In Vivo and In Silico Investigation of the Anti-Obesity Effects of Lactiplantibacillus plantarum Combined with Chia Seeds, Green Tea, and Chitosan in Alleviating Hyperlipidemia and Inflammation
Abstract
1. Introduction
2. Results and Discussion
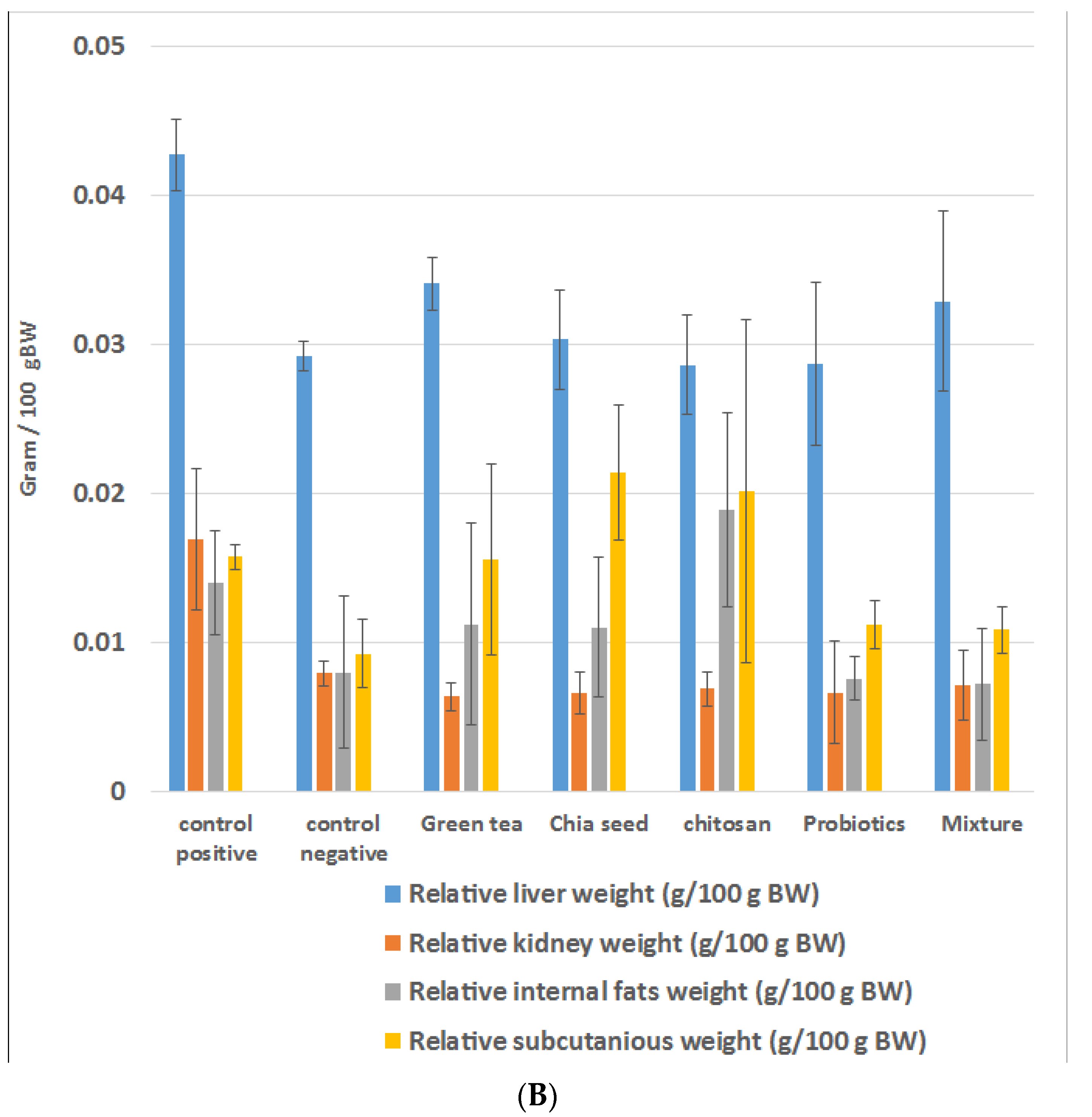
2.1. Effects of Prebiotic and Probiotic on the Weight of HFD Rats
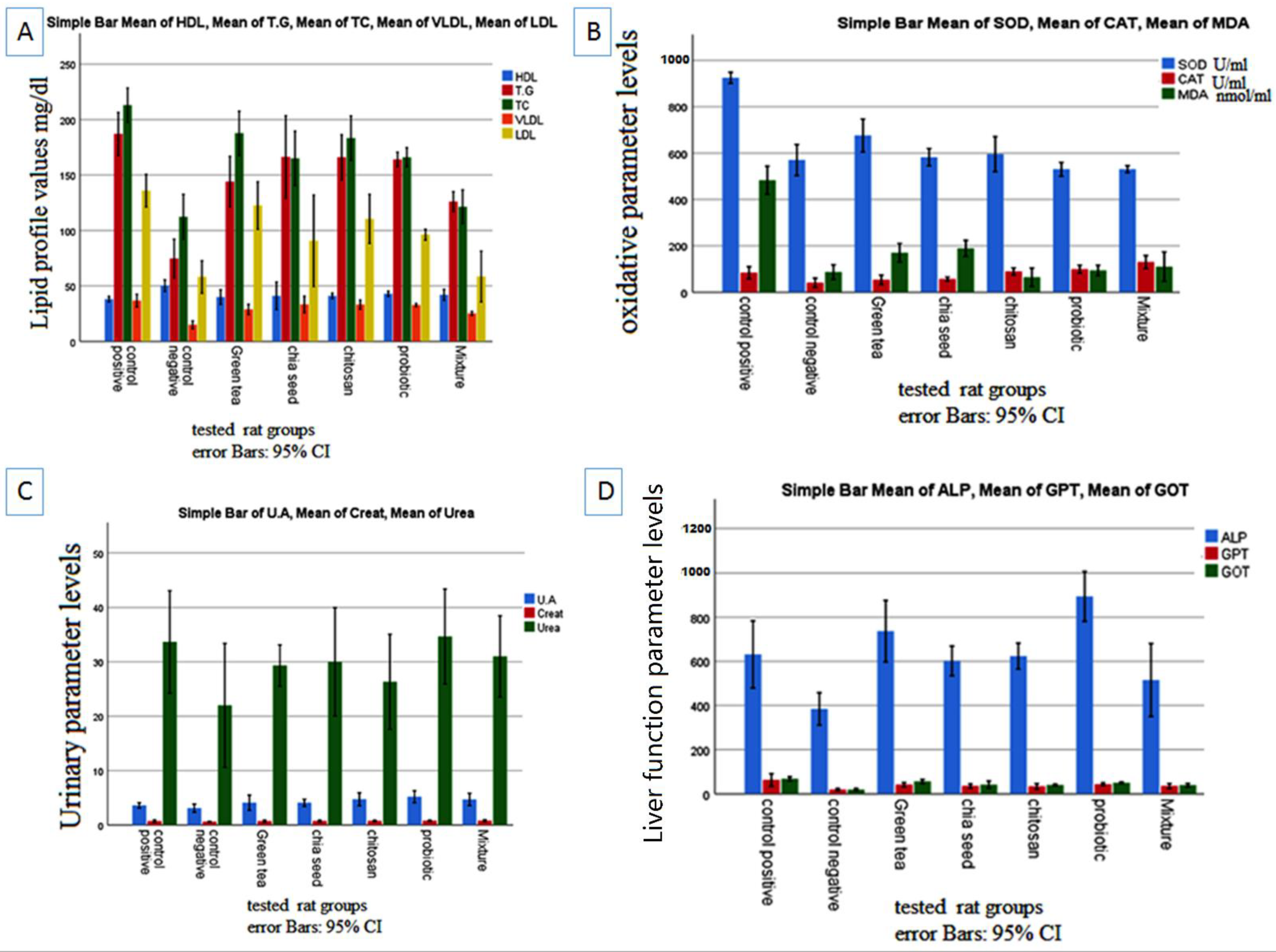
2.2. Effects of Prebiotics and Probiotic on the Lipid Profile
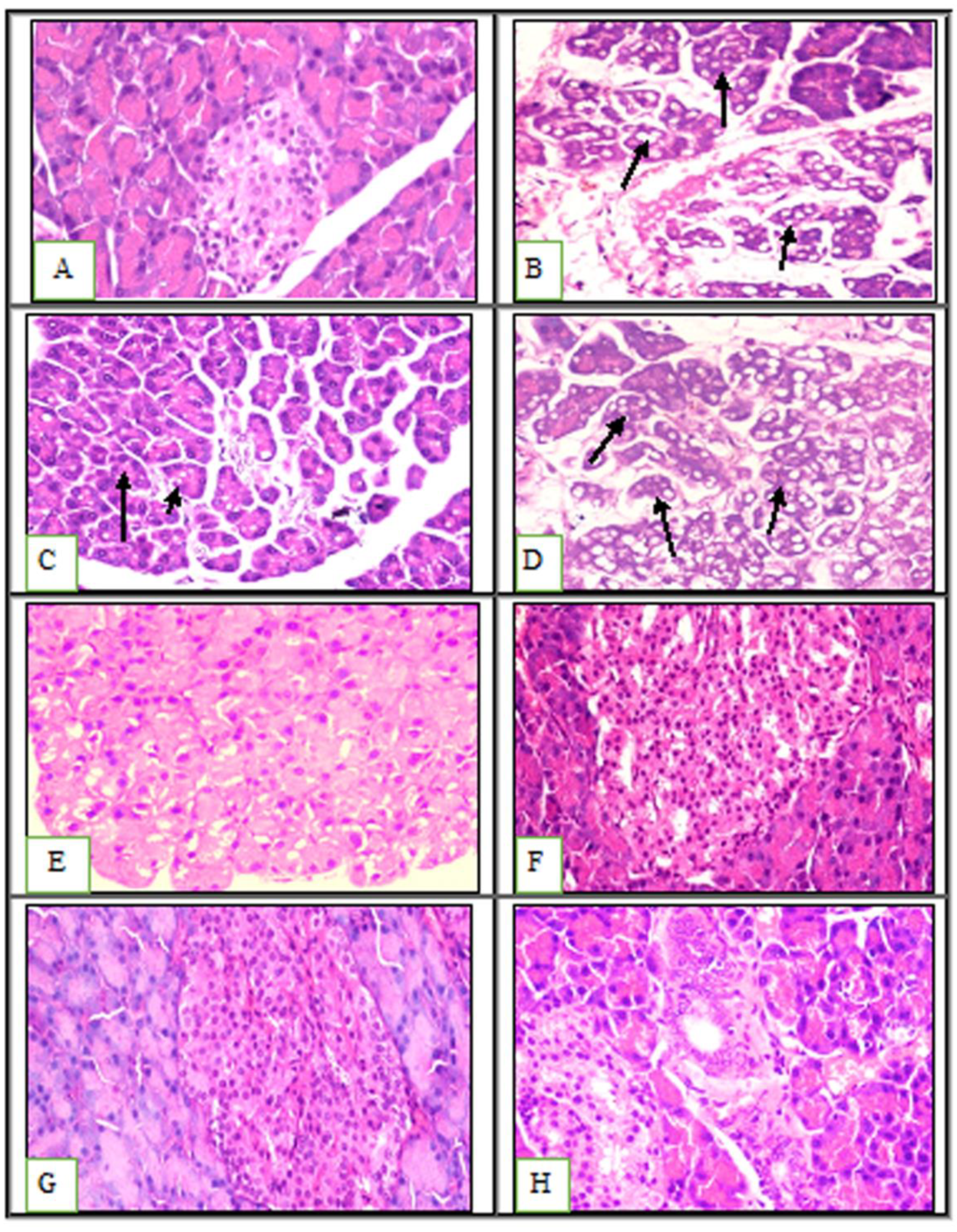
2.3. Effects of Prebiotics and Probiotics on Internal Organ Damage
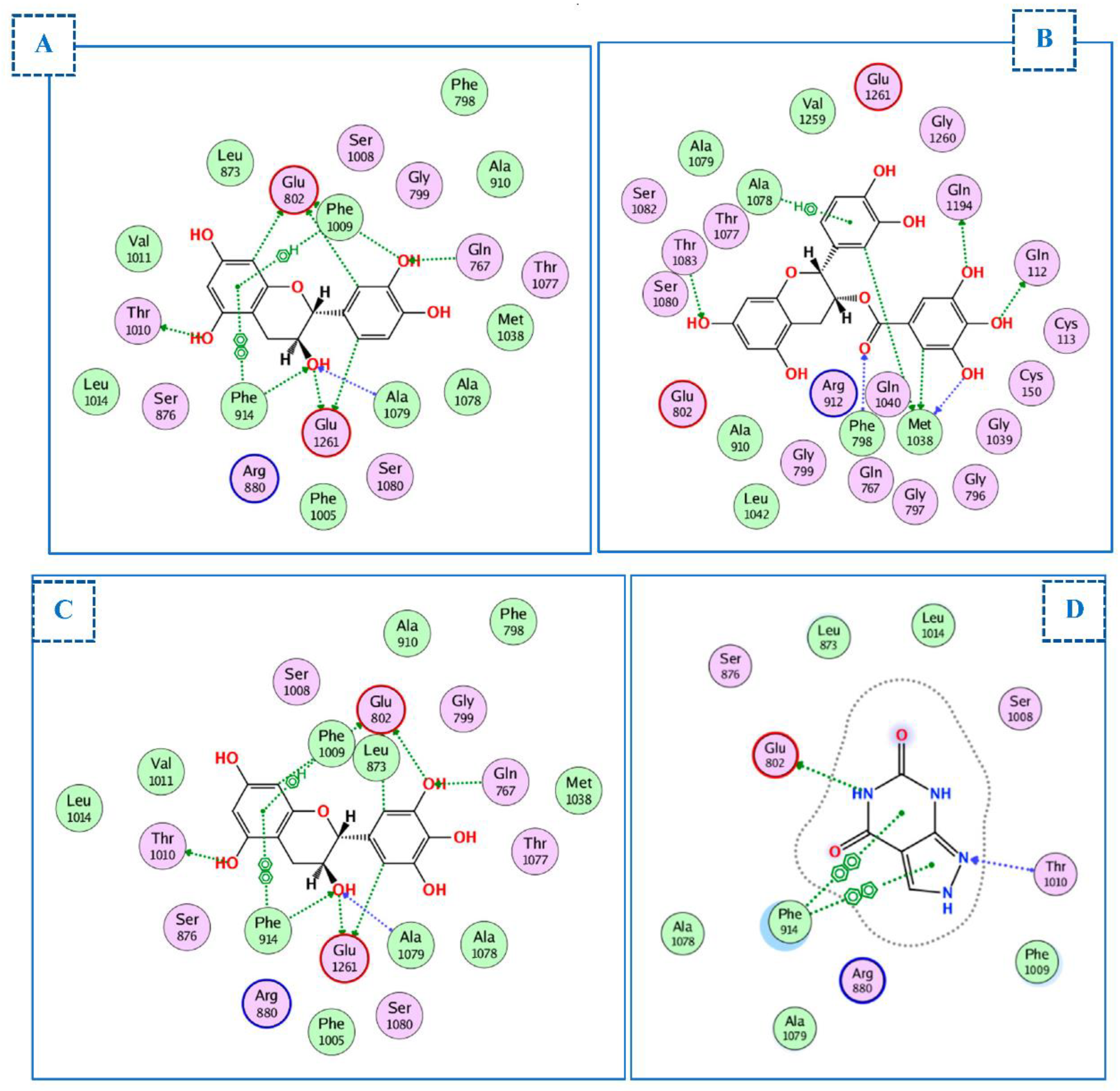
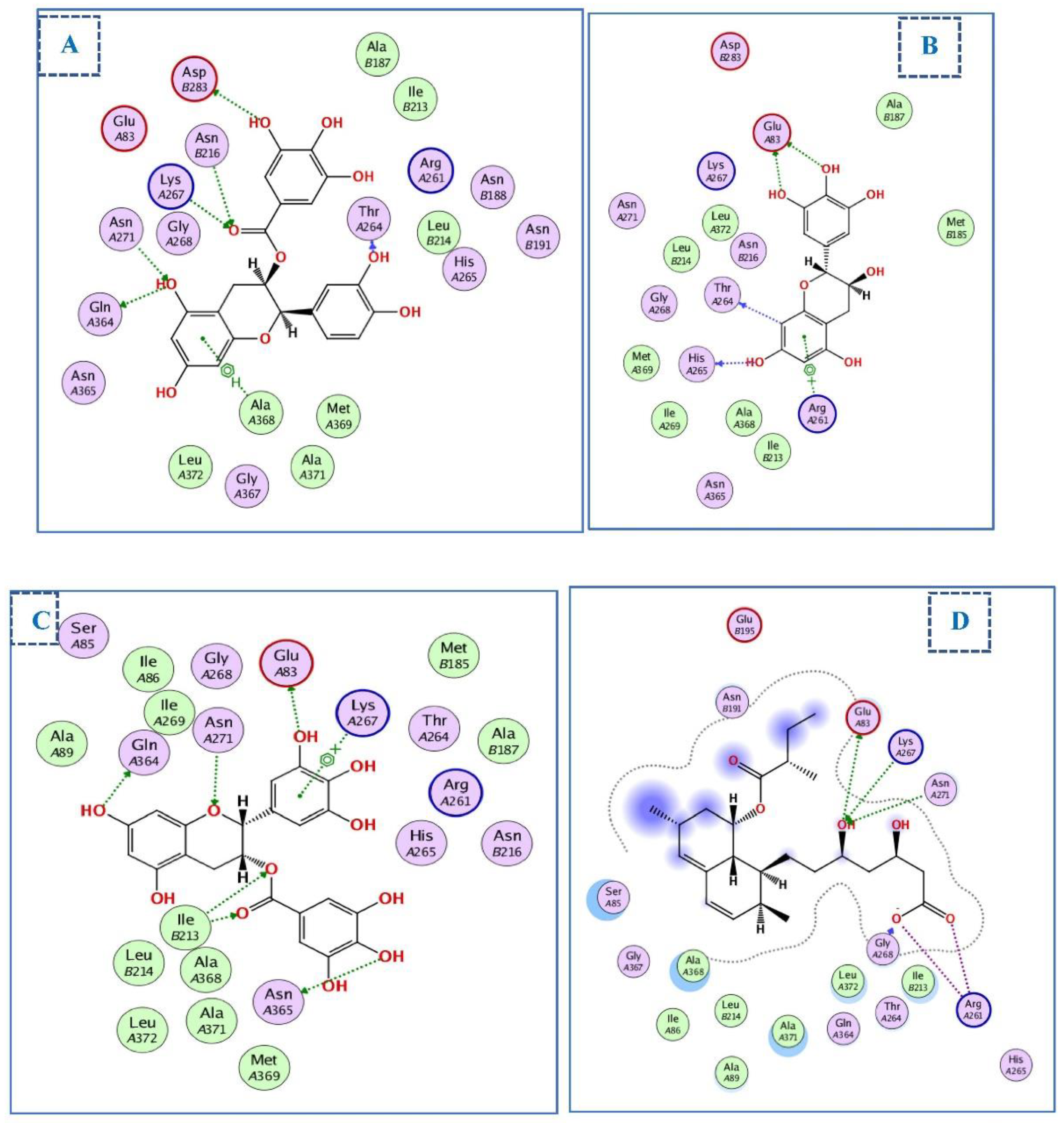
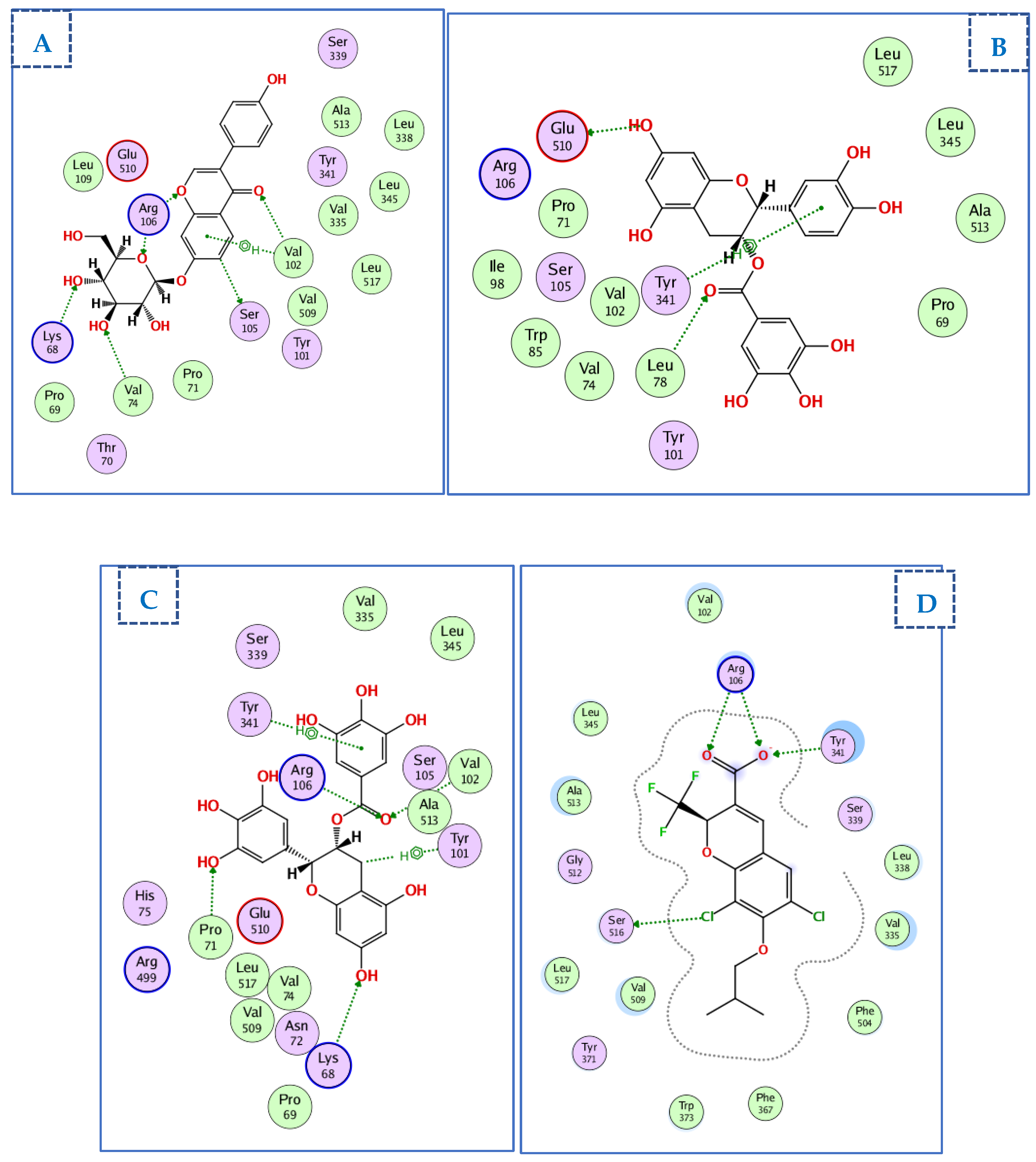
2.4. Molecular Docking Studies
2.4.1. Docking of Compounds into Xanthine Oxidase (XO) Binding Site
2.4.2. Docking of Compounds into the HMG-CoA Reductase Binding Site
2.4.3. Docking of Compounds into the COX-2 Binding Site
3. Materials and Methods
3.1. Materials
3.1.1. Procurement, Preparation, Purification, and Activation of the Probiotics (L. plantarum)
3.1.2. Procurement and Preparation of the Prebiotics
3.2. Methods
3.2.1. Animal Models
3.2.2. Sampling and Evaluation of Biological Parameters
3.2.3. Biochemical Analysis Tests
3.2.4. Histological Examination
3.2.5. Statistical Analysis
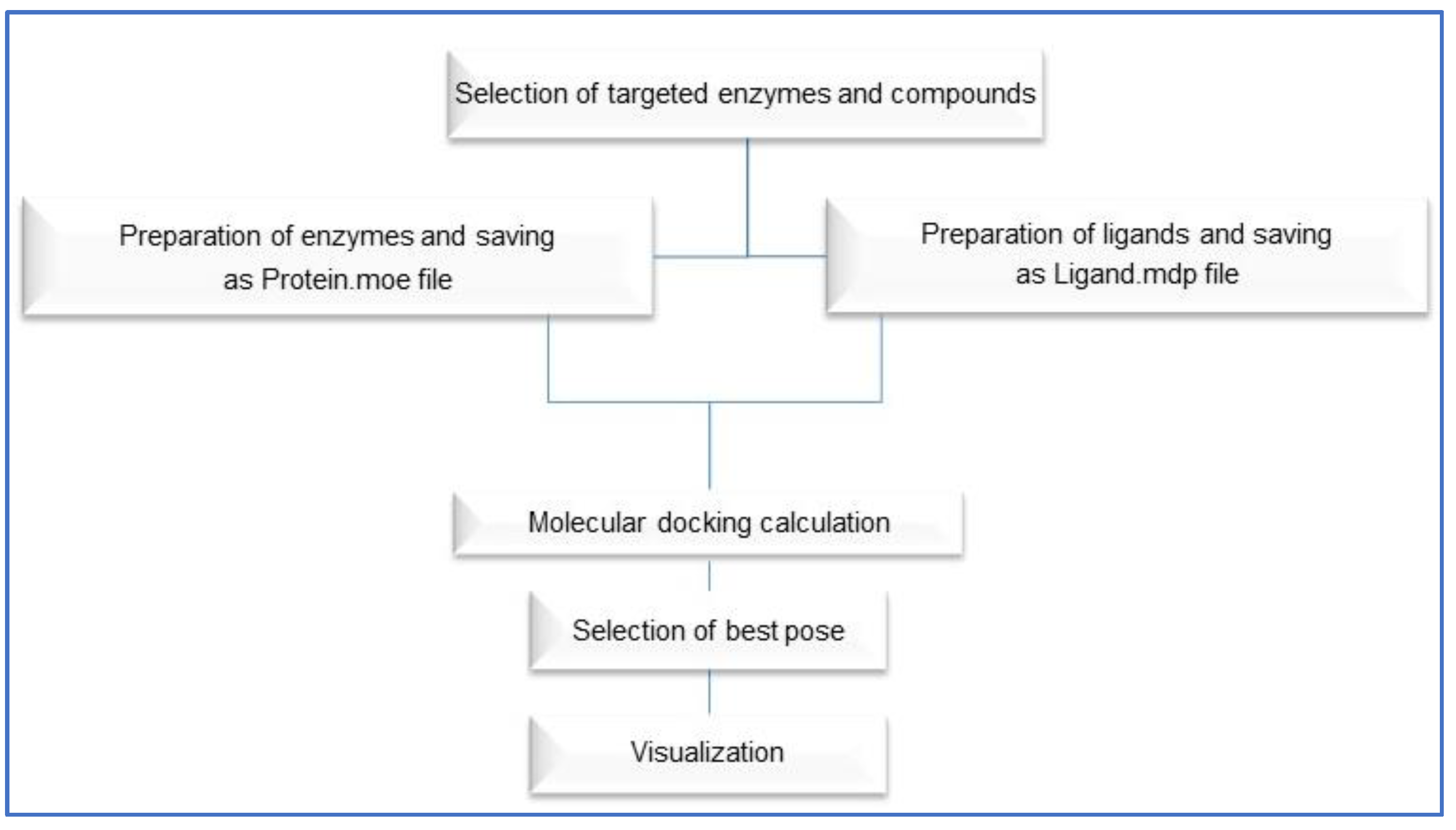
3.2.6. Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Adeloye, D.; Ige-Elegbede, J.O.; Ezejimofor, M.; Owolabi, E.O.; Ezeigwe, N.; Omoyele, C.; Mpazanje, R.G.; Dewan, M.T.; Agogo, E.; Gadanya, M.A. Estimating the prevalence of overweight and obesity in Nigeria in 2020: A systematic review and meta-analysis. Ann. Med. 2021, 53, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Antoniak, K.; Hansdorfer-Korzon, R.; Mrugacz, M.; Zorena, K. Adipose Tissue and Biological Factors. Possible Link between Lymphatic System Dysfunction and Obesity. Metabolites 2021, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45. [Google Scholar] [PubMed]
