Cancer Stem Cells in Hepatocellular Carcinoma: Intrinsic and Extrinsic Molecular Mechanisms in Stemness Regulation
Abstract
1. Introduction
2. Intrinsic Molecules and Involved Signaling Pathways
2.1. Cell Surface Markers of Hepatic CSCs
| CSC Markers | Source of Identification | Phenotypes | Signaling Involved in CSCs | Clinical Predictive Value | Refs. |
|---|---|---|---|---|---|
| CD133 | Cell lines, Primary tissues | Self-renewal, Tumorigenicity, Chemoresistance, Invasiveness, Cell proliferation, Radioresistance | Wnt/β-catenin signaling, IL-6/STAT3 signaling, IL-8/MAPK signaling, AKT/PKB pathway, MEK/ERK signaling, STAT3/SOX4 signaling, ANXA3/JNK signaling, TGF-β gaining, TLR4/NANOG and STAT3 signaling | Diagnostic, Therapeutic, Prognostic | [12,13,14,15,16,17,18,19,20,21,22,23,36,61,62,63] |
| CD44 | Cell lines, Primary tissues | Sphere formation, Tumorigenicity, Targeted drug resistance, TGF-β-mediated mesenchymal phenotype, EMT | TGF-β signaling, JAK/STAT signaling, IL-6/STAT3 signaling, AKT/GSK-3β/β-catenin signaling | Therapeutic, Prognostic | [24,25,26,27,64,65,66,67] |
| EpCAM | Cell lines, Primary tissues | Self-renewal, Differentiation, Drug resistance | Wnt/β-catenin signaling, TNF-α/NF-κB, IL-6/STAT3 signaling | Diagnostic, Therapeutic, Prognostic | [28,29,68] |
| CD90 | Cell lines, Primary tissues | Sphere formation, Tumorigenicity, Metastasis, Cell migration and invasion, Cell proliferation, Chemoresistance | AKT/EphA2 signaling, IL-6/JAK2 signaling, AKT and mTOR signaling, MAP3K8 signaling | Diagnostic, Therapeutic, Prognostic | [30,31,32,33,34,69,70] |
| CD13 | Cell lines, Primary tissues | Sphere formation, Tumorigenicity, Chemoresistance, Angiogenesis, ROS-induced DNA damage | Wnt/β-catenin signaling, YAP1 signaling, NF-kB signaling, Aerobic metabolism of tyrosine signaling | Therapeutic, Prognostic | [35,36,37,38,71] |
| CD24 | Cell lines, Primary tissues | Sphere formation, Stemness gene expression, Tumorigenicity, Chemoresistance, Cell migration and invasion | IL-6/STAT3 signaling, STAT3-mediated NANOG regulation, iNOS-mediated TACE-ADAM17-NOTCH signaling | Therapeutic, Prognostic | [28,39,40,65,72] |
| CD47 | Cell lines, Primary tissues | Tumor initiation, Self-renewal, Tumorigenicity, Chemoresistance, Metastasis | CTSS–PAR2 signaling | Therapeutic, Prognostic | [41] |
| CD54/ ICAM1 | Cell lines, Primary tissues | Sphere formation, Tumorigenicity, Metastasis | NANOG dysregulation | Therapeutic, Prognostic | [42,43] |
| LGR5 | Cell lines | Sphere formation, Tumorigenicity, Organoid initiation, Tumor growth, Drug resistance | Wnt/β-catenin signaling | Therapeutic, Prognostic | [44,45,46,47] |
| OV6 | Cell lines, Primary tissues | Tumorigenicity, Chemoresistance, Invasion, Metastasis | β-catenin signaling | Therapeutic, Prognostic | [48,49,73] |
| CD34 | Cell lines | Self-renewal, Tumorigenicity | OCT4, SOX2, NAONG, KLF4, c-MYC upregulation | Prognostic | [50,51,52] |
| Calcium channel α2δ1 subunit | Cell lines | Self-renewal, Tumorigenicity | ERK1/2 phosphorylation | Therapeutic | [53,54,55] |
| DLK1m | Cell lines | Chemoresistance, Colony formation, Spheroid formation, Tumorigenicity | Not reported | Therapeutic | [56,57,58] |
| CD73 | Cell lines | Sphere formation, Lenvatinib resistance, Stemness gene expression | AKT signaling, SOX9 upregulation | Therapeutic, Prognostic | [59] |
| CD206 | Cell lines | Cell migration and invasion | Not reported | Prognostic | [60] |
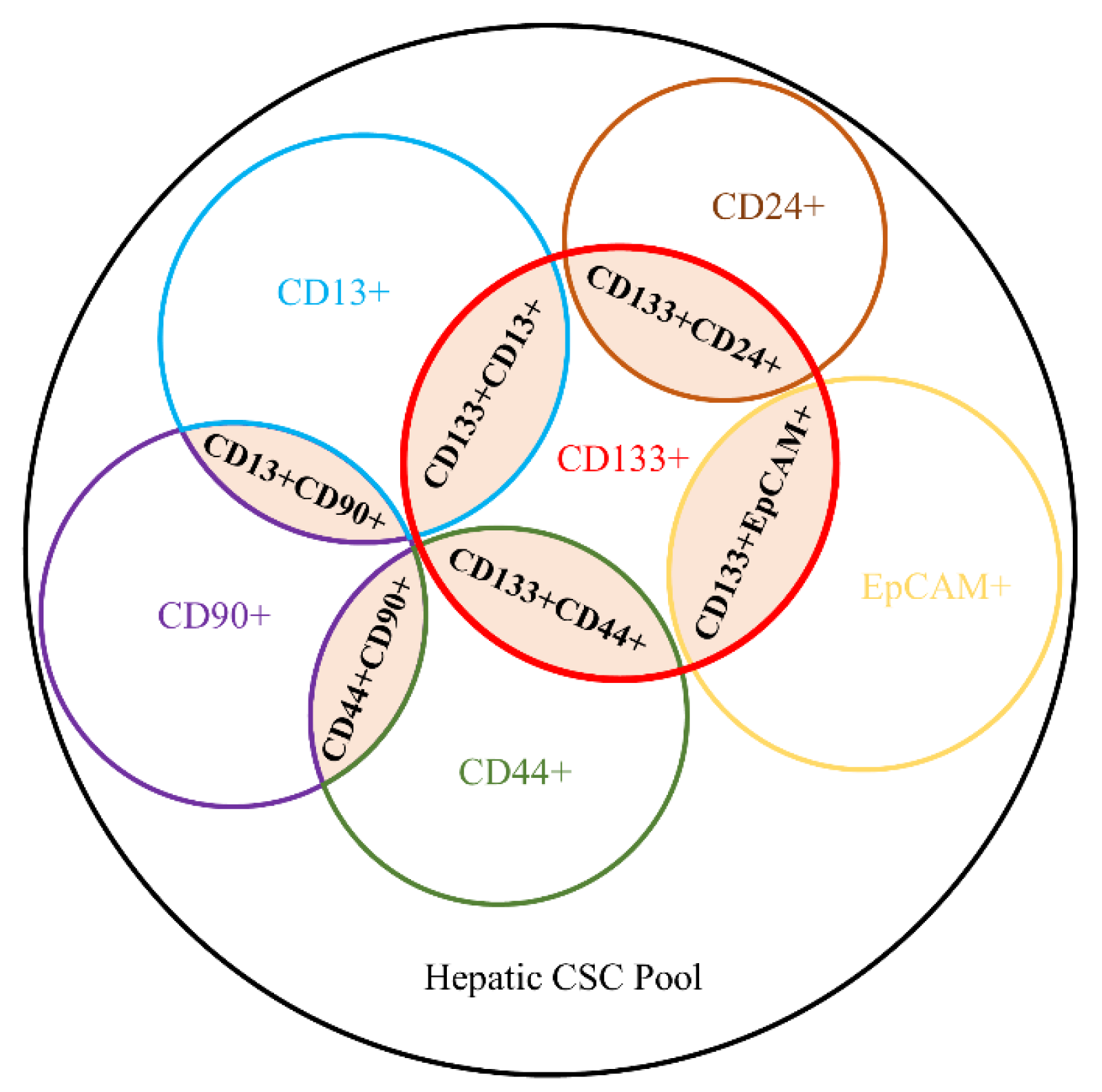
2.2. Heterogeneous Patterns of Hepatic CSC Surface Markers and Phenotypes
3. Extrinsic Cellular Components in the Tumor Microenvironment
4. CSCs-Targeted Therapy in HCC
4.1. Targeting Surface Markers of Hepatic CSCs
4.2. Hampering Cellular Supports for Hepatic CSCs in TME
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Yoh, T.; Seo, S.; Taura, K.; Iguchi, K.; Ogiso, S.; Fukumitsu, K.; Ishii, T.; Kaido, T.; Uemoto, S. Surgery for Recurrent Hepatocellular Carcinoma: Achieving Long-Term Survival. Ann. Surg. 2021, 273, 792–799. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Liang, J.; Chen, W.; Ye, J.; Ni, C.; Zhai, W. Single-Cell Transcriptomics Analysis Reveals Intratumoral Heterogeneity and Identifies a Gene Signature Associated with Prognosis of Hepatocellular Carcinoma. Biosci. Rep. 2022, 42, BSR20212560. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, L.; Zhong, Y.; Zhou, K.; Hou, Y.; Wang, Z.; Zhang, Z.; Xie, J.; Wang, C.; Chen, D.; et al. Single-Cell Landscape of the Ecosystem in Early-Relapse Hepatocellular Carcinoma. Cell 2021, 184, 404–421. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Wang, L.; Feng, Z.; Peng, C. Single-Cell Sequencing of Hepatocellular Carcinoma Reveals Cell Interactions and Cell Heterogeneity in the Microenvironment. Int. J. Gen. Med. 2021, 14, 10141–10153. [Google Scholar] [CrossRef]
- Ho, D.W.; Tsui, Y.M.; Chan, L.K.; Sze, K.M.; Zhang, X.; Cheu, J.W.; Chiu, Y.T.; Lee, J.M.; Chan, A.C.; Cheung, E.T.; et al. Single-Cell RNA Sequencing Shows the Immunosuppressive Landscape and Tumor Heterogeneity of HBV-associated Hepatocellular Carcinoma. Nat. Commun. 2021, 12, 3684. [Google Scholar] [CrossRef]
- Ma, L.; Hernandez, M.O.; Zhao, Y.; Mehta, M.; Tran, B.; Kelly, M.; Rae, Z.; Hernandez, J.M.; Davis, J.L.; Martin, S.P.; et al. Tumor Cell Biodiversity Drives Microenvironmental Reprogramming in Liver Cancer. Cancer Cell 2019, 36, 418–430. [Google Scholar] [CrossRef]
