Metabolic Regulation and Lipidomic Remodeling in Relation to Spermidine-induced Stress Tolerance to High Temperature in Plants
Abstract
1. Introduction
2. Results
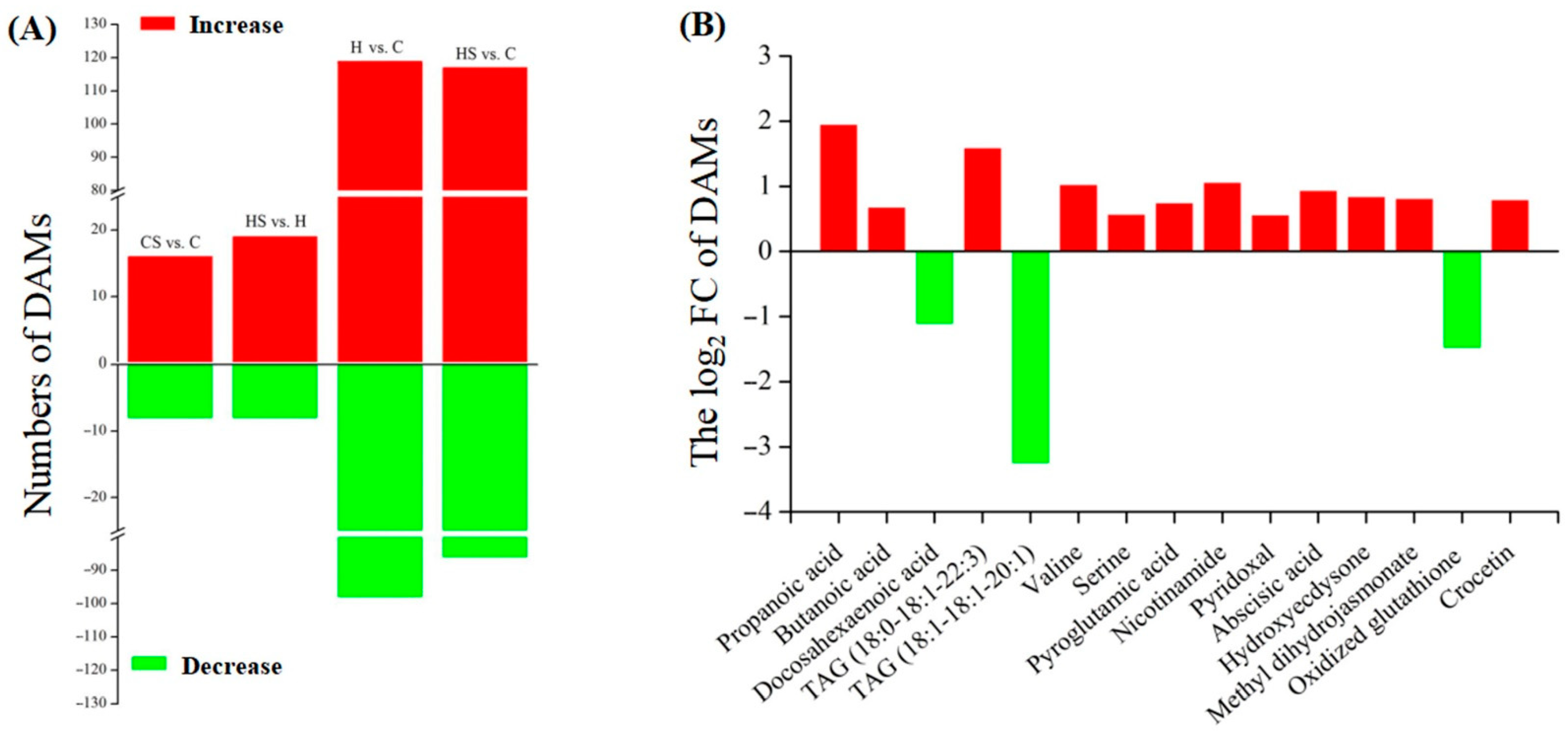
2.1. Stress Tolerance and Metabolomics Regulated by Spermidine in White Clover under Heat Stress
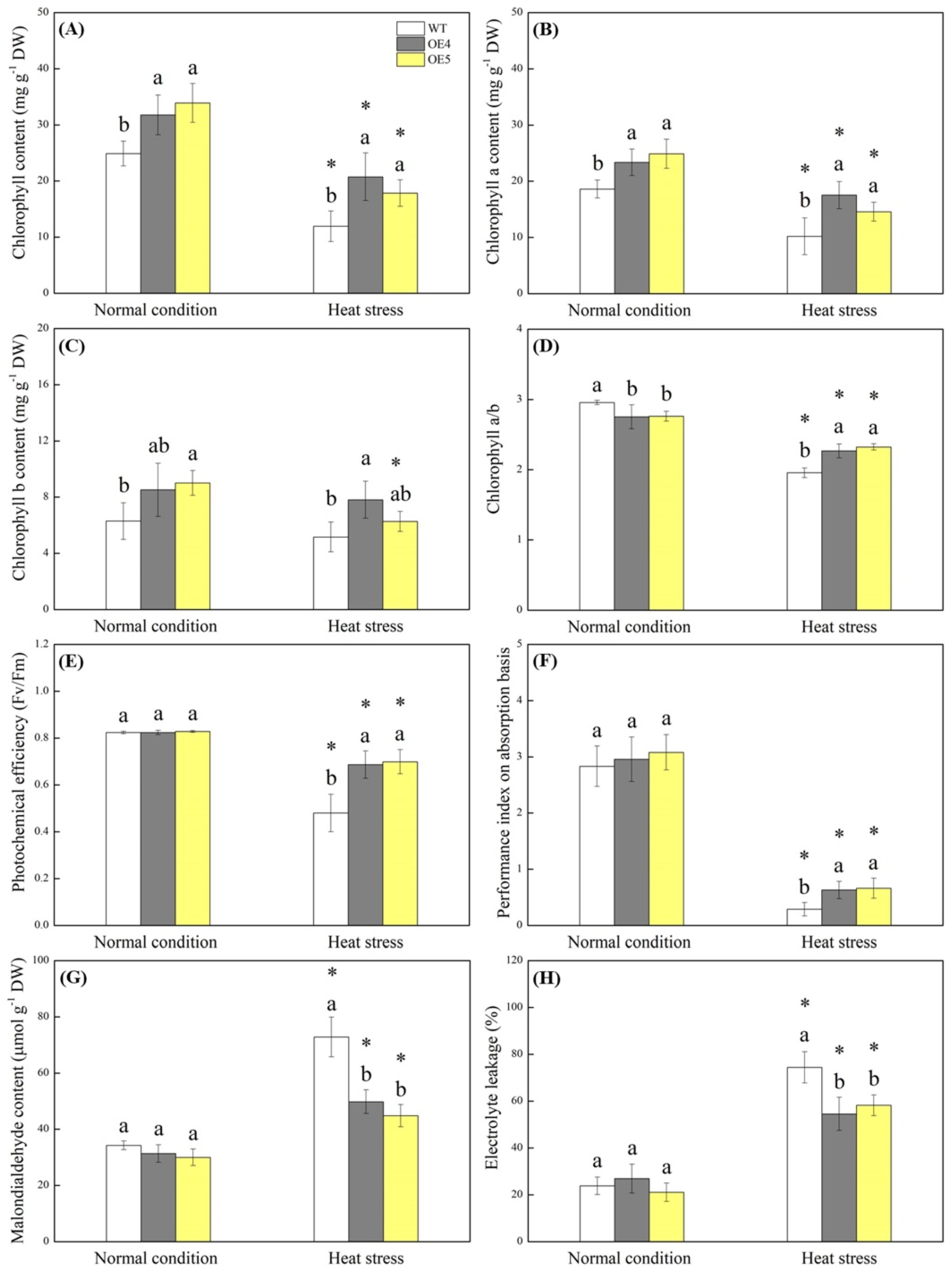
2.2. Endogenous Spermidine Level and Stress Tolerance Regulated by TrSAMS Overexpression in Arabidopsis thaliana under Heat Conditions
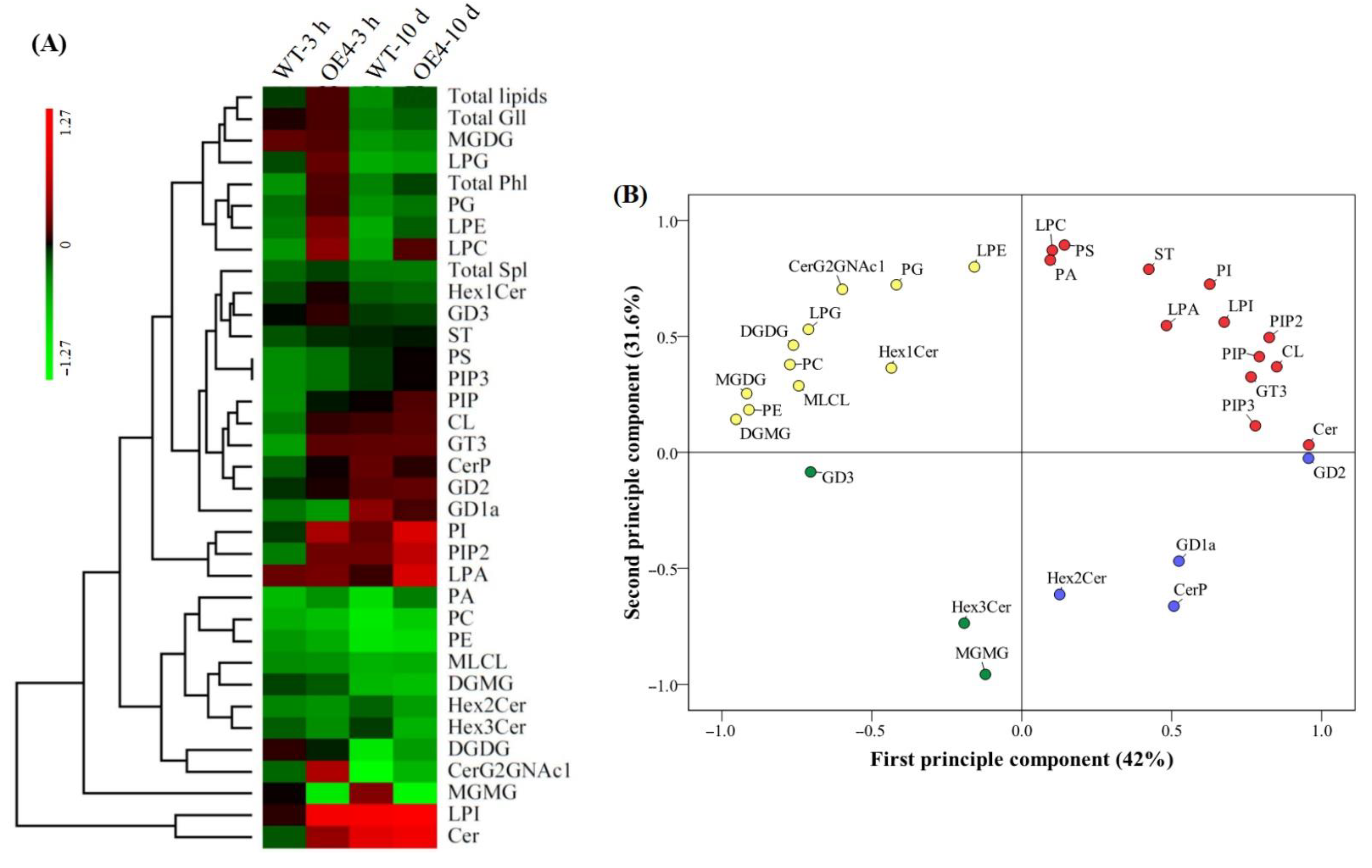
2.3. Heat-Map and Principal Component Analysis Based on 31 Lipid Classes in Arabidopsis thaliana in Response to Spermidine and Heat Stress
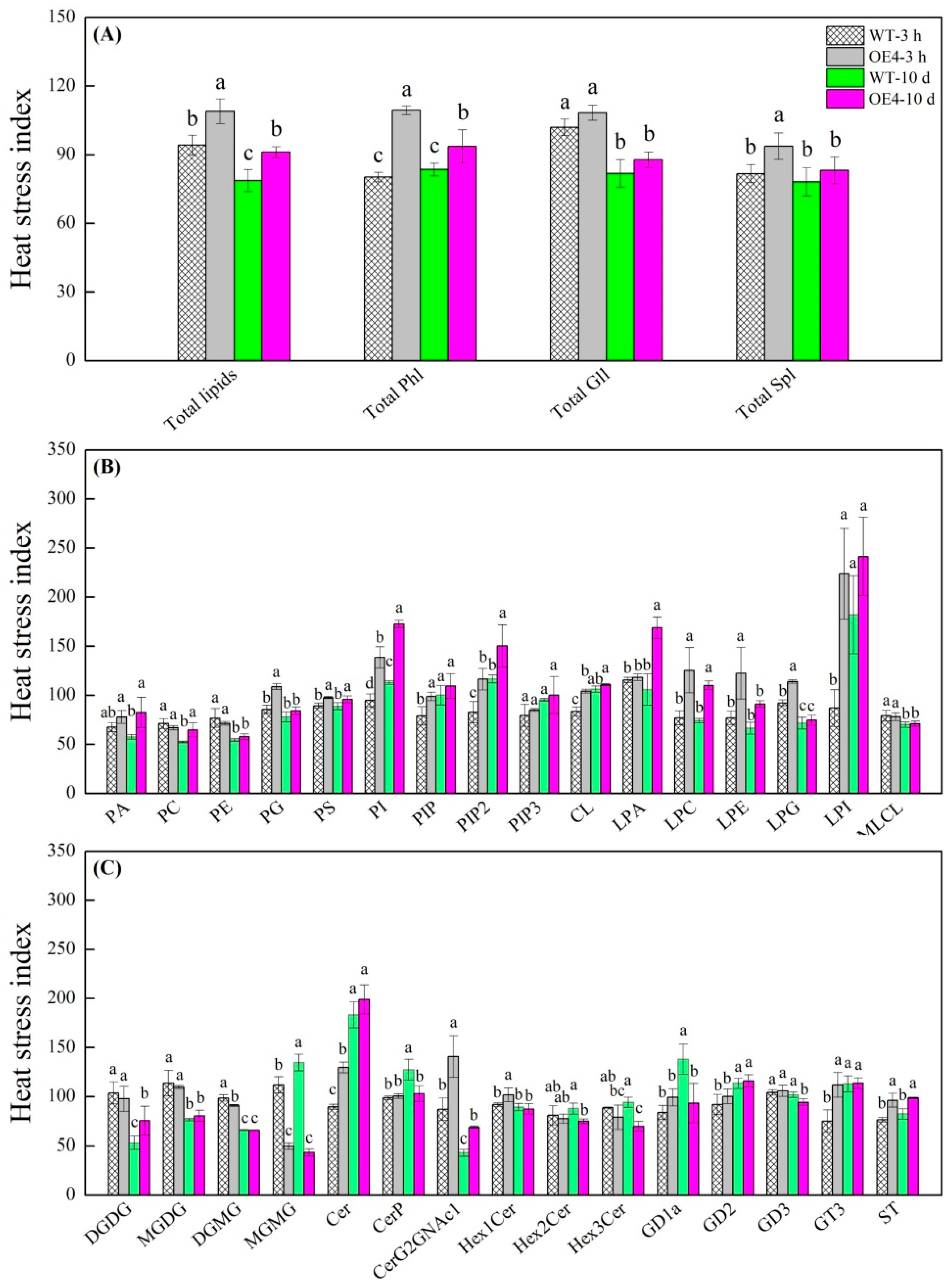
2.4. Changes in Total Lipids, Phospholipids, Glycoglycerolipids, and Sphingolipids Regulated by Spermidine in Arabidopsis thaliana under Heat Stress
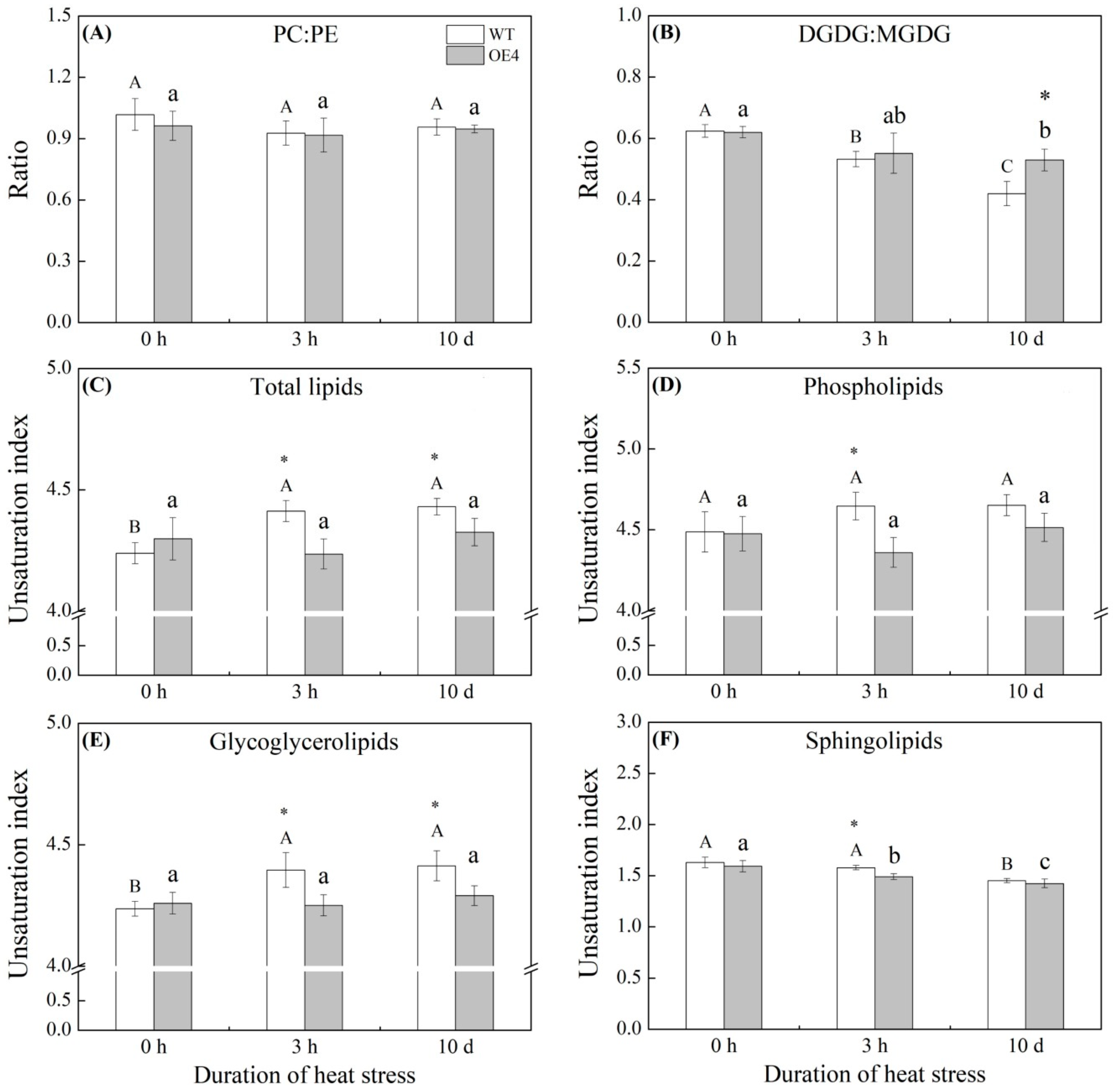
2.5. Lipid Molecular Species, Ratios of PC:PE and DGDG:MGDG, and the Unsaturation Index of Lipids Regulated by Spermidine in Arabidopsis thaliana under Heat Stress
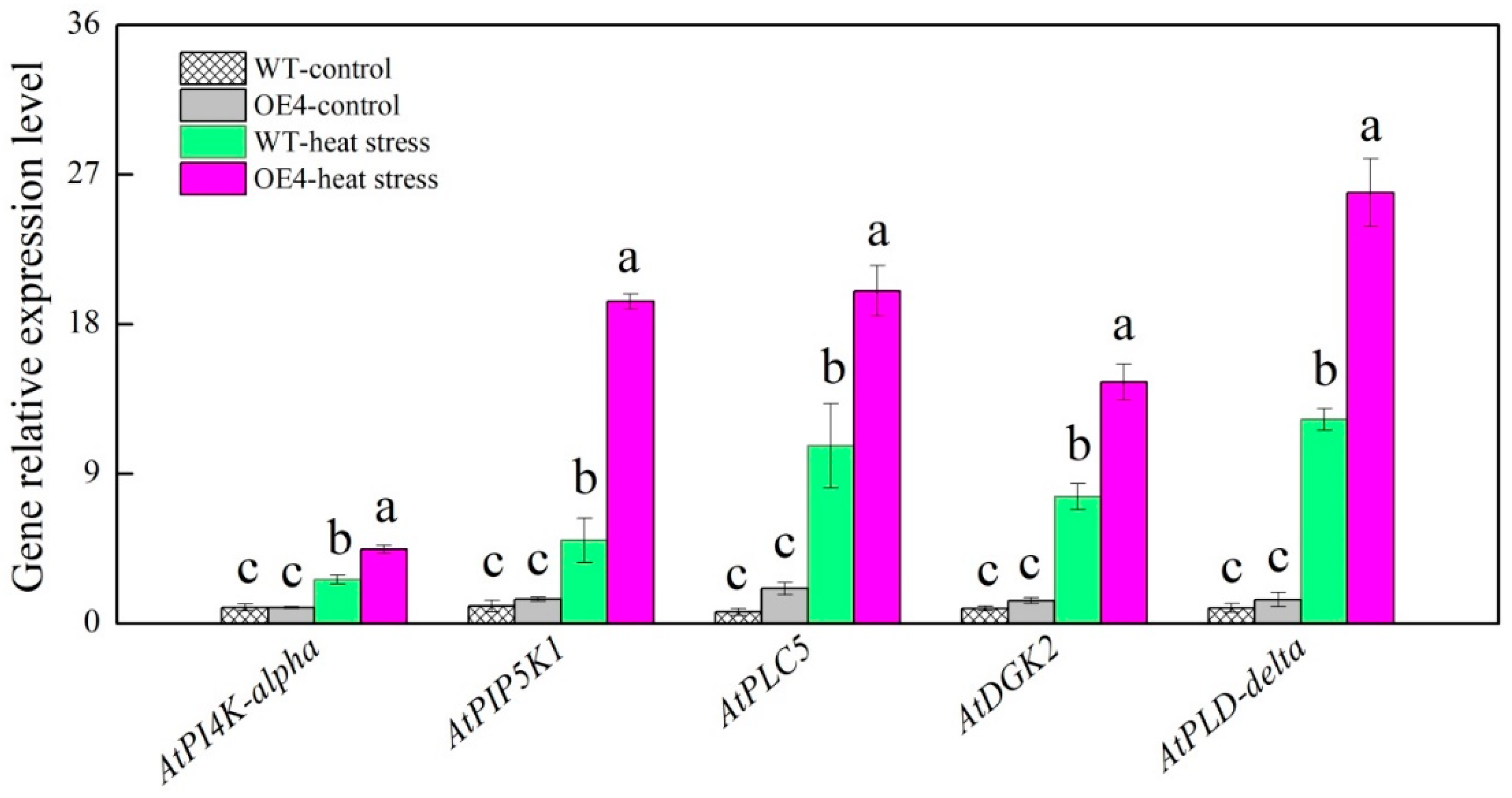
2.6. Key Genes Involved in PI and PA Metabolic Pathways Regulated by Spermidine in Arabidopsis thaliana under Heat Stress
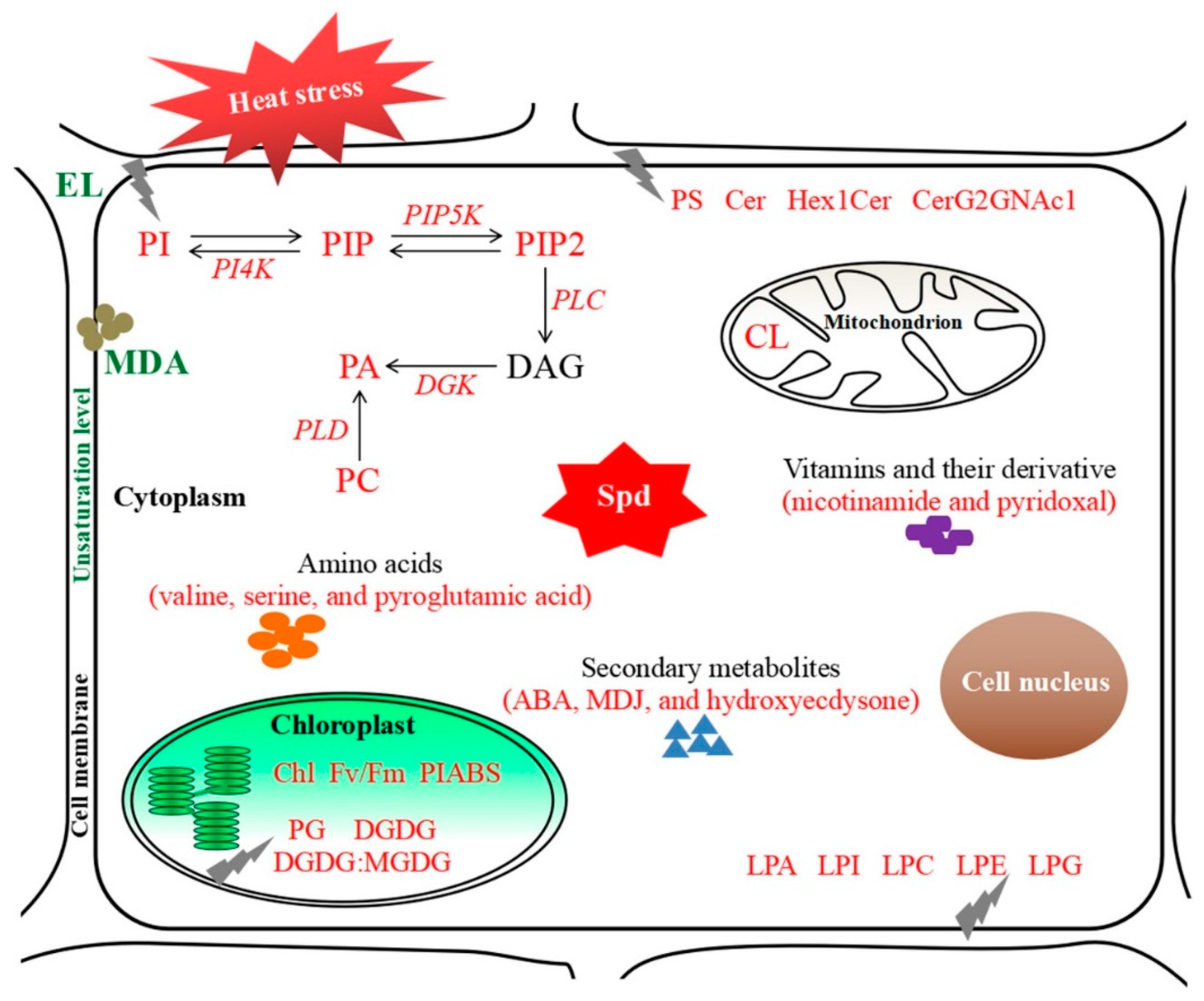
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Growth and Physiological Measurements
4.3. Measurements of Endogenous Spermidine
4.4. Metabolomics Analysis
4.5. Lipidomics Analysis
4.6. Gene Expression Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Gourdji, S.M.; Sibley, A.M.; Lobell, D.B. Global crop exposure to critical high temperatures in the reproductive period: Historical trends and future projections. Environ. Res. Lett. 2013, 8, 024041. [Google Scholar] [CrossRef]
- Bokszczanin, K. Perspectives on deciphering mechanisms underlying plant heat stress response and thermotolerance. Front. Plant 2013, 4, 315. [Google Scholar] [CrossRef]
- Takashi, H.; Katsutomo, T.; Ken-Ichi, S.; Yasuyuki, Y. Molecular cloning of plant spermidine synthases. Plant Cell Physiol. 1998, 39, 73–79. [Google Scholar]
- de Sousa Araújo, S.; dos Santos, A.L.W.; Duque, A.S. Engineering polyamine metabolic pathways for abiotic stress tolerance in plants. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants: Recent Advances and Future Perspectives; Hossain, M.A., Kumar, V., Burritt, D.J., Fujita, M., Mäkelä, P.S.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 287–318. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Li, Y.P.; Zhang, X.Q.; Ma, X.; Huang, L.K.; Yan, Y.H.; Peng, Y. Chitosan and spermine enhance drought resistance in white clover, associated with changes in endogenous phytohormones and polyamines, and antioxidant metabolism. Funct. Plant Biol. 2018, 45, 1205–1222. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Sun, J.; Guo, S. Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar] [CrossRef]
