Genomic Basis for Individual Differences in Susceptibility to the Neurotoxic Effects of Diesel Exhaust
Abstract
1. Introduction
1.1. Air Pollution Produces Multi-Organ System Disease
1.2. Particulate Components of Air Pollution and Diesel Exhaust Are Particularly Troublesome
1.3. Diesel Exhaust Exposure Carries Risk for Neurological Disease
1.4. Neurological Effects of Exposure to Diesel Exhaust: Not Everyone Is Equally Susceptible
2. Results
2.1. Proinflammatory Cytokine Gene Expression
2.2. Whole-Genome Gene Expression by RNA-seq
3. Discussion
3.1. Support for the Hypothesis That Individual Differences in Susceptibility to the Adverse Health Effects of Exposure to Diesel Exhaust Are Related to Genetics
3.2. RNA-seq Identifies Genes and Pathways That May Confer Differential Susceptibility to the Neurotoxic Effects of Diesel Exhaust
3.3. Overall Impact of This Study
3.4. Why Mice?
3.5. Summary
4. Materials and Methods
4.1. Animals
4.2. Diesel Exhaust Exposures
4.3. Brain Tissue Harvest
4.4. RNA Isolation, cDNA Synthesis, and rtPCR
4.5. Data Analysis
4.6. RNA-seq Analysis for Genome-Wide Transcript Abundance
4.7. Library Preparation and Sequencing
4.8. Data Quality Control and Filtering
4.9. Alignment to the Reference Genome
4.10. Quantification of Gene Expression Level
4.11. Differential Expression Analysis
4.12. Gene Set Enrichment Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Ee, N.; Peters, J.; Booth, A.; Mudway, I.; Anstey, K.J. Air Pollution and Dementia: A Systematic Review. J. Alzheimer’s Dis. JAD 2019, 70, S145–S163. [Google Scholar] [CrossRef]
- Arias-Pérez, R.D.; Taborda, N.A.; Gómez, D.M.; Narvaez, J.F.; Porras, J.; Hernandez, J.C. Inflammatory effects of particulate matter air pollution. Environ. Sci. Pollut. Res. Int. 2020, 27, 42390–42404. [Google Scholar] [CrossRef] [PubMed]
- Giannadaki, D.; Lelieveld, J.; Pozzer, A. Implementing the US air quality standard for PM2.5 worldwide can prevent millions of premature deaths per year. Environ. Health 2016, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Sobus, J.R.; Pleil, J.D.; Madden, M.C. Controlled human exposures to diesel exhaust. Swiss Med. Wkly. 2012, 31, 142. [Google Scholar] [CrossRef]
- Valavanidis, A.; Fiotakis, K.; Vlahogianni, T.; Papadimitriou, V.; Pantikaki, V. Determination of selective quinones and quinoid radicals in airborne particulate matter and vehicular exhaust particles. Environ. Chem. 2006, 3, 118–123. [Google Scholar] [CrossRef]
- Clayton, P.T. Inherited disorders of transition metal metabolism: An update. J. Inherit. Metab. Dis. 2017, 40, 519–529. [Google Scholar] [CrossRef]
- Gehling, W.; Khachatryan, L.; Dellinger, B. Hydroxyl radical generation from environmentally persistent free radicals (EPFRs) in PM2.5. Environ. Sci. Technol. 2014, 48, 4266–4272. [Google Scholar] [CrossRef]
- Noël, A.; Charbonneau, M.; Cloutier, Y.; Tardif, R.; Truchon, G. Rat pulmonary responses to inhaled nano-TiO2: Effect of primary particle size and agglomeration state. Part. Fibre Toxicol. 2013, 10, 48. [Google Scholar] [CrossRef]
- United States, Environmental Protection Agency (US EPA). Available online: https://www.epa.gov/pm-pollution/national-ambient-air-quality-standards-naaqs-pm (accessed on 10 January 2022).
- American Lung Association, State of the Air Report (2021). Available online: https://www.lung.org/getmedia/17c6cb6c-8a38-42a7-a3b0-6744011da370/sota-2021.pdf (accessed on 10 January 2022).
- Corfa, E.; Maury, F.; Segers, P.; Fresneau, A.; Albergel, A. Short-range evaluation of air pollution near bus and railway stations. Sci. Total Environ. 2004, 334, 223–230. [Google Scholar] [CrossRef]
- Buzzard, N.A.; Clark, N.N.; Guffey, S.E. Investigation into pedestrian exposure to near-vehicle exhaust emissions. Environ. Health 2009, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Our World in Data.org. 2017. Available online: https://ourworldindata.org/data-review-air-pollution-deaths (accessed on 10 January 2022).