- Zheng, H.; Pomyen, Y.; Hernandez, M.O.; Li, C.; Livak, F.; Tang, W.; Dang, H.; Greten, T.F.; Davis, J.L.; Zhao, Y.; et al. Single-Cell Analysis Reveals Cancer Stem Cell Heterogeneity in Hepatocellular Carcinoma. Hepatology 2018, 68, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Walcher, L.; Kistenmacher, A.K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauss, A.; Blaudszun, A.R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Lin, J.; Qin, F.; Yang, Z.; Ding, Y.; Zhang, Y.; Han, L.; Zhu, X.; Zhang, Q. LncAPC Drives Wnt/beta-catenin Activation and Liver TIC Self-Renewal through EZH2 Mediated APC Transcriptional Inhibition. Mol. Carcinog. 2018, 57, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Cheng, Z.; Liu, H.; Wang, X.; Han, T.; Sun, W.; Li, X.; Yang, W.; Chen, C.; Xia, M.; et al. Shp2 Promotes Liver Cancer Stem Cell Expansion by Augmenting Beta-Catenin Signaling and Predicts Chemotherapeutic Response of Patients. Hepatology 2017, 65, 1566–1580. [Google Scholar] [CrossRef]
- Chai, S.; Ng, K.Y.; Tong, M.; Lau, E.Y.; Lee, T.K.; Chan, K.W.; Yuan, Y.F.; Cheung, T.T.; Cheung, S.T.; Wang, X.Q.; et al. Octamer 4/microRNA-1246 Signaling Axis Drives Wnt/beta-catenin Activation in Liver Cancer Stem Cells. Hepatology 2016, 64, 2062–2076. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Du, Y.; Zhu, P.; Huang, G.; Luo, J.; Yan, X.; Ye, B.; Li, C.; Xia, P.; et al. The Long Noncoding RNA lncTCF7 Promotes Self-Renewal of Human Liver Cancer Stem Cells through Activation of Wnt Signaling. Cell Stem Cell 2015, 16, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Won, C.; Kim, B.H.; Yi, E.H.; Choi, K.J.; Kim, E.K.; Jeong, J.M.; Lee, J.H.; Jang, J.J.; Yoon, J.H.; Jeong, W.I.; et al. Signal Transducer and Activator of Transcription 3-Mediated CD133 Up-Regulation Contributes to Promotion of Hepatocellular Carcinoma. Hepatology 2015, 62, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Tsukamoto, H.; Liu, J.C.; Kashiwabara, C.; Feldman, D.; Sher, L.; Dooley, S.; French, S.W.; Mishra, L.; Petrovic, L.; et al. Reciprocal Regulation by TLR4 and TGF-beta in Tumor-Initiating Stem-Like Cells. J. Clin. Investig. 2013, 123, 2832–2849. [Google Scholar] [CrossRef]
- Ma, S.; Lee, T.K.; Zheng, B.J.; Chan, K.W.; Guan, X.Y. CD133+ HCC Cancer Stem Cells Confer Chemoresistance by Preferential Expression of the Akt/PKB Survival Pathway. Oncogene 2008, 27, 1749–1758. [Google Scholar] [CrossRef]
- Tang, K.H.; Ma, S.; Lee, T.K.; Chan, Y.P.; Kwan, P.S.; Tong, C.M.; Ng, I.O.; Man, K.; To, K.F.; Lai, P.B.; et al. CD133(+) Liver Tumor-Initiating Cells Promote Tumor Angiogenesis, Growth, and Self-Renewal through neurotensin/interleukin-8/CXCL1 Signaling. Hepatology 2012, 55, 807–820. [Google Scholar] [CrossRef]
- Uthaya, K.D.; Chen, C.L.; Liu, J.C.; Feldman, D.E.; Sher, L.S.; French, S.; DiNorcia, J.; French, S.W.; Naini, B.V.; Junrungsee, S.; et al. TLR4 Signaling via NANOG Cooperates with STAT3 to Activate Twist1 and Promote Formation of Tumor-Initiating Stem-Like Cells in Livers of Mice. Gastroenterology 2016, 150, 707–719. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Huang, L.; Wu, Y.H.; Zhai, W.J.; Zhu, P.P.; Gao, Y.F. LncSox4 Promotes the Self-Renewal of Liver Tumour-Initiating Cells through Stat3-mediated Sox4 Expression. Nat. Commun. 2016, 7, 12598. [Google Scholar] [CrossRef]
- Chan, L.H.; Zhou, L.; Ng, K.Y.; Wong, T.L.; Lee, T.K.; Sharma, R.; Loong, J.H.; Ching, Y.P.; Yuan, Y.F.; Xie, D.; et al. PRMT6 Regulates RAS/RAF Binding and MEK/ERK-Mediated Cancer Stemness Activities in Hepatocellular Carcinoma through CRAF Methylation. Cell Rep. 2018, 25, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Fung, T.M.; Luk, S.T.; Ng, K.Y.; Lee, T.K.; Lin, C.H.; Yam, J.W.; Chan, K.W.; Ng, F.; Zheng, B.J.; et al. ANXA3/JNK Signaling Promotes Self-Renewal and Tumor Growth, and its Blockade Provides a Therapeutic Target for Hepatocellular Carcinoma. Stem Cell Rep. 2015, 5, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Park, N.R.; Cha, J.H.; Jang, J.W.; Bae, S.H.; Jang, B.; Kim, J.H.; Hur, W.; Choi, J.Y.; Yoon, S.K. Synergistic Effects of CD44 and TGF-β1 through AKT/GSK-3β/β-catenin Signaling During Epithelial-Mesenchymal Transition in Liver Cancer Cells. Biochem. Biophys. Res. Commun. 2016, 477, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Toh, T.B.; Lim, J.J.; Hooi, L.; Rashid, M.; Chow, E.K. Targeting Jak/Stat Pathway as a Therapeutic Strategy Against SP/CD44+ Tumorigenic Cells in Akt/β-catenin-driven Hepatocellular Carcinoma. J. Hepatol. 2020, 72, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, C.; Ungerleider, N.; Chen, W.; Song, K.; Wang, Y.; Kwon, H.; Ma, W.; Wu, T. A Transforming Growth Factor-Beta and H19 Signaling Axis in Tumor-Initiating Hepatocytes that Regulates Hepatic Carcinogenesis. Hepatology 2019, 69, 1549–1563. [Google Scholar] [CrossRef]
- Wan, S.; Zhao, E.; Kryczek, I.; Vatan, L.; Sadovskaya, A.; Ludema, G.; Simeone, D.M.; Zou, W.; Welling, T.H. Tumor-Associated Macrophages Produce Interleukin 6 and Signal Via STAT3 to Promote Expansion of Human Hepatocellular Carcinoma Stem Cells. Gastroenterology 2014, 147, 1393–1404. [Google Scholar] [CrossRef]
- Wang, X.; Sun, W.; Shen, W.; Xia, M.; Chen, C.; Xiang, D.; Ning, B.; Cui, X.; Li, H.; Li, X.; et al. Long Non-Coding RNA DILC Regulates Liver Cancer Stem Cells Via IL-6/STAT3 Axis. J. Hepatol. 2016, 64, 1283–1294. [Google Scholar] [CrossRef]
