Relationships between Inflammation and Age-Related Neurocognitive Changes
Abstract
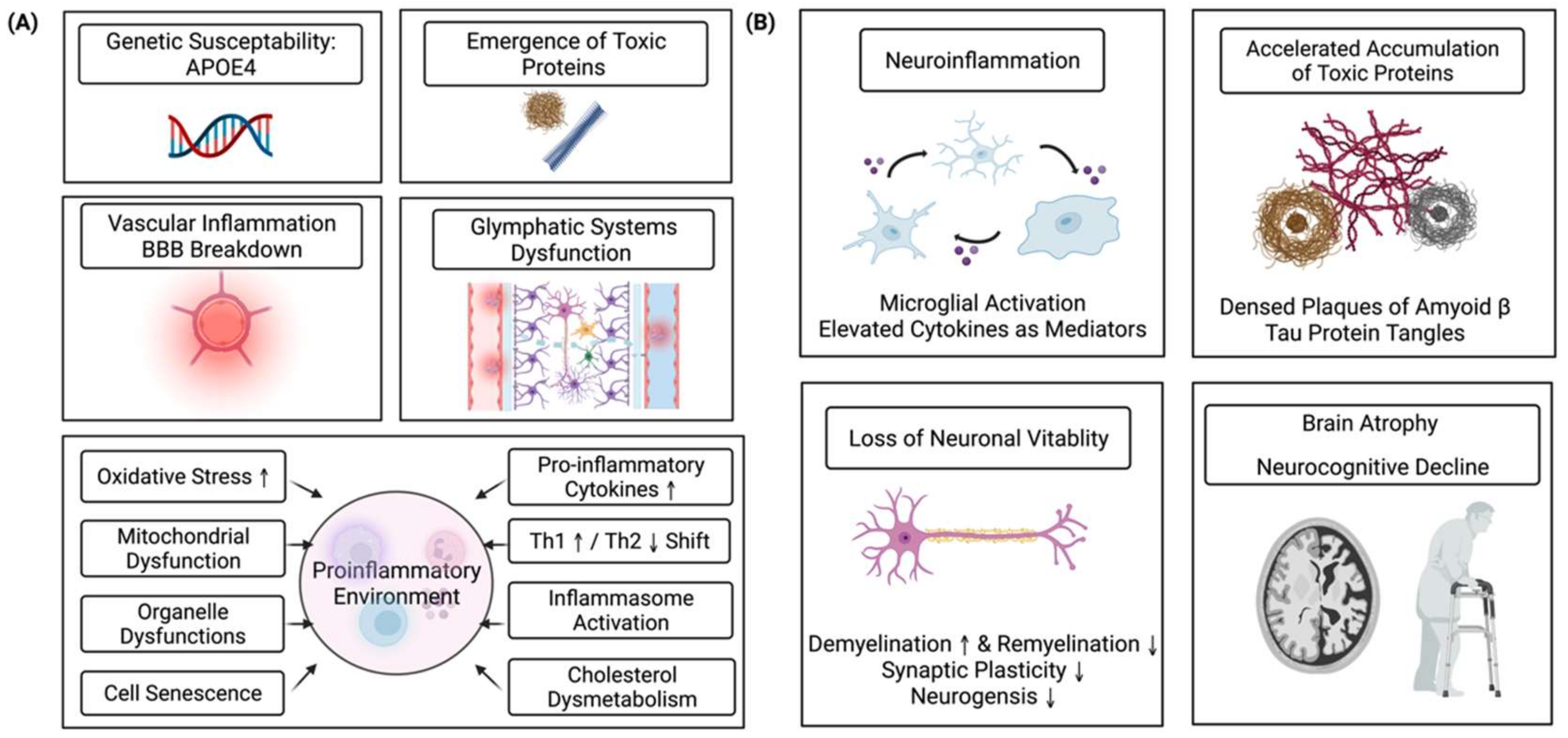
1. Introduction
2. Inflammation and Cognitive Aging
3. Key Molecules Bridging Inflammation and Cognition
3.1. IL-6
3.2. IL-12
3.3. IL-1β and IL-18
3.4. Interferons
4. Neuroinflammation
5. Organelle Dysfunction and Abnormal Lipid Metabolism
6. Glymphatic System
7. Potential Interventions
7.1. Pharmacological Approaches
7.2. Lifestyle Management
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. World Population Ageing 2019 Highlights; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Borgoni, S.; Kudryashova, K.S.; Burka, K.; de Magalhães, J.P. Targeting immune dysfunction in aging. Ageing Res. Rev. 2021, 70, 101410. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, S.; Müller, L.; Wenger, E.; Düzel, S.; Pawelec, G. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci. Biobehav. Rev. 2017, 75, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.L.; Savage, J.C.; Hui, C.W.; Bisht, K.; Tremblay, M.-È. Microglia across the lifespan: From origin to function in brain development, plasticity and cognition. J. Physiol. 2017, 595, 1929–1945. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Corsi, A.; Lauretani, F.; Bandinelli, S.; Bartali, B.; Taub, D.D.; Guralnik, J.M.; Longo, D.L. The origins of age-related proinflammatory state. Blood 2005, 105, 2294–2299. [Google Scholar] [CrossRef]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. Source of Chronic Inflammation in Aging. Front. Cardiovasc. Med. 2018, 5, 12. [Google Scholar] [CrossRef]
- Vatner, S.F.; Zhang, J.; Oydanich, M.; Berkman, T.; Naftalovich, R.; Vatner, D.E. Healthful aging mediated by inhibition of oxidative stress. Ageing Res. Rev. 2020, 64, 101194. [Google Scholar] [CrossRef]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef]
- Uciechowski, P.; Kahmann, L.; Plümäkers, B.; Malavolta, M.; Mocchegiani, E.; Dedoussis, G.; Herbein, G.; Jajte, J.; Fulop, T.; Rink, L. TH1 and TH2 cell polarization increases with aging and is modulated by zinc supplementation. Exp. Gerontol. 2008, 43, 493–498. [Google Scholar] [CrossRef]
- Fotuhi, M.; Hachinski, V.; Whitehouse, P.J. Changing perspectives regarding late-life dementia. Nat. Rev. Neurol. 2009, 5, 649–658. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; Van Der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Fard, M.T.; Stough, C. A Review and Hypothesized Model of the Mechanisms That Underpin the Relationship Between Inflammation and Cognition in the Elderly. Front. Aging Neurosci. 2019, 11, 56. [Google Scholar] [CrossRef]
- Cunningham, C.; Campion, S.; Lunnon, K.; Murray, C.L.; Woods, J.F.; Deacon, R.M.; Rawlins, J.N.P.; Perry, V.H. Systemic Inflammation Induces Acute Behavioral and Cognitive Changes and Accelerates Neurodegenerative Disease. Biol. Psychiatry 2009, 65, 304–312. [Google Scholar] [CrossRef]
- Lin, T.; Liu, G.A.; Perez, E.; Rainer, R.D.; Febo, M.; Cruz-Almeida, Y.; Ebner, N.C. Systemic Inflammation Mediates Age-Related Cognitive Deficits. Front. Aging Neurosci. 2018, 10, 236. [Google Scholar] [CrossRef]
- Darweesh, S.K.L.; Wolters, F.J.; Ikram, M.A.; de Wolf, F.; Bos, D.; Hofman, A. Inflammatory markers and the risk of dementia and Alzheimer’s disease: A meta-analysis. Alzheimers Dement. 2018, 14, 1450–1459. [Google Scholar] [CrossRef]
- Bradburn, S.; Murgatroyd, C.; Ray, N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Ageing Res. Rev. 2019, 50, 1–8. [Google Scholar] [CrossRef]
- Tao, Q.; Ang, T.F.A.; DeCarli, C.; Auerbach, S.H.; Devine, S.; Stein, T.D.; Zhang, X.; Massaro, J.; Au, R.; Qiu, W.Q. Association of Chronic Low-grade Inflammation with Risk of Alzheimer Disease in ApoE4 Carriers. JAMA Netw. Open 2018, 1, e183597. [Google Scholar] [CrossRef]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Wang, F.; Fan, Y.-X.; Ping, G.-F.; Yang, J.-Y.; Wu, C.-F. Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behav. Brain Res. 2013, 236, 270–282. [Google Scholar] [CrossRef]
- Williams, A.E. Immunology: Mucosal and Body Surface Defences; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ershler, W.B.; Sun, W.H.; Binkley, N.; Gravenstein, S.; Volk, M.J.; Kamoske, G.; Klopp, R.G.; Roecker, E.B.; Daynes, R.A.; Weindruch, R. Interleukin-6 and aging: Blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res. 1993, 12, 225–230. [Google Scholar]
- Stevenson, A.J.; McCartney, D.L.; Harris, S.E.; Taylor, A.M.; Redmond, P.; Starr, J.M.; Zhang, Q.; McRae, A.F.; Wray, N.R.; Spires-Jones, T.L.; et al. Trajectories of inflammatory biomarkers over the eighth decade and their associations with immune cell profiles and epigenetic ageing. Clin. Epigenetics 2018, 10, 159. [Google Scholar] [CrossRef]
- Koelman, L.; Pivovarova-Ramich, O.; Pfeiffer, A.F.H.; Grune, T.; Aleksandrova, K. Cytokines for evaluation of chronic inflammatory status in ageing research: Reliability and phenotypic characterisation. Immun. Ageing 2019, 16, 11. [Google Scholar] [CrossRef]
