A Hydrogen-Sulfide-Repressed Methionine Synthase SlMS1 Acts as a Positive Regulator for Fruit Ripening in Tomato
Abstract
1. Introduction
2. Results
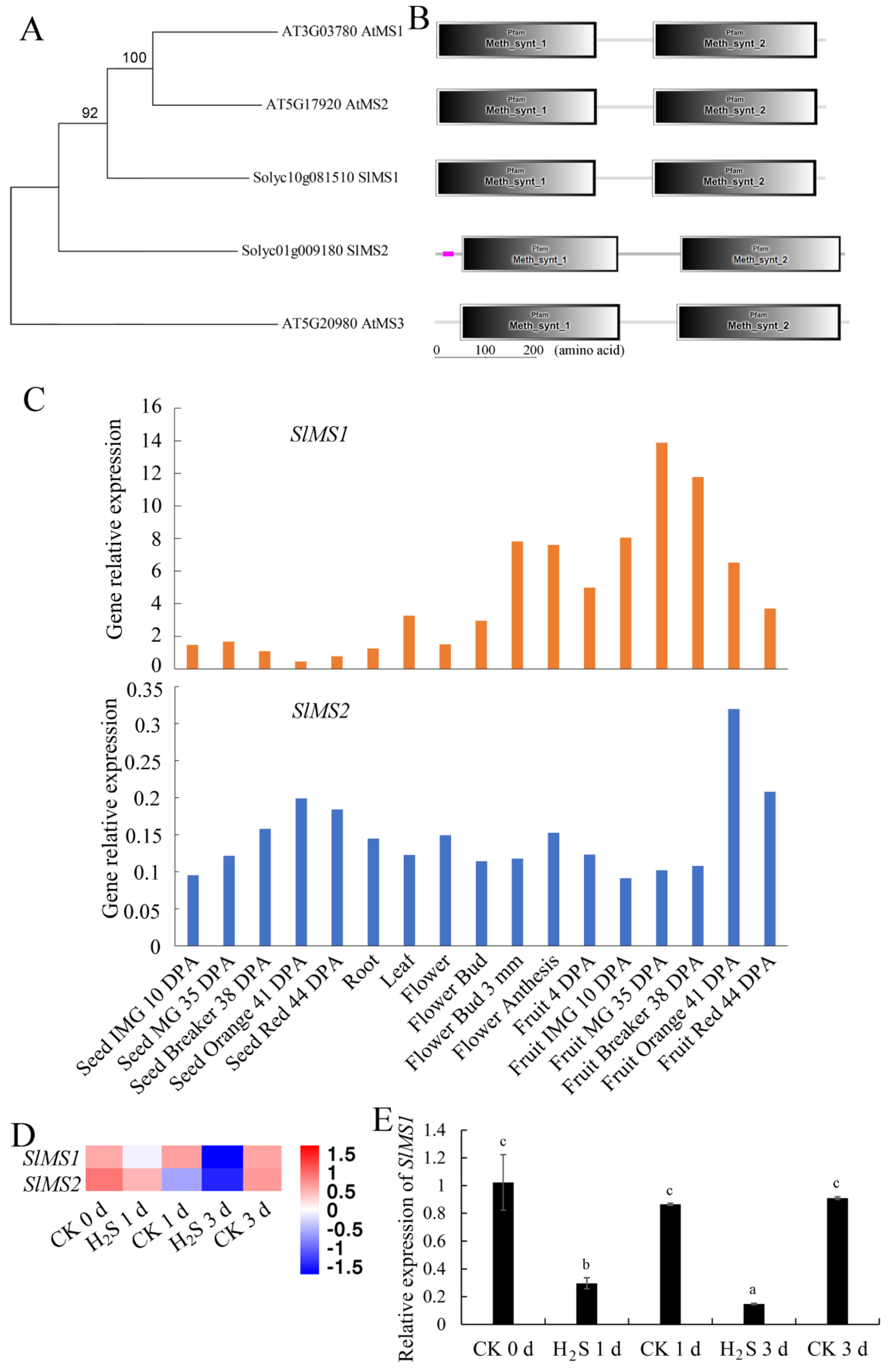
2.1. Identification and Bioinformatics Analyses of Methionine Synthase Genes in Tomato Genome
2.2. Expression Patterns of Tomato MS Gene in Different Tissues of Tomato Plants and in Response to Hydrogen Sulfide Treatment
2.3. Subcellular Localization of SlMS1
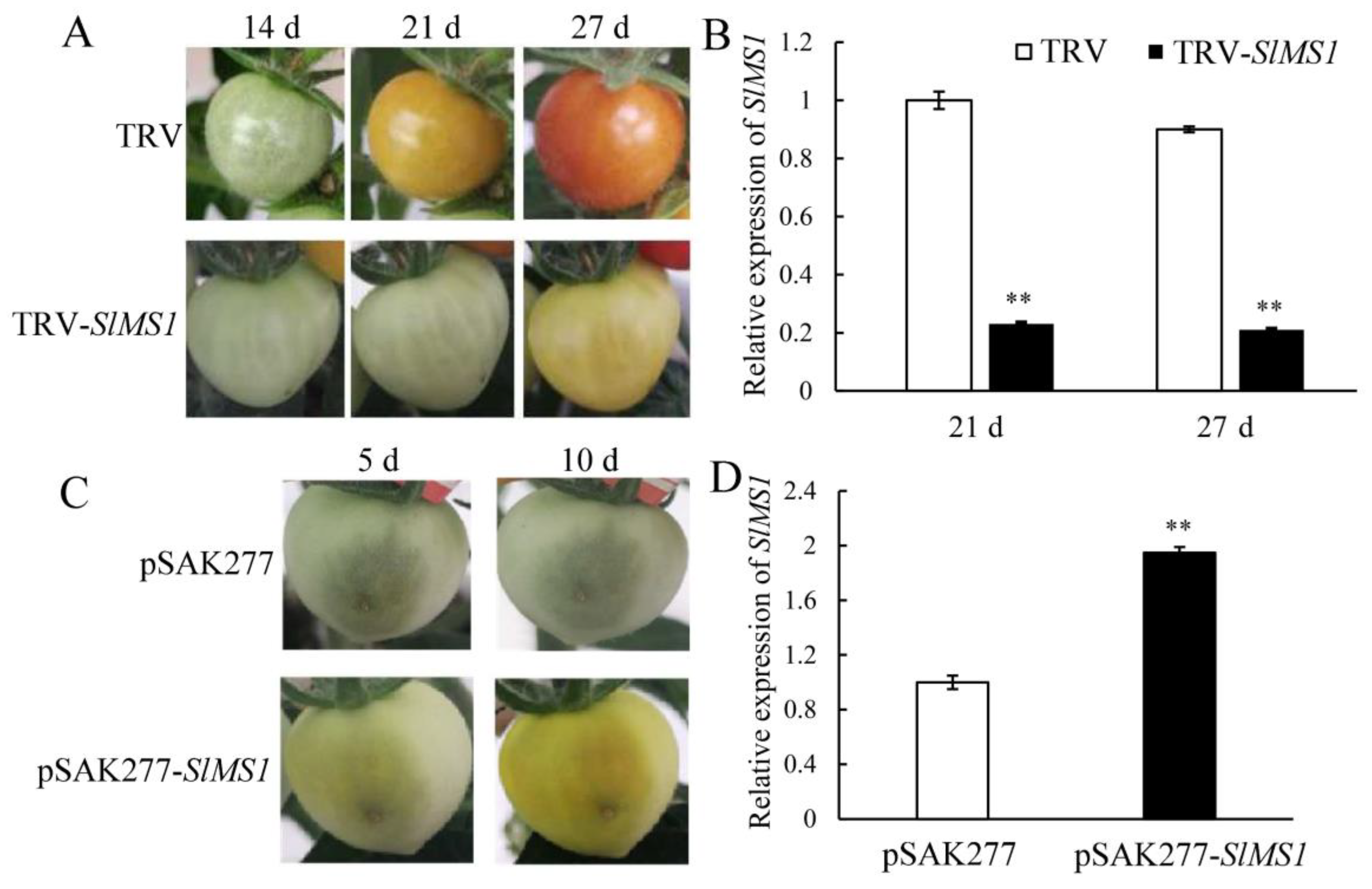
2.4. Effects of Transient Silencing and Gene Expression of SlMS1 on Tomato Fruit Ripening
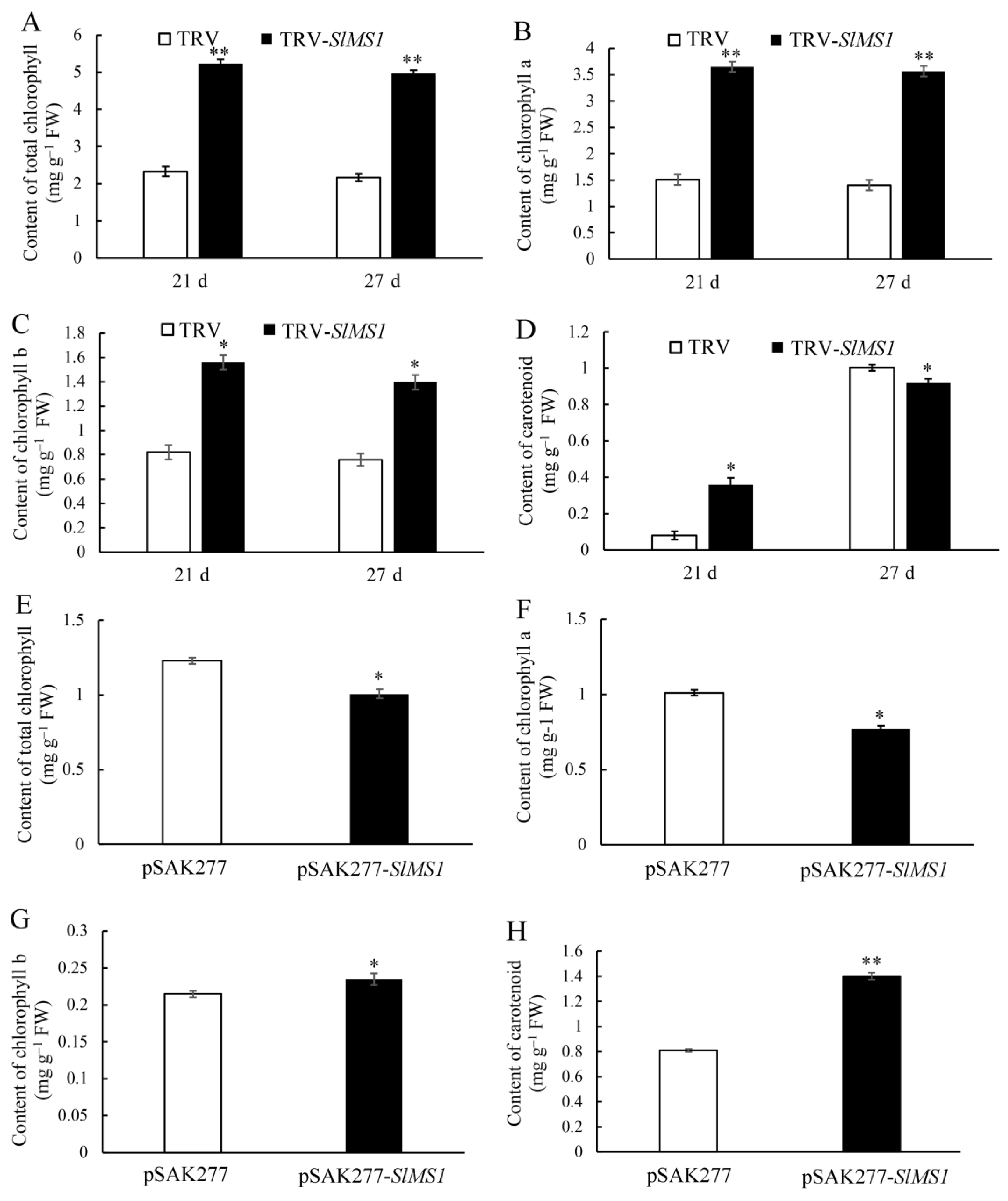
2.5. Effect of Gene Silencing and Overexpression of SlMS1 on the Metabolism of Chlorophyll and Carotenoid in Tomato Fruit
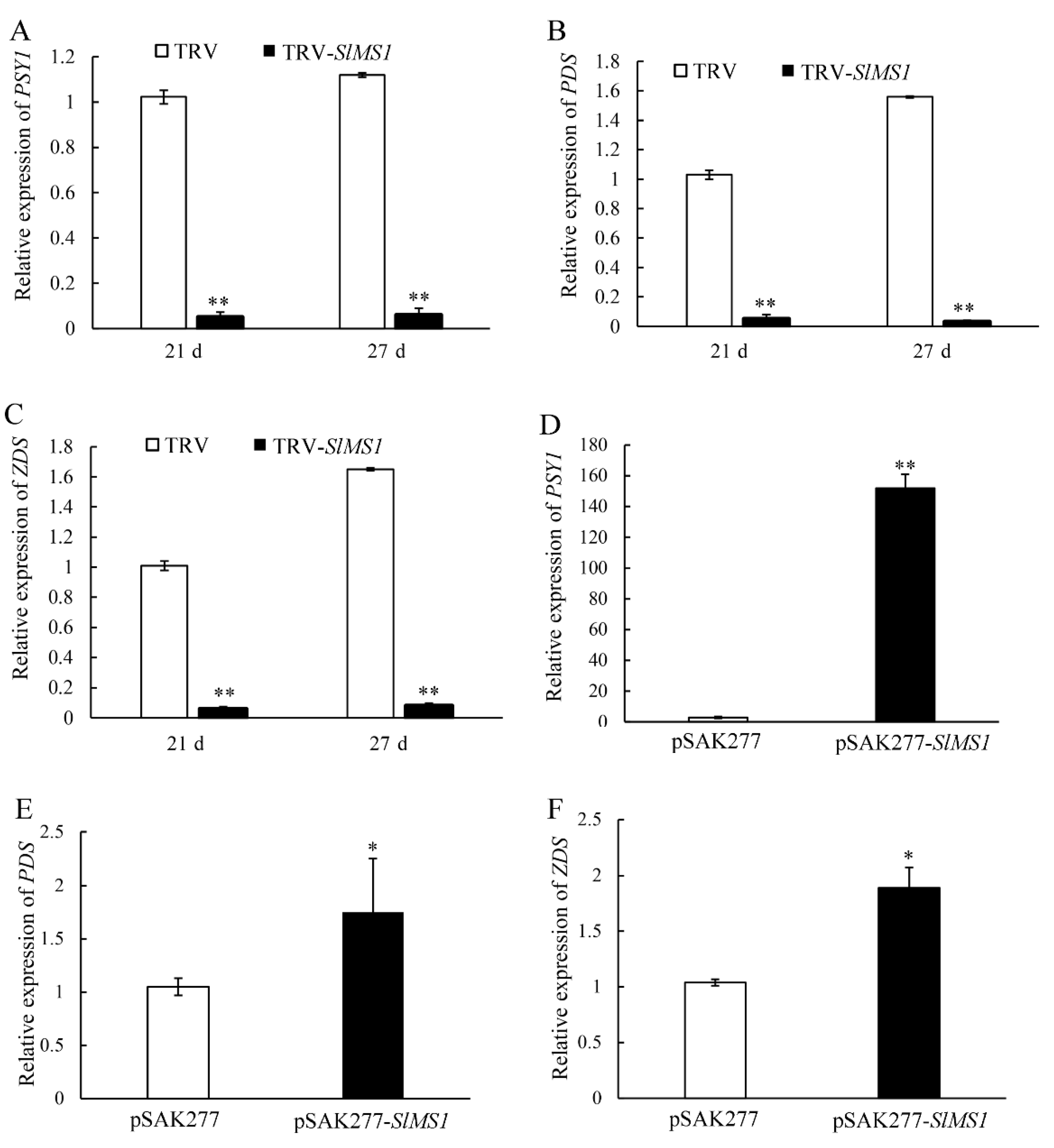
2.6. Effect of Gene Silencing and Overexpression of SlMS1 on the Expression of Carotenoid Synthesis Genes and Chlorophyll Degradation Genes
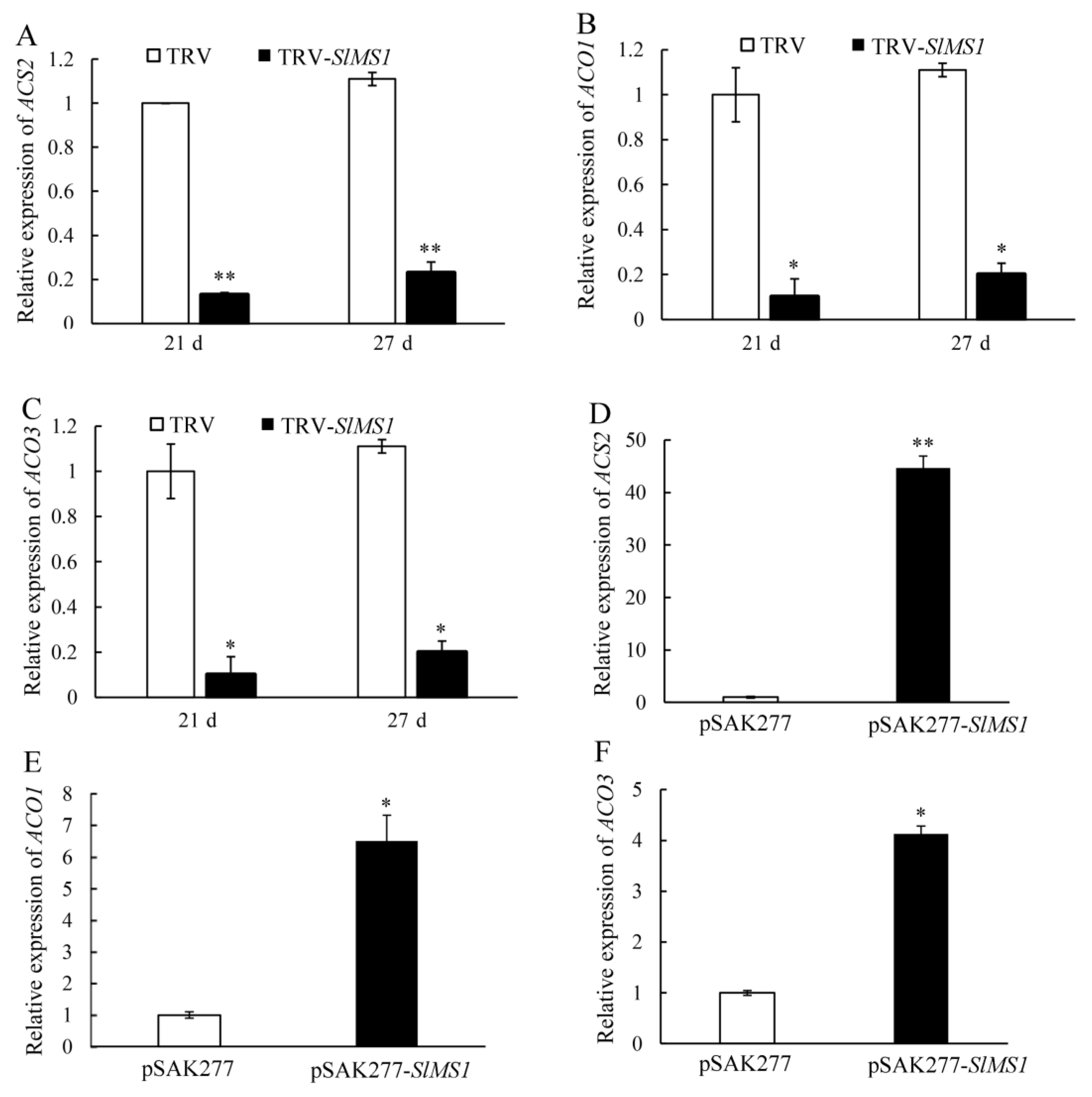
2.7. Effect of Gene Silencing and Overexpression of SlMS1 on the Expression of Ethylene Biosynthesis Genes
2.8. Effect of Gene Silencing and Overexpression of SlMS1 on the Expression of Cell-Wall-Metabolism-Related Genes in Tomato Fruit