- Leggio, M.; Lombardi, M.; Caldarone, E.; Severi, P.; D’Emidio, S.; Armeni, M.; Bravi, V.; Bendini, M.G.; Mazza, A. The relationship between obesity and hypertension: An updated comprehensive overview on vicious twins. Hypertens. Res. 2017, 40, 947–963. [Google Scholar] [CrossRef]
- Bouchard, C. Genetics of obesity: What we have learned over decades of research. Obesity 2021, 29, 802–820. [Google Scholar] [CrossRef]
- da Silva Pérez, E.M.; de Alencar, N.M.N.; de Figueiredo, I.S.T.; Aragão, K.S.; Gaban, S.V.F. Effect of safflower oil (Carthamus tinctorius L.) supplementation in the abdominal adipose tissues and body weight of male Wistar rats undergoing exercise training. Food Chem. Mol. Sci. 2022, 4, 100083. [Google Scholar] [CrossRef]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef]
- Levi, J.; Wang, J.; Venter, F.; Hill, A. 891. Minimum Manufacturing Costs and National Prices for Weight Loss Treatments, as Potential Mitigation for Anti-Retroviral Related Weight Gain in HIV. Open Forum Infect. Dis. 2021, 8, S536–S537. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body mass index: Obesity, BMI, and health: A critical review. Nutr. Today 2015, 50, 117. [Google Scholar] [CrossRef]
- Collaboration, P.S. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef]
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus—Present and future perspectives. Nat. Rev. Endocrinol. 2012, 8, 228–236. [Google Scholar] [CrossRef]
- Huang, T.-W.; Chang, C.-L.; Kao, E.-S.; Lin, J.-H. Effect of Hibiscus sabdariffa extract on high fat diet–induced obesity and liver damage in hamsters. J. Food Nutr. Res. 2015, 59, 29018. [Google Scholar] [CrossRef]