- Alboni, S.; Cervia, D.; Sugama, S.; Conti, B. Interleukin 18 in the CNS. J. Neuroinflamm. 2010, 7, 9. [Google Scholar] [CrossRef]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a Major Cytokine in the Central Nervous System. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef]
- Monteiro, S.; Roque, S.; Marques, F.; Correia-Neves, M.; Cerqueira, J.J. Brain interference: Revisiting the role of IFNγ in the central nervous system. Prog. Neurobiol. 2017, 156, 149–163. [Google Scholar] [CrossRef]
- McAfoose, J.; Baune, B. Evidence for a cytokine model of cognitive function. Neurosci. Biobehav. Rev. 2009, 33, 355–366. [Google Scholar] [CrossRef]
- Borsini, A.; Zunszain, P.A.; Thuret, S.; Pariante, C.M. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 2015, 38, 145–157. [Google Scholar] [CrossRef]
- Wilson, C.J.; Finch, C.E.; Cohen, H.J. Cytokines and Cognition-The Case for A Head-to-Toe Inflammatory Paradigm. J. Am. Geriatr. Soc. 2002, 50, 2041–2056. [Google Scholar] [CrossRef]
- Yankner, B.A.; Lu, T.; Loerch, P. The aging brain. Annu. Rev. Pathol. 2008, 3, 41–66. [Google Scholar] [CrossRef]
- Lai, K.S.P.; Liu, C.S.; Rau, A.; Lanctôt, K.L.; Köhler, C.A.; Pakosh, M.; Carvalho, A.F.; Herrmann, N. Peripheral inflammatory markers in Alzheimer’s disease: A systematic review and meta-analysis of 175 studies. J. Neurol. Neurosurg. Psychiatry 2017, 88, 876–882. [Google Scholar] [CrossRef]
- Blank, T.; Prinz, M. Type I interferon pathway in CNS homeostasis and neurological disorders. Glia 2017, 65, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.; Baune, B. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: A systematic review of biomarker studies. Neurosci. Biobehav. Rev. 2014, 42, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Kalliolias, G.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2015, 12, 49–62. [Google Scholar] [CrossRef]
- Becher, B.; Spath, S.; Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2016, 17, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.R.; Karavitis, J.; Palmer, J.L.; Faunce, U.E.; Ramirez, L.; Nomellini, V.; Kovacs, E.J. Interleukin-6 Contributes to Age-Related Alteration of Cytokine Production by Macrophages. Mediat. Inflamm. 2010, 2010, 475139. [Google Scholar] [CrossRef]
- Bradburn, S.; Sarginson, J.; Murgatroyd, C. Association of Peripheral Interleukin-6 with Global Cognitive Decline in Non-demented Adults: A Meta-Analysis of Prospective Studies. Front. Aging Neurosci. 2018, 9, 438. [Google Scholar] [CrossRef]
- Wennberg, A.M.V.; Hagen, C.E.; Machulda, M.M.; Knopman, D.S.; Petersen, R.C.; Mielke, M.M. The Cross-sectional and Longitudinal Associations Between IL-6, IL-10, and TNFalpha and Cognitive Outcomes in the Mayo Clinic Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1289–1295. [Google Scholar] [CrossRef]
- Qi, D.; Wong, N.M.; Shao, R.; Man, I.S.; Wong, C.H.; Yuen, L.P.; Chan, C.C.; Lee, T.M. Qigong exercise enhances cognitive functions in the elderly via an interleukin-6-hippocampus pathway: A randomized active-controlled trial. Brain Behav. Immun. 2021, 95, 381–390. [Google Scholar] [CrossRef]
- E Silva, N.M.L.; Gonçalves, R.A.; Pascoal, T.A.; Lima-Filho, R.A.S.; Resende, E.D.P.F.; Vieira, E.L.M.; Teixeira, A.L.; de Souza, L.C.; Peny, J.A.; Fortuna, J.T.S.; et al. Pro-inflammatory interleukin-6 signaling links cognitive impairments and peripheral metabolic alterations in Alzheimer’s disease. Transl. Psychiatry 2021, 11, 251. [Google Scholar] [CrossRef]
- Tehranian, R.; Hasanvan, H.; Iverfeldt, K.; Post, C.; Schultzberg, M. Early induction of interleukin-6 mRNA in the hippocampus and cortex of APPsw transgenic mice Tg2576. Neurosci. Lett. 2001, 301, 54–58. [Google Scholar] [CrossRef]
- Ringheim, G.E.; Szczepanik, A.M.; Petko, W.; Burgher, K.L.; Zu Zhu, S.; Chao, C.C. Enhancement of beta-amyloid precursor protein transcription and expression by the soluble interleukin-6 receptor/interleukin-6 complex. Mol. Brain Res. 1998, 55, 35–44. [Google Scholar] [CrossRef]
- Vignali, D.A.; Kuchroo, V.K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef]
- Wojno, E.T.; Hunter, C.A.; Stumhofer, J.S. The Immunobiology of the Interleukin-12 Family: Room for Discovery. Immunity 2019, 50, 851–870. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, L.; Zhang, B.; Gao, J.; Zhao, T.; Fang, S. Higher levels of C-reactive protein in the acute phase of stroke indicate an increased risk for post-stroke depression: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 134, 104309. [Google Scholar] [CrossRef]
- Sun, L.; He, C.; Nair, L.; Yeung, J.; Egwuagu, C.E. Interleukin 12 (IL-12) family cytokines: Role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine 2015, 75, 249–255. [Google Scholar] [CrossRef]
- Lin, E.; Kuo, P.-H.; Liu, Y.-L.; Yang, A.C.; Tsai, S.-J. Association and Interaction Effects of Interleukin-12 Related Genes and Physical Activity on Cognitive Aging in Old Adults in the Taiwanese Population. Front. Neurol. 2019, 10, 1065. [Google Scholar] [CrossRef]
