Reciprocal Alterations in Osteoprogenitor and Immune Cell Populations in Rheumatoid Synovia
Abstract
1. Introduction
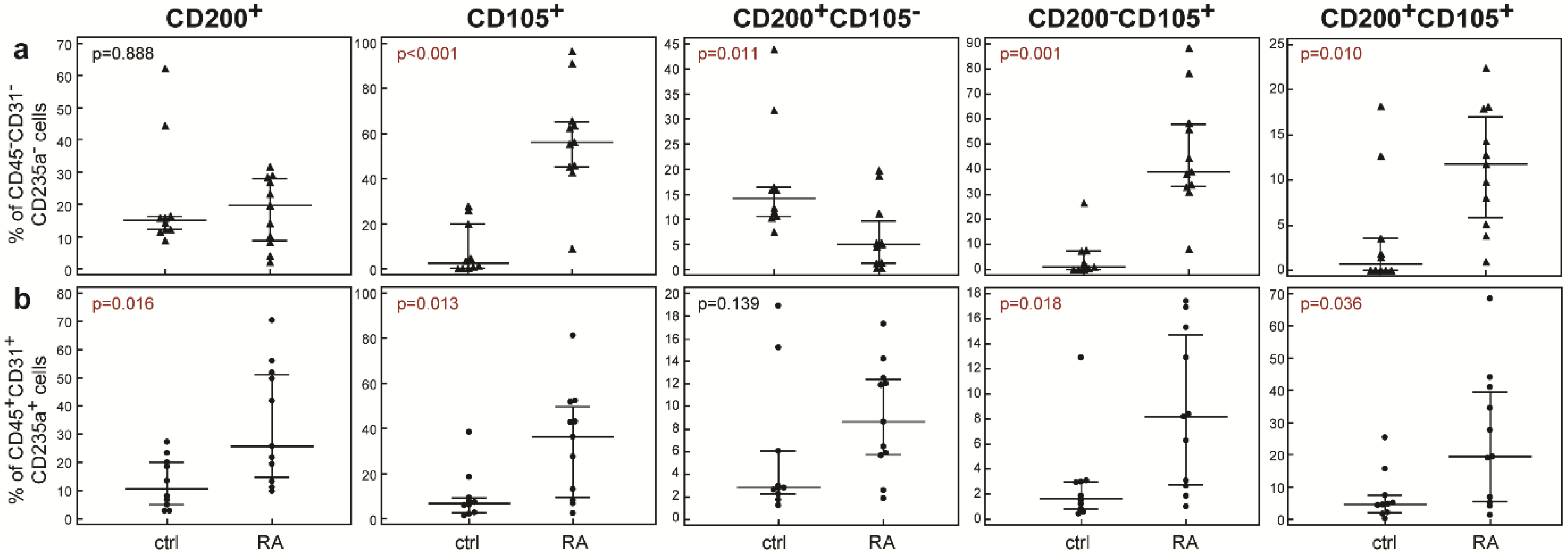
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Flow Cytometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Komatsu, N.; Takayanagi, H. Mechanisms of joint destruction in rheumatoid arthritis-immune cell-fibroblast-bone interactions. Nat. Rev. Rheumatol. 2022, 18, 415–429. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Malemud, C.J. Dysfunctional Immune-Mediated Inflammation in Rheumatoid Arthritis Dictates that Development of Anti-Rheumatic Disease Drugs Target Multiple Intracellular Signaling Pathways. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2011, 10, 78–84. [Google Scholar] [CrossRef]
- Bottini, N.; Firestein, G.S. Duality of fibroblast-like synoviocytes in RA: Passive responders and imprinted aggressors. Nat. Rev. Rheumatol. 2013, 9, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Schett, G. Osteoimmunology in rheumatic diseases. Arthritis Res. Ther. 2009, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Šućur, A.; Katavić, V.; Kelava, T.; Jajić, Z.; Kovačić, N.; Grčević, D. Induction of osteoclast progenitors in inflammatory conditions: Key to bone destruction in arthritis. Int. Orthop. 2014, 38, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- Šućur, A.; Jajić, Z.; Artuković, M.; Matijašević, M.I.; Anić, B.; Flegar, D.; Markotić, A.; Kelava, T.; Ivčević, S.; Kovačić, N.; et al. Chemokine signals are crucial for enhanced homing and differentiation of circulating osteoclast progenitor cells. Arthritis Res. Ther. 2017, 19, 142. [Google Scholar] [CrossRef]
- Berardi, S.; Corrado, A.; Maruotti, N.; Cici, D.; Cantatore, F.P. Osteoblast role in the pathogenesis of rheumatoid arthritis. Mol. Biol. Rep. 2021, 48, 2843–2852. [Google Scholar] [CrossRef]
- Lazić, E.; Jelušić, M.; Grčević, D.; Marušić, A.; Kovačić, N. Osteoblastogenesis from synovial fluid-derived cells is related to the type and severity of juvenile idiopathic arthritis. Arthritis Res. Ther. 2012, 14, R139. [Google Scholar] [CrossRef]
- Gilbert, L.; He, X.; Farmer, P.; Boden, S.; Kozlowski, M.; Rubin, J.; Nanes, M.S. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology 2000, 141, 3956–3964. [Google Scholar] [CrossRef]
- Gilbert, L.; He, X.; Farmer, P.; Rubin, J.; Drissi, H.; van Wijnen, A.J.; Lian, J.B.; Stein, G.S.; Nanes, M.S. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J. Biol. Chem. 2002, 277, 2695–2701. [Google Scholar] [CrossRef]