- White, P.; Cercato, L.; Batista, V.; Camargo, E.; De Lucca Jr, W.; Oliveira, A.; Silva, F.; Goes, T.; Oliveira, E.; Moraes, V. Aqueous extract of Chrysobalanus icaco leaves, in lower doses, prevent fat gain in obese high-fat fed mice. J. Ethnopharmacol. 2016, 179, 92–100. [Google Scholar] [CrossRef]
- Heck, A.M.; Yanovski, J.A.; Calis, K.A. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2000, 20, 270–279. [Google Scholar] [CrossRef]
- Ballinger, A. Orlistat in the treatment of obesity. Expert Opin. Pharmacother. 2000, 1, 841–847. [Google Scholar] [CrossRef]
- Cava, E.; Marzullo, P.; Farinelli, D.; Gennari, A.; Saggia, C.; Riso, S.; Prodam, F. Breast Cancer Diet “BCD”: A Review of Healthy Dietary Patterns to Prevent Breast Cancer Recurrence and Reduce Mortality. Nutrients 2022, 14, 476. [Google Scholar] [CrossRef]
- Le Barz, M.; Anhê, F.F.; Varin, T.V.; Desjardins, Y.; Levy, E.; Roy, D.; Urdaci, M.C.; Marette, A. Probiotics as complementary treatment for metabolic disorders. Diabetes Metab. J. 2015, 39, 291–303. [Google Scholar] [CrossRef]
- Krentz, A.; Fujioka, K.; Hompesch, M. Evolution of pharmacological obesity treatments: Focus on adverse side-effect profiles. Diabetes Obes. Metab. 2016, 18, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Xing, Z.; Li, C.; Wang, J.; Wang, Y. Molecular mechanisms and in vitro antioxidant effects of Lactobacillus plantarum MA2. Food Chem. 2017, 221, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Prete, R.; Garcia-Gonzalez, N.; Di Mattia, C.D.; Corsetti, A.; Battista, N. Food-borne Lactiplantibacillus plantarum protect normal intestinal cells against inflammation by modulating reactive oxygen species and IL-23/IL-17 axis. Sci. Rep. 2020, 10, 16340. [Google Scholar] [CrossRef] [PubMed]
- Gillor, O.; Etzion, A.; Riley, M. The dual role of bacteriocins as anti-and probiotics. Appl. Microbiol. Biotechnol. 2008, 81, 591–606. [Google Scholar] [CrossRef]