2.9. Correlation Analysis among Different Physiological Indices and Ripening-Related Gene Expression
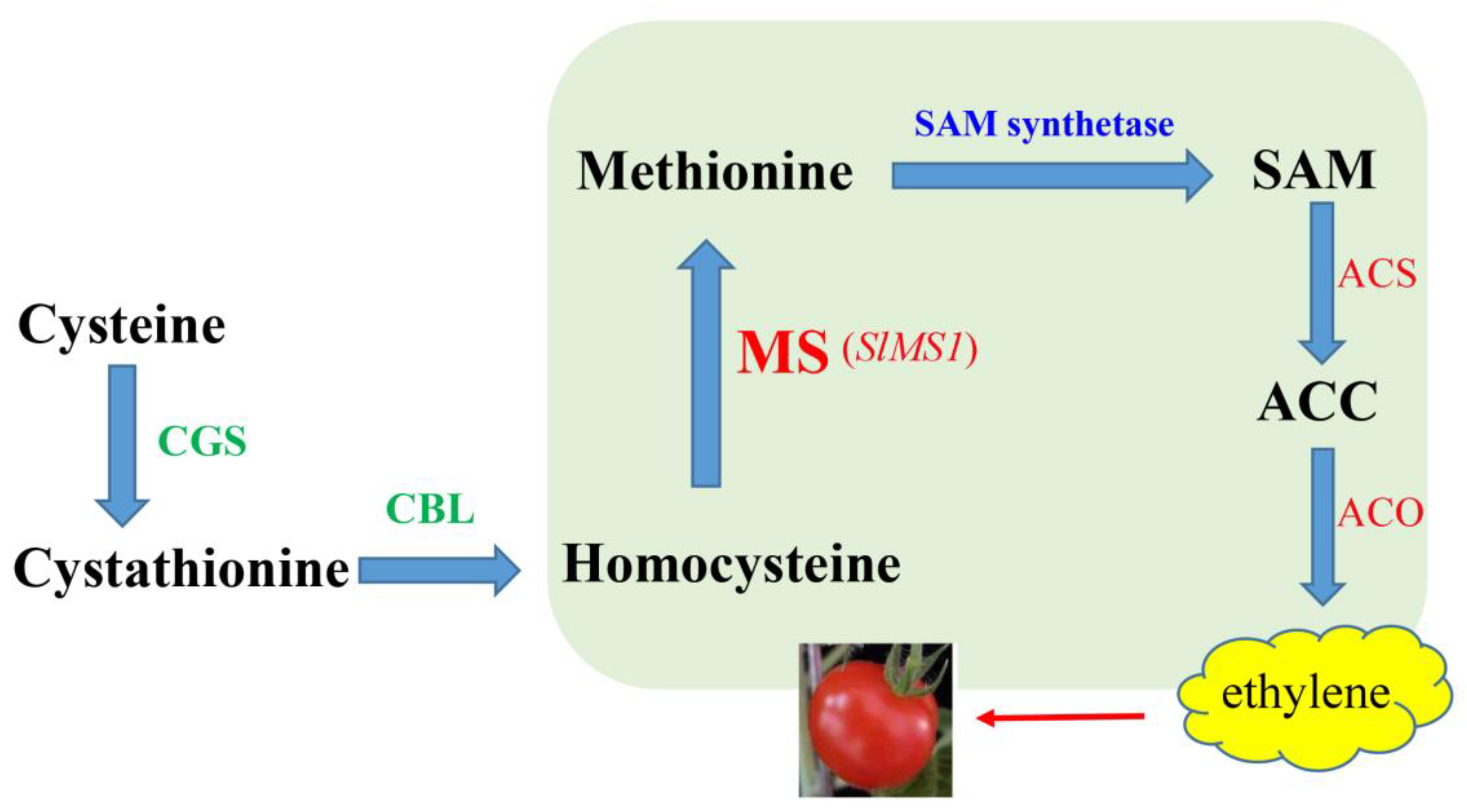
3. Discussion
4. Materials and Methods
4.1. Identification, Phylogenetic Analysis and Data Exploration of Methionine Synthases in Plants
4.2. Subcellular Localization Analysis
4.3. VIGS of SlMS1 and Transient Overexpression of SlMS1 in Tomato Fruit
4.4. Determination of the Levels of Chlorophyll and Carotenoids in Tomato Fruit
4.5. RNA Extraction and RT-qPCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Qin, G.; Tian, S. Regulatory network of fruit ripening: Current understanding and future challenges. New Phytol. 2020, 228, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Horie, Y.; Ito, H.; Kusaba, M.; Tanaka, R.; Tanaka, A. Participation of chlorophyll b reductase in the initial step of the degradation of light-harvesting chlorophyll a/b-protein complexes in Arabidopsis. J. Biol. Chem. 2009, 284, 17449–17456. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, S.; Jiao, B.; Duan, M.; Meng, Q.; Ma, N.; Lv, W. SlSGRL, a tomato SGR-like protein, promotes chlorophyll degradation downstream of the ABA signaling pathway. Plant Physiol. Biochem. 2020, 157, 316–327. [Google Scholar] [CrossRef]