- Yamashita, T.; Ji, J.; Budhu, A.; Forgues, M.; Yang, W.; Wang, H.Y.; Jia, H.; Ye, Q.; Qin, L.X.; Wauthier, E.; et al. EpCAM-positive Hepatocellular Carcinoma Cells are Tumor-Initiating Cells with Stem/Progenitor Cell Features. Gastroenterology 2009, 136, 1012–1024. [Google Scholar] [CrossRef]
- Zhang, K.; Che, S.; Su, Z.; Zheng, S.; Zhang, H.; Yang, S.; Li, W.; Liu, J. CD90 Promotes Cell Migration, Viability and Sphere Forming Ability of Hepatocellular Carcinoma Cells. Int. J. Mol. Med. 2018, 41, 946–954. [Google Scholar] [CrossRef]
- Asakura, N.; Nakamura, N.; Muroi, A.; Nojima, Y.; Yamashita, T.; Kaneko, S.; Ikeda, K.; Koshikawa, N.; Suzuki, T. Expression of Cancer Stem Cell Markers EpCAM and CD90 is Correlated with Anti- and Pro-Oncogenic EphA2 Signaling in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 8652. [Google Scholar] [CrossRef]
- Zhang, K.; Che, S.; Pan, C.; Su, Z.; Zheng, S.; Yang, S.; Zhang, H.; Li, W.; Wang, W.; Liu, J. The SHH/Gli Axis Regulates CD90-mediated Liver Cancer Stem Cell Function by Activating the IL6/JAK2 Pathway. J. Cell. Mol. Med. 2018, 22, 3679–3690. [Google Scholar] [CrossRef]
- Moustafa, M.; Dähling, K.K.; Günther, A.; Riebandt, L.; Smit, D.J.; Riecken, K.; Schröder, C.; Zhuang, R.; Krech, T.; Kriegs, M.; et al. Combined Targeting of AKT and mTOR Inhibits Tumor Formation of EpCAM(+) and CD90(+) Human Hepatocellular Carcinoma Cells in an Orthotopic Mouse Model. Cancers 2022, 14, 1882. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, P.; Shuai, L.; Chen, K.; Li, Z.; Zhang, Y.; Jiang, Y.; Li, X. MiR-589-5p Inhibits MAP3K8 and Suppresses CD90(+) Cancer Stem Cells in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2016, 35, 176. [Google Scholar] [CrossRef]
- Hu, B.; Xu, Y.; Li, Y.C.; Huang, J.F.; Cheng, J.W.; Guo, W.; Yin, Y.; Gao, Y.; Wang, P.X.; Wu, S.Y.; et al. CD13 Promotes Hepatocellular Carcinogenesis and Sorafenib Resistance by Activating HDAC5-LSD1-NF-kappaB Oncogenic Signaling. Clin. Transl. Med. 2020, 10, e233. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, Y.; Wu, J.; Huang, G.; Liu, B.; Ye, B.; Du, Y.; Gao, G.; Tian, Y.; He, L.; et al. LncBRM Initiates YAP1 Signalling Activation to Drive Self-Renewal of Liver Cancer Stem Cells. Nat. Commun. 2016, 7, 13608. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, Y.; Huang, G.; Ye, B.; Liu, B.; Wu, J.; Du, Y.; He, L.; Fan, Z. Lnc-beta-Catm Elicits EZH2-dependent Beta-Catenin Stabilization and Sustains Liver CSC Self-Renewal. Nat. Struct. Mol. Biol. 2016, 23, 631–639. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, L.; Chen, J.; Li, C.; Sun, H.; Wang, J.; Xiao, H. Activation of Tyrosine Metabolism in CD13+ Cancer Stem Cells Drives Relapse in Hepatocellular Carcinoma. Cancer Res. Treat. 2020, 52, 604–621. [Google Scholar] [CrossRef]
- Lee, T.K.; Castilho, A.; Cheung, V.C.; Tang, K.H.; Ma, S.; Ng, I.O. CD24(+) Liver Tumor-Initiating Cells Drive Self-Renewal and Tumor Initiation through STAT3-mediated NANOG Regulation. Cell Stem Cell 2011, 9, 50–63. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Tsung, A.; Huang, H.; Du, Q.; Yang, M.; Deng, M.; Xiong, S.; Wang, X.; Zhang, L.; et al. INOS Promotes CD24(+) CD133(+) Liver Cancer Stem Cell Phenotype through a TACE/ADAM17-dependent Notch Signaling Pathway. Proc. Natl. Acad. Sci. USA 2018, 115, E10127–E10136. [Google Scholar] [CrossRef]
- Lee, T.K.; Cheung, V.C.; Lu, P.; Lau, E.Y.; Ma, S.; Tang, K.H.; Tong, M.; Lo, J.; Ng, I.O. Blockade of CD47-mediated Cathepsin S/protease-activated Receptor 2 Signaling Provides a Therapeutic Target for Hepatocellular Carcinoma. Hepatology 2014, 60, 179–191. [Google Scholar] [CrossRef]
- Guo, W.; Liu, S.; Cheng, Y.; Lu, L.; Shi, J.; Xu, G.; Li, N.; Cheng, K.; Wu, M.; Cheng, S.; et al. ICAM-1-Related Noncoding RNA in Cancer Stem Cells Maintains ICAM-1 Expression in Hepatocellular Carcinoma. Clin. Cancer Res. 2016, 22, 2041–2050. [Google Scholar] [CrossRef]
- Liu, S.; Li, N.; Yu, X.; Xiao, X.; Cheng, K.; Hu, J.; Wang, J.; Zhang, D.; Cheng, S.; Liu, S. Expression of Intercellular Adhesion Molecule 1 by Hepatocellular Carcinoma Stem Cells and Circulating Tumor Cells. Gastroenterology 2013, 144, 1031–1041. [Google Scholar] [CrossRef]
- Lei, Z.J.; Wang, J.; Xiao, H.L.; Guo, Y.; Wang, T.; Li, Q.; Liu, L.; Luo, X.; Fan, L.L.; Lin, L.; et al. Lysine-Specific Demethylase 1 Promotes the Stemness and Chemoresistance of Lgr5(+) Liver Cancer Initiating Cells by Suppressing Negative Regulators of Beta-Catenin Signaling. Oncogene 2015, 34, 3188–3198. [Google Scholar] [CrossRef]
- Akbari, S.; Kunter, I.; Azbazdar, Y.; Ozhan, G.; Atabey, N.; Firtina, K.Z.; Erdal, E. LGR5/R-Spo1/Wnt3a Axis Promotes Stemness and Aggressive Phenotype in Hepatoblast-Like Hepatocellular Carcinoma Cell Lines. Cell. Signal. 2021, 82, 109972. [Google Scholar] [CrossRef]
- Ang, C.H.; Hsu, S.H.; Guo, F.; Tan, C.T.; Yu, V.C.; Visvader, J.E.; Chow, P.; Fu, N.Y. Lgr5(+) Pericentral Hepatocytes are Self-Maintained in Normal Liver Regeneration and Susceptible to Hepatocarcinogenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 19530–19540. [Google Scholar] [CrossRef]
- Cao, W.; Li, M.; Liu, J.; Zhang, S.; Noordam, L.; Verstegen, M.; Wang, L.; Ma, B.; Li, S.; Wang, W.; et al. LGR5 Marks Targetable Tumor-Initiating Cells in Mouse Liver Cancer. Nat. Commun. 2020, 11, 1961. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.D.; Cheng, P.; Huang, H.; Dong, W.; Zhang, W.W.; Li, P.P.; Lin, C.; Pan, Z.Y.; Wu, M.C.; et al. Hepatitis B Virus X Protein Promotes the Stem-Like Properties of OV6(+) Cancer Cells in Hepatocellular Carcinoma. Cell Death Dis. 2017, 8, e2560. [Google Scholar] [CrossRef]