- Diarra, D.; Stolina, M.; Polzer, K.; Zwerina, J.; Ominsky, M.S.; Dwyer, D.; Korb, A.; Smolen, J.; Hoffmann, M.; Scheinecker, C.; et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 2007, 13, 156–163. [Google Scholar] [CrossRef]
- Matzelle, M.M.; Shaw, A.T.; Baum, R.; Maeda, Y.; Li, J.; Karmakar, S.; Manning, C.A.; Walsh, N.C.; Rosen, V.; Gravallese, E.M. Inflammation in arthritis induces expression of BMP3, an inhibitor of bone formation. Scand. J. Rheumatol. 2016, 45, 379–383. [Google Scholar] [CrossRef]
- Lukač, N.; Katavić, V.; Novak, S.; Šućur, A.; Filipović, M.; Kalajzić, I.; Grčević, D.; Kovačić, N. What do we know about bone morphogenetic proteins and osteochondroprogenitors in inflammatory conditions? Bone 2020, 137, 115403. [Google Scholar] [CrossRef]
- Chan, C.K.; Seo, E.Y.; Chen, J.Y.; Lo, D.; McArdle, A.; Sinha, R.; Tevlin, R.; Seita, J.; Vincent-Tompkins, J.; Wearda, T.; et al. Identification and specification of the mouse skeletal stem cell. Cell 2015, 160, 285–298. [Google Scholar] [CrossRef]
- Lazić Mosler, E.; Lukač, N.; Flegar, D.; Fadljević, M.; Radanović, I.; Cvija, H.; Kelava, T.; Ivčević, S.; Šućur, A.; Markotić, A.; et al. Fas receptor induces apoptosis of synovial bone and cartilage progenitor populations and promotes bone loss in antigen-induced arthritis. FASEB J. 2019, 33, 3330–3342. [Google Scholar] [CrossRef]
- Cui, W.; Cuartas, E.; Ke, J.; Zhang, Q.; Einarsson, H.B.; Sedgwick, J.D.; Li, J.; Vignery, A. CD200 and its receptor, CD200R, modulate bone mass via the differentiation of osteoclasts. Proc. Natl. Acad. Sci. USA 2007, 104, 14436–14441. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, B.; Yin, Y.; Leng, X.; Jiang, Y.; Zhang, L.; Li, Y.; Li, X.; Zhang, F.; He, W.; et al. Aberrant CD200/CD200R1 expression and its potential role in Th17 cell differentiation, chemotaxis and osteoclastogenesis in rheumatoid arthritis. Rheumatology 2015, 54, 712–721. [Google Scholar] [CrossRef]
- Middleton, J.; Americh, L.; Gayon, R.; Julien, D.; Mansat, M.; Mansat, P.; Anract, P.; Cantagrel, A.; Cattan, P.; Reimund, J.M.; et al. A comparative study of endothelial cell markers expressed in chronically inflamed human tissues: MECA-79, Duffy antigen receptor for chemokines, von Willebrand factor, CD31, CD34, CD105 and CD146. J. Pathol. 2005, 206, 260–268. [Google Scholar] [CrossRef]
- Wright, G.J.; Cherwinski, H.; Foster-Cuevas, M.; Brooke, G.; Puklavec, M.J.; Bigler, M.; Song, Y.; Jenmalm, M.; Gorman, D.; McClanahan, T.; et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J. Immunol. 2003, 171, 3034–3046. [Google Scholar] [CrossRef]