- Zhu, F.; Wen, W.; Cheng, Y.; Fernie, A.R. The metabolic changes that effect fruit quality during tomato fruit ripening. Mol. Hortic. 2022, 2, 2. [Google Scholar] [CrossRef]
- Li, S.; Chen, K.; Grierson, D. Molecular and hormonal mechanisms regulating fleshy fruit ripening. Cells 2021, 10, 1136. [Google Scholar] [CrossRef]
- Brummell, D.A.; Harpster, M.H.; Civello, P.M.; Palys, J.M.; Bennett, A.B.; Dunsmuir, P. Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. Plant Cell 1999, 11, 2203–2216. [Google Scholar] [CrossRef]
- Alexander, L.; Grierson, D. Ethylene biosynthesis and action in tomato: A model for climacteric fruit ripening. J. Exp. Bot. 2002, 53, 2039–2055. [Google Scholar] [CrossRef]
- Karlova, R.; Chapman, N.; David, K.; Angenent, G.C.; Seymour, G.B.; de Maagd, R.A. Transcriptional control of fleshy fruit development and ripening. J. Exp. Bot. 2014, 65, 4527–4541. [Google Scholar] [CrossRef]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.P.; Bouzayen, M. Ethylene control of fruit ripening: Revisiting the complex network of transcriptional regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef]
