and 1% Penicillin/Streptomycin (Gibco Life Technologies, Paisley, UK).
The UM-SCC47 was cultured in DMEM supplemented with 10% FBS, 2% L-Glutamine, 1% Penicillin/Streptomycin, and 1% non-essential amino acids (Gibco Life Technologies, Paisley, UK).
The UPCI-SCC154 cell line was grown in Minimum Essential Medium (MEM, Gibco Life Technologies, Paisley, UK) supplemented with 10% FBS, 2% L-Glutamine, 1% Penicillin/Streptomycin, and 1% non-essential amino acids (Gibco Life Technologies, Paisley, UK).
Routine cell culture was carried out at 37 °C in a humidified cell incubator under 5% CO2.
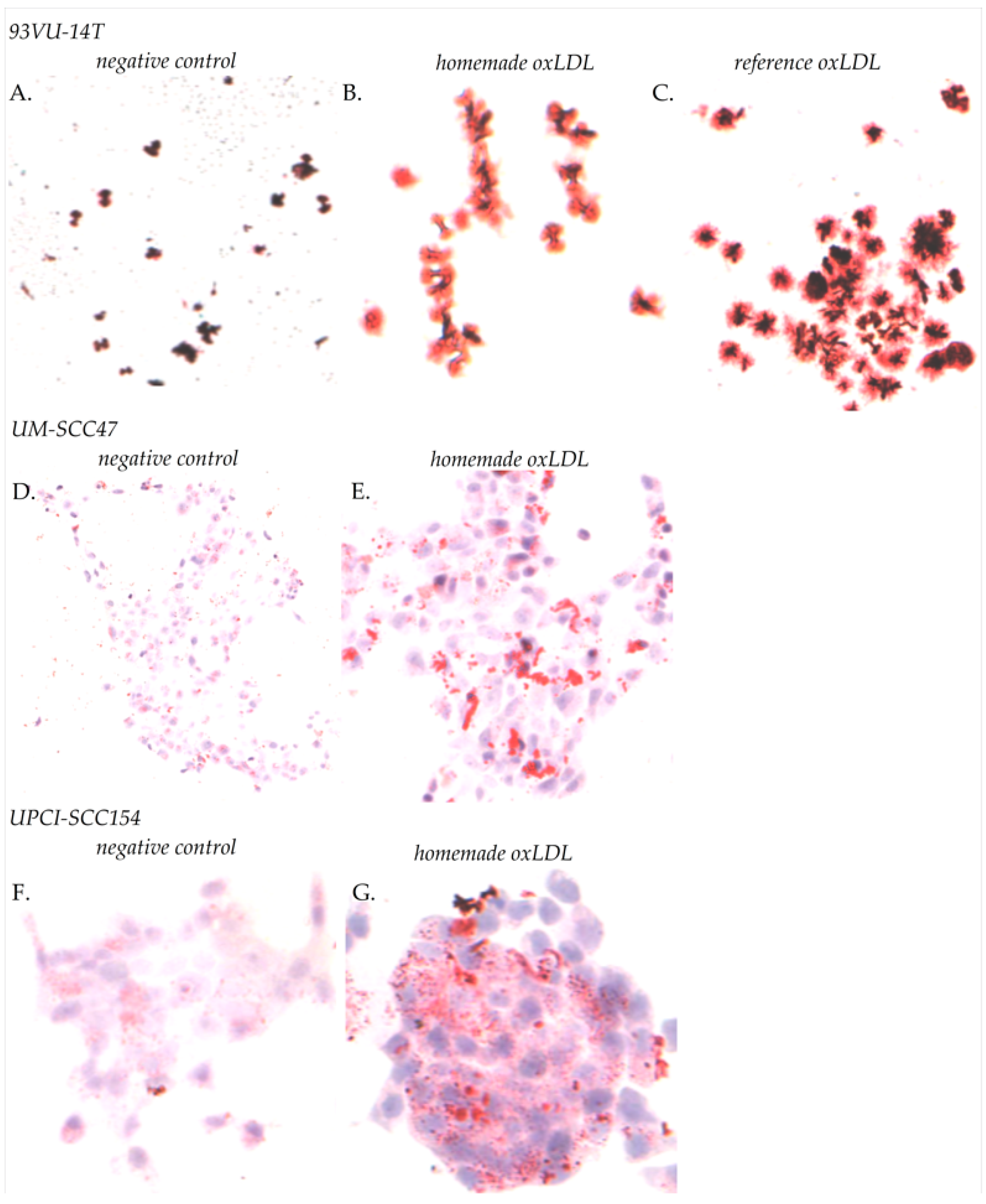
4.3. Oil Red O Staining
The amount of oxLDL internalization in cancer cells was evaluated by Oil Red staining. HNCC were plated on sterile round glass coverslips in a 12-wells dish at 60,000 cells/well during 24 h. For 93VU-147T cells, the medium was replaced by fresh medium with 30 µg/mL reference oxLDL, or 30 µg/mL home-made oxLDL, or fresh medium alone, for 48 h. For UM-SCC47 and UPCI-SCC154 cells, the medium was replaced by fresh medium with 30 µg/mL home-made oxLDL or fresh medium alone. The medium used was prepared with 10% Charcoal Stripped FBS (Labconsult SA-NV, Brussels, Belgium), which is delipidated with active carbon, instead of standard, decomplemented FBS. Cells were fixed with 4% paraformaldehyde in Dulbecco’s Phosphate-Buffer (DPBS, Lonza, Verviers, Belgium), then rinsed with DPBS and stained with Oil Red O (Merk Sigma, Darmstadt, Germany) for 15 min at room temperature (RT). After three rinsing steps with distilled water, cells were mounted with Aquatex