- Magalhães, T.N.C.; Weiler, M.; Teixeira, C.V.L.; Hayata, T.; Moraes, A.S.; Boldrini, V.O.; dos Santos, L.M.; de Campos, B.M.; de Rezende, T.J.R.; Joaquim, H.P.G.; et al. Systemic Inflammation and Multimodal Biomarkers in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease. Mol. Neurobiol. 2017, 55, 5689–5697. [Google Scholar] [CrossRef]
- Choi, J.K.; Dambuza, I.M.; He, C.; Yu, C.-R.; Uche, A.N.; Mattapallil, M.J.; Caspi, R.R.; Egwuagu, C.E. IL-12p35 Inhibits Neuroinflammation and Ameliorates Autoimmune Encephalomyelitis. Front. Immunol. 2017, 8, 1285. [Google Scholar] [CrossRef]
- Yang, H.S.; Zhang, C.; Carlyle, B.C.; Zhen, S.Y.; Trombetta, B.A.; Schultz, A.P.; Pruzin, J.J.; Fitzpatrick, C.D.; Yau, W.W.; Kirn, D.R.; et al. Plasma IL-12/IFN-gamma axis predicts cognitive trajectories in cognitively unimpaired older adults. Alzheimers Dement. 2022, 18, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Vom Berg, J.; Prokop, S.; Miller, K.R.; Obst, J.; Kalin, R.E.; Lopategui-Cabezas, I.; Wegner, A.; Mair, F.; Schipke, C.G.; Peters, O.; et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat. Med. 2012, 18, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Corbo, R.M.; Businaro, R.; Scarabino, D. Leukocyte telomere length and plasma interleukin-1beta and interleukin-18 levels in mild cognitive impairment and Alzheimer’s disease: New biomarkers for diagnosis and disease progression? Neural Regen. Res. 2021, 16, 1397–1398. [Google Scholar] [PubMed]
- Orhan, F.; Fatouros-Bergman, H.; Schwieler, L.; Cervenka, S.; Flyckt, L.; Sellgren, C.M.; Engberg, G.; Erhardt, S. First-episode psychosis patients display increased plasma IL-18 that correlates with cognitive dysfunction. Schizophr. Res. 2018, 195, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Dong, R.; Song, C.; Li, X.; Zhang, L.; Shi, M.; Lv, C.; Wang, L.; Kou, J.; Xie, H.; et al. Mediation Effects of IL-1beta and IL-18 on the Association Between Vitamin D Levels and Mild Cognitive Impairment Among Chinese Older Adults: A Case-Control Study in Taiyuan, China. Front. Aging Neurosci. 2022, 14, 836311. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Singhal, G.; Jaehne, E.J.; Corrigan, F.; Toben, C.; Baune, B.T. Inflammasomes in neuroinflammation and changes in brain function: A focused review. Front. Neurosci. 2014, 8, 315. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Curran, B.; O’Connor, J. The pro-inflammatory cytokine interleukin-18 impairs long-term potentiation and NMDA receptor-mediated transmission in the rat hippocampus in vitro. Neuroscience 2001, 108, 83–90. [Google Scholar] [CrossRef]
- Prieto, G.A.; Tong, L.; Smith, E.D.; Cotman, C.W. TNFalpha and IL-1beta but not IL-18 Suppresses Hippocampal Long-Term Potentiation Directly at the Synapse. Neurochem. Res. 2019, 44, 49–60. [Google Scholar] [CrossRef]
- Burke, S.N.; Barnes, C.A. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006, 7, 30–40. [Google Scholar] [CrossRef]
- Rosenzweig, E.S.; Barnes, C.A. Impact of aging on hippocampal function: Plasticity, network dynamics, and cognition. Prog. Neurobiol. 2003, 69, 143–179. [Google Scholar] [CrossRef]
- Lambert, M.P.; Barlow, A.K.; Chromy, B.A.; Edwards, C.; Freed, R.; Liosatos, M.; Morgan, T.E.; Rozovsky, I.; Trommer, B.; Viola, K.L.; et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA 1998, 95, 6448–6453. [Google Scholar] [CrossRef]
- Isaacs, A.; Lindenmann, J. Virus interference. I. The interferon. Proc. R. Soc. Lond. Ser. B 1957, 147, 258–267. [Google Scholar]
- Walter, M.R. The Role of Structure in the Biology of Interferon Signaling. Front. Immunol. 2020, 11, 606489. [Google Scholar] [CrossRef]
- Rota, E.; Bellone, G.; Rocca, P.; Bergamasco, B.; Emanuelli, G.; Ferrero, P. Increased intrathecal TGF-beta1, but not IL-12, IFN-gamma and IL-10 levels in Alzheimer’s disease patients. Neurol. Sci. 2006, 27, 33–39. [Google Scholar] [CrossRef]
- Popko, B.; Corbin, J.G.; Baerwald, K.D.; DuPree, J.; Garcia, A.M. The effects of interferon-γ on the central nervous system. Mol. Neurobiol. 1997, 14, 19–35. [Google Scholar] [CrossRef]
- Ottum, P.A.; Arellano, G.; Reyes, L.I.; Iruretagoyena, M.; Naves, R. Opposing Roles of Interferon-Gamma on Cells of the Central Nervous System in Autoimmune Neuroinflammation. Front. Immunol. 2015, 6, 539. [Google Scholar] [CrossRef]
- Deczkowska, A.; Baruch, K.; Schwartz, M. Type I/II Interferon Balance in the Regulation of Brain Physiology and Pathology. Trends Immunol. 2016, 37, 181–192. [Google Scholar] [CrossRef]
- Zhang, J.; He, H.; Qiao, Y.; Zhou, T.; He, H.; Yi, S.; Zhang, L.; Mo, L.; Li, Y.; Jiang, W.; et al. Priming of microglia with IFN-gamma impairs adult hippocampal neurogenesis and leads to depression-like behaviors and cognitive defects. Glia 2020, 68, 2674–2692. [Google Scholar] [CrossRef]
- Alasmari, F.; Alshammari, M.A.; Alasmari, A.F.; Alanazi, W.A.; Alhazzani, K. Neuroinflammatory Cytokines Induce Amyloid Beta Neurotoxicity through Modulating Amyloid Precursor Protein Levels/Metabolism. BioMed Res. Int. 2018, 2018, 3087475. [Google Scholar] [CrossRef]