- Yang, W.; Wang, C.; Lin, Y.; Liu, Q.; Yu, L.X.; Tang, L.; Yan, H.X.; Fu, J.; Chen, Y.; Zhang, H.L.; et al. OV6+ Tumor-Initiating Cells Contribute to Tumor Progression and Invasion in Human Hepatocellular Carcinoma. J. Hepatol. 2012, 57, 613–620. [Google Scholar] [CrossRef]
- Park, S.C.; Zeng, C.; Tschudy-Seney, B.; Nguyen, N.T.; Eun, J.R.; Zhang, Y.; Ramsamooj, R.; Zhang, Y.; Zhao, M.; Theise, N.D.; et al. Clonogenically Culturing and Expanding CD34+ Liver Cancer Stem Cells in Vitro. Stem Cells Dev. 2015, 24, 1506–1514. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, Y.; Park, S.C.; Eun, J.R.; Nguyen, N.T.; Tschudy-Seney, B.; Jung, Y.J.; Theise, N.D.; Zern, M.A.; Duan, Y. CD34(+) Liver Cancer Stem Cells were Formed by Fusion of Hepatobiliary Stem/Progenitor Cells with Hematopoietic Precursor-Derived Myeloid Intermediates. Stem Cells Dev. 2015, 24, 2467–2478. [Google Scholar] [CrossRef]
- Park, S.C.; Nguyen, N.T.; Eun, J.R.; Zhang, Y.; Jung, Y.J.; Tschudy-Seney, B.; Trotsyuk, A.; Lam, A.; Ramsamooj, R.; Zhang, Y.; et al. Identification of Cancer Stem Cell Subpopulations of CD34(+) PLC/PRF/5 that Result in Three Types of Human Liver Carcinomas. Stem Cells Dev. 2015, 24, 1008–1021. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Han, H.; Li, S.; Chen, D.; Zhang, Z. MicroRNA-31 Suppresses the Self-Renewal Capability of Alpha2delta1(+) Liver Tumor-Initiating Cells by Targeting ISL1. Oncotarget 2017, 8, 87647–87657. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, L.; Han, H.; Jin, K.; Lin, N.; Guo, T.; Chen, Y.; Cheng, H.; Lu, F.; Fang, W.; et al. 1B50-1, a mAb Raised Against Recurrent Tumor Cells, Targets Liver Tumor-Initiating Cells by Binding to the Calcium Channel Alpha2delta1 Subunit. Cancer Cell 2013, 23, 541–556. [Google Scholar] [CrossRef]
- Zhao, W.; Lv, M.; Yang, X.; Zhou, J.; Xing, B.; Zhang, Z. Liver Tumor-Initiating Cells Initiate the Formation of a Stiff Cancer Stem Cell Microenvironment Niche by Secreting LOX. Carcinogenesis 2022, 43, 766–778. [Google Scholar] [CrossRef]
- Yanai, H.; Nakamura, K.; Hijioka, S.; Kamei, A.; Ikari, T.; Ishikawa, Y.; Shinozaki, E.; Mizunuma, N.; Hatake, K.; Miyajima, A. Dlk-1, a Cell Surface Antigen On Foetal Hepatic Stem/Progenitor Cells, is Expressed in Hepatocellular, Colon, Pancreas and Breast Carcinomas at a High Frequency. J. Biochem. 2010, 148, 85–92. [Google Scholar] [CrossRef]
- Xu, X.; Liu, R.F.; Zhang, X.; Huang, L.Y.; Chen, F.; Fei, Q.L.; Han, Z.G. DLK1 as a Potential Target Against Cancer Stem/Progenitor Cells of Hepatocellular Carcinoma. Mol. Cancer Ther. 2012, 11, 629–638. [Google Scholar] [CrossRef]
- Grassi, E.S.; Pietras, A. Emerging Roles of DLK1 in the Stem Cell Niche and Cancer Stemness. J. Histochem. Cytochem. 2022, 70, 17–28. [Google Scholar] [CrossRef]
- Ma, X.; Hu, B.; Tang, W.; Xie, S.; Ren, N.; Guo, L.; Lu, R. CD73 Sustained Cancer-Stem-Cell Traits by Promoting SOX9 Expression and Stability in Hepatocellular Carcinoma. J. Hematol. Oncol. 2020, 13, 11. [Google Scholar] [CrossRef]
- Fan, W.; Yang, X.; Huang, F.; Tong, X.; Zhu, L.; Wang, S. Identification of CD206 as a Potential Biomarker of Cancer Stem-Like Cells and Therapeutic Agent in Liver Cancer. Oncol. Lett. 2019, 18, 3218–3226. [Google Scholar] [CrossRef]
- Jun, S.Y.; Jeon, S.J.; Yoon, J.Y.; Lee, J.J.; Yoon, H.R.; Choi, M.H.; Halder, D.; Lee, K.; Kim, N.S. The Positive Correlation of TIPRL with LC3 and CD133 Contributes to Cancer Aggressiveness: Potential Biomarkers for Early Liver Cancer. Sci. Rep. 2019, 9, 16802. [Google Scholar] [CrossRef]
- Piao, L.S.; Hur, W.; Kim, T.K.; Hong, S.W.; Kim, S.W.; Choi, J.E.; Sung, P.S.; Song, M.J.; Lee, B.C.; Hwang, D.; et al. CD133+ Liver Cancer Stem Cells Modulate Radioresistance in Human Hepatocellular Carcinoma. Cancer Lett. 2012, 315, 129–137. [Google Scholar] [CrossRef]
- Ma, S.; Chan, K.W.; Hu, L.; Lee, T.K.; Wo, J.Y.; Ng, I.O.; Zheng, B.J.; Guan, X.Y. Identification and Characterization of Tumorigenic Liver Cancer Stem/Progenitor Cells. Gastroenterology 2007, 132, 2542–2556. [Google Scholar] [CrossRef]
- Wuputra, K.; Hsiao, P.J.; Chang, W.T.; Wu, P.H.; Chen, L.A.; Huang, J.W.; Su, W.L.; Yang, Y.H.; Wu, D.C.; Yokoyama, K.K.; et al. FOXM1-CD44 Signaling is Critical for the Acquisition of Regorafenib Resistance in Human Liver Cancer Cells. Int. J. Mol. Sci. 2022, 23, 7782. [Google Scholar] [CrossRef]
- Ho, D.W.; Tsui, Y.M.; Sze, K.M.; Chan, L.K.; Cheung, T.T.; Lee, E.; Sham, P.C.; Tsui, S.K.; Lee, T.K.; Ng, I.O. Single-Cell Transcriptomics Reveals the Landscape of Intra-Tumoral Heterogeneity and Stemness-Related Subpopulations in Liver Cancer. Cancer Lett. 2019, 459, 176–185. [Google Scholar] [CrossRef]
- Lee, D.; Na, J.; Ryu, J.; Kim, H.J.; Nam, S.H.; Kang, M.; Jung, J.W.; Lee, M.S.; Song, H.E.; Choi, J.; et al. Interaction of Tetraspan(In) TM4SF5 with CD44 Promotes Self-Renewal and Circulating Capacities of Hepatocarcinoma Cells. Hepatology 2015, 61, 1978–1997. [Google Scholar] [CrossRef]
- Mima, K.; Okabe, H.; Ishimoto, T.; Hayashi, H.; Nakagawa, S.; Kuroki, H.; Watanabe, M.; Beppu, T.; Tamada, M.; Nagano, O.; et al. CD44s Regulates the TGF-beta-mediated Mesenchymal Phenotype and is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma. Cancer Res. 2012, 72, 3414–3423. [Google Scholar] [CrossRef]
- Zhou, K.; Nguyen, R.; Qiao, L.; George, J. Single Cell RNA-seq Analysis Identifies a Noncoding RNA Mediating Resistance to Sorafenib Treatment in HCC. Mol. Cancer 2022, 21, 6. [Google Scholar] [CrossRef]