® (Merk Sigma, Darmstadt, Germany) and observed by phase-contrast microscopy using a Zeiss axioplan microscope equipped with a color charge-coupled device (CCD) camera (ProgRes C10plus, Jenoptik, Jena, Germany). Five fields were captured at ×10 magnification. Total cells and cells having accumulated lipids were counted using ImageJ (Research Services Branch of the National Institute of Health, NIH, Bethesda, MD, USA) [
32].
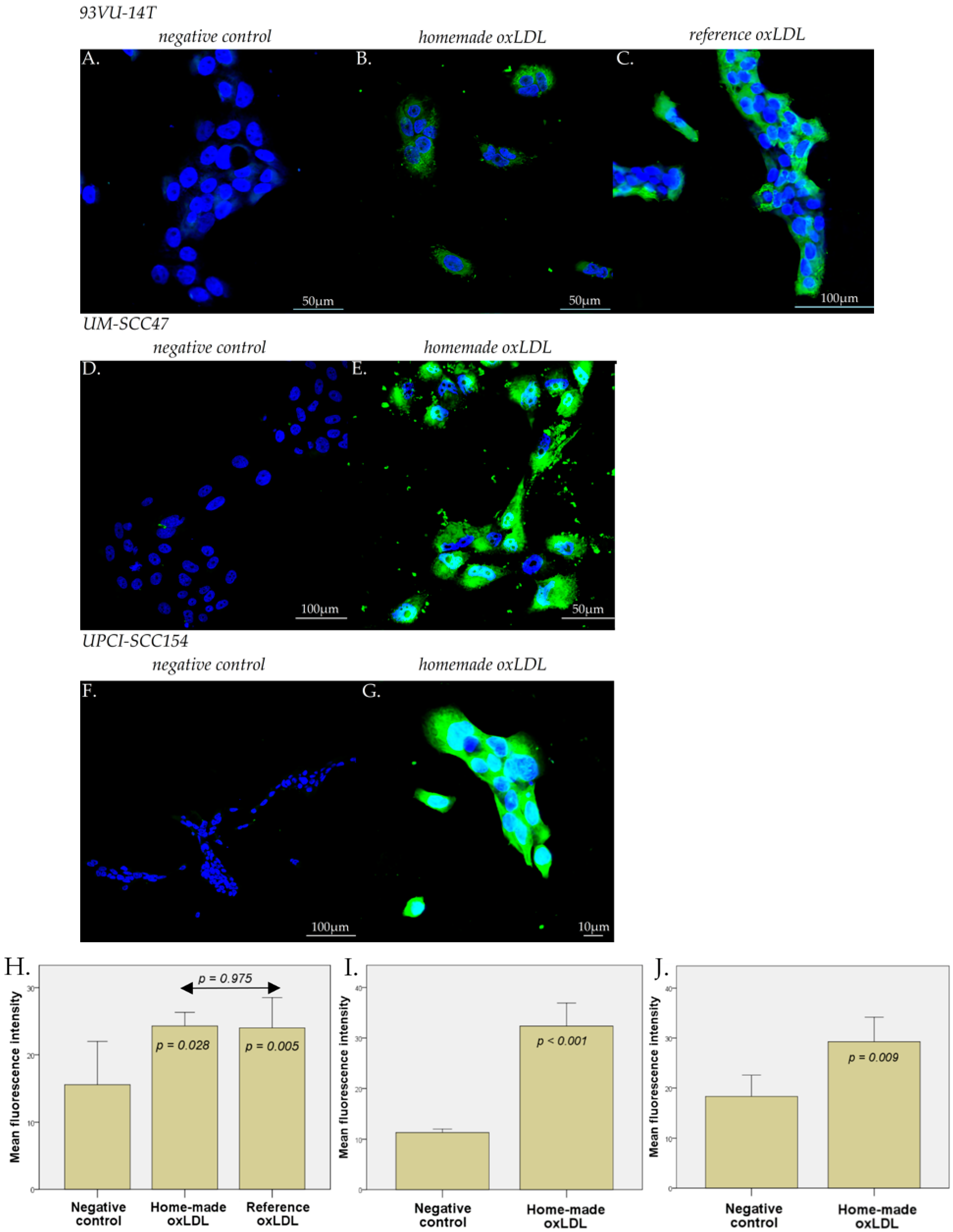
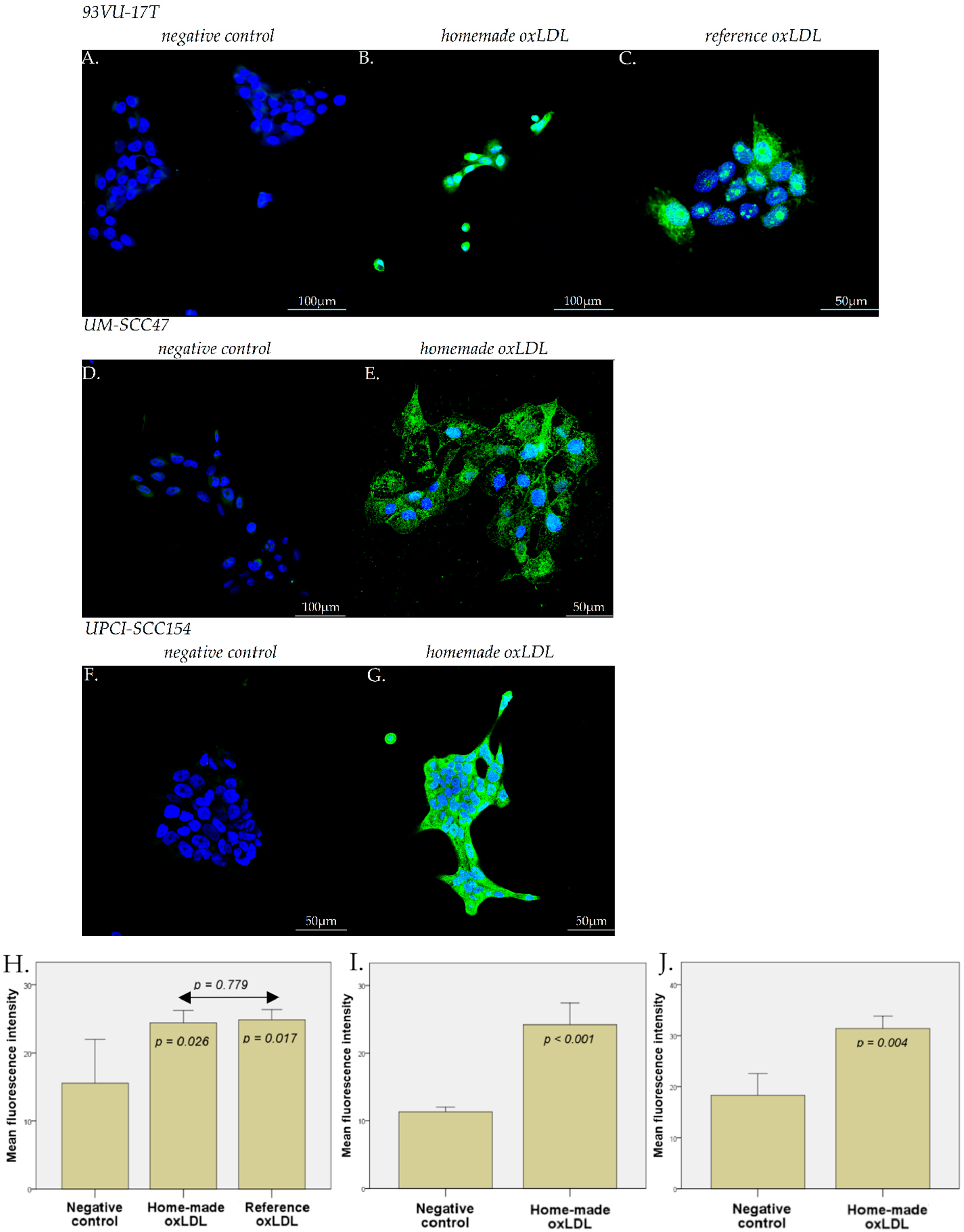
4.4. Immunofluorescence Microscopy
HNCC were plated on sterile round glass coverslips in a 12-well dish at 60,000 cells/well during 24 h. For 93VU-147T cells, the medium was replaced by fresh stripped medium with 30 µg/mL reference oxLDL, or 30 µg/mL home-made oxLDL, or fresh stripped medium alone, for 48 h. For UM-SCC47 and UPCI-SCC154 cells, the medium was replaced by fresh stripped medium with 30 µg/mL home-made oxLDL or fresh stripped medium alone. After 48 h, cells were fixed with 4% paraformaldehyde in PBS for 10 min at 4 °C and 5 min at RT.