- Browne, T.C.; McQuillan, K.; McManus, R.M.; O’Reilly, J.A.; Mills, K.H.; Lynch, M.A. IFN-γ Production by amyloid β-specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer’s disease. J. Immunol. 2013, 190, 2241–2251. [Google Scholar] [CrossRef]
- He, Z.; Yang, Y.; Xing, Z.; Zuo, Z.; Wang, R.; Gu, H.; Qi, F.; Yao, Z. Intraperitoneal injection of IFN-γ restores microglial autophagy, promotes amyloid-β clearance and improves cognition in APP/PS1 mice. Cell Death Dis. 2020, 11, 440. [Google Scholar] [CrossRef]
- Angelova, D.M.; Brown, D.R. Microglia and the aging brain: Are senescent microglia the key to neurodegeneration? J. Neurochem. 2019, 151, 676–688. [Google Scholar] [CrossRef]
- Wu, K.-M.; Zhang, Y.-R.; Huang, Y.-Y.; Dong, Q.; Tan, L.; Yu, J.-T. The role of the immune system in Alzheimer’s disease. Ageing Res. Rev. 2021, 70, 101409. [Google Scholar] [CrossRef]
- Disabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. 2), 136–153. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar] [PubMed]
- Lavisse, S.; Guillermier, M.; Herard, A.-S.; Petit, F.; Delahaye, M.; Van Camp, N.; Ben Haim, L.; Lebon, V.; Remy, P.; Dollé, F.; et al. Reactive Astrocytes Overexpress TSPO and Are Detected by TSPO Positron Emission Tomography Imaging. J. Neurosci. 2012, 32, 10809–10818. [Google Scholar] [CrossRef] [PubMed]
- Motta, C.; Finardi, A.; Toniolo, S.; Di Lorenzo, F.; Scaricamazza, E.; Loizzo, S.; Mercuri, N.B.; Furlan, R.; Koch, G.; Martorana, A. Protective Role of Cerebrospinal Fluid Inflammatory Cytokines in Patients with Amnestic Mild Cognitive Impairment and Early Alzheimer’s Disease Carrying Apolipoprotein E4 Genotype. J. Alzheimer’s Dis. 2020, 76, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, H.; Bai, F.; Zhang, Z.; Ren, Q. Remyelination: A Potential Therapeutic Strategy for Alzheimer’s Disease? J. Alzheimers Dis. 2017, 58, 597–612. [Google Scholar] [CrossRef]
- Ruckh, J.M.; Zhao, J.-W.; Shadrach, J.L.; van Wijngaarden, P.; Rao, T.N.; Wagers, A.J.; Franklin, R.J. Rejuvenation of Regeneration in the Aging Central Nervous System. Cell Stem Cell 2012, 10, 96–103. [Google Scholar] [CrossRef]
- Shen, S.; Sandoval, J.; Swiss, V.A.; Li, J.; Dupree, J.; Franklin, R.J.M.; Casaccia-Bonnefil, P. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat. Neurosci. 2008, 11, 1024–1034. [Google Scholar] [CrossRef]
- Domingues, H.S.; Portugal, C.C.; Socodato, R.; Relvas, J.B. Oligodendrocyte, Astrocyte, and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front. Cell Dev. Biol. 2016, 4, 71. [Google Scholar]
- Perry, V.H.; Nicoll, J.A.R.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201. [Google Scholar] [CrossRef]
- Hughes, A.N.; Appel, B. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. 2020, 23, 1055–1066. [Google Scholar] [CrossRef]
- Han, F.; Perrin, R.J.; Wang, Q.; Wang, Y.; Perlmutter, J.S.; Morris, J.C.; Benzinger, T.L.S.; Xu, J. Neuroinflammation and Myelin Status in Alzheimer’s Disease, Parkinson’s Disease, and Normal Aging Brains: A Small Sample Study. Parkinson’s Dis. 2019, 2019, 7975407. [Google Scholar] [CrossRef]
- Srinivasan, K.; Friedman, B.; Larson, J.L.; Lauffer, B.E.; Goldstein, L.D.; Appling, L.L.; Borneo, J.; Poon, C.; Ho, T.; Cai, F.; et al. Untangling the brain’s neuroinflammatory and neurodegenerative transcriptional responses. Nat. Commun. 2016, 7, 11295. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Li, A.; Grutzendler, J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci. 2018, 21, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.H.; Lee, G.J.; Raven, E.P.; Tingus, K.; Khoo, T.; Thompson, P.; Bartzokis, G. Age-related slowing in cognitive processing speed is associated with myelin integrity in a very healthy elderly sample. J. Clin. Exp. Neuropsychol. 2011, 33, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Caso, F.; Agosta, F.; Filippi, M. Insights into White Matter Damage in Alzheimer’s Disease: From Postmortem to in vivo Diffusion Tensor MRI Studies. Neurodegener. Dis. 2016, 16, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lam, C.L.; Lee, T.M. White matter microstructural abnormalities in amnestic mild cognitive impairment: A meta-analysis of whole-brain and ROI-based studies. Neurosci. Biobehav. Rev. 2017, 83, 405–416. [Google Scholar] [CrossRef]
- Chamberlain, J.D.; Turney, I.C.; Goodman, J.T.; Hakun, J.G.; Dennis, N.A. Fornix white matter microstructure differentially predicts false recollection rates in older and younger adults. Neuropsychologia 2021, 157, 107848. [Google Scholar] [CrossRef]
- Bubb, E.J.; Metzler-Baddeley, C.; Aggleton, J.P. The cingulum bundle: Anatomy, function, and dysfunction. Neurosci. Biobehav. Rev. 2018, 92, 104–127. [Google Scholar] [CrossRef]
- Von Der Heide, R.J.; Skipper, L.M.; Klobusicky, E.; Olson, I.R. Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain 2013, 136, 1692–1707. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Elahy, M.; Jackaman, C.; Mamo, J.C.; Lam, V.; Dhaliwal, S.S.; Giles, C.; Nelson, D.; Takechi, R. Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun. Ageing 2015, 12, 2. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef]