- Pope, C.A., III. Respiratory disease associated with community air pollution and a steel mill, Utah Valley. Am. J. Public Health 1989, 79, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., III. What do epidemiologic findings tell us about the health effects of environmental aerosols? J. Aerosol. Med. 2000, 13, 335–354. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., III. Epidemiology of fine particulate air pollution and human health: Biologic mechanisms and who’s at risk? Environ. Health Perspect. 2000, 108 (Suppl. 4), 713–723. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Anderson, H.R.; Sunyer, J.; Ayres, J.; Baccini, M.; Vonk, J.M.; Boumghar, A.; Forastiere, F.; Forsberg, B.; Touloumi, G.; et al. Acute effects of particulate air pollution on respiratory admissions: Results from APHEA 2 project. Air Pollution and Health: A European Approach. Am. J. Respir. Crit. Care Med. 2001, 164, 1860–1866. [Google Scholar] [CrossRef]
- Kim, J.J.; Smorodinsky, S.; Lipsett, M.; Singer, B.C.; Hodgson, A.T.; Ostro, B. Traffic-related air pollution near busy roads: The East Bay Children’s Respiratory Health Study. Am. J. Respir. Crit. Care Med. 2004, 170, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J. Air pollution and children’s health. Pediatrics 2004, 113, 1037–1043. [Google Scholar] [CrossRef]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Nel, A. The role of reactive oxygen species and oxidative stress in mediating particulate matter injury. Clin. Occup. Environ. Med. 2006, 5, 817–836. [Google Scholar] [PubMed]
- Wegesser, T.C.; Franzi, L.M.; Mitloehner, F.M.; Eiguren-Fernandez, A.; Last, J.A. Lung antioxidant and cytokine responses to coarse and fine particulate matter from the great California wildfires of 2008. Inhal. Toxicol. 2010, 22, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Seaton, A.; Macnee, W.; Donaldson, K.; Godden, D. Particulate air pollution and acute health effects. Lancet 1995, 345, 176–178. [Google Scholar] [CrossRef]
