Short Peptides of Innate Immunity Protein Tag7 Inhibit the Production of Cytokines in CFA-Induced Arthritis
Abstract
1. Introduction
2. Results
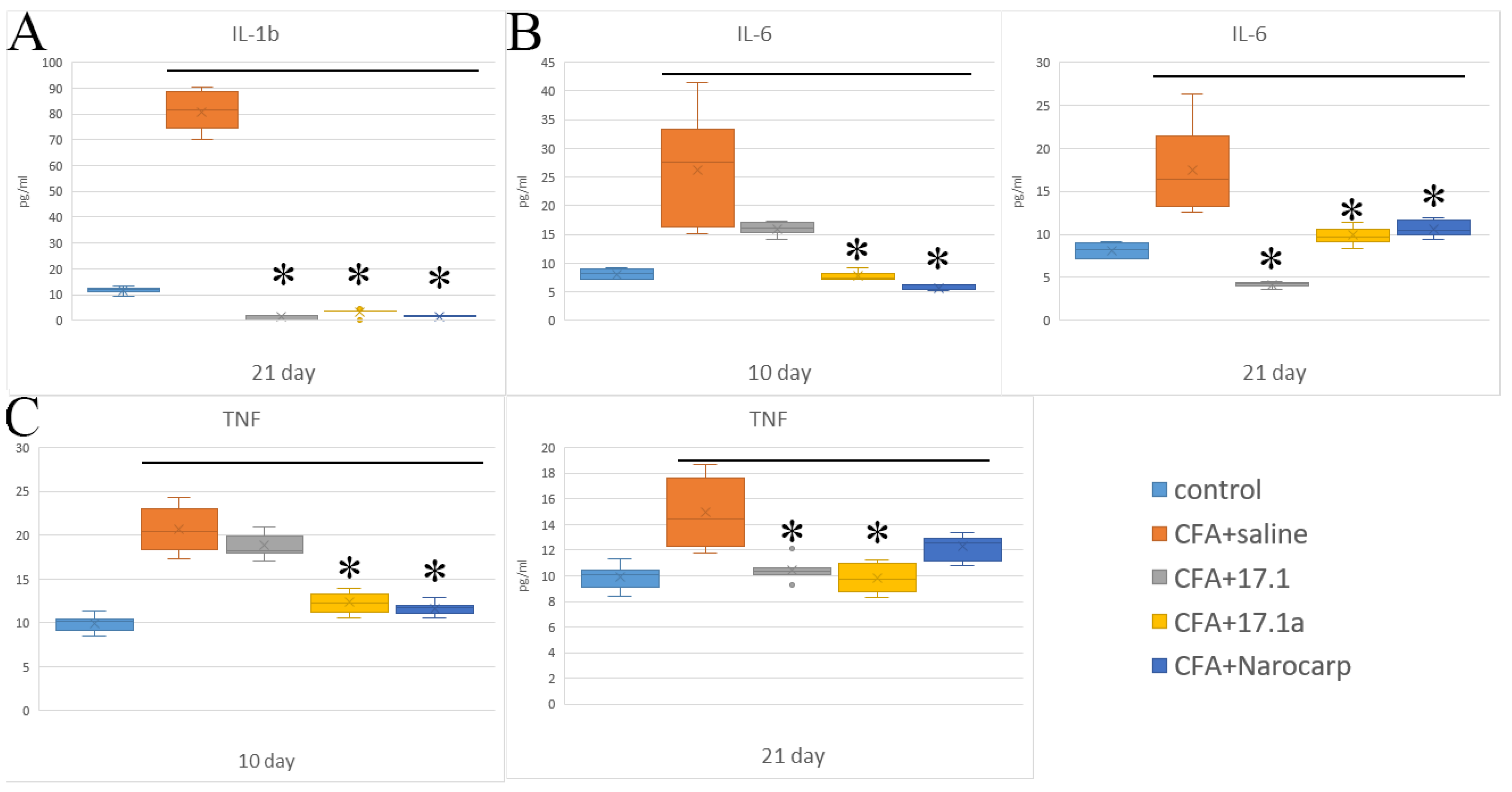
2.1. Pro-Inflammatory Cytokines
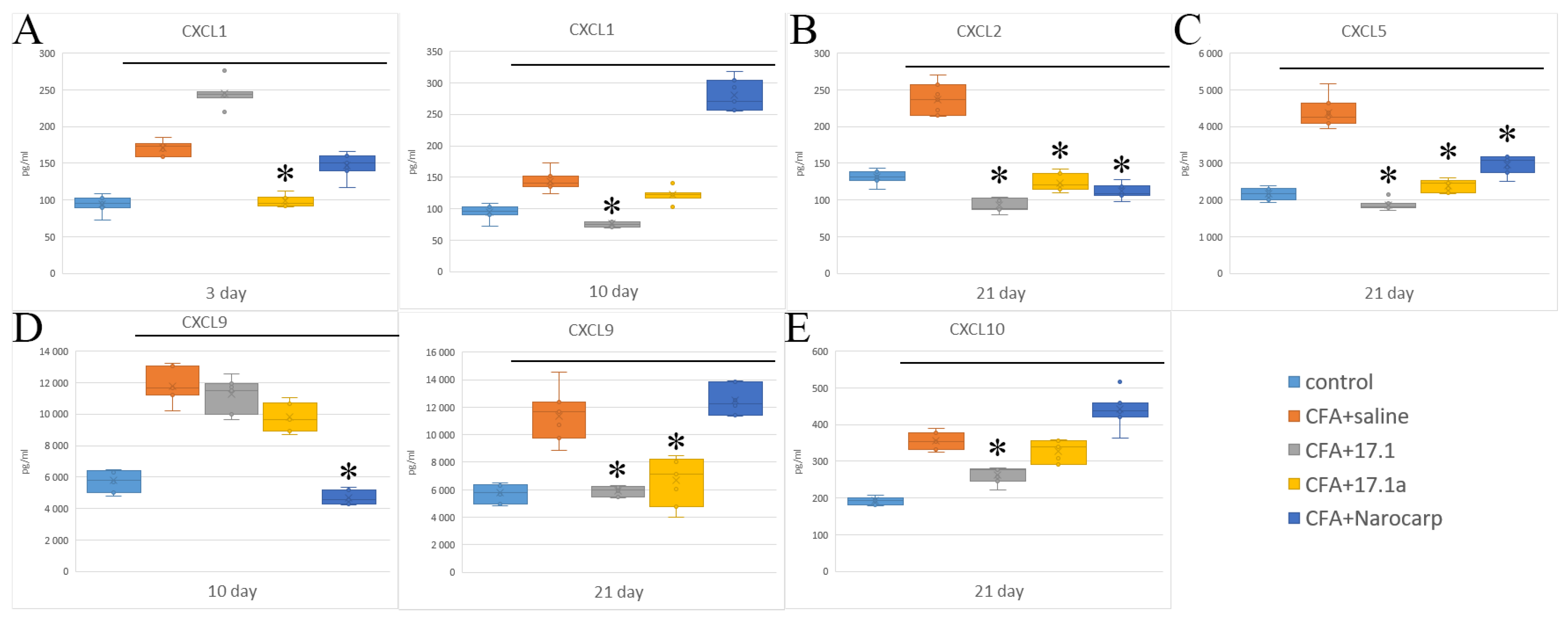
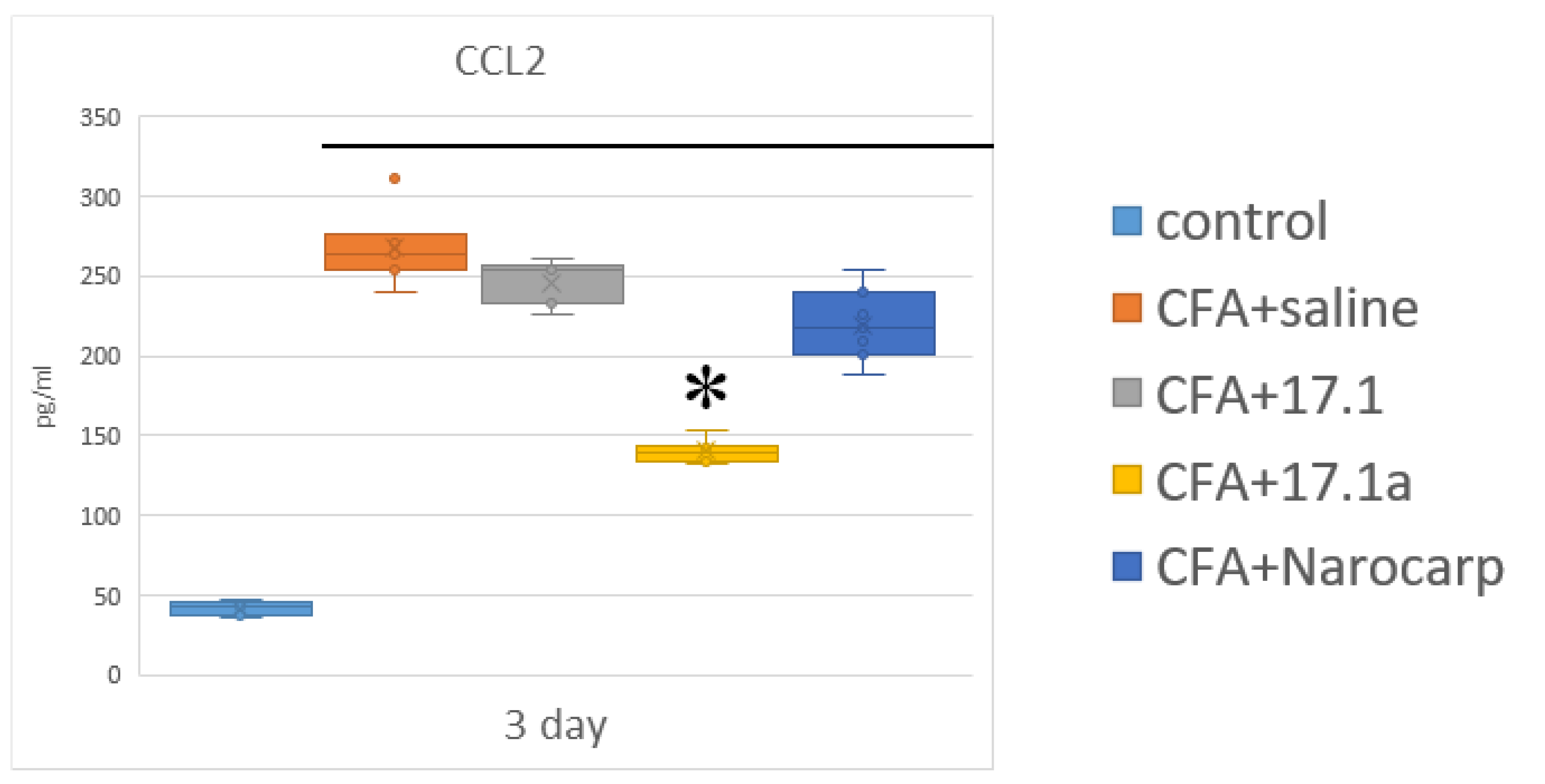
2.2. Chemokines
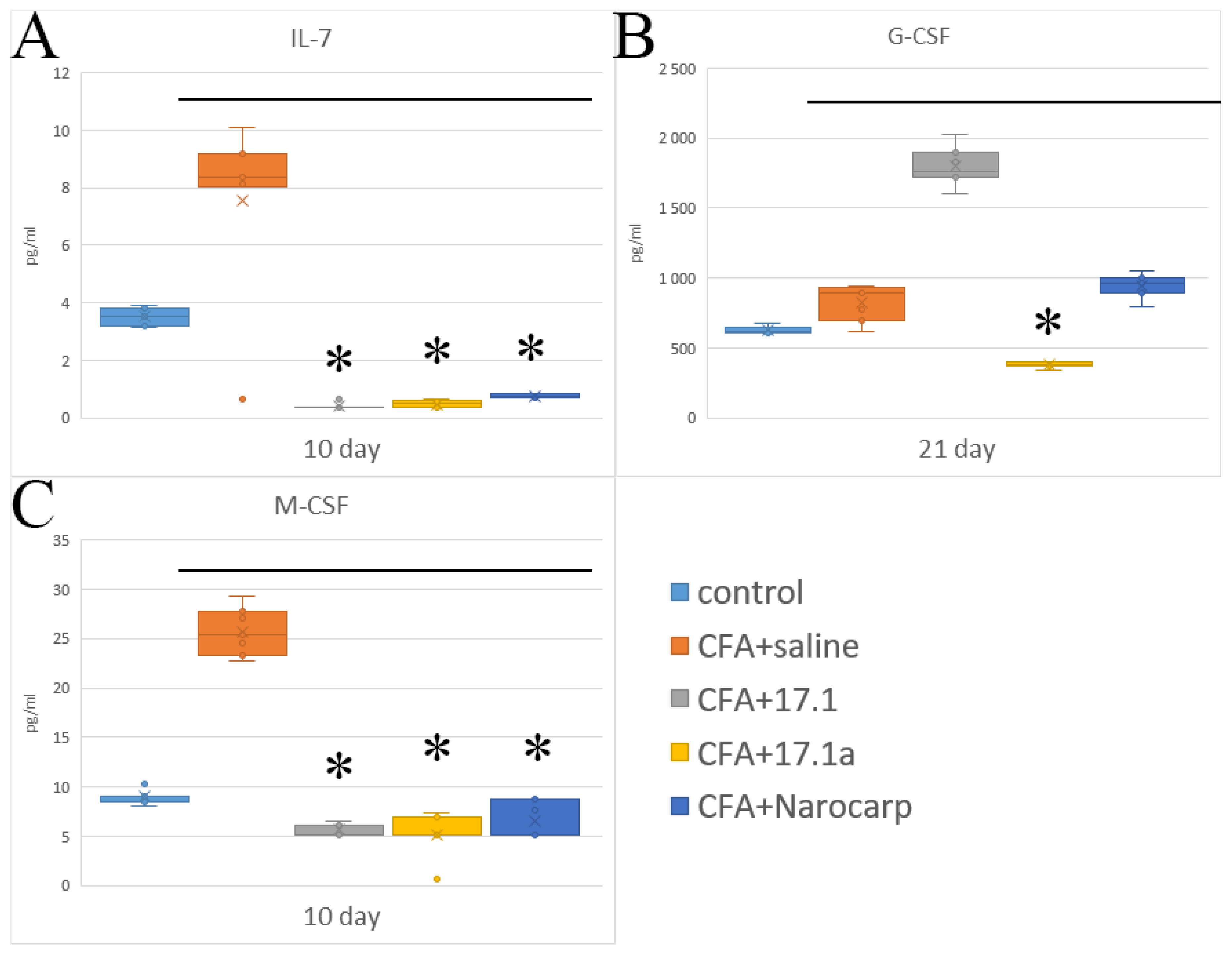
2.3. Hematopoietic Cytokines and Growth Factors
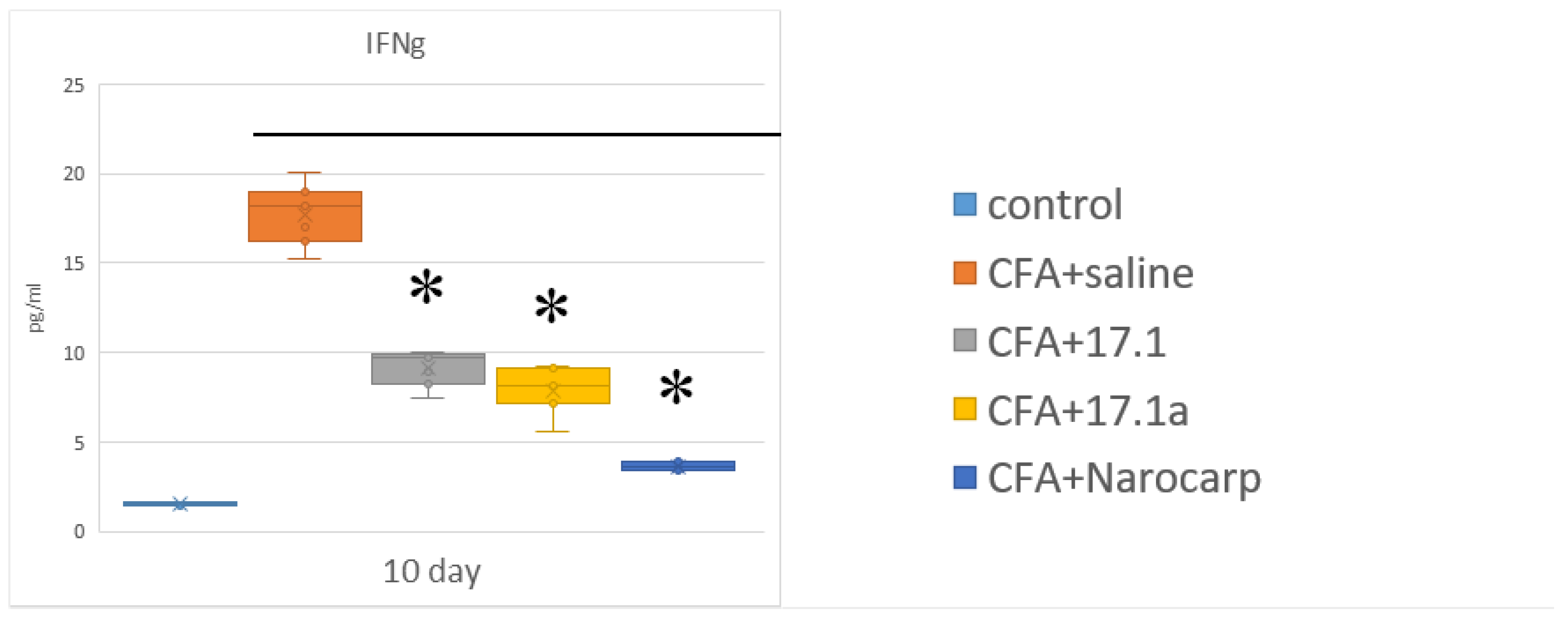
2.4. Cytokines of the Effector Phase of Immune Response
3. Discussion
- (1)
- Under the effect of CFA, there is an increase in the levels of all pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF, all chemokines tested (except CCL11), and IFN-γ, as well as IL-7, G-CSF, and M-CSF.
- (2)
- Peptide 17.1 and its truncated variant 17.1a caused a decrease in the levels of above-mentioned cytokines and chemokines, which was most pronounced by days 10 and 21. In our previous work, we have shown that administration of peptide 17.1 may reduce the effect of CFA-induced arthritis on mouse cartilage and bone tissue [24].
4. Materials and Methods
4.1. Animals
4.2. Experimental Groups
4.3. CFA-Induced Arthritis Model
4.4. The Assay of Chemokines and Cytokines
4.5. Peptides
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ridgley, L.A.; Anderson, A.E.; Pratt, A.G. What Are the Dominant Cytokines in Early 383 Rheumatoid Arthritis? Curr. Opin. Rheumatol. 2018, 30, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 10922. [Google Scholar] [CrossRef]