- Rajeev, V.; Fann, D.Y.; Dinh, Q.N.; Kim, H.A.; De Silva, T.M.; Lai, M.K.P.; Chen, C.L.-H.; Drummond, G.R.; Sobey, C.G.; Arumugam, T.V. Pathophysiology of blood brain barrier dysfunction during chronic cerebral hypoperfusion in vascular cognitive impairment. Theranostics 2022, 12, 1639–1658. [Google Scholar] [CrossRef]
- Daniels, B.P.; Klein, R.S. Knocking on Closed Doors: Host Interferons Dynamically Regulate Blood-Brain Barrier Function during Viral Infections of the Central Nervous System. PLOS Pathog. 2015, 11, e1005096. [Google Scholar] [CrossRef]
- Skelly, D.T.; Hennessy, E.; Dansereau, M.A.; Cunningham, C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1β, TNF-α and IL-6 challenges in C57BL/6 mice. PLoS ONE 2013, 8, e69123. [Google Scholar] [CrossRef]
- Voirin, A.-C.; Perek, N.; Roche, F. Inflammatory stress induced by a combination of cytokines (IL-6, IL-17, TNF-α) leads to a loss of integrity on bEnd.3 endothelial cells in vitro BBB model. Brain Res. 2020, 1730, 146647. [Google Scholar] [CrossRef]
- Propson, N.E.; Roy, E.R.; Litvinchuk, A.; Köhl, J.; Zheng, H. Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging. J. Clin. Investig. 2021, 131, e140966. [Google Scholar] [CrossRef]
- Senatorov, V.V.; Friedman, A.R.; Milikovsky, D.Z.; Ofer, J.; Saar-Ashkenazy, R.; Charbash, A.; Jahan, N.; Chin, G.; Mihaly, E.; Lin, J.M.; et al. Blood-brain barrier dysfunction in aging induces hyperactivation of TGFβ signaling and chronic yet reversible neural dysfunction. Sci. Transl. Med. 2019, 11, eaaw8283. [Google Scholar] [CrossRef]
- Toniolo, S.; Sen, A.; Husain, M. Modulation of Brain Hyperexcitability: Potential New Therapeutic Approaches in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 9318. [Google Scholar] [CrossRef]
- Tweedy, C.; Kindred, N.; Curry, J.; Williams, C.; Taylor, J.-P.; Atkinson, P.; Randall, F.; Erskine, D.; Morris, C.M.; Reeve, A.K.; et al. Hippocampal network hyperexcitability in young transgenic mice expressing human mutant alpha-synuclein. Neurobiol. Dis. 2020, 149, 105226. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Han, R.T.; Kim, R.D.; Molofsky, A.V.; Liddelow, S.A. Astrocyte-immune cell interactions in physiology and pathology. Immunity 2021, 54, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I. Oligodendrogliopathy in neurodegenerative diseases with abnormal protein aggregates: The forgotten partner. Prog. Neurobiol. 2018, 169, 24–54. [Google Scholar] [CrossRef] [PubMed]
- Di Cara, F.; Andreoletti, P.; Trompier, D.; Vejux, A.; Bülow, M.H.; Sellin, J.; Lizard, G.; Cherkaoui-Malki, M.; Savary, S. Peroxisomes in Immune Response and Inflammation. Int. J. Mol. Sci. 2019, 20, 3877. [Google Scholar] [CrossRef] [PubMed]
- Di Cara, F.; Sheshachalam, A.; Braverman, N.E.; Rachubinski, R.A.; Simmonds, A.J. Peroxisome-Mediated Metabolism Is Required for Immune Response to Microbial Infection. Immunity 2017, 47, 93–106.e7. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, C.M.; Lodhi, I.J. Peroxisomal Dysfunction in Age-Related Diseases. Trends Endocrinol. Metab. 2017, 28, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Ivashchenko, O.; Van Veldhoven, P.P.; Brees, C.; Ho, Y.-S.; Terlecky, S.R.; Fransen, M. Intraperoxisomal redox balance in mammalian cells: Oxidative stress and interorganellar cross-talk. Mol. Biol. Cell 2011, 22, 1440–1451. [Google Scholar] [CrossRef]
- Jo, D.S.; Cho, D.-H. Peroxisomal dysfunction in neurodegenerative diseases. Arch. Pharmacal Res. 2019, 42, 393–406. [Google Scholar] [CrossRef]
- Beckers, L.; Geric, I.; Stroobants, S.; Beel, S.; Van Damme, P.; D’Hooge, R.; Baes, M. Microglia lacking a peroxisomal β-oxidation enzyme chronically alter their inflammatory profile without evoking neuronal and behavioral deficits. J. Neuroinflamm. 2019, 16, 61. [Google Scholar] [CrossRef]
- Youdim, K.A.; Martin, A.; Joseph, J.A. Essential fatty acids and the brain: Possible health implications. Int. J. Dev. Neurosci. 2000, 18, 383–399. [Google Scholar] [CrossRef]
- Wang, H.; Kulas, J.A.; Holtzman, D.M.; Ferris, H.A.; Hansen, S.B. Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc. Natl. Acad. Sci. USA 2021, 118, e2102191118. [Google Scholar] [CrossRef]
- Zarrouk, A.; Hammouda, S.; Ghzaiel, I.; Hammami, S.; Khamlaoui, W.; Ahmed, S.H.; Lizard, G.; Hammami, M. Association Between Oxidative Stress and Altered Cholesterol Metabolism in Alzheimer’s Disease Patients. Curr. Alzheimer Res. 2021, 17, 823–834. [Google Scholar] [CrossRef]
- Testa, G.; Staurenghi, E.; Zerbinati, C.; Gargiulo, S.; Iuliano, L.; Giaccone, G.; Fantò, F.; Poli, G.; Leonarduzzi, G.; Gamba, P. Changes in brain oxysterols at different stages of Alzheimer’s disease: Their involvement in neuroinflammation. Redox Biol. 2016, 10, 24–33. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, J.-H.; Woo, J.H.; Jou, I. 25-Hydroxycholesterol suppress IFN-γ-induced inflammation in microglia by disrupting lipid raft formation and caveolin-mediated signaling endosomes. Free Radic. Biol. Med. 2021, 179, 252–265. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Nedergaard, M. The Glymphatic System: A Novel Component of Fundamental Neurobiology. J. Neurosci. 2021, 41, 7698–7711. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef]