- Noël, A.; Xiao, R.; Perveen, Z.; Zaman, H.M.; Rouse, R.L.; Paulsen, D.B.; Penn, A.L. Incomplete lung recovery following sub-acute inhalation of combustion-derived ultrafine particles in mice. Part. Fibre Toxicol. 2016, 13, 10. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoet, P.H.; Dinsdale, D.; Vermylen, J.; Hoylaerts, M.F.; Nemery, B. Diesel exhaust particles in lung acutely enhance experimental peripheral thrombosis. Circulation 2003, 107, 1202–1208. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoet, P.H.; Vandervoort, P.; Dinsdale, D.; Nemery, B.; Hoylaerts, M.F. Enhanced peripheral thrombogenicity after lung inflammation is mediated by platelet-leukocyte activation: Role of P-selectin. J. Thromb. Haemost. 2007, 5, 1217–1226. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoet, P.H.; Vanquickenborne, B.; Dinsdale, D.; Thomeer, M.; Hoylaerts, M.F.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of inhaled particles into the blood circulation in humans. Circulation 2002, 105, 411–414. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoylaerts, M.F.; Hoet, P.H.; Nemery, B. Possible mechanisms of the cardiovascular effects of inhaled particles: Systemic translocation and prothrombotic effects. Toxicol. Lett. 2004, 149, 243–253. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoylaerts, M.F.; Hoet, P.H.; Dinsdale, D.; Smith, T.; Xu, H.; Vermylen, J.; Nemery, B. Ultrafine particles affect experimental thrombosis in an in vivo hamster model. Am. J. Respir. Crit. Care Med. 2002, 166, 998–1004. [Google Scholar] [CrossRef]
- Sun, Q.; Hong, X.; Wold, L.E. Cardiovascular effects of ambient particulate air pollution exposure. Circulation 2010, 121, 2755–2765. [Google Scholar] [CrossRef]
- Franchini, M.; Mannucci, P.M. Thrombogenicity and cardiovascular effects of ambient air pollution. Blood 2011, 118, 2405–2412. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Lunts, A.; Kreyling, W.; Cox, C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J. Toxicol. Environ. Health A 2002, 65, 1531–1543. [Google Scholar] [CrossRef]
- Takenaka, S.; Karg, E.; Roth, C.; Schulz, H.; Ziesenis, A.; Heinzmann, U.; Schramel, P.; Heyder, J. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ. Health Perspect. 2001, 109 (Suppl. 4), 547–551. [Google Scholar]