- Choi, W.J.; Dong, H.J.; Jeong, H.U.; Jung, H.H.; Kim, Y.-H.; Kim, T.H. Antiobesity effects of Lactobacillus plantarum LMT1-48 accompanied by inhibition of Enterobacter cloacae in the intestine of diet-induced obese mice. J. Med. Food 2019, 22, 560–566. [Google Scholar] [CrossRef]
- Vuksan, V.; Jenkins, A.; Brissette, C.; Choleva, L.; Jovanovski, E.; Gibbs, A.; Bazinet, R.; Au-Yeung, F.; Zurbau, A.; Ho, H. Salba-chia (Salvia hispanica L.) in the treatment of overweight and obese patients with type 2 diabetes: A double-blind randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 138–146. [Google Scholar] [CrossRef]
- Grancieri, M.; Martino, H.S.D.; Gonzalez de Mejia, E. Chia seed (Salvia hispanica L.) as a source of proteins and bioactive peptides with health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 480–499. [Google Scholar] [CrossRef]
- Sharangi, A. Medicinal and therapeutic potentialities of tea (Camellia sinensis L.)–A review. Food Res. Int. 2009, 42, 529–535. [Google Scholar] [CrossRef]
- Wolfram, S.; Wang, Y.; Thielecke, F. Anti-obesity effects of green tea: From bedside to bench. Mol. Nutr. Food Res. 2006, 50, 176–187. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Wu, T.; Dai, S.; Xu, J.; Zhou, Z. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015, 6, 296–303. [Google Scholar] [CrossRef]
- Chandy, T.; Sharma, C.P. Chitosan-as a biomaterial. Biomater. Artif. Cells Artif. Organs 1990, 18, 1–24. [Google Scholar] [CrossRef]
- Walsh, A.M.; Sweeney, T.; Bahar, B.; O’Doherty, J.V. Multi-functional roles of chitosan as a potential protective agent against obesity. PLoS ONE 2013, 8, e53828. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Ye, Z.; Cao, H.; Bai, Y.; Che, Q.; Guo, J.; Su, Z. Chitosan oligosaccharide ameliorated obesity by reducing endoplasmic reticulum stress in diet-induced obese rats. Food Funct. 2020, 11, 6285–6296. [Google Scholar] [CrossRef] [PubMed]