- Giovanelli, J.; Mudd, S.H.; Datko, A.H. Quantitative analysis of pathways of methionine metabolism and their regulation in lemna. Plant Physiol. 1985, 78, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Ravanel, S.; Gakière, B.; Job, D.; Douce, R. The specific features of methionine biosynthesis and metabolism in plants. Proc. Natl. Acad. Sci. USA 1998, 95, 7805–7812. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Kong, D.; Lee, Y.; Ge, G.; Song, Y.; Liu, J.; Kwak, J.M. Methionine synthase 1 provides methionine for activation of the GLR3.5 Ca2+ channel and regulation of germination in Arabidopsis. J. Exp. Bot. 2020, 71, 178–187. [Google Scholar] [CrossRef]
- Zegzouti, H.; Jones, B.; Frasse, P.; Marty, C.; Maitre, B.; Latch, A.; Pech, J.C.; Bouzayen, M. Ethylene-regulated gene expression in tomato fruit: Characterization of novel ethylene-responsive and ripening-related genes isolated by differential display. Plant J. 1999, 18, 589–600. [Google Scholar] [CrossRef]
- Hu, L.Y.; Hu, S.L.; Wu, J.; Li, Y.H.; Zheng, J.L.; Wei, Z.J.; Liu, J.; Wang, H.L.; Liu, Y.S.; Zhang, H. Hydrogen sulfide prolongs postharvest shelf life of strawberry and plays an antioxidative role in fruits. J. Agric. Food Chem. 2012, 60, 8684–8693. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.F.; Wei, Z.Z.; Li, T.T.; Tang, J.; Huang, Z.Q.; Yang, F.; Li, Y.H.; Han, Z.; Hu, F.; Hu, L.Y.; et al. Modulation of enhanced antioxidant activity by hydrogen sulfide antagonization of ethylene in tomato fruit ripening. J. Agric. Food Chem. 2018, 66, 10380–10387. [Google Scholar] [CrossRef]
- Huo, J.; Huang, D.; Zhang, J.; Fang, H.; Wang, B.; Wang, C.; Liao, W. Hydrogen sulfide: A gaseous molecule in postharvest freshness. Front. Plant Sci. 2018, 9, 1172. [Google Scholar] [CrossRef]
- Hu, K.D.; Zhang, X.Y.; Yao, G.F.; Rong, Y.L.; Ding, C.; Tang, J.; Yang, F.; Huang, Z.Q.; Xu, Z.M.; Chen, X.Y.; et al. A nuclear-localized cysteine desulfhydrase plays a role in fruit ripening in tomato. Hortic. Res. 2020, 7, 211. [Google Scholar] [CrossRef]
- Ravanel, S.; Block, M.A.; Rippert, P.; Jabrin, S.; Curien, G.; Rébeillé, F.; Douce, R. Methionine metabolism in plants: Chloroplasts are autonomous for de novo methionine synthesis and can import S-adenosylmethionine from the cytosol. J. Biol. Chem. 2004, 279, 22548–22557. [Google Scholar] [CrossRef]