- Li, S.; Jin, H.; Zhang, Q. The Effect of exogenous spermidine concentration on polyamine metabolism and salt tolerance in Zoysiagrass (Zoysia japonica Steud) subjected to short-term salinity stress. Front. Plant Sci. 2016, 7, 1221. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, H.; Li, L.; Liu, X.; Chen, L.; Chen, W.; Ding, Y. Exogenous spermidine enhances the photosynthetic and antioxidant capacity of rice under heat stress during early grain-filling period. Funct. Plant Biol. 2018, 45, 911–921. [Google Scholar] [CrossRef]
- Fu, Y.; Gu, Q.; Dong, Q.; Zhang, Z.; Lin, C.; Hu, W.; Pan, R.; Guan, Y.; Hu, J. Spermidine enhances heat tolerance of rice seeds by modulating endogenous starch and polyamine metabolism. Molecules 2019, 24, 1395. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zou, Y.; Ding, S.; Zhang, J.; Yu, X.; Cao, J.; Gang, L. Polyamine accumulation in transgenic tomato enhances the tolerance to high temperature stress. J. Integr. Plant Biol. 2010, 51, 489–499. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, R.R.; Wang, F.Y.; Peng, Z.; Kong, F.L.; Wu, J.; Cao, J.S.; Lu, G. Spermidine affects the transcriptome responses to high temperature stress in ripening tomato fruit. J. Zhejiang Univ. SCIENCE B 2012, 13, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.L.; Cao, Y.Q.; Wang, D.; Peng, Y.; Zhang, Y.; Li, Z. Spermine alleviates heat-induced senescence in creeping bentgrass by regulating water and oxidative balance, photosynthesis, and heat shock proteins. Biol. Plant. 2021, 65, 184–192. [Google Scholar] [CrossRef]
- Luo, L.; Li, Z.; Tang, M.Y.; Cheng, B.Z.; Zeng, W.H.; Peng, Y.; Nie, G.; Zhang, X.Q. Metabolic regulation of polyamines and γ-aminobutyric acid in relation to spermidine-induced heat tolerance in white clover. Plant Biol. 2020, 22, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Qian, P.; Jin, R.; Ali, S.; Khan, M.; Aziz, R.; Tian, T.; Zhou, W. Physiological and ultra-structural changes in Brassica napus seedlings induced by cadmium stress. Biol. Plant. 2014, 58, 131–138. [Google Scholar] [CrossRef]
- Ali, B.; Gill, R.A.; Yang, S.; Gill, M.B.; Ali, S.; Rafiq, M.T.; Zhou, W. Hydrogen sulfide alleviates cadmium-induced morpho-physiological and ultrastructural changes in Brassica napus. Ecotoxicol. Environ. Saf. 2014, 110, 197–207. [Google Scholar] [CrossRef]