- Tamura, R.; Yoshida, K.; Toda, M. Current understanding of lymphatic vessels in the central nervous system. Neurosurg. Rev. 2019, 43, 1055–1064. [Google Scholar] [CrossRef]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 2015, 11, 457–470, Erratum in Nat. Rev. Neurol. 2016, 12, 248. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Boland, B.; Yu, W.H.; Corti, O.; Mollereau, B.; Henriques, A.; Bezard, E.; Pastores, G.M.; Rubinsztein, D.C.; Nixon, R.A.; Duchen, M.; et al. Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2018, 17, 660–688. [Google Scholar] [CrossRef]
- Mader, S.; Brimberg, L. Aquaporin-4 Water Channel in the Brain and Its Implication for Health and Disease. Cells 2019, 8, 90. [Google Scholar] [CrossRef]
- Wang, J.; Gu, B.J.; Masters, C.L.; Wang, Y.J. A systemic view of Alzheimer disease—Insights from amyloid-beta metabolism beyond the brain. Nat. Rev. Neurol. 2017, 13, 612–623. [Google Scholar] [CrossRef]
- Mogensen, F.; Delle, C.; Nedergaard, M. The Glymphatic System (En)during Inflammation. Int. J. Mol. Sci. 2021, 22, 7491. [Google Scholar] [CrossRef]
- Troili, F.; Cipollini, V.; Moci, M.; Morena, E.; Palotai, M.; Rinaldi, V.; Romano, C.; Ristori, G.; Giubilei, F.; Salvetti, M.; et al. Perivascular Unit: This Must Be the Place. The Anatomical Crossroad Between the Immune, Vascular and Nervous System. Front. Neuroanat. 2020, 14, 17. [Google Scholar] [CrossRef]
- Zeppenfeld, D.M.; Simon, M.; Haswell, J.D.; D’Abreo, D.; Murchison, C.; Quinn, J.F.; Grafe, M.R.; Woltjer, R.L.; Kaye, J.; Iliff, J.J. Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol. 2017, 74, 91–99. [Google Scholar] [CrossRef]
- Cunningham, C.; Skelly, D.T. Non-steroidal anti-inflammatory drugs and cognitive function: Are prostaglandins at the heart of cognitive impairment in dementia and delirium? J. Neuroimmune Pharmacol. 2012, 7, 60–73. [Google Scholar] [CrossRef]
- Kazberuk, A.; Zareba, I.; Palka, J.; Surazynski, A. A novel plausible mechanism of NSAIDs-induced apoptosis in cancer cells: The implication of proline oxidase and peroxisome proliferator-activated receptor. Pharmacol. Rep. 2020, 72, 1152–1160. [Google Scholar] [CrossRef]
- Calvo-Rodríguez, M.; Núñez, L.; Villalobos, C. Non-steroidal anti-inflammatory drugs (NSAIDs) and neuroprotection in the elderly: A view from the mitochondria. Neural Regen. Res. 2015, 10, 1371–1372. [Google Scholar] [CrossRef]
- Grodstein, F.; Skarupski, K.A.; Bienias, J.L.; Wilson, R.S.; Bennett, D.A.; Evans, D.A. Anti-Inflammatory Agents and Cognitive Decline in a Bi-Racial Population. Neuroepidemiology 2008, 30, 45–50. [Google Scholar] [CrossRef]
- Custodero, C.; Mankowski, R.T.; Lee, S.A.; Chen, Z.; Wu, S.; Manini, T.M.; Echeverri, J.H.; Sabbà, C.; Beavers, D.P.; Cauley, J.A.; et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2018, 46, 42–59. [Google Scholar] [CrossRef]
- Holmes, C. The Role of Adaptive and Innate Immunity in Alzheimer’s Disease. In Textbook of Immunopsychiatry; Bullmore, E., Khandaker, G., Harrison, N., Dantzer, R., Eds.; Cambridge University Press: Cambridge, UK, 2021; pp. 213–232. [Google Scholar]
- Liu, X.; Ma, Y.; Ouyang, R.; Zeng, Z.; Zhan, Z.; Lu, H.; Cui, Y.; Dai, Z.; Luo, L.; He, C.; et al. The relationship between inflammation and neurocognitive dysfunction in obstructive sleep apnea syndrome. J. Neuroinflamm. 2020, 17, 229. [Google Scholar] [CrossRef]
- Imbimbo, B.P.; Solfrizzi, V.; Panza, F. Are NSAIDs useful to treat Alzheimer’s disease or mild cognitive impairment? Front. Aging Neurosci. 2010, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Sastre, M.; Gentleman, S.M. NSAIDs: How they work and their prospects as therapeutics in Alzheimer’s disease. Front. Aging Neurosci. 2010, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Jordan, F.; Quinn, T.J.; McGuinness, B.; Passmore, P.; Kelly, J.P.; Smith, C.T.; Murphy, K.; Devane, D. Aspirin and other non-steroidal anti-inflammatory drugs for the prevention of dementia. Cochrane Database Syst. Rev. 2020, 4, CD011459. [Google Scholar] [CrossRef] [PubMed]
- Jaturapatporn, D.; Isaac, M.G.; McCleery, J.; Tabet, N. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. Cochrane Database Syst. Rev. 2012, 2, CD006378. [Google Scholar] [CrossRef]
- Szczechowiak, K.; Diniz, B.S.; Leszek, J. Diet and Alzheimer’s dementia—Nutritional approach to modulate inflammation. Pharmacol. Biochem. Behav. 2019, 184, 172743. [Google Scholar] [CrossRef]
- Travica, N.; D’Cunha, N.M.; Naumovski, N.; Kent, K.; Mellor, D.; Firth, J.; Georgousopoulou, E.N.; Dean, O.M.; Loughman, A.; Jacka, F.; et al. The effect of blueberry interventions on cognitive performance and mood: A systematic review of randomized controlled trials. Brain Behav. Immun. 2019, 85, 96–105. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S. The gut microbiota in anxiety and depression—A systematic review. Clin. Psychol. Rev. 2020, 83, 101943. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Béguet-Crespel, F.; Marinelli, L.; Jamet, A.; LeDue, F.; Blottière, H.M.; Lapaque, N. Butyrate produced by gut commensal bacteria activates TGF-beta1 expression through the transcription factor SP1 in human intestinal epithelial cells. Sci. Rep. 2018, 8, 9742. [Google Scholar] [CrossRef]