- Kotwica-Mojzych, K.; Jodłowska-Jędrych, B.; Mojzych, M. CD200:CD200R Interactions and Their Importance in Immunoregulation. Int. J. Mol. Sci. 2021, 22, 1602. [Google Scholar] [CrossRef] [PubMed]
- Gorczynski, R.M.; Chen, Z.; Yu, K.; Hu, J. CD200 Immunoadhesin Suppresses Collagen-Induced Arthritis in Mice. Clin. Immunol. 2001, 101, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Pietilä, M.; Lehtonen, S.; Tuovinen, E.; Lähteenmäki, K.; Laitinen, S.; Leskelä, H.V.; Nätynki, A.; Pesälä, J.; Nordström, K.; Lehenkari, P. CD200 positive human mesenchymal stem cells suppress TNF-alpha secretion from CD200 receptor positive macrophage-like cells. PLoS ONE 2012, 7, e31671. [Google Scholar] [CrossRef] [PubMed]
- Schoonderwoerd, M.J.A.; Goumans, M.T.H.; Hawinkels, L.J.A.C. Endoglin: Beyond the Endothelium. Biomolecules 2020, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Barry, F.P.; Boynton, R.E.; Haynesworth, S.; Murphy, J.M.; Zaia, J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105). Biochem. Biophys. Res. Commun. 1999, 265, 134–139. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Anderson, P.; Carrillo-Gálvez, A.B.; García-Pérez, A.; Cobo, M.; Martín, F. CD105 (endoglin)-negative murine mesenchymal stromal cells define a new multipotent subpopulation with distinct differentiation and immunomodulatory capacities. PLoS ONE 2013, 8, e76979. [Google Scholar] [CrossRef]
- Hieu Pham, L.; Bich Vu, N.; Van Pham, P. The subpopulation of CD105 negative mesenchymal stem cells show strong immunomodulation capacity compared to CD105 positive mesenchymal stem cells. Biomed. Res. Ther. 2019, 6, 3131–3140. [Google Scholar] [CrossRef]
- Lukač, N.; Katavić, V.; Šućur, A.; Filipović, M.; Grčević, D.; Kovačić, N. RNA sequencing data from osteochondroprogenitor populations in synovial joints of mice during murine model of rheumatoid arthritis. Data Brief 2020, 33, 106570. [Google Scholar] [CrossRef]


| Parent Population | Subpopulation | CD19+ * | CD3+ * | CD11b+CD14− * | CD11b+CD14+ * | CRP ** | ESR *** | |
|---|---|---|---|---|---|---|---|---|
| Non- hematopoietic cells (CD45− CD31− CD235a−) | Total CD200+ | ρ p | 0.251 0.2717 | 0.433 0.0498 | −0.036 0.8778 | 0.021 0.9287 | −0.240 0.3895 | −0.127 0.6278 |
| Total CD105+ | ρ p | 0.624 0.0025 | 0.515 0.0169 | 0.364 0.1044 | 0.279 0.2201 | 0.558 0.0306 | 0.365 0.1503 | |
| CD200+CD105− | ρ p | −0.157 0.4980 | −0.050 0.8295 | −0.375 0.0941 | −0.272 0.2338 | −0.601 0.0178 | −0.370 0.1432 | |