- Qi, H.; Kang, D.; Zeng, W.; Jawad Hassan, M.; Peng, Y.; Zhang, X.; Zhang, Y.; Feng, G.; Li, Z. Alterations of endogenous hormones, antioxidant metabolism, and aquaporin gene expression in relation to γ-aminobutyric acid-regulated thermotolerance in white clover. Antioxidants 2021, 10, 1099. [Google Scholar] [CrossRef]
- Ali, B.; Gill, R.A.; Yang, S.; Gill, M.B.; Farooq, M.A.; Liu, D.; Daud, M.K.; Ali, S.; Zhou, W. Regulation of cadmium-induced proteomic and metabolic changes by 5-aminolevulinic acid in leaves of Brassica napus L. PLoS ONE 2015, 10, e0123328. [Google Scholar] [CrossRef]
- Watson, A.D. Thematic review series: Systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: A global approach to lipid analysis in biological systems. J. Lipid Res. 2006, 47, 2101–2111. [Google Scholar] [CrossRef]
- Liu, X.; Ma, D.; Zhang, Z.; Wang, S.; Du, S.; Deng, X.; Yin, L. Plant lipid remodeling in response to abiotic stresses. Environ. Exp. Bot. 2019, 165, 174–184. [Google Scholar] [CrossRef]
- Orešič, M.; Hänninen, V.A.; Vidal-Puig, A. Lipidomics: A new window to biomedical frontiers. Trends Biotechnol. 2008, 26, 647–652. [Google Scholar] [CrossRef]
- Hsu, F.F. Mass spectrometry-based shotgun lipidomics—A critical review from the technical point of view. Anal. Bioanal. Chem. 2018, 410, 6387–6409. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Zoong Lwe, Z.; Gandhi, N.; Welti, R.; Fallen, B.; Smith, J.; Rustgi, S. Comparative lipidomic analysis reveals heat stress responses of two soybean genotypes differing in temperature sensitivity. Plants 2020, 9, 457. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Okazaki, Y.; Myouga, F.; Shinozaki, K.; Saito, K. Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana. Sci. Rep. 2015, 5, 10533. [Google Scholar] [CrossRef]
- Dureja-Munjal, I.; Acharya, M.K.; Guha-Mukherjee, S. Effect of hormones and spermidine on the turnover of inositolphospholipids in Brassica seedlings. Phytochemistry 1992, 31, 1161–1163. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Lee, I.J. Regulation of salicylic acid, jasmonic acid and fatty acids in cucumber (Cucumis sativus L.) by spermidine promotes plant growth against salt stress. Acta Physiol. Plant. 2013, 35, 3315–3322. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Way, D.A.; Sharkey, T.D. Plant heat stress: Concepts directing future research. Plant Cell Environ. 2021, 44, 1992–2005. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Yoshida, N.; Fujita, M. Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul. 2014, 73, 31–44. [Google Scholar] [CrossRef]