- Mateen, S.; Zafar, A.; Moin, S.; Khan, A.Q.; Zubair, S. Understanding the Role of Cytokines in the Pathogenesis of Rheumatoid Arthritis. Clin. Chim. Acta Int. J. Clin. Chem. 2016, 455, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Rasool, M. Underpinning IL-6 Biology and Emphasizing Selective JAK Blockade as the Potential Alternate Therapeutic Intervention for Rheumatoid Arthritis. Life Sci. 2022, 298, 120516. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Kanneganti, T.D. Function and Regulation of IL-1α in Inflammatory Diseases and 394 Cancer. Immunol. Rev. 2018, 281, 124–137. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Achuthan, A. Colony Stimulating Factors and Myeloid Cell Biology in Health and Disease. Trends Immunol. 2013, 34, 81–89. [Google Scholar] [CrossRef]
- Crotti, C.; Agape, E.; Becciolini, A.; Biggioggero, M.; Favalli, E.G. Targeting Granulocyte-Monocyte Colony-Stimulating Factor Signaling in Rheumatoid Arthritis: Future Prospects. Drugs 2019, 79, 1741–1755. [Google Scholar] [CrossRef]
- Christensen, A.D.; Haase, C.; Cook, A.D.; Hamilton, J.A. Granulocyte Colony-Stimulating Factor (G-CSF) Plays an Important Role in Immune Complex-Mediated Arthritis. Eur. J. Immunol. 2016, 46, 1235–1245. [Google Scholar] [CrossRef]
- Alves, C.H.; Farrell, E.; Vis, M.; Colin, E.M.; Lubberts, E. Animal Models of Bone Loss in Inflammatory Arthritis: From Cytokines in the Bench to Novel Treatments for Bone Loss in the Bedside-a Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 51, 27–47. [Google Scholar] [CrossRef]
- van Hamburg, J.P.; Tas, S.W. Molecular Mechanisms Underpinning T Helper 17 Cell Heterogeneity and Functions in Rheumatoid Arthritis. J. Autoimmun. 2018, 87, 69–81. [Google Scholar] [CrossRef]