| CD200−CD105+ | ρ p | 0.568 0.0072 | 0.454 0.0388 | 0.401 0.0720 | 0.265 0.2452 | 0.687 0.0047 | 0.395 0.1168 | |
| CD200+CD105+ | ρ p | 0.515 0.0169 | 0.667 0.0010 | 0.275 0.2285 | 0.242 0.2909 | 0.224 0.4218 | 0.237 0.3594 | |
| Hematopoietic cells (CD45+ CD31+ CD235a+) | Total CD200+ | ρ p | 0.569 0.0071 | 0.671 0.0009 | 0.674 0.0008 | 0.835 < 0.0001 | 0.366 0.1792 | 0.047 0.8585 |
| Total CD105+ | ρ p | 0.484 0.0261 | 0.608 0.0035 | 0.630 0.0022 | 0.790 < 0.0001 | 0.372 0.1724 | 0.103 0.6929 | |
| CD200+CD105− | ρ p | 0.504 0.0198 | 0.499 0.0213 | 0.305 0.1794 | 0.314 0.1661 | 0.138 0.6248 | 0.114 0.6633 | |
| CD200−CD105+ | ρ p | 0.446 0.0426 | 0.494 0.0229 | 0.673 0.0008 | 0.595 0.0044 | 0.495 0.0606 | 0.193 0.4572 | |
| CD200+CD105+ | ρ p | 0.406 0.0675 | 0.539 0.0117 | 0.571 0.0068 | 0.734 0.0002 | 0.263 0.3441 | 0.028 0.9141 |
| Characteristic | Control | RA |
|---|---|---|
| Number of subjects | 10 | 11 |
| Male/female | 5/5 | 0/11 |
| Age, years | 56 [53–58] | 57 [46.25–70.5] |
| Diagnosis (ICD-10) | M05 (N = 7), M06 (N = 3), M08 (N = 1) | M23 (N = 10) |
| ESR | 6 [5–7.75], (N = 7) | 18 [12–42] (N = 10) * |
| CRP | 1.40 [0.85–3.63] (N = 7) | 6.85 [4.20–41.65] (N = 8) * |
| Affected joints | Knee (N = 10) | Hand and wrist (N = 6), elbow (N = 1), knee (N = 3), hip (N = 1) |
| NSAIDs | n/a | 6/11 |
| DMARDs | n/a | 6/11 |
| Corticosteroids | n/a | 9/11 |
| Biological therapy | n/a | 3/11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbarić Starčević, K.; Lukač, N.; Jelić, M.; Šućur, A.; Grčević, D.; Kovačić, N. Reciprocal Alterations in Osteoprogenitor and Immune Cell Populations in Rheumatoid Synovia. Int. J. Mol. Sci. 2022, 23, 12379. https://doi.org/10.3390/ijms232012379
Barbarić Starčević K, Lukač N, Jelić M, Šućur A, Grčević D, Kovačić N. Reciprocal Alterations in Osteoprogenitor and Immune Cell Populations in Rheumatoid Synovia. International Journal of Molecular Sciences. 2022; 23(20):12379. https://doi.org/10.3390/ijms232012379
Chicago/Turabian StyleBarbarić Starčević, Katarina, Nina Lukač, Mislav Jelić, Alan Šućur, Danka Grčević, and Nataša Kovačić. 2022. "Reciprocal Alterations in Osteoprogenitor and Immune Cell Populations in Rheumatoid Synovia" International Journal of Molecular Sciences 23, no. 20: 12379. https://doi.org/10.3390/ijms232012379
APA StyleBarbarić Starčević, K., Lukač, N., Jelić, M., Šućur, A., Grčević, D., & Kovačić, N. (2022). Reciprocal Alterations in Osteoprogenitor and Immune Cell Populations in Rheumatoid Synovia. International Journal of Molecular Sciences, 23(20), 12379. https://doi.org/10.3390/ijms232012379