For the anti-CD36 primary antibody (Merk Sigma, Darmstadt, Germany), the fixed cells were preincubated for 20 min at RT in a solution containing 2% Bovine Serum Albumin (BSA) in PBS to prevent non-specific adsorption of immunoglobulins. Then, anti-CD36 was diluted at 1/100 in the same solution and applied on cells overnight at 4 °C.
For the anti-LOX-1 primary antibody (ThermoFisher Scientific, Waltham, MA, USA), the fixed cells were pre-incubated for 1 h at RT in a solution containing 5% Normal Goat Serum (NGS)/Triton-X100 0.3% in PBS. Then, anti-LOX-1 was diluted at 1/1000 in the same solution and applied on cells overnight at 4 °C.
After several rinses with PBS, cells were treated with an anti-rabbit IgG antibody coupled with Alexa 488 for 30 min (ThermoFisher Scientific, Waltham, MA, USA), diluted in their respective solution.
After the final rinses with PBS and deionized water, the coverslips were mounted onto glass slides using a commercial anti-fading medium (ProLong™ Gold antifade reagent with DAPI, Invitrogen, ThermoFisher Scientific, Waltham, MA, USA). Confocal microscopy observations were carried out using an Olympus FV1000D laser scanning inverted microscope (LD559) (Olympus, Tokyo, Japan).
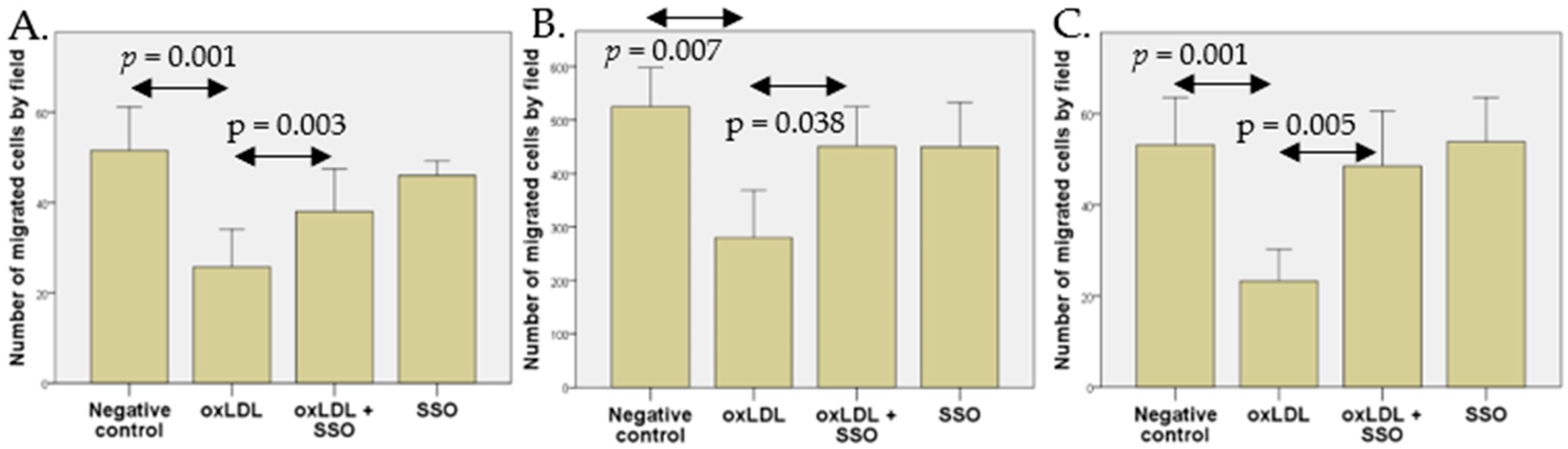
4.5. Cell Migration Assay
A Boyden Chamber assay was used to determine cell migration. A 24-wells plate containing fresh complete stripped medium (as described in