- Maciejewska Rodrigues, H.; Jüngel, A.; Gay, R.E.; Gay, S. Innate Immunity, Epigenetics and Autoimmunity in Rheumatoid Arthritis. Mol. Immunol. 2009, 47, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Troshina, E.A. The role of cytokines in the processes of adaptive integration of immune and neuroendocrine reactions of the human body. Probl. Endokrinol. 2021, 67, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Sandoghchian Shotorbani, S.; Zhang, Y.; Baidoo, S.E.; Xu, H.; Ahmadi, M. IL-4 Can Inhibit IL-17 Production in Collagen Induced Arthritis. Iran. J. Immunol. 2011, 8, 209–217. [Google Scholar] [PubMed]
- Kruglov, A.; Drutskaya, M.; Schlienz, D.; Gorshkova, E.; Kurz, K.; Morawietz, L.; Nedospasov, S. Contrasting Contributions of TNF from Distinct Cellular Sources in Arthritis. Ann. Rheum. Dis. 2020, 79, 1453–1459. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF Biology, Pathogenic Mechanisms and Emerging Therapeutic Strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef]
- Yashin, D.V.; Ivanova, O.K.; Soshnikova, N.V.; Sheludchenkov, A.A.; Romanova, E.A.; Dukhanina, E.A.; Tonevitsky, A.G.; Gnuchev, N.V.; Gabibov, A.G.; Georgiev, G.P.; et al. Tag7 (PGLYRP1) in Complex with Hsp70 Induces Alternative Cytotoxic Processes in Tumor Cells via TNFR1 Receptor. J. Biol. Chem. 2015, 290, 21724–21731. [Google Scholar] [CrossRef]
- Kang, D.; Liu, G.; Lundström, A.; Gelius, E.; Steiner, H. A Peptidoglycan Recognition Protein in Innate Immunity Conserved from Insects to Humans. Proc. Natl. Acad. Sci. USA 1998, 95, 10078–10082. [Google Scholar] [CrossRef]