- Pattyn, J.; Vaughan-Hirsch, J.; Van de Poel, B. The regulation of ethylene biosynthesis: A complex multilevel control circuitry. New Phytol. 2021, 229, 770–782. [Google Scholar] [CrossRef]
- Eichel, J.; González, J.C.; Hotze, M.; Matthews, R.G.; Schröder, J. Vitamin-B12-Independent methionine synthase from a higher plant (Catharanthus roseus). Molecular characterization, regulation, heterologous expression, and enzyme properties. Eur. J. Biochem. 1995, 230, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Eckermann, C.; Eichel, J.; Schröder, J. Plant methionine synthase: New insights into properties and expression. Biol. Chem. 2000, 381, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, L.; Wang, J.; Zhao, X.; Cheng, J.; Yu, W.; Jin, D.; Li, Q.; Gong, Z. METHIONINE ADENOSYLTRANSFERASE4 mediates DNA and histone methylation. Plant Physiol. 2018, 177, 652–670. [Google Scholar] [CrossRef]
- Liu, D.; Lu, J.; Li, H.; Wang, J.; Pei, Y. Characterization of the O-acetylserine(thiol)lyase gene family in Solanum lycopersicum L. Plant Mol. Biol. 2019, 99, 123–134. [Google Scholar] [CrossRef] [PubMed]