Section 2.2) was used as lower chamber and cell culture inserts (Falcon™ Cell Culture Inserts, ThermoFisher Scientific, Waltham, MA, USA) containing fresh serum-free medium were used as upper chambers. Lower and upper chamber were separated by a polycarbonate membrane (8 µm pore size). After 1 h of membrane rehydration with serum-free medium, 93VU-147T and UPCI-SCC154 cells were plated with 300,000 cells/inserts, and UM-SCC47 cells were plated at 120,000 cells/insert. The following day, the lower chamber was replaced with a complete stripped medium with or without 30 µg/mL home-made oxLDL. On 93VU-147T cells, an additional condition with 30 µg/mL of reference oxLDL was tested.
After 48 h, cells were wiped from the upper surface of the cell culture inserts with a cotton-tipped swab and the migrating cells were stained with crystal violet, after fixation with 4% paraformaldehyde. Migration was assessed by counting migrated cells over five microscopic fields (×10 magnification) using a Panthera C2 Classic Motic microscope (Motic®Europe, Barcelona, Spain).
An inhibition of CD36 was realized with sulfosuccinimidyl oleate (SSO), an irreversible inhibitor of CD36 that leads to a reduction in oxLDL uptake [
33]. Cells were seeded in cell culture inserts as previously described. The following day, cells were treated with 1 µM of SSO (Cayman chemical, Ann Arbor, MI, USA) for 4 h in a serum-free medium. Then, the medium containing SSO was replaced by fresh serum-free medium. The medium in the lower chambers was replaced with completed stripped medium with or without 30 µg/mL oxLDL. After 48 h, the cells were fixed with 4% paraformaldehyde and stained with crystal violet. The numbers of migrated cells were evaluated over five microscopic fields.