- Telle-Hansen, V.H.; Holven, K.B.; Ulven, S.M. Impact of a Healthy Dietary Pattern on Gut Microbiota and Systemic Inflammation in Humans. Nutrients 2018, 10, 1783. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Mishra, S.P.; Craft, S.; Yadav, H. Gut mycobiome and its interaction with diet, gut bacteria and alzheimer’s disease markers in subjects with mild cognitive impairment: A pilot study. EBioMedicine 2020, 59, 102950. [Google Scholar] [CrossRef]
- Ju, Y.-E.; Lucey, B.; Holtzman, D.M. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat. Rev. Neurol. 2013, 10, 115–119. [Google Scholar] [CrossRef]
- Lowe, C.J.; Safati, A.; Hall, P.A. The neurocognitive consequences of sleep restriction: A meta-analytic review. Neurosci. Biobehav. Rev. 2017, 80, 586–604. [Google Scholar] [CrossRef]
- Song, D.; Zhou, J.; Ma, J.; Chang, J.; Qiu, Y.; Zhuang, Z.; Xiao, H.; Zeng, L. Sleep disturbance mediates the relationship between depressive symptoms and cognitive function in older adults with mild cognitive impairment. Geriatr. Nurs. 2021, 42, 1019–1023. [Google Scholar] [CrossRef]
- Atienza, M.; Ziontz, J.; Cantero, J.L. Low-grade inflammation in the relationship between sleep disruption, dysfunctional adiposity, and cognitive decline in aging. Sleep Med. Rev. 2018, 42, 171–183. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Wang, Z.; Zhang, X.; Deng, C.; Ma, B.; Yang, J.; Lu, Q.; Zhao, Y. Sleep Duration and Frailty Risk among Older Adults: Evidence from a Retrospective, Population-Based Cohort Study. J. Nutr. Heal. Aging 2022, 26, 383–390. [Google Scholar] [CrossRef]
- Siow, T.Y.; Toh, C.H.; Hsu, J.-L.; Liu, G.-H.; Lee, S.-H.; Chen, N.-H.; Fu, C.J.; Castillo, M.; Fang, J.-T. Association of Sleep, Neuropsychological Performance, and Gray Matter Volume with Glymphatic Function in Community-Dwelling Older Adults. Neurology 2021, 98, e829–e838. [Google Scholar] [CrossRef]
- Christensen, J.; Yamakawa, G.R.; Shultz, S.R.; Mychasiuk, R. Is the glymphatic system the missing link between sleep impairments and neurological disorders? Examining the implications and uncertainties. Prog. Neurobiol. 2020, 198, 101917. [Google Scholar] [CrossRef]
- Benveniste, H.; Heerdt, P.M.; Fontes, M.; Rothman, D.L.; Volkow, N.D. Glymphatic System Function in Relation to Anesthesia and Sleep States. Anesthesia Analg. 2019, 128, 747–758. [Google Scholar] [CrossRef]
- Nedergaard, M.; Goldman, S.A. Glymphatic failure as a final common pathway to dementia. Science 2020, 370, 50–56. [Google Scholar] [CrossRef]
- Fultz, N.E.; Bonmassar, G.; Setsompop, K.; Stickgold, R.A.; Rosen, B.R.; Polimeni, J.R.; Lewis, L.D. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 2019, 366, 628–631. [Google Scholar] [CrossRef]
- Małkiewicz, M.A.; Szarmach, A.; Sabisz, A.; Cubała, W.J.; Szurowska, E.; Winklewski, P.J. Blood-brain barrier permeability and physical exercise. J. Neuroinflamm. 2019, 16, 15. [Google Scholar] [CrossRef]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Speisman, R.B.; Kumar, A.; Rani, A.; Foster, T.C.; Ormerod, B.K. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain Behav. Immun. 2012, 28, 25–43. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Tucker, D.; Wang, R.; Ahmed, M.E.; Brann, D.; Zhang, Q. Treadmill Exercise Exerts Neuroprotection and Regulates Microglial Polarization and Oxidative Stress in a Streptozotocin-Induced Rat Model of Sporadic Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 56, 1469–1484. [Google Scholar] [CrossRef]
- Lee, T.M.; Wong, M.L.; Lau, B.W.-M.; Lee, J.C.-D.; Yau, S.-Y.; So, K.-F. Aerobic exercise interacts with neurotrophic factors to predict cognitive functioning in adolescents. Psychoneuroendocrinology 2014, 39, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Prata, L.G.L.; Ovsyannikova, I.G.; Tchkonia, T.; Kirkland, J.L. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin. Immunol. 2018, 40, 101275. [Google Scholar] [CrossRef] [PubMed]
- Coleman, L.G., Jr.; Zou, J.; Crews, F.T. Microglial depletion and repopulation in brain slice culture normalizes sensitized proinflammatory signaling. J. Neuroinflamm. 2020, 17, 1–20. [Google Scholar] [CrossRef]
- Stojiljkovic, M.R.; Schmeer, C.; Witte, O.W. Pharmacological Depletion of Microglia Leads to a Dose-Dependent Reduction in Inflammation and Senescence in the Aged Murine Brain. Neuroscience 2022, 488, 1–9. [Google Scholar] [CrossRef]
- Jin, W.-N.; Shi, K.; He, W.; Sun, J.-H.; Van Kaer, L.; Shi, F.-D.; Liu, Q. Neuroblast senescence in the aged brain augments natural killer cell cytotoxicity leading to impaired neurogenesis and cognition. Nat. Neurosci. 2020, 24, 61–73. [Google Scholar] [CrossRef]
- Papismadov, N.; Krizhanovsky, V. Natural killers of cognition. Nat. Neurosci. 2020, 24, 2–4. [Google Scholar] [CrossRef]