- Hildebrandt, T.M. Synthesis versus degradation: Directions of amino acid metabolism during Arabidopsis abiotic stress response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Peng, Y.; Huang, B. Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci. Rep. 2016, 6, 30338. [Google Scholar] [CrossRef]
- Takeuchi, S.; Kono, Y.; Kawarada, A.; Ota, Y.; Nakayama, M. Nicotinamide as a plant growth regulator isolated from rice hulls. Agric. Biol. Chem. 1975, 39, 859–861. [Google Scholar] [CrossRef]
- Hassanein, R.A.; Bassiouny, F.; Barakat, D.M.; Khalil, R. Physiological effects of nicotinamide and ascorbic acid on Zea mays plant grown under salinity stress. I-Changes in growth, some relevant metabolic activities and oxidative defense systems. Res. J. Agric. Biol. Sci. 2009, 31, 72–80. [Google Scholar]
- Abdelhamid, M.T.; Sadak, M.S.; Schmidhalter, U.R.S.; El-Saady, A.K.M. Interactive effects of salinity stress and nicotinamide on physiological and biochemical parameters of faba bean plant. Acta Biológica Colomb. 2013, 18, 499–509. [Google Scholar]
- Colinas, M.; Eisenhut, M.; Tohge, T.; Pesquera, M.; Fernie, A.; Weber, A.; Fitzpatrick, T. Balancing of B6 vitamers is essential for plant development and metabolism in Arabidopsis. Plant Cell 2016, 28, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Vanderschuren, H.; Boycheva, S.; Li, K.T.; Szydlowski, N.; Gruissem, W.; Fitzpatrick, T.B. Strategies for vitamin B6 biofortification of plants: A dual role as a micronutrient and a stress protectant. Front. Plant Sci. 2013, 4, 143. [Google Scholar] [CrossRef]
- Larkindale, J.; Knight, M.R. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [CrossRef]
- Ding, W.; Song, L.; Wang, X.; Bi, Y. Effect of abscisic acid on heat stress tolerance in the calli from two ecotypes of Phragmites communis. Biol. Plant. 2010, 54, 607–613. [Google Scholar] [CrossRef]
- Thussagunpanit, J.; Jutamanee, K.; Kaveeta, L.; Chai-arree, W.; Pankean, P.; Suksamrarn, A. Effects of a brassinosteroid and an ecdysone analogue on pollen germination of rice under heat stress. J. Pestic. Sci. 2013, 38, 105–111. [Google Scholar] [CrossRef]
- Qian, Y.; Zhu, C.; Xia, K.; Gan, L. Effects of methyl dihydrojasmonate on seedling growth and drought resistance in tall fescue. J. Nanjing Agric. Univ. 2009, 32, 47–51. [Google Scholar]