- Jull, A.B.; Mhurchu, C.N.; Bennett, D.A.; Dunshea-Mooij, C.A.; Rodgers, A. Chitosan for overweight or obesity. Cochrane Database Syst. Rev. 2008, 6, CD003892. [Google Scholar] [CrossRef]
- Alard, J.; Cudennec, B.; Boutillier, D.; Peucelle, V.; Descat, A.; Decoin, R.; Kuylle, S.; Jablaoui, A.; Rhimi, M.; Wolowczuk, I. Multiple selection criteria for probiotic strains with high potential for obesity management. Nutrients 2021, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Bezzina, R.; Hinch, E.; Lewandowski, P.A.; Cameron-Smith, D.; Mathai, M.L.; Jois, M.; Sinclair, A.J.; Begg, D.P.; Wark, J.D. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Nutr. Res. 2009, 29, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Ayerza, R., Jr.; Coates, W. Effect of dietary α-linolenic fatty acid derived from chia when fed as ground seed, whole seed and oil on lipid content and fatty acid composition of rat plasma. Ann. Nutr. Metab. 2007, 51, 27–34. [Google Scholar] [CrossRef]
- Chicco, A.G.; D’Alessandro, M.E.; Hein, G.J.; Oliva, M.E.; Lombardo, Y.B. Dietary chia seed (Salvia hispanica L.) rich in α-linolenic acid improves adiposity and normalises hypertriacylglycerolaemia and insulin resistance in dyslipaemic rats. Br. J. Nutr. 2008, 101, 41–50. [Google Scholar] [CrossRef]
- Konuma, K.; Itoh, M.; Suganami, T.; Kanai, S.; Nakagawa, N.; Sakai, T.; Kawano, H.; Hara, M.; Kojima, S.; Izumi, Y. Eicosapentaenoic acid ameliorates non-alcoholic steatohepatitis in a novel mouse model using melanocortin 4 receptor-deficient mice. PLoS ONE 2015, 10, e0121528. [Google Scholar] [CrossRef]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Txicology Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Kimura, Y. Low molecular weight chitosan inhibits obesity induced by feeding a high-fat diet long-term in mice. J. Pharm. Pharmacol. 2006, 58, 201–207. [Google Scholar] [CrossRef]