- González, B.; Vera, P. Folate metabolism interferes with plant immunity through 1C methionine synthase-directed genome-wide DNA methylation enhancement. Mol. Plant 2019, 12, 1227–1242. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, S.; Hasebe, M.; Tomita, M.; Yanagawa, H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Natl. Acad. Sci. USA 2009, 106, 10171–10176. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Fu, D.Q.; Zhu, B.Z.; Zhu, H.L.; Jiang, W.B.; Luo, Y.B. Virus-induced gene silencing in tomato fruit. Plant J. 2005, 43, 299–308. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]




| Gene | Accession Number | Subcellular Localization Prediction | Number of Amino Acids | Molecular Weight | Theoretical pI |
|---|---|---|---|---|---|
| AtMS1 | AT3G03780 | cyto: 7, chlo: 4, mito: 2, nucl: 1 | 765 | 84583.84 | 6.09 |
| AtMS2 | AT5G17920 | cyto: 5, mito: 4, chlo: 3, nucl: 1, pero: 1 | 765 | 84356.64 | 6.09 |
| AtMS3 | AT5G20980 | mito: 9, chlo: 5 | 812 | 90594.12 | 8.17 |
| SlMS1 | Solyc10g081510 | cyto: 7, chlo: 3, mito: 2, nucl: 1, pero: 1 | 765 | 84724.91 | 6.01 |