- He, M.; Ding, N.Z. Plant unsaturated fatty acids: Multiple roles in stress response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- Hu, T.; Sun, X.Y.; Zhao, Z.J.; Amombo, E.; Fu, J.M. High temperature damage to fatty acids and carbohydrate metabolism in tall fescue by coupling deep transcriptome and metabolome analysis. Ecotoxicol. Environ. Saf. 2020, 203, 110943. [Google Scholar] [CrossRef]
- Higashi, Y.; Saito, K. Lipidomic studies of membrane glycerolipids in plant leaves under heat stress. Prog. Lipid Res. 2019, 75, 100990. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.; Ishii, T.; El-Sayed, M.; Tran, L.S.P. Heat sensing and lipid reprograming as a signaling switch for heat stress responses in wheat. Plant Cell Physiol. 2020, 61, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Larkindale, J.; Huang, B. Changes of lipid composition and saturation level in leaves and roots for heat-stressed and heat-acclimated creeping bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2004, 51, 57–67. [Google Scholar] [CrossRef]
- Routaboul, J.M.; Skidmore, C.; Wallis, J.G.; Browse, J. Arabidopsis mutants reveal that short- and long-term thermotolerance have different requirements for trienoic fatty acids. J. Exp. Bot. 2011, 63, 1435–1443. [Google Scholar] [CrossRef]
- Navarro-Reig, M.; Tauler, R.; Iriondo-Frias, G.; Jaumot, J. Untargeted lipidomic evaluation of hydric and heat stresses on rice growth. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, I. Phosphoinositide signaling in plant development. Development 2016, 143, 2044–2055. [Google Scholar] [CrossRef]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid signalling in plant responses to abiotic stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef]
- Wang, X.; Huang, B. Lipid- and calcium-signaling regulation of HsfA2c-mediated heat tolerance in tall fescue. Environ. Exp. Bot. 2017, 136, 59–67. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Huang, B. Lipidomic reprogramming associated with drought stress priming-enhanced heat tolerance in tall fescue (Festuca arundinacea). Plant Cell Environ. 2019, 42, 947–958. [Google Scholar] [CrossRef]
- Liu, Y.N.; Lu, X.X.; Ren, A.; Shi, L.; Zhu, J.; Jiang, A.L.; Yu, H.S.; Zhao, M.W. Conversion of phosphatidylinositol (PI) to PI4-phosphate (PI4P) and then to PI(4, 5)P2 is essential for the cytosolic Ca2+ concentration under heat stress in Ganoderma lucidum. Environ. Microbiol. 2018, 20, 2456–2468. [Google Scholar] [CrossRef]
- Vincent, P.; Maneta-Peyret, L.; Cassagne, C.; Moreau, P. Phosphatidylserine delivery to endoplasmic reticulum-derived vesicles of plant cells depends on two biosynthetic pathways. FEBS Lett. 2001, 498, 32–36. [Google Scholar] [CrossRef]