- Deuchi, K.; Kanauchi, O.; Imasato, Y.; Kobayashi, E. Effect of the viscosity or deacetylation degree of chitosan on fecal fat excreted from rats fed on a high-fat diet. Biosci. Biotechnol. Biochem. 1995, 59, 781–785. [Google Scholar] [CrossRef]
- Han, L.; Kimura, Y.; Okuda, H. Reduction in fat storage during chitin-chitosan treatment in mice fed a high-fat diet. Int. J. Obes. 1999, 23, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Gębalski, J.; Gołębiewski, J.; Malinowski, B. Probiotics for the treatment of overweight and obesity in humans—A review of clinical trials. Microorganisms 2020, 8, 1148. [Google Scholar] [CrossRef] [PubMed]
- Barengolts, E. Gut microbiota, prebiotics, probiotics, and synbiotics in management of obesity and prediabetes: Review of randomized controlled trials. Endocr. Pract. 2016, 22, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Joung, H.; Chu, J.; Kim, B.-K.; Choi, I.-S.; Kim, W.; Park, T.-S. Probiotics ameliorate chronic low-grade inflammation and fat accumulation with gut microbiota composition change in diet-induced obese mice models. Appl. Microbiol. Biotechnol. 2021, 105, 1203–1213. [Google Scholar] [CrossRef]
- Pell, L.G.; Horne, R.G.; Huntley, S.; Rahman, H.; Kar, S.; Islam, M.S.; Evans, K.C.; Saha, S.K.; Campigotto, A.; Morris, S.K. Antimicrobial susceptibilities and comparative whole genome analysis of two isolates of the probiotic bacterium Lactiplantibacillus plantarum, strain ATCC 202195. Sci. Rep. 2021, 11, 15893. [Google Scholar] [CrossRef]
- Jitpakdee, J.; Kantachote, D.; Kanzaki, H.; Nitoda, T. Potential of lactic acid bacteria to produce functional fermented whey beverage with putative health promoting attributes. LWT 2022, 160, 113269. [Google Scholar] [CrossRef]