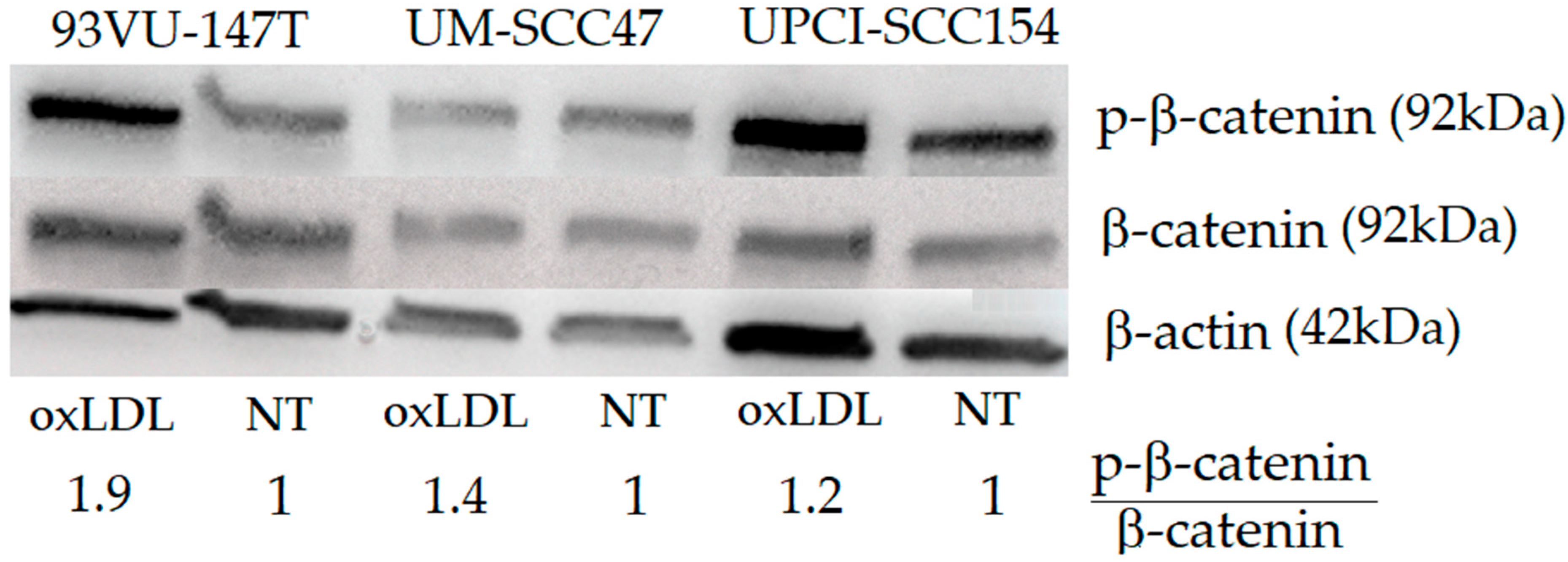
4.6. Western Blotting
Given its importance in cell motility and migration [
34], the Wnt/b-catenin pathway was further evaluated. Once cells reached 70–80% confluence on T75 cell culture flask, (ThermoFisher Scientific, Waltham, MA, USA), 93VU-147T, UM-SCC47, and UPCI-SCC154 cells were treated, or not, with 30 µg/mL home-made oxLDL for 48 h. Afterward, a lysis buffer (M-PER Mammalian Protein Extraction Reagent, ThermoFisher Scientific, Waltham, MA, USA) supplemented with protease and phosphatase inhibitors (Halt Protease and Phosphatase inhibitor cocktail, ThermoFisher Scientific, Waltham, MA, USA) were applied to lyse cells that were then scraped and centrifuged at 13,000 rpm for 10 min to collect protein lysates. Protein concentrations were assessed by a BCA protein assay (Pierce, ThermoFisher Scientific, Waltham, MA, USA) using bovine serum albumin as the standard. An amount of 25 µg of extracted proteins were loaded on 4–20% Mini-PROTEAN TGX gels (PAGE-SDS) (Bio-Rad Laboratories, München, Germany) and separated using electrophoresis at 120 V during 1 h. Then, proteins in the gel were electrotransferred onto nitrocellulose membranes (iBlot
® Dry Blotting System, Life Technologies-Invitrogen, Ghent, Belgium).