- Vance, J.E.; Steenbergen, R. Metabolism and functions of phosphatidylserine. Prog. Lipid Res. 2005, 44, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, F.; Zhang, J.; Zhao, M.; Hui, Z.; Wang, W. Overaccumulation of glycine betaine enhances tolerance of the photosynthetic apparatus to drought and heat stress in wheat. Photosynthetica 2010, 48, 30–41. [Google Scholar] [CrossRef]
- Pineau, B.; Bourge, M.; Marion, J.; Mauve, C.; Gilard, F.; Maneta-Peyret, L.; Moreau, P.; Satiat-Jeunemaître, B.; Brown, S.C.; De Paepe, R. The importance of cardiolipin synthase for mitochondrial ultrastructure, respiratory function, plant development, and stress responses in Arabidopsis. Plant Cell 2013, 25, 4195–4208. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Jones, A.D.; Hu, J. Cardiolipin-mediated mitochondrial dynamics and stress response in Arabidopsis. Plant Cell 2014, 26, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Suh, S.; Kim, S.; Crain, R.C.; Kwak, J.M.; Nam, H.G.; Lee, Y. Systemic elevation of phosphatidic acid and lysophospholipid levels in wounded plants. Plant J. 1997, 12, 547–556. [Google Scholar] [CrossRef]
- Wi, S.J.; yeon Seo, S.; Cho, K.; Nam, M.H.; Park, K.Y. Lysophosphatidylcholine enhances susceptibility in signaling pathway against pathogen infection through biphasic production of reactive oxygen species and ethylene in tobacco plants. Phytochemistry 2014, 104, 48–59. [Google Scholar] [CrossRef]
- Viehweger, K.; Dordschbal, B.; Roos, W. Elicitor-activated phospholipase A2 generates lysophosphatidylcholines that mobilize the vacuolar H+ pool for pH signaling via the activation of Na+-dependent proton fluxes. Plant Cell 2002, 14, 1509–1525. [Google Scholar] [CrossRef]
- Lee, B.H.; Choi, S.H.; Kim, H.J.; Jung, S.W.; Kim, H.K.; Nah, S.Y. Plant lysophosphatidic acids: A rich source for bioactive lysophosphatidic acids and their pharmacological applications. Biol. Pharm. Bull. 2016, 39, 156–162. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Qi, L.; Yin, L.; Deng, X. Galactolipid remodeling is involved in drought-induced leaf senescence in maize. Environ. Exp. Bot. 2018, 150, 57–68. [Google Scholar] [CrossRef]
- Narayanan, S.; Tamura, P.J.; Roth, M.R.; Prasad, P.V.; Welti, R. Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alterations. Plant Cell Environ. 2016, 39, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Dörmann, P.; Benning, C. Galactolipids rule in seed plants. Trends Plant Sci. 2002, 7, 112–118. [Google Scholar] [CrossRef]