- Zapata, A.; Ramirez-Arcos, S. A Comparative Study of McFarland Turbidity Standards and the Densimat Photometer to Determine Bacterial Cell Density. Curr. Microbiol. 2015, 70, 907–909. [Google Scholar] [CrossRef]
- Samadi, S.; Fard, F.R. Phytochemical properties, antioxidant activity and mineral content (Fe, Zn and Cu) in Iranian produced black tea, green tea and roselle calyces. Biocatal. Agric. Biotechnol. 2020, 23, 101472. [Google Scholar] [CrossRef]
- Rasoulzadehzali, M.; Namazi, H. Facile preparation of antibacterial chitosan/graphene oxide-Ag bio-nanocomposite hydrogel beads for controlled release of doxorubicin. Int. J. Biol. Macromol. 2018, 116, 54–63. [Google Scholar] [CrossRef]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharm. Pharm. 2010, 1, 87–93. [Google Scholar] [CrossRef]
- Abdel Mageed, S.S.; Ammar, R.M.; Nassar, N.N.; Moawad, H.; Kamel, A.S. Role of PI3K/Akt axis in mitigating hippocampal ischemia-reperfusion injury via CB1 receptor stimulation by paracetamol and FAAH inhibitor in rat. Neuropharmacology 2022, 207, 108935. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.A. Clinical Guide to Laboratory Tests, 3rd ed.; Tietz, N.W., Ed.; W.B. Saunders: Philadelphia, PA, USA, 1995; Volume 35, p. 972. [Google Scholar] [CrossRef]
- Uniprot Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- Young, D. Effects of disease on Clinical Lab. Tests 4th Ed AACC. Clin. Chem. 2001, 48, 682–683. [Google Scholar]
- Knight, J.A.; Anderson, S.; Rawle, J.M. Chemical basis of the sulfo-phospho-vanillin reaction for estimating total serum lipids. Clin. Chem. 1972, 18, 199–202. [Google Scholar] [CrossRef]
- Sugiuchi, H. History of development and technical details of the homogenous assay for HDL and LDL cholesterol. Eng. J. Med. 2005, 1, 4–11. [Google Scholar]