- Jia, Q.; Zhang, X.; Deng, T.; Gao, J. Positive Correlation of Oct4 and ABCG2 to Chemotherapeutic Resistance in CD90(+) CD133(+) Liver Cancer Stem Cells. Cell. Reprogram. 2013, 15, 143–150. [Google Scholar] [CrossRef]
- Yang, Z.F.; Ngai, P.; Ho, D.W.; Yu, W.C.; Ng, M.N.; Lau, C.K.; Li, M.L.; Tam, K.H.; Lam, C.T.; Poon, R.T.; et al. Identification of Local and Circulating Cancer Stem Cells in Human Liver Cancer. Hepatology 2008, 47, 919–928. [Google Scholar] [CrossRef]
- Yamanaka, C.; Wada, H.; Eguchi, H.; Hatano, H.; Gotoh, K.; Noda, T.; Yamada, D.; Asaoka, T.; Kawamoto, K.; Nagano, H.; et al. Clinical Significance of CD13 and Epithelial Mesenchymal Transition (EMT) Markers in Hepatocellular Carcinoma. Jpn. J. Clin. Oncol. 2018, 48, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hou, J.; Lin, Z.; Zhuo, H.; Chen, D.; Zhang, X.; Chen, Y.; Sun, B. Attenuated Listeria Monocytogenes as a Cancer Vaccine Vector for the Delivery of CD24, a Biomarker for Hepatic Cancer Stem Cells. Cell. Mol. Immunol. 2014, 11, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yu, H.; Chen, S.; Yang, P.; Dong, Z.; Ling, Y.; Tang, H.; Bai, S.; Yang, W.; Tang, L.; et al. Prognostic Significance of Combining High Mobility Group Box-1 and OV-6 Expression in Hepatocellular Carcinoma. Sci. China Life Sci. 2018, 61, 912–923. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zheng, X.; Ji, J. Liver Cancer Stem Cells as a Hierarchical Society: Yes Or No? Acta Biochim. Biophys. Sin. 2020, 52, 723–735. [Google Scholar] [CrossRef]
- Yamashita, T.; Honda, M.; Nakamoto, Y.; Baba, M.; Nio, K.; Hara, Y.; Zeng, S.S.; Hayashi, T.; Kondo, M.; Takatori, H.; et al. Discrete Nature of EpCAM+ and CD90+ Cancer Stem Cells in Human Hepatocellular Carcinoma. Hepatology 2013, 57, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Hao, X.; Yan, M.; Yao, M.; Ge, C.; Gu, J.; Li, J. Cancer Stem/Progenitor Cells are Highly Enriched in CD133+CD44+ Population in Hepatocellular Carcinoma. Int. J. Cancer 2010, 126, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Ho, D.W.; Ng, M.N.; Lau, C.K.; Yu, W.C.; Ngai, P.; Chu, P.W.; Lam, C.T.; Poon, R.T.; Fan, S.T. Significance of CD90+ Cancer Stem Cells in Human Liver Cancer. Cancer Cell 2008, 13, 153–166. [Google Scholar] [CrossRef]
- Haraguchi, N.; Ishii, H.; Mimori, K.; Tanaka, F.; Ohkuma, M.; Kim, H.M.; Akita, H.; Takiuchi, D.; Hatano, H.; Nagano, H.; et al. CD13 is a Therapeutic Target in Human Liver Cancer Stem Cells. J. Clin. Investig. 2010, 120, 3326–3339. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, K.H.; Wong, T.L.; Zhang, Z.; Chan, C.H.; Loong, J.H.; Che, N.; Yu, H.J.; Tan, K.V.; Tong, M.; et al. Lineage Tracing and Single-Cell Analysis Reveal Proliferative Prom1+ Tumour-Propagating Cells and their Dynamic Cellular Transition During Liver Cancer Progression. Gut 2022, 71, 1656–1668. [Google Scholar] [CrossRef]
- Oshimori, N.; Guo, Y.; Taniguchi, S. An Emerging Role for Cellular Crosstalk in the Cancer Stem Cell Niche. J. Pathol. 2021, 254, 384–394. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, C.; Xu, M. Linking Tumor Microenvironment to Plasticity of Cancer Stem Cells: Mechanisms and Application in Cancer Therapy. Front. Oncol. 2021, 11, 678333. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Tunger, A.; Plesca, I.; Wehner, R.; Temme, A.; Westphal, D.; Meier, F.; Bachmann, M.; Schmitz, M. Bidirectional Crosstalk Between Cancer Stem Cells and Immune Cell Subsets. Front. Immunol. 2020, 11, 140. [Google Scholar] [CrossRef]
- Lau, E.Y.; Lo, J.; Cheng, B.Y.; Ma, M.K.; Lee, J.M.; Ng, J.K.; Chai, S.; Lin, C.H.; Tsang, S.Y.; Ma, S.; et al. Cancer-Associated Fibroblasts Regulate Tumor-Initiating Cell Plasticity in Hepatocellular Carcinoma through c-Met/FRA1/HEY1 Signaling. Cell Rep. 2016, 15, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Wang, R.; Chen, Q.; Luo, J.; Wang, J.; Zhao, Z.; Li, Y.; Wang, Y.; Wang, X.; Cheng, B. Cancer-Associated Fibroblasts Promote Stem Cell-Like Properties of Hepatocellular Carcinoma Cells through IL-6/STAT3/Notch Signaling. Am. J. Cancer Res. 2018, 8, 302–316. [Google Scholar]
- Fang, T.; Lv, H.; Lv, G.; Li, T.; Wang, C.; Han, Q.; Yu, L.; Su, B.; Guo, L.; Huang, S.; et al. Tumor-Derived Exosomal miR-1247-3p Induces Cancer-Associated Fibroblast Activation to Foster Lung Metastasis of Liver Cancer. Nat. Commun. 2018, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, R.; Xiong, S.; Wang, X.; Zhao, Z.; Bai, S.; Wang, Y.; Zhao, Y.; Cheng, B. Cancer-Associated Fibroblasts Promote the Stemness of CD24(+) Liver Cells Via Paracrine Signaling. J. Mol. Med. 2019, 97, 243–255. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Wang, L.; Yao, B.; Chen, T.; Li, Q.; Liu, Z.; Liu, R.; Niu, Y.; Song, T.; et al. Resolvin D1 Prevents Epithelial-Mesenchymal Transition and Reduces the Stemness Features of Hepatocellular Carcinoma by Inhibiting Paracrine of Cancer-Associated Fibroblast-Derived COMP. J. Exp. Clin. Cancer Res. 2019, 38, 170. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C.; Wang, Y.; Sun, L.; Liu, Z.; Wang, L.; Song, T.; Yao, Y.; Liu, Q.; Tu, K. HSCs-derived COMP Drives Hepatocellular Carcinoma Progression by Activating MEK/ERK and PI3K/AKT Signaling Pathways. J. Exp. Clin. Cancer Res. 2018, 37, 231. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Bai, S.; Wang, R.; Xiong, S.; Li, Y.; Wang, X.; Chen, W.; Cheng, B. Cancer-Associated Fibroblasts Endow Stem-Like Qualities to Liver Cancer Cells by Modulating Autophagy. Cancer Manag. Res. 2019, 11, 5737–5744. [Google Scholar] [CrossRef]