- Kreyling, W.G.; Semmler-Behnke, M.; Seitz, J.; Scymczak, W.; Wenk, A.; Mayer, P.; Takenaka, S.; Oberdorster, G. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal. Toxicol. 2009, 21 (Suppl. 1), 55–60. [Google Scholar] [CrossRef]
- Cole, T.B.; Coburn, J.; Dao, K.; Roqué, P.; Chang, Y.C.; Kalia, V.; Guilarte, T.R.; Dziedzic, J.; Costa, L.G. Sex and genetic differences in the effects of acute diesel exhaust exposure on inflammation and oxidative stress in mouse brain. Toxicology 2016, 374, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.C.; Coburn, J.; Garrick, J.M. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol. Ther. 2020, 210, 107523. [Google Scholar] [CrossRef] [PubMed]
- Ajmani, G.S.; Suh, H.H.; Pinto, J.M. Effects of Ambient Air Pollution Exposure on Olfaction: A Review. Environ. Health Perspect. 2016, 11, 1683–1693. [Google Scholar] [CrossRef]
- Maher, B.A.; Ahmed, I.A.; Karloukovski, V.; MacLaren, D.A.; Foulds, P.G.; Allsop, D.; Mann, D.M.; Torres-Jardón, R.; Calderon-Garciduenas, L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA 2016, 113, 10797–10801. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Zhang, X. The impact of exposure to air pollution on cognitive performance. Proc. Natl. Acad. Sci. USA 2018, 115, 9193–9197. [Google Scholar] [CrossRef]
- Gatto, N.M.; Henderson, V.W.; Hodis, H.N.; St John, J.A.; Lurmann, F.; Chen, J.C.; Mack, W.J. Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology 2014, 40, 1–7. [Google Scholar] [CrossRef]
- Salinas-Rodríguez, A.; Fernández-Niño, J.A.; Manrique-Espinoza, B.; Moreno-Banda, G.L.; Sosa-Ortiz, A.L.; Qian, Z.M.; Lin, H. Exposure to ambient PM2.5 concentrations and cognitive function among older Mexican adults. Environ. Int. 2018, 117, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hüls, A.; Vierkötter, A.; Sugiri, D.; Abramson, M.J.; Ranft, U.; Krämer, U.; Schikowski, T. The role of air pollution and lung function in cognitive impairment. Eur. Respir. J. 2018, 51, 1701963. [Google Scholar] [CrossRef]