- Chen, J.; Burke, J.J.; Xin, Z.; Xu, C.; Velten, J. Characterization of the Arabidopsis thermosensitive mutant atts02 reveals an important role for galactolipids in thermotolerance. Plant Cell Environ. 2006, 29, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Michaelson, L.V.; Napier, J.A.; Molino, D.; Faure, J.D. Plant sphingolipids: Their importance in cellular organization and adaption. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2016, 1861, 1329–1335. [Google Scholar] [CrossRef]
- Berkey, R.; Bendigeri, D.; Xiao, S. Sphingolipids and plant defense/disease: The “death” connection and beyond. Front. Plant Sci. 2012, 3, 68. [Google Scholar] [CrossRef]
- Hassan, M.J.; Qi, H.; Cheng, B.; Hussain, S.; Peng, Y.; Liu, W.; Feng, G.; Zhao, J.; Li, Z. Enhanced adaptability to limited water supply regulated by diethyl aminoethyl hexanoate (DA-6) associated with lipidomic reprogramming in two white clover genotypes. Front. Plant Sci. 2022, 13, 879331. [Google Scholar] [CrossRef]
- Hogland, C.; Arnon, D. The solution-culture method for growing plants without soil. Calif. Agric. Exp. Circ. 1950, 247, 16–21. [Google Scholar]
- Li, Z.; Hou, J.; Zhang, Y.; Zeng, W.; Cheng, B.; Hassan, M.; Zhang, Y.; Pu, Q.; Peng, Y. Spermine regulates water balance associated with Ca2+-dependent aquaporin (TrTIP2-1, TrTIP2-2 and TrPIP2-7) expression in plants under water stress. Plant Cell Physiol. 2020, 61, 1576–1589. [Google Scholar] [CrossRef]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Dhindsa, R.; Plumb-Dhindsa, P.; Thorpe, T. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Duan, J.; Li, J.; Guo, S.; Kang, Y. Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J. Plant Physiol. 2008, 165, 1620–1635. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Bremer, D.J.; Jeannotte, R.; Welti, R.; Yang, C. Membrane lipid composition and heat tolerance in cool-season turfgrasses, including a hybrid bluegrass. J. Am. Soc. Hortic. Sci. 2009, 134, 511–520. [Google Scholar] [CrossRef]
- Gutierrez, L.; Mauriat, M.; Guénin, S.; Pelloux, J.; Lefebvre, J.F.; Louvet, R.; Rusterucci, C.; Moritz, T.; Guerineau, F.; Bellini, C.; et al. The lack of a systematic validation of reference genes: A serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnol. J. 2008, 6, 609–618. [Google Scholar] [CrossRef]