- Kustikova, O.S.; Kiselev, S.L.; Borodulina, O.R.; Senin, V.M.; Afanas’eva, A.V.; Kabishev, A.A. Cloning of the Tag7 gene expressed in metastatic mouse tumors. Genetika 1996, 32, 621–628. [Google Scholar]
- Michel, T.; Reichhart, J.M.; Hoffmann, J.A.; Royet, J. Drosophila Toll Is Activated by Gram-Positive Bacteria through a Circulating Peptidoglycan Recognition Protein. Nature 2001, 414, 756–759. [Google Scholar] [CrossRef]
- Dziarski, R.; Gupta, D. Mammalian PGRPs: Novel Antibacterial Proteins. Cell. Microbiol. 2006, 8, 1059–1069. [Google Scholar] [CrossRef]
- Sharapova, T.N.; Ivanova, O.K.; Soshnikova, N.V.; Romanova, E.A.; Sashchenko, L.P.; Yashin, D.V. Innate Immunity Protein Tag7 Induces 3 Distinct Populations of Cytotoxic Cells That Use Different Mechanisms to Exhibit Their Antitumor Activity on Human Leukocyte Antigen-Deficient Cancer Cells. J. Innate Immun. 2017, 9, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Romanova, E.A.; Sharapova, T.N.; Telegin, G.B.; Minakov, A.N.; Chernov, A.S.; Ivanova, O.K.; Bychkov, M.L.; Sashchenko, L.P.; Yashin, D.V. A 12-Mer Peptide of Tag7 (PGLYRP1) Forms a Cytotoxic Complex with Hsp70 and Inhibits TNF-Alpha Induced Cell Death. Cells 2020, 9, 488. [Google Scholar] [CrossRef] [PubMed]
- Telegin, G.B.; Chernov, A.S.; Kazakov, V.A.; Romanova, E.A.; Sharapova, T.N.; Yashin, D.V.; Gabibov, A.G.; Sashchenko, L.P. A 8-Mer Peptide of PGLYRP1/Tag7 Innate Immunity Protein Binds to TNFR1 Receptor and Inhibits TNFα-Induced Cytotoxic Effect and Inflammation. Front. Immunol. 2021, 12, 622471. [Google Scholar] [CrossRef] [PubMed]