- Song, M.; He, J.; Pan, Q.Z.; Yang, J.; Zhao, J.; Zhang, Y.J.; Huang, Y.; Tang, Y.; Wang, Q.; He, J.; et al. Cancer-Associated Fibroblast-Mediated Cellular Crosstalk Supports Hepatocellular Carcinoma Progression. Hepatology 2021, 73, 1717–1735. [Google Scholar] [CrossRef]
- Liu, B.; Fang, X.; Kwong, D.L.; Zhang, Y.; Verhoeft, K.; Gong, L.; Zhang, B.; Chen, J.; Yu, Q.; Luo, J.; et al. Targeting TROY-mediated P85a/AKT/TBX3 Signaling Attenuates Tumor Stemness and Elevates Treatment Response in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 182. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.J.; Li, T.W.; Zhou, L.; Wong, T.L.; Liu, X.; Ma, V.; Lo, C.M.; Man, K.; Lee, T.K.; Ning, W.; et al. FSTL1 Secreted by Activated Fibroblasts Promotes Hepatocellular Carcinoma Metastasis and Stemness. Cancer Res. 2021, 81, 5692–5705. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Liu, N.; Lin, M.C.; Zheng, J. Positive Feedback Loop Between Cancer Stem Cells and Angiogenesis in Hepatocellular Carcinoma. Cancer Lett. 2016, 379, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Xia, Y.; Ding, S.; Lu, P.; Sun, L.; Fan, Y.; Li, X.; Wang, Y.; Tian, D.A.; Liu, M. Potential Role of CXCL9 Induced by Endothelial cells/CD133+ Liver Cancer Cells Co-Culture System in Tumor Transendothelial Migration. Genes Cancer 2016, 7, 254–259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, Y.; Shi, D.; Liang, Z.; Liu, Y.; Li, Y.; Xing, Y.; Liu, W.; Ai, Z.; Zhuang, J.; Chen, X.; et al. IL-17A Secreted From Lymphatic Endothelial Cells Promotes Tumorigenesis by Upregulation of PD-L1 in Hepatoma Stem Cells. J. Hepatol. 2019, 71, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Firtina, K.Z.; Koc, D.; Sahin, E.; Avci, S.T.; Yilmaz, M.; Atabey, N.; Erdal, E. Effect of Adipocyte-Secreted Factors On EpCAM+/CD133+ Hepatic Stem Cell Population. Biochem. Biophys. Res. Commun. 2016, 474, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.M.; Jing, Y.Y.; Yu, G.F.; Kou, X.R.; Ye, F.; Gao, L.; Li, R.; Zhao, Q.D.; Yang, Y.; Lu, Z.H.; et al. Tumor-Associated Macrophages Promote Cancer Stem Cell-Like Properties Via Transforming Growth Factor-Beta1-Induced Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma. Cancer Lett. 2014, 352, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Li, X.; Chen, S.; Zeng, Q.; Zhao, Y.; Luo, F. Tumor-Associated Macrophage Or Chemokine Ligand CCL17 Positively Regulates the Tumorigenesis of Hepatocellular Carcinoma. Med. Oncol. 2016, 33, 17. [Google Scholar] [CrossRef]
- Li, X.F.; Chen, C.; Xiang, D.M.; Qu, L.; Sun, W.; Lu, X.Y.; Zhou, T.F.; Chen, S.Z.; Ning, B.F.; Cheng, Z.; et al. Chronic Inflammation-Elicited Liver Progenitor Cell Conversion to Liver Cancer Stem Cell with Clinical Significance. Hepatology 2017, 66, 1934–1951. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, H.; Zhou, C.; Su, Q.; Lin, Y.; Xie, Y.; Huang, Y.; Qiu, Q.; Lin, J.; Huang, X.; et al. TNF-alpha Derived From M2 Tumor-Associated Macrophages Promotes Epithelial-Mesenchymal Transition and Cancer Stemness through the Wnt/beta-catenin Pathway in SMMC-7721 Hepatocellular Carcinoma Cells. Exp. Cell Res. 2019, 378, 41–50. [Google Scholar] [CrossRef]
- Wei, R.; Zhu, W.W.; Yu, G.Y.; Wang, X.; Gao, C.; Zhou, X.; Lin, Z.F.; Shao, W.Q.; Wang, S.H.; Lu, M.; et al. S100 Calcium-Binding Protein A9 From Tumor-Associated Macrophage Enhances Cancer Stem Cell-Like Properties of Hepatocellular Carcinoma. Int. J. Cancer 2021, 148, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.L.; Yin, D.; Hu, Z.Q.; Luo, C.B.; Zhou, Z.J.; Xin, H.Y.; Yang, X.R.; Shi, Y.H.; Wang, Z.; Huang, X.W.; et al. A Positive Feedback Loop Between Cancer Stem-Like Cells and Tumor-Associated Neutrophils Controls Hepatocellular Carcinoma Progression. Hepatology 2019, 70, 1214–1230. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Shiota, G. Immune Evasion by Cancer Stem Cells. Regen. Ther. 2021, 17, 20–33. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Ren, X. Interaction Between Regulatory T Cells and Cancer Stem Cells. Int. J. Cancer 2012, 131, 1491–1498. [Google Scholar] [CrossRef]
- Shi, C.; Chen, Y.; Chen, Y.; Yang, Y.; Bing, W.; Qi, J. CD4(+) CD25(+) Regulatory T Cells Promote Hepatocellular Carcinoma Invasion Via TGF-beta1-induced Epithelial-Mesenchymal Transition. Onco Targets Ther. 2019, 12, 279–289. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Li, S.; Lv, M.; Yang, X.; Li, M.; Zhang, Z. CXCL11 Promotes Self-Renewal and Tumorigenicity of Alpha2delta1(+) Liver Tumor-Initiating Cells through CXCR3/ERK1/2 Signaling. Cancer Lett. 2019, 449, 163–171. [Google Scholar] [CrossRef]
- Song, M.; Ping, Y.; Zhang, K.; Yang, L.; Li, F.; Zhang, C.; Cheng, S.; Yue, D.; Maimela, N.R.; Qu, J.; et al. Low-Dose IFNgamma Induces Tumor Cell Stemness in Tumor Microenvironment of Non-Small Cell Lung Cancer. Cancer Res. 2019, 79, 3737–3748. [Google Scholar] [CrossRef]
- Xiang, T.; Long, H.; He, L.; Han, X.; Lin, K.; Liang, Z.; Zhuo, W.; Xie, R.; Zhu, B. Interleukin-17 Produced by Tumor Microenvironment Promotes Self-Renewal of CD133+ Cancer Stem-Like Cells in Ovarian Cancer. Oncogene 2015, 34, 165–176. [Google Scholar] [CrossRef]
- Zhang, Y.; Zoltan, M.; Riquelme, E.; Xu, H.; Sahin, I.; Castro-Pando, S.; Montiel, M.F.; Chang, K.; Jiang, Z.; Ling, J.; et al. Immune Cell Production of Interleukin 17 Induces Stem Cell Features of Pancreatic Intraepithelial Neoplasia Cells. Gastroenterology 2018, 155, 210–223. [Google Scholar] [CrossRef]