| SlMS2 | Solyc01g009180 | mito: 9, chlo: 5 | 830 | 92074.57 | 6.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, Z.-K.; Ma, L.; Rong, Y.-L.; Li, W.-J.; Yao, G.-F.; Zhang, H.; Hu, K.-D. A Hydrogen-Sulfide-Repressed Methionine Synthase SlMS1 Acts as a Positive Regulator for Fruit Ripening in Tomato. Int. J. Mol. Sci. 2022, 23, 12239. https://doi.org/10.3390/ijms232012239
Geng Z-K, Ma L, Rong Y-L, Li W-J, Yao G-F, Zhang H, Hu K-D. A Hydrogen-Sulfide-Repressed Methionine Synthase SlMS1 Acts as a Positive Regulator for Fruit Ripening in Tomato. International Journal of Molecular Sciences. 2022; 23(20):12239. https://doi.org/10.3390/ijms232012239
Chicago/Turabian StyleGeng, Zhi-Kun, Lin Ma, Yu-Lei Rong, Wan-Jie Li, Gai-Fang Yao, Hua Zhang, and Kang-Di Hu. 2022. "A Hydrogen-Sulfide-Repressed Methionine Synthase SlMS1 Acts as a Positive Regulator for Fruit Ripening in Tomato" International Journal of Molecular Sciences 23, no. 20: 12239. https://doi.org/10.3390/ijms232012239
APA StyleGeng, Z.-K., Ma, L., Rong, Y.-L., Li, W.-J., Yao, G.-F., Zhang, H., & Hu, K.-D. (2022). A Hydrogen-Sulfide-Repressed Methionine Synthase SlMS1 Acts as a Positive Regulator for Fruit Ripening in Tomato. International Journal of Molecular Sciences, 23(20), 12239. https://doi.org/10.3390/ijms232012239