- Sharapova, T.N.; Romanova, E.A.; Chernov, A.S.; Minakov, A.N.; Kazakov, V.A.; Kudriaeva, A.A.; Belogurov, A.A.; Ivanova, O.K.; Gabibov, A.G.; Telegin, G.B.; et al. Protein PGLYRP1/Tag7 Peptides Decrease the Proinflammatory Response in Human Blood Cells and Mouse Model of Diffuse Alveolar Damage of Lung through Blockage of the TREM-1 and TNFR1 Receptors. Int. J. Mol. Sci. 2021, 22, 11213. [Google Scholar] [CrossRef] [PubMed]
- Telegin, G.B.; Chernov, A.S.; Konovalov, N.A.; Belogurov, A.A.; Balmasova, I.P.; Gabibov, A.G. Cytokine Profile as a Marker of Cell Damage and Immune Dysfunction after Spinal Cord Injury. Acta Nat. 2020, 12, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Berke, M.S.; Fensholdt, L.K.D.; Hestehave, S.; Kalliokoski, O.; Abelson, K.S.P. Effects of buprenorphine on model development in an adjuvant-induced monoarthritis rat model. PLoS ONE 2022, 17, e0260356. [Google Scholar] [CrossRef]
- Abdel Jaleel, G.A.; Azab, S.S.; El-Bakly, W.M.; Hassan, A. Methyl palmitate attenuates adjuvant induced arthritis in rats by decrease of CD68 synovial macrophages. Biomed. Pharmacother. 2021, 137, 111347. [Google Scholar] [CrossRef]
- Liu, M.; Guo, S.; Hibbert, J.M.; Jain, V.; Singh, N.; Wilson, N.O.; Stiles, J.K. CXCL10/IP-10 in Infectious Diseases Pathogenesis and Potential Therapeutic Implications. Cytokine Growth Factor Rev. 2011, 22, 121–130. [Google Scholar] [CrossRef]
- Woodell-May, J.E.; Sommerfeld, S.D. Role of Inflammation and the Immune System in the Progression of Osteoarthritis. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2020, 38, 253–257. [Google Scholar] [CrossRef]