- Schikowski, T.; Vossoughi, M.; Vierkötter, A.; Schulte, T.; Teichert, T.; Sugiri, D.; Fehsel, K.; Tzivian, L.; Bae, I.S.; Ranft, U.; et al. Association of air pollution with cognitive functions and its modification by APOE gene variants in elderly women. Environ. Res. 2015, 142, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Coburn, J.L.; Cole, T.B.; Dao, K.T.; Costa, L.G. Acute exposure to diesel exhaust impairs adult neurogenesis in mice: Prominence in males and protective effect of pioglitazone. Arch. Toxicol. 2018, 92, 1815–1829. [Google Scholar] [CrossRef]
- Hüls, A.; Krämer, U.; Herder, C.; Fehsel, K.; Luckhaus, C.; Stolz, S.; Vierkötter, A.; Schikowski, T. Genetic susceptibility for air pollution-induced airway inflammation in the SALIA study. Environ. Res. 2017, 152, 43–50. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, J.P.; Miller, D.B. Neuroinflammation disorders exacerbated by environmental stressors. Metabolism 2019, 100S, 153951. [Google Scholar] [CrossRef]
- Genc, S.; Zadeoglulari, Z.; Fuss, S.H.; Genc, K. The adverse effects of air pollution on the nervous system. J. Toxicol. 2012, 2012, 782462. [Google Scholar] [CrossRef]
- Müller, L.; Chehrazi, C.V.; Henderson, M.W.; Noah, T.L.; Jaspers, I. Diesel exhaust particles modify natural killer cell function and cytokine release. Part. Fibre Toxicol. 2013, 10, 16. [Google Scholar] [CrossRef]
- Pawlak, E.A.; Noah, T.L.; Zhou, H.; Chehrazi, C.; Robinette, C.; Diaz-Sanchez, D.; Müller, L.; Jaspers, I. Diesel exposure suppresses natural killer cell function and resolution of eosinophil inflammation: A randomized controlled trial of exposure in allergic rhinitis. Part. Fibre Toxicol. 2016, 13, 24. [Google Scholar] [CrossRef]
- Park, E.J.; Park, K. Induction of pro-inflammatory signals by 1-nitropyrene in cultured BEAS-2B cells. Toxicol. Lett. 2009, 184, 126–133. [Google Scholar] [CrossRef]
- Cho, Y.; Lim, J.H.; Song, M.K.; Jeong, S.C.; Lee, K.; Heo, Y.; Kim, T.S.; Ryu, J.C. Toxicogenomic analysis of the pulmonary toxic effects of hexanal in F344 rat. Environ. Toxicol. 2017, 32, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.L.; He, S.W.; Zhang, Y.D.; Duan, H.X.; Huang, T.; Huang, Y.C.; Li, G.F.; Wang, P.; Ma, L.J.; Zhou, G.B.; et al. Air pollution and DNA methylation alterations in lung cancer: A systematic and comparative study. Oncotarget 2017, 8, 1369–1391. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Y.; Gao, N.; Sun, H.; Lu, R.; Yang, H.; Zhang, C.; Meng, Q.; Wu, S.; Li, A.Q.; et al. microRNA-802/Rnd3 pathway imposes on carcinogenesis and metastasis of fine particulate matter exposure. Oncotarget 2016, 7, 35026–35043. [Google Scholar] [CrossRef]
- Wang, H.; Quan, L.; Liang, J.; Shi, J.; Qiu, T.; Zhang, Y.; Wang, Y.; Hui, Q.; Zhang, Y.; Tao, K. Gene expression profiling analysis of keloids with and without hydrocortisone treatment. Exp. Ther. Med. 2017, 14, 5283–5288. [Google Scholar] [CrossRef]
- Fu, J.; Guo, O.; Zhen, Z.; Zhen, J. Essential Functions of the Transcription Factor Npas4 in Neural Circuit Development, Plasticity, and Diseases. Front. Neurosci. 2020, 14, 603373. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Nagai, H.; Lin, T.M.; Peterson, R.E.; Tohyama, C.; Kobayashi, T.; Nohara, K. Organic Chemicals Adsorbed onto Diesel Exhaust Particles Directly Alter the Differentiation of Fetal Thymocytes Through Aryl hydrocarbon Receptor but Not Oxidative Stress Responses. J. Immunotoxicol. 2006, 3, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef]
- Ermann, J.; Glimcher, L.H. After GWAS: Mice to the rescue? Curr. Opin. Immunol. 2012, 24, 564–570. [Google Scholar] [CrossRef]
- Jones, L.C.; Earley, C.J.; Allen, R.P.; Jones, B.C. Of mice and men, periodic limb movements and iron: How the human genome informs the mouse genome. Genes Brain Behav. 2008, 7, 513–514. [Google Scholar] [CrossRef]
- Ashbrook, D.G.; Arends, D.; Prins, P.; Mulligan, M.K.; Roy, S.; Williams, E.G.; Lutz, C.M.; Valenzuela, A.; Bohl, C.J.; Ingels, J.F.; et al. A platform for experimental precision medicine: The extended BXD mouse family. Cell Syst. 2021, 12, 235–247.e9. [Google Scholar] [CrossRef]