- Nong, J.; Glassman, P.M.; Muzykantov, V.R. Targeting vascular inflammation through emerging methods and drug carriers. Adv. Drug Deliv. Rev. 2022, 184, 114180. [Google Scholar] [CrossRef]
- Luster, A.D.; Alon, R.; Von Andrian, U.H. Immune cell migration in inflammation: Present and future therapeutic targets. Nat. Immunol. 2005, 6, 1182–1190. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Myers, G.L.; Rifai, N.; Tracy, R.P.; Roberts, W.L.; Alexander, R.W.; Biasucci, L.M.; Catravas, J.D.; Cole, T.G.; Cooper, G.R.; Khan, B.V.; et al. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: Report from the laboratory science discussion group. Circulation 2004, 110, e545–e549. [Google Scholar] [CrossRef]
- Lassale, C.; Batty, G.D.; Steptoe, A.; Cadar, D.; Akbaraly, T.N.; Kivimäki, M.; Zaninotto, P. Association of 10-Year C-Reactive Protein Trajectories with Markers of Healthy Aging: Findings from the English Longitudinal Study of Aging. J. Gerontol. Ser. A 2018, 74, 195–203. [Google Scholar] [CrossRef]
- Trollor, J.N.; Smith, E.; Baune, B.T.; Kochan, N.A.; Campbell, L.; Samaras, K.; Crawford, J.; Brodaty, H.; Sachdev, P. Systemic Inflammation Is Associated with MCI and Its Subtypes: The Sydney Memory and Aging Study. Dement. Geriatr. Cogn. Disord. 2010, 30, 569–578. [Google Scholar] [CrossRef]
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R.C. IL-1beta, IL-6, TNF- alpha and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 12050. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Jansson, D.; Smyth, L.C.; Dragunow, M. Brain Pericytes as Mediators of Neuroinflammation. Trends Pharmacol. Sci. 2017, 38, 291–304. [Google Scholar] [CrossRef]
- Haruwaka, K.; Ikegami, A.; Tachibana, Y.; Ohno, N.; Konishi, H.; Hashimoto, A.; Matsumoto, M.; Kato, D.; Ono, R.; Kiyama, H.; et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 2019, 10, 5816. [Google Scholar] [CrossRef]
- Pan, J.; Ma, N.; Zhong, J.; Yu, B.; Wan, J.; Zhang, W. Age-associated changes in microglia and astrocytes ameliorate blood-brain barrier dysfunction. Mol. Ther.-Nucleic Acids 2021, 26, 970–986. [Google Scholar] [CrossRef]
- Sumi, N.; Nishioku, T.; Takata, F.; Matsumoto, J.; Watanabe, T.; Shuto, H.; Yamauchi, A.; Dohgu, S.; Kataoka, Y. Lipopolysaccharide-Activated Microglia Induce Dysfunction of the Blood-Brain Barrier in Rat Microvascular Endothelial Cells Co-Cultured with Microglia. Cell. Mol. Neurobiol. 2010, 30, 247–253. [Google Scholar] [CrossRef]
- Chi, G.C.; Fitzpatrick, A.L.; Sharma, M.; Jenny, N.S.; Lopez, O.L.; DeKosky, S. Inflammatory Biomarkers Predict Domain-Specific Cognitive Decline in Older Adults. J. Gerontol. Ser. A 2016, 72, 796–803. [Google Scholar] [CrossRef]
- Sharma, M.; Fitzpatrick, A.L.; Arnold, A.M.; Chi, G.; Lopez, O.L.; Jenny, N.S.; DeKosky, S.T. Inflammatory Biomarkers and Cognitive Decline: The Ginkgo Evaluation of Memory Study. J. Am. Geriatr. Soc. 2016, 64, 1171–1177. [Google Scholar] [CrossRef]
- Trollor, J.N.; Smith, E.; Agars, E.; Kuan, S.A.; Baune, B.T.; Campbell, L.; Samaras, K.; Crawford, J.; Lux, O.; Kochan, N.A.; et al. The association between systemic inflammation and cognitive performance in the elderly: The Sydney Memory and Ageing Study. AGE 2011, 34, 1295–1308. [Google Scholar] [CrossRef]
- Walker, K.A.; Windham, B.G.; Power, M.C.; Hoogeveen, R.; Folsom, A.R.; Ballantyne, C.M.; Knopman, D.S.; Selvin, E.; Jack, C.R.; Gottesman, R. The association of mid-to late-life systemic inflammation with white matter structure in older adults: The Atherosclerosis Risk in Communities Study. Neurobiol. Aging 2018, 68, 26–33. [Google Scholar] [CrossRef]
- Marsland, A.L.; Gianaros, P.J.; Kuan, D.C.-H.; Sheu, L.K.; Krajina, K.; Manuck, S.B. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav. Immun. 2015, 48, 195–204. [Google Scholar] [CrossRef]
- Dev, S.I.; Moore, R.C.; Soontornniyomkij, B.; Achim, C.L.; Jeste, D.V.; Eyler, L.T. Peripheral inflammation related to lower fMRI activation during a working memory task and resting functional connectivity among older adults: A preliminary study. Int. J. Geriatr. Psychiatry 2016, 32, 341–349. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, R.; Chan, A.K.Y.; Wu, J.; Lee, T.M.C. Relationships between Inflammation and Age-Related Neurocognitive Changes. Int. J. Mol. Sci. 2022, 23, 12573. https://doi.org/10.3390/ijms232012573
Jin R, Chan AKY, Wu J, Lee TMC. Relationships between Inflammation and Age-Related Neurocognitive Changes. International Journal of Molecular Sciences. 2022; 23(20):12573. https://doi.org/10.3390/ijms232012573
Chicago/Turabian StyleJin, Run, Aidan Kai Yeung Chan, Jingsong Wu, and Tatia Mei Chun Lee. 2022. "Relationships between Inflammation and Age-Related Neurocognitive Changes" International Journal of Molecular Sciences 23, no. 20: 12573. https://doi.org/10.3390/ijms232012573
APA StyleJin, R., Chan, A. K. Y., Wu, J., & Lee, T. M. C. (2022). Relationships between Inflammation and Age-Related Neurocognitive Changes. International Journal of Molecular Sciences, 23(20), 12573. https://doi.org/10.3390/ijms232012573