- Jiang, Y.X.; Yang, S.W.; Li, P.A.; Luo, X.; Li, Z.Y.; Hao, Y.X.; Yu, P.W. The Promotion of the Transformation of Quiescent Gastric Cancer Stem Cells by IL-17 and the Underlying Mechanisms. Oncogene 2017, 36, 1256–1264. [Google Scholar] [CrossRef]
- Shokouhifar, A.; Firouzi, J.; Nouri, M.; Sarab, G.A.; Ebrahimi, M. NK Cell Upraise in the Dark World of Cancer Stem Cells. Cancer Cell Int. 2021, 21, 682. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Zhang, Y.; Rosenblatt, J.D. B Cell Regulation of the Anti-Tumor Response and Role in Carcinogenesis. J. Immunother. Cancer 2016, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Tsunedomi, R.; Yoshimura, K.; Matsukuma, S.; Shindo, Y.; Matsui, H.; Tokumitsu, Y.; Yoshida, S.; Iida, M.; Suzuki, N.; et al. Immune Evasion of Hepatoma Cancer Stem-Like Cells from Natural Killer Cells. Ann. Surg. Oncol. 2022. [Google Scholar] [CrossRef]
- Zhong, M.; Zhong, C.; Cui, W.; Wang, G.; Zheng, G.; Li, L.; Zhang, J.; Ren, R.; Gao, H.; Wang, T.; et al. Induction of Tolerogenic Dendritic Cells by Activated TGF-beta/Akt/Smad2 Signaling in RIG-I-deficient Stemness-High Human Liver Cancer Cells. BMC Cancer 2019, 19, 439. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.G.M.; Bresnahan, E.; Molina-Sanchez, P.; Lindblad, K.E.; Maier, B.; Sia, D.; Puigvehi, M.; Miguela, V.; Casanova-Acebes, M.; Dhainaut, M.; et al. Beta-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019, 9, 1124–1141. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Q.; Greten, T.F. MDSCs in Liver Cancer: A Critical Tumor-Promoting Player and a Potential Therapeutic Target. Cell. Immunol. 2021, 361, 104295. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, Z.; Song, J.; Lan, X.; Lu, S.; Chen, M.; Wang, Z.; Chen, W.; Fan, X.; Wu, F.; et al. Interactions Between Interleukin-6 and Myeloid-Derived Suppressor Cells Drive the Chemoresistant Phenotype of Hepatocellular Cancer. Exp. Cell Res. 2017, 351, 142–149. [Google Scholar] [CrossRef]
- Peng, D.; Tanikawa, T.; Li, W.; Zhao, L.; Vatan, L.; Szeliga, W.; Wan, S.; Wei, S.; Wang, Y.; Liu, Y.; et al. Myeloid-Derived Suppressor Cells Endow Stem-Like Qualities to Breast Cancer Cells through IL6/STAT3 and NO/NOTCH Cross-Talk Signaling. Cancer Res. 2016, 76, 3156–3165. [Google Scholar] [CrossRef]
- Cao, G.D.; He, X.B.; Sun, Q.; Chen, S.; Wan, K.; Xu, X.; Feng, X.; Li, P.P.; Chen, B.; Xiong, M.M. The Oncolytic Virus in Cancer Diagnosis and Treatment. Front. Oncol. 2020, 10, 1786. [Google Scholar] [CrossRef]
- Bach, P.; Abel, T.; Hoffmann, C.; Gal, Z.; Braun, G.; Voelker, I.; Ball, C.R.; Johnston, I.C.; Lauer, U.M.; Herold-Mende, C.; et al. Specific Elimination of CD133+ Tumor Cells with Targeted Oncolytic Measles Virus. Cancer Res. 2013, 73, 865–874. [Google Scholar] [CrossRef]
- Kleinlutzum, D.; Hanauer, J.; Muik, A.; Hanschmann, K.M.; Kays, S.K.; Ayala-Breton, C.; Peng, K.W.; Muhlebach, M.D.; Abel, T.; Buchholz, C.J. Enhancing the Oncolytic Activity of CD133-Targeted Measles Virus: Receptor Extension or Chimerism with Vesicular Stomatitis Virus are Most Effective. Front. Oncol. 2017, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, C.; Wang, Y.; Lv, H.; Guo, Y.; Dai, H.; Wicha, M.S.; Chang, A.E.; Li, Q. Cytokine-Induced Killer (CIK) Cells Bound with anti-CD3/anti-CD133 Bispecific Antibodies Target CD133(high) Cancer Stem Cells in Vitro and in Vivo. Clin. Immunol. 2013, 149, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Tong, C.; Shi, D.; Chen, M.; Guo, Y.; Chen, D.; Han, X.; Wang, H.; Wang, Y.; Shen, P. Efficacy and Biomarker Analysis of CD133-directed CAR T Cells in Advanced Hepatocellular Carcinoma: A Single-Arm, Open-Label, Phase II Trial. Oncoimmunology 2020, 9, 1846926. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, M.; Wu, Z.; Tong, C.; Dai, H.; Guo, Y.; Liu, Y.; Huang, J.; Lv, H.; Luo, C.; et al. CD133-directed CAR T Cells for Advanced Metastasis Malignancies: A Phase I Trial. Oncoimmunology 2018, 7, e1440169. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; Pan, K.; Chen, M.S.; Wang, Q.J.; Wang, H.; Ma, H.Q.; Li, Y.Q.; Liang, X.T.; Li, J.J.; Zhao, J.J.; et al. Dendritic Cells-Mediated CTLs Targeting Hepatocellular Carcinoma Stem Cells. Cancer Biol. Ther. 2010, 10, 368–375. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, Y.J.; Park, S.J.; Park, Y.S.; Park, H.S.; Yang, K.M.; Heo, K. EpCAM Peptide-Primed Dendritic Cell Vaccination Confers Significant Anti-Tumor Immunity in Hepatocellular Carcinoma Cells. PLoS ONE 2018, 13, e190638. [Google Scholar] [CrossRef]
- Ogawa, K.; Tanaka, S.; Matsumura, S.; Murakata, A.; Ban, D.; Ochiai, T.; Irie, T.; Kudo, A.; Nakamura, N.; Tanabe, M.; et al. EpCAM-targeted Therapy for Human Hepatocellular Carcinoma. Ann. Surg. Oncol. 2014, 21, 1314–1322. [Google Scholar] [CrossRef]
- Ibis, K.; Nalbat, E.; Caliskan, B.; Kahraman, D.C.; Cetin-Atalay, R.; Banoglu, E. Synthesis and Biological Evaluation of Novel Isoxazole-Piperazine Hybrids as Potential Anti-Cancer Agents with Inhibitory Effect On Liver Cancer Stem Cells. Eur. J. Med. Chem. 2021, 221, 113489. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Q.; Zhou, Y.; Zhu, H.; Li, T.; Du, F. A Novel Nitidine Chloride Nanoparticle Overcomes the Stemness of CD133(+)EPCAM(+) Huh7 Hepatocellular Carcinoma Cells for Liver Cancer Therapy. BMC Pharmacol. Toxicol. 2022, 23, 48. [Google Scholar] [CrossRef]