After a preincubation with PBS/Tween 0.1%/Milk powder 5% to prevent non-specific adsorption of immunoglobulins, the immunodetection was performed using an anti-β-catenin antibody (1/1000), an anti-P-β-catenin antibody (to detect the phosphorylation of β-catenin at the S675; 1/1000) (Cell Signaling, Danvers, MA, USA), and an anti-actin antibody (1/1000) (Pierce, ThermoFisher Scientific, Waltham, MA, USA).
A peroxidase-labeled anti-rabbit IgG antibody (1/5000) (Amersham Pharmacia Biotech, Roosendaal, the Netherlands) and a peroxidase-labeled anti-mouse IgG antibody (1/2000) were used as the secondary antibodies. The bound peroxidase activity was detected using the SuperSignal® West Pico Chemiluminescent Substrate (Pierce, ThermoFisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions, and chemiluminescence was detected using a CCD camera (Fusion FX, Vilber, Marne-la-Vallée, France). Band intensities were quantified by measuring their pixel density using ImageJ software to calculate a P-β-catenin/β-catenin relative ratio.
4.7. Statistical Analysis
SPSS® Statistics version 23 software (IBM®, Armonk, NY, USA) was used for the statistical analysis. Normality was checked using the Shapiro-Wilk test, confirming the applicability of parametric analyses using the Student’s t-test. Data were expressed as means ± SD with a p ≤ 0.05 value to indicate a statistically significant difference.