- Yanai, S.; Endo, S. Functional Aging in Male C57BL/6J Mice Across the Life-Span: A Systematic Behavioral Analysis of Motor, Emotional, and Memory Function to Define an Aging Phenotype. Front. Aging Neurosci. 2021, 13, 697621. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, J.P.; Kelly, K.A.; Locker, A.R.; Miller, D.B.; Lasley, S.M. Corticosterone primes the neuroinflammatory response to DFP in mice: Potential animal model of Gulf War Illness. J. Neurochem. 2015, 133, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Locker, A.R.; Michalovicz, L.T.; Kelly, K.A.; Miller, J.V.; Miller, D.B.; O’Callaghan, J.P. Corticosterone primes the neuroinflammatory response to Gulf War-relevant organophosphates independently of acetylcholinesterase inhibition. J. Neurochem. 2017, 142, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Pattwell, S.S.; Holland, E.C.; Bolouri, H. Variability in estimated gene expression among commonly used RNA-seq pipelines. Sci. Rep. 2020, 10, 2734. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. Feature Counts: An efficient general-purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Wang, J.; Vasaikar, S.; Shi, Z.; Greer, M.; Zhang, B. WebGestalt 2017: A more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 2017, 45, W130–W137. [Google Scholar] [CrossRef]
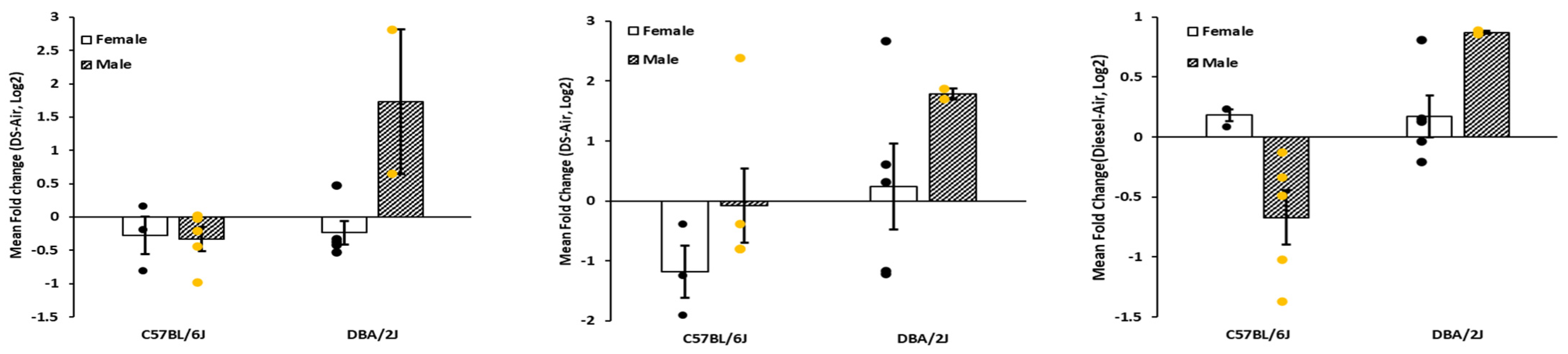
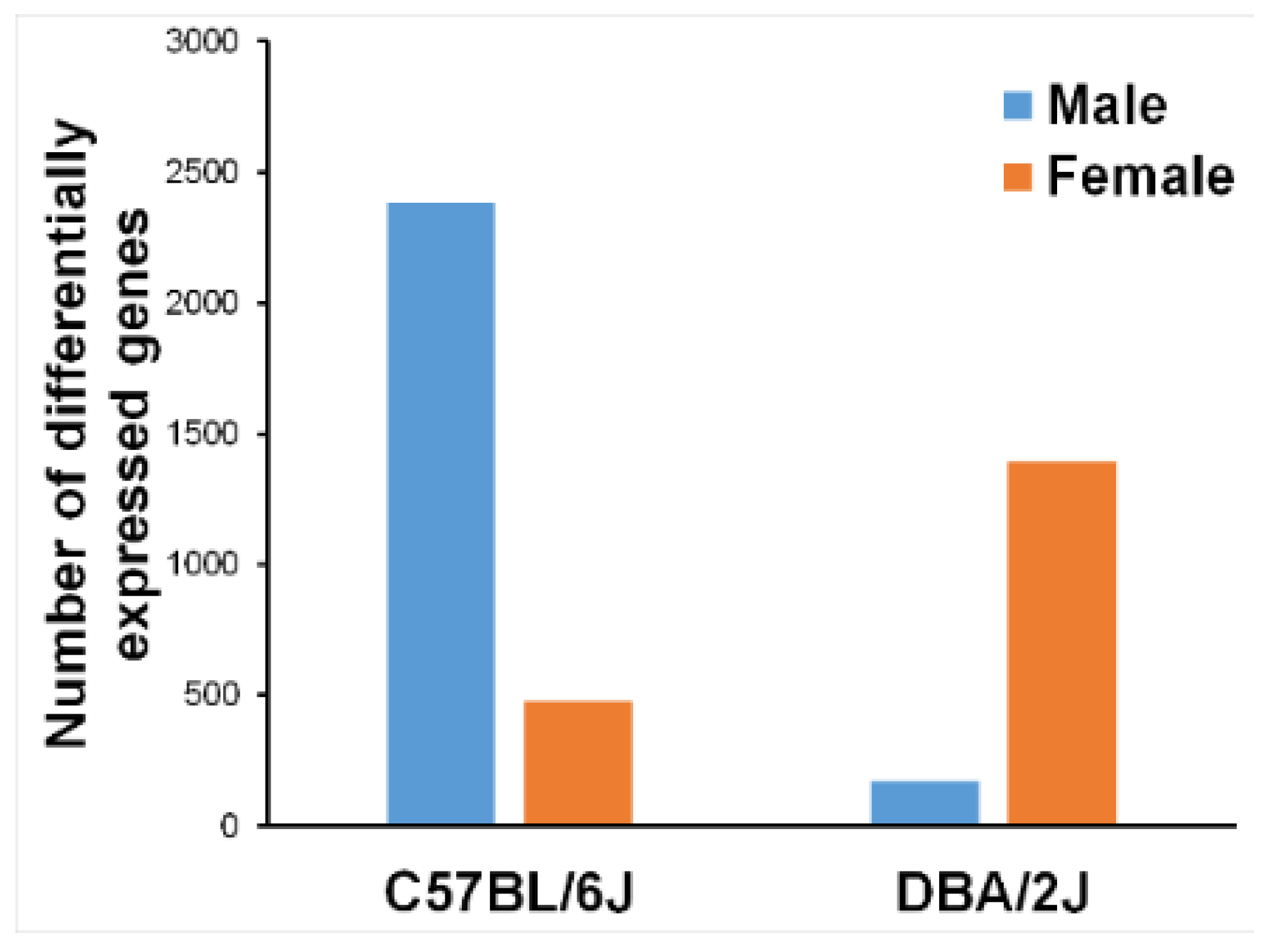

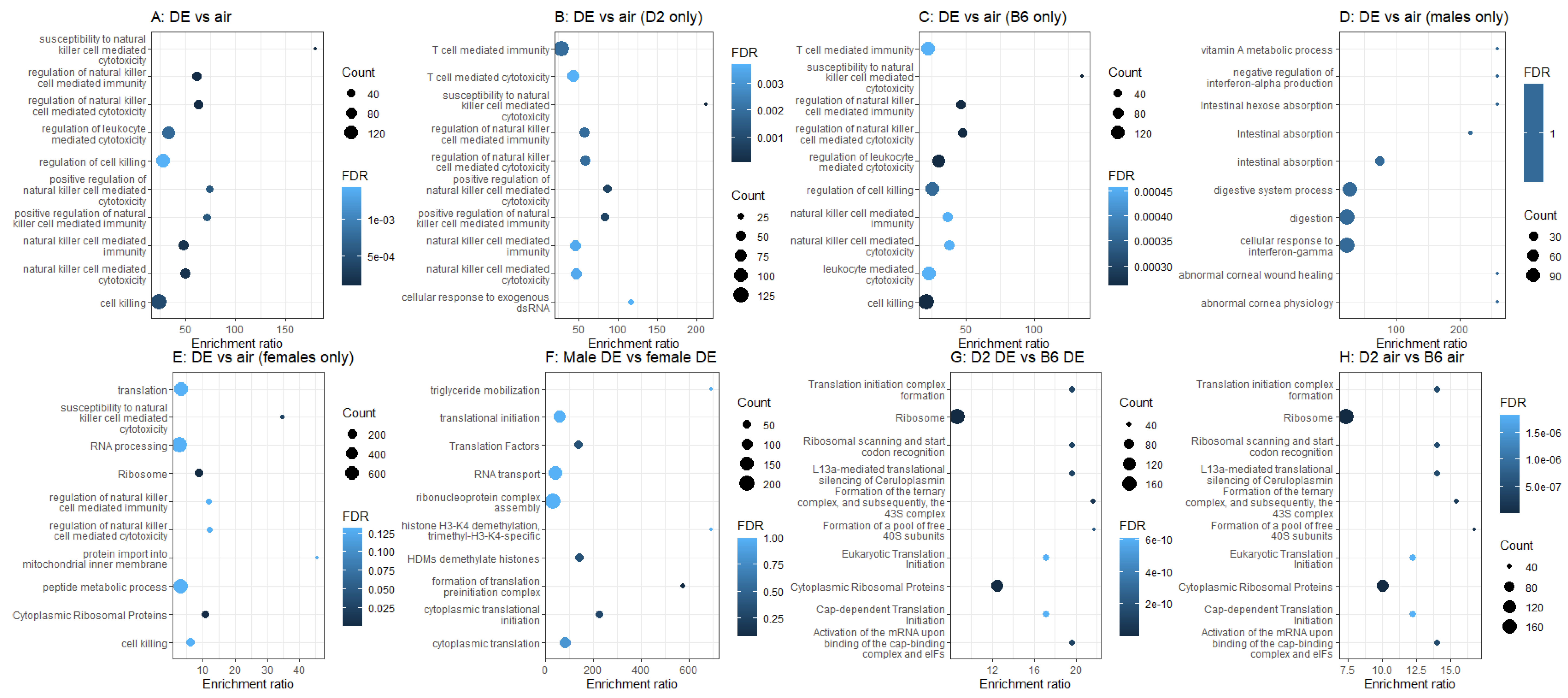
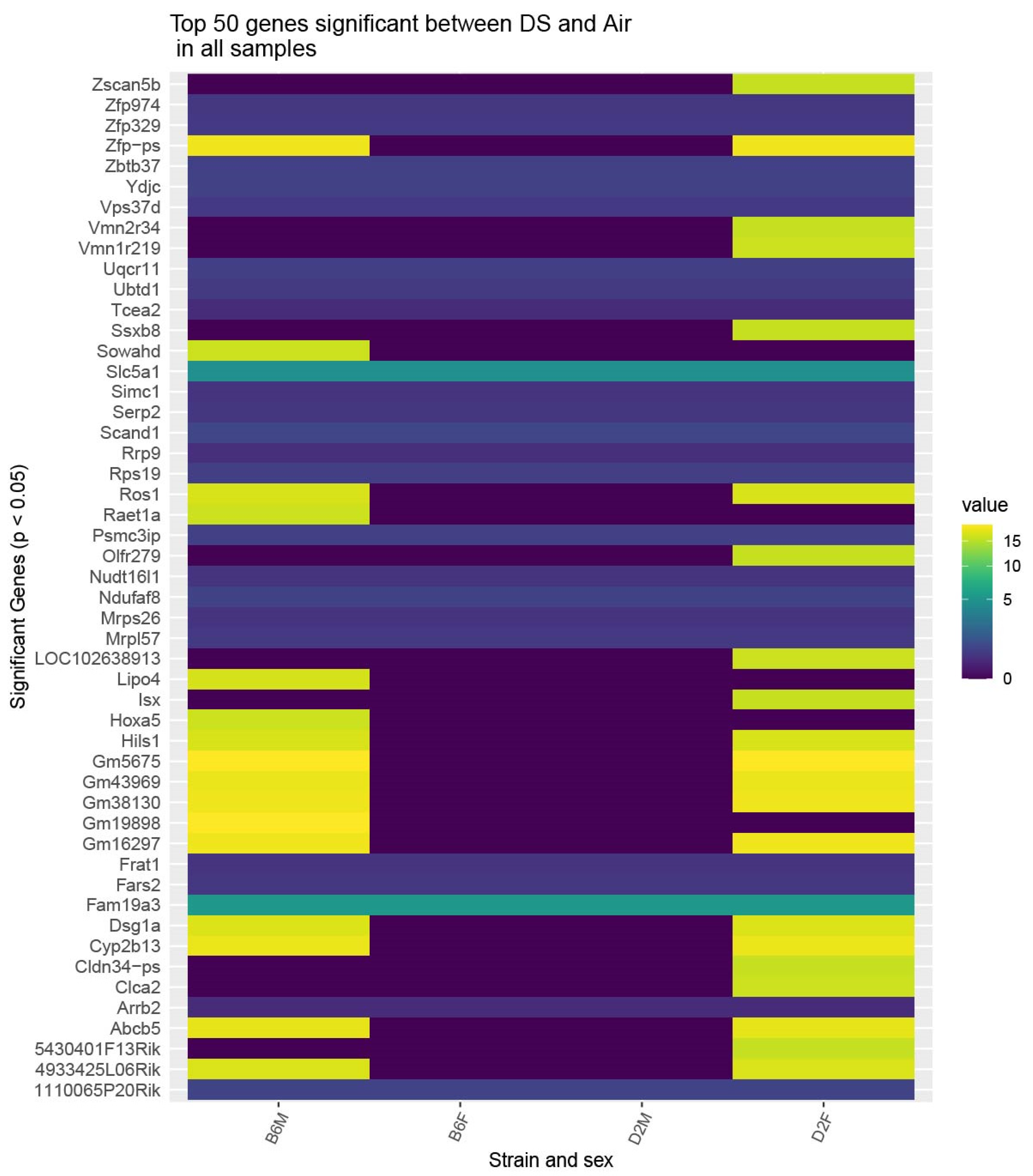

| Strain | Sex | Gene | Mean Fold Change | s.e.m. | n |
|---|---|---|---|---|---|
| C57 | F | Il1b | 0.001 | 5.22 × 10−5 | 3 |
| M | Il1b | 0.001 | 0.00001 | 5 | |
| D2 | F | Il1b | 0.002 | 0.00002 | 5 |
| M | Il1b | 0.001 | 4.97 × 10−5 | 2 | |
| B6 | F | Il6 | 3.76 × 10−5 | 1.34 × 10−5 | 3 |
| M | Il6 | 1.43 × 10−5 | 2.46 × 10−6 | 5 | |
| D2 | F | Il6 | 1.47 × 10−5 | 3.13 × 10−6 | 5 |
| M | Il6 | 2.61 × 10−6 | 1.09 × 10−6 | 2 | |
| B6 | F | Tnfa | 0.007 | 0.00004 | 3 |
| M | Tnfa | 0.006 | 0.0014 | 5 | |
| D2 | F | Tnfa | 0.008 | 0.00077 | 5 |
| M | Tnfa | 0.006 | 0.0006 | 2 |
| Filtered Air | Diesel Exhaust | |
|---|---|---|
| Temperature (°C ± SEM) | 22.5 ± 0.5 | 22.8 ± 1.0 |
| Humidity (%RH ± SEM) | 36.9 ± 3.7 | 26.4 ± 0.9 |
| CO (PPM ± SEM) | --- | 9.27 ± 1.21 |
| Average total particulate matter (TPM) concentration measured throughout the daily exposures (mg/m3 ± SEM) | --- | 0.95 ± 0.3 |