- De Filippo, K.; Dudeck, A.; Hasenberg, M.; Nye, E.; van Rooijen, N.; Hartmann, K.; Gunzer, M.; Roers, A.; Hogg, N. Mast Cell and Macrophage Chemokines CXCL1/CXCL2 Control the Early Stage of Neutrophil Recruitment during Tissue Inflammation. Blood 2013, 121, 4930–4937. [Google Scholar] [CrossRef]
- Schoenborn, J.R.; Wilson, C.B. Regulation of Interferon-Gamma During Innate and Adaptive Immune Responses. Adv. Immunol. 2007, 96, 41–101. [Google Scholar] [PubMed]
- Haas, C.S.; Martinez, R.J.; Attia, N.; Haines, G.K.; Campbell, P.L.; Koch, A.E. Chemokine Receptor Expression in Rat Adjuvant-Induced Arthritis. Arthritis Rheum. 2005, 52, 3718–3730. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Wanchu, A.; Bhatnagar, A. Interaction Between Oxidative Stress and Chemokines: Possible Pathogenic Role in Systemic Lupus Erythematosus and Rheumatoid Arthritis. Immunobiology 2011, 216, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.Y.; Feige, U.; Sarosi, I.; Bolon, B.; Tafuri, A.; Morony, S.; Capparelli, C.; Li, J.; Elliott, R.; McCabe, S.; et al. Activated T Cells Regulate Bone Loss and Joint Destruction in Adjuvant Arthritis through Osteoprotegerin Ligand. Nature 1999, 402, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Pettit, A.R.; Ji, H.; von Stechow, D.; Müller, R.; Goldring, S.R.; Choi, Y.; Benoist, C.; Gravallese, E.M. TRANCE/RANKL Knockout Mice Are Protected from Bone Erosion in a Serum Transfer Model of Arthritis. Am. J. Pathol. 2001, 159, 1689–1699. [Google Scholar] [CrossRef]