- Gómez-Merino, F.C.; Arana-Ceballos, F.A.; Trejo-Téllez, L.I.; Skirycz, A.; Brearley, C.A.; Dörmann, P.; Mueller-Roeber, B. Arabidopsis AtDGK7, the smallest member of plant diacylglycerol kinases (DGKs), displays unique biochemical features and saturates at low substrate concentration: The DGK inhibitor R59022 differentially affects AtDGK2 and AtDGK7 activity in vitro and alters plant growth and development. J. Biol. Chem. 2005, 280, 34888–34899. [Google Scholar]
- Vaz Dias, F.; Serrazina, S.; Vitorino, M.; Marchese, D.; Heilmann, I.; Godinho, M.; Rodrigues, M.; Malhó, R. A role for diacylglycerol kinase 4 in signalling crosstalk during Arabidopsis pollen tube growth. New Phytol. 2019, 222, 1434–1446. [Google Scholar] [CrossRef]
- Wada, Y.; Kusano, H.; Tsuge, T.; Aoyama, T. Phosphatidylinositol phosphate 5-kinase genes respond to phosphate deficiency for root hair elongation in Arabidopsis thaliana. Plant J. 2015, 81, 426–437. [Google Scholar] [CrossRef]
- Hunt, L.; Otterhag, L.; Lee, J.; Lasheen, T.; Hunt, J.; Seki, M.; Shinozaki, K.; Sommarin, M.; Gilmour, D.; Pical, C. Gene-specific expression and calcium activation of Arabidopsis thaliana phospholipase C isoforms. New Phytol. 2004, 162, 643–654. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bouslama, M.; Schapaugh, W.T., Jr. Stress tolerance in soybeans. I. Evaluation of three screening techniques for heat and drought tolerance. Crop Sci. 1984, 24, 933–937. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Cheng, B.; Zhao, Y.; Luo, L.; Zhang, Y.; Feng, G.; Han, L.; Peng, Y.; Zhang, X. Metabolic Regulation and Lipidomic Remodeling in Relation to Spermidine-induced Stress Tolerance to High Temperature in Plants. Int. J. Mol. Sci. 2022, 23, 12247. https://doi.org/10.3390/ijms232012247
Li Z, Cheng B, Zhao Y, Luo L, Zhang Y, Feng G, Han L, Peng Y, Zhang X. Metabolic Regulation and Lipidomic Remodeling in Relation to Spermidine-induced Stress Tolerance to High Temperature in Plants. International Journal of Molecular Sciences. 2022; 23(20):12247. https://doi.org/10.3390/ijms232012247
Chicago/Turabian StyleLi, Zhou, Bizhen Cheng, Yue Zhao, Lin Luo, Yan Zhang, Guangyan Feng, Liebao Han, Yan Peng, and Xinquan Zhang. 2022. "Metabolic Regulation and Lipidomic Remodeling in Relation to Spermidine-induced Stress Tolerance to High Temperature in Plants" International Journal of Molecular Sciences 23, no. 20: 12247. https://doi.org/10.3390/ijms232012247
APA StyleLi, Z., Cheng, B., Zhao, Y., Luo, L., Zhang, Y., Feng, G., Han, L., Peng, Y., & Zhang, X. (2022). Metabolic Regulation and Lipidomic Remodeling in Relation to Spermidine-induced Stress Tolerance to High Temperature in Plants. International Journal of Molecular Sciences, 23(20), 12247. https://doi.org/10.3390/ijms232012247