| Particle surface area (μm2/cm3 ± SEM) | --- | 8.99 ± 3.47 |
| Gene | Orientation | Labeled Name | Sequence |
|---|---|---|---|
| IL1beta | Downstream | mIL1bpro38Le | AGT TGA CGG ACC CCA AAA G |
| Upstream | mIL1bpro38Ri | AGC TGG ATG CTC TCA TCA GG | |
| IL6 | Downstream | mIL6pro55Le | CGC TAT GAA GTT CCT CTC TGC |
| Upstream | mIL6pro55Ri | TTG GGA GTG GTA TCC TCT GTG | |
| TNFa | Downstream | mTNFapro25le | CTG TAG CCC ACG TCG TAG C |
| Upstream | mTNFapro25ri | TTG AGA TCC ATG CCG TTG | |
| TBP | Downstream | mTBPp107le | TCT GGG TTA TCT TCA CAC ACC A |
| Upstream | mTBPp107Ri | GGG GAG CTG TGA TGT GAA GT |
| B6 | D2 | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Air | 3 | 5 | 5 | 2 |
| Diesel | 3 | 5 | 5 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noël, A.; Ashbrook, D.G.; Xu, F.; Cormier, S.A.; Lu, L.; O’Callaghan, J.P.; Menon, S.K.; Zhao, W.; Penn, A.L.; Jones, B.C. Genomic Basis for Individual Differences in Susceptibility to the Neurotoxic Effects of Diesel Exhaust. Int. J. Mol. Sci. 2022, 23, 12461. https://doi.org/10.3390/ijms232012461
Noël A, Ashbrook DG, Xu F, Cormier SA, Lu L, O’Callaghan JP, Menon SK, Zhao W, Penn AL, Jones BC. Genomic Basis for Individual Differences in Susceptibility to the Neurotoxic Effects of Diesel Exhaust. International Journal of Molecular Sciences. 2022; 23(20):12461. https://doi.org/10.3390/ijms232012461
Chicago/Turabian StyleNoël, Alexandra, David G. Ashbrook, Fuyi Xu, Stephania A. Cormier, Lu Lu, James P. O’Callaghan, Shyam K. Menon, Wenyuan Zhao, Arthur L. Penn, and Byron C. Jones. 2022. "Genomic Basis for Individual Differences in Susceptibility to the Neurotoxic Effects of Diesel Exhaust" International Journal of Molecular Sciences 23, no. 20: 12461. https://doi.org/10.3390/ijms232012461
APA StyleNoël, A., Ashbrook, D. G., Xu, F., Cormier, S. A., Lu, L., O’Callaghan, J. P., Menon, S. K., Zhao, W., Penn, A. L., & Jones, B. C. (2022). Genomic Basis for Individual Differences in Susceptibility to the Neurotoxic Effects of Diesel Exhaust. International Journal of Molecular Sciences, 23(20), 12461. https://doi.org/10.3390/ijms232012461