- Binder, N.B.; Puchner, A.; Niederreiter, B.; Hayer, S.; Leiss, H.; Blüml, S.; Kreindl, R.; Smolen, J.S.; Redlich, K. Tumor Necrosis Factor-Inhibiting Therapy Preferentially Targets Bone Destruction but Not Synovial Inflammation in a Tumor Necrosis Factor-Driven Model of Rheumatoid Arthritis. Arthritis Rheum. 2013, 65, 608–617. [Google Scholar] [CrossRef]
- Ma, H.; Xu, M.; Song, Y.; Zhang, T.; Yin, H.; Yin, S. Interferon-γ Facilitated Adjuvant-Induced Arthritis at Early Stage. Scand. J. Immunol. 2019, 89, e12757. [Google Scholar] [CrossRef]
- Intriago, M.; Maldonado, G.; Cárdenas, J.; Ríos, C. Clinical Characteristics in Patients with Rheumatoid Arthritis: Differences between Genders. Sci. World J. 2019, 2019, 8103812. [Google Scholar] [CrossRef]
- Almoallim, H.; Al Saleh, J.; Badsha, H.; Ahmed, H.M.; Habjoka, S.; Menassa, J.A.; El-Garf, A.A. Review of the Prevalence and Unmet Needs in the Management of Rheumatoid Arthritis in Africa and the Middle East. Rheumatol Ther. 2021, 8, 1–16. [Google Scholar] [CrossRef]
- Kim, J.R.; Kim, H.A. Molecular Mechanisms of Sex-Related Differences in Arthritis and Associated Pain. Int. J. Mol. Sci. 2020, 21, 7938. [Google Scholar] [CrossRef]
- Yu, C.; Liu, C.; Jiang, J.; Li, H.; Chen, J.; Chen, T.; Zhan, X. Gender Differences in Rheumatoid Arthritis: Interleukin-4 Plays an Important Role. J. Immunol. Res. 2020, 2020, 4121524. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Karagoz, H.; Herneisey, M.; Zor, F.; Komatsu, T.; Loftus, S.; Janjic, B.M.; Gorantla, V.S.; Janjic, J.M. Sex Differences Revealed in a Mouse CFA Inflammation Model with Macrophage Targeted Nanotheranostics. Theranostics 2020, 10, 1694–1707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. Flare-up of cytokines in rheumatoid arthritis and their role in triggering depression: Shared common function and their possible applications in treatment (Review). Biomed. Rep. 2021, 14, 16. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telegin, G.B.; Chernov, A.S.; Minakov, A.N.; Balmasova, I.P.; Romanova, E.A.; Sharapova, T.N.; Sashchenko, L.P.; Yashin, D.V. Short Peptides of Innate Immunity Protein Tag7 Inhibit the Production of Cytokines in CFA-Induced Arthritis. Int. J. Mol. Sci. 2022, 23, 12435. https://doi.org/10.3390/ijms232012435
Telegin GB, Chernov AS, Minakov AN, Balmasova IP, Romanova EA, Sharapova TN, Sashchenko LP, Yashin DV. Short Peptides of Innate Immunity Protein Tag7 Inhibit the Production of Cytokines in CFA-Induced Arthritis. International Journal of Molecular Sciences. 2022; 23(20):12435. https://doi.org/10.3390/ijms232012435
Chicago/Turabian StyleTelegin, Georgii B., Aleksandr S. Chernov, Alexey N. Minakov, Irina P. Balmasova, Elena A. Romanova, Tatiana N. Sharapova, Lidia P. Sashchenko, and Denis V. Yashin. 2022. "Short Peptides of Innate Immunity Protein Tag7 Inhibit the Production of Cytokines in CFA-Induced Arthritis" International Journal of Molecular Sciences 23, no. 20: 12435. https://doi.org/10.3390/ijms232012435
APA StyleTelegin, G. B., Chernov, A. S., Minakov, A. N., Balmasova, I. P., Romanova, E. A., Sharapova, T. N., Sashchenko, L. P., & Yashin, D. V. (2022). Short Peptides of Innate Immunity Protein Tag7 Inhibit the Production of Cytokines in CFA-Induced Arthritis. International Journal of Molecular Sciences, 23(20), 12435. https://doi.org/10.3390/ijms232012435