- Chen, W.C.; Chang, Y.S.; Hsu, H.P.; Yen, M.C.; Huang, H.L.; Cho, C.Y.; Wang, C.Y.; Weng, T.Y.; Lai, P.T.; Chen, C.S.; et al. Therapeutics Targeting CD90-integrin-AMPK-CD133 Signal Axis in Liver Cancer. Oncotarget 2015, 6, 42923–42937. [Google Scholar] [CrossRef]
- Dou, C.; Fang, C.; Zhao, Y.; Fu, X.; Zhang, Y.; Zhu, D.; Wu, H.; Liu, H.; Zhang, J.; Xu, W.; et al. BC-02 Eradicates Liver Cancer Stem Cells by Upregulating the ROS-dependent DNA Damage. Int. J. Oncol. 2017, 51, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Wada, H.; Eguchi, H.; Ogawa, H.; Yamada, D.; Noda, T.; Asaoka, T.; Kawamoto, K.; Gotoh, K.; Umeshita, K.; et al. A CD13 Inhibitor, Ubenimex, Synergistically Enhances the Effects of Anticancer Drugs in Hepatocellular Carcinoma. Int. J. Oncol. 2016, 49, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.B.; Gong, J.H.; Liu, X.J.; Li, Y.; Zhen, Y.S. A CD13-targeting Peptide Integrated Protein Inhibits Human Liver Cancer Growth by Killing Cancer Stem Cells and Suppressing Angiogenesis. Mol. Carcinog. 2017, 56, 1395–1404. [Google Scholar] [CrossRef]
- Toshiyama, R.; Konno, M.; Eguchi, H.; Takemoto, H.; Noda, T.; Asai, A.; Koseki, J.; Haraguchi, N.; Ueda, Y.; Matsushita, K.; et al. Poly(Ethylene Glycol)-Poly(Lysine) Block Copolymer-Ubenimex Conjugate Targets Aminopeptidase N and Exerts an Antitumor Effect in Hepatocellular Carcinoma Stem Cells. Oncogene 2019, 38, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.N.; Zhang, B.; Wang, H.Y.; Wang, D.X.; Zhang, M.M.; Zhang, M.; Wang, X.K.; Fan, S.Y.; Xu, Y.C.; Zeng, Q.; et al. A Functional Screening Identifies a New Organic Selenium Compound Targeting Cancer Stem Cells: Role of c-Myc Transcription Activity Inhibition in Liver Cancer. Adv. Sci. 2022, 9, e2201166. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, F.; Zhang, X.; Wang, T.; Jiang, J.; Cai, J.; Gao, Q.; Hezam, K.; Liu, Y.; Xie, J.; et al. CD24 Targeting Bi-Specific Antibody that Simultaneously Stimulates NKG2D Enhances the Efficacy of Cancer Immunotherapy. J. Cancer Res. Clin. Oncol. 2019, 145, 1179–1190. [Google Scholar] [CrossRef]
- Rodriguez, M.M.; Fiore, E.; Bayo, J.; Atorrasagasti, C.; Garcia, M.; Onorato, A.; Dominguez, L.; Malvicini, M.; Mazzolini, G. 4Mu Decreases CD47 Expression on Hepatic Cancer Stem Cells and Primes a Potent Antitumor T Cell Response Induced by Interleukin-12. Mol. Ther. 2018, 26, 2738–2750. [Google Scholar] [CrossRef]
- Lo, J.; Lau, E.Y.; Ching, R.H.; Cheng, B.Y.; Ma, M.K.; Ng, I.O.; Lee, T.K. Nuclear Factor Kappa B-mediated CD47 Up-Regulation Promotes Sorafenib Resistance and its Blockade Synergizes the Effect of Sorafenib in Hepatocellular Carcinoma in Mice. Hepatology 2015, 62, 534–545. [Google Scholar] [CrossRef]
- Bilusic, M.; Heery, C.R.; Collins, J.M.; Donahue, R.N.; Palena, C.; Madan, R.A.; Karzai, F.; Marte, J.L.; Strauss, J.; Gatti-Mays, M.E.; et al. Phase I Trial of HuMax-IL8 (BMS-986253), an anti-IL-8 Monoclonal Antibody, in Patients with Metastatic Or Unresectable Solid Tumors. J. Immunother. Cancer 2019, 7, 240. [Google Scholar] [CrossRef]


| Approach | Agent or Inhibitor | Target | Applied Model | Refs. |
|---|---|---|---|---|
| Oncolytic viruses | MV-CD133 VSV-CD133 | CD133 | Cell lines Mouse Models | [119,120,121] |
| Antibody and cell-based | BsAb-CIK | CD133 | Cell lines Mouse Models | [122] |
| CAR T | CAR-CD133 | CD133 | Clinical Trials | [123,124] |
| CAR-EpCAM | EpCAM | Clinical Trials | / | |
| Vaccines | DC vaccines | CD133 CD44 EpCAM | Cell lines Mouse Models | [125,126] |
| Compounds | VB4-845 | EpCAM | Cell lines Mouse Models | [127] |
| isoxazole-piperazine analogue | CD133 EpCAM | Cell lines | [128] | |
| TPGS-FA/NC | CD133 EpCAM | Cell lines Mouse Models | [129] | |
| OSU-CG5 | CD90 | Cell lines Mouse Models | [130] | |
| Bestatin | CD13 | Cell lines | [131,132] | |
| NGR-LDP-AE | CD13 | Cell lines Mouse Models | [133] | |
| PEG-b-PLys (Ube) | CD13 | Cell lines Mouse Models | [134] | |
| CU27 | CD133 CD13 | Cell lines Mouse Models | [135] | |
| 4Mu | CD47 | Cell lines Mouse Models | [137] | |
| Resolvin D1 | CAFs-derived COMP | Cell lines Mouse Models | [87] | |
| Antibodies | anti-CD44 antibody | CD44 CD90 | Cell lines Mouse Models | [77] |
| BsAb cG7-MICA | CD24 | Cell lines Mouse Models | [136] | |
| anti-CD47 antibody | CD47 | Cell lines Mouse Models | [138] | |
| 1B50-1 | α2δ1 | Cell lines Mouse Models | [54] | |
| Tocilizumab | CD44 | Cell lines Mouse Models | [27] | |
| FSTL1 neutralizing antibody | CAFs-derived FSTL1 | Cell lines Mouse Models | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Yan, Q.; Liu, S.; Guan, X.-Y. Cancer Stem Cells in Hepatocellular Carcinoma: Intrinsic and Extrinsic Molecular Mechanisms in Stemness Regulation. Int. J. Mol. Sci. 2022, 23, 12327. https://doi.org/10.3390/ijms232012327
Fang X, Yan Q, Liu S, Guan X-Y. Cancer Stem Cells in Hepatocellular Carcinoma: Intrinsic and Extrinsic Molecular Mechanisms in Stemness Regulation. International Journal of Molecular Sciences. 2022; 23(20):12327. https://doi.org/10.3390/ijms232012327
Chicago/Turabian StyleFang, Xiaona, Qian Yan, Shan Liu, and Xin-Yuan Guan. 2022. "Cancer Stem Cells in Hepatocellular Carcinoma: Intrinsic and Extrinsic Molecular Mechanisms in Stemness Regulation" International Journal of Molecular Sciences 23, no. 20: 12327. https://doi.org/10.3390/ijms232012327
APA StyleFang, X., Yan, Q., Liu, S., & Guan, X.-Y. (2022). Cancer Stem Cells in Hepatocellular Carcinoma: Intrinsic and Extrinsic Molecular Mechanisms in Stemness Regulation. International Journal of Molecular Sciences, 23(20), 12327. https://doi.org/10.3390/ijms232012327