- Fukuyama, N.; Homma, K.; Wakana, N.; Kudo, K.; Suyama, A.; Ohazama, H.; Tsuji, C.; Ishiwata, K.; Eguchi, Y.; Nakazawa, H. Validation of the Friedewald equation for evaluation of plasma LDL-cholesterol. J. Clin. Biochem. Nutr. 2007, 43, 1–5. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P. A modified spectrophotometric assay of superoxide dismutase. NISCAIR-CSIR 1984, 37, 201–204. [Google Scholar]
- Kale, M.; Rathore, N.; John, S.; Bhatnagar, D. Lipid peroxidative damage on pyrethroid exposure and alterations in antioxidant status in rat erythrocytes: A possible involvement of reactive oxygen species. Toxicol. Lett. 1999, 105, 197–205. [Google Scholar] [CrossRef]
- Thayer, W.S. Serum lipid peroxides in rats treated chronically with adriamycin. Biochem. Pharmacol. 1984, 33, 2259–2263. [Google Scholar] [CrossRef]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Vilar, S.; Cozza, G.; Moro, S. Medicinal chemistry and the molecular operating environment (MOE): Application of QSAR and molecular docking to drug discovery. Curr. Top. Med. Chem. 2008, 8, 1555–1572. [Google Scholar] [CrossRef]
- El Hassab, M.A.; Eldehna, W.M.; Al-Rashood, S.T.; Alharbi, A.; Eskandrani, R.O.; Alkahtani, H.M.; Elkaeed, E.B.; Abou-Seri, S.M. Multi-stage structure-based virtual screening approach towards identification of potential SARS-CoV-2 NSP13 helicase inhibitors. J. Enzym. Inhib. Med. Chem. 2022, 37, 563–572. [Google Scholar] [CrossRef]
- Wu, L.J.; Long, L.; Sun, J.Y.; Bu, L.L.; Cao, J.L.; Luo, Y.; Liu, H.J.; Wu, Y.; Meng, X. Exploring the antioxidant effect of Lactobacillus plantarum SCS2 on mice with type 2 diabetes. J. Food Biochem. 2021, 45, e13781. [Google Scholar] [CrossRef]
- Bock, P.M.; Telo, G.H.; Ramalho, R.; Sbaraini, M.; Leivas, G.; Martins, A.F.; Schaan, B.D. The effect of probiotics, prebiotics or synbiotics on metabolic outcomes in individuals with diabetes: A systematic review and meta-analysis. Diabetologia 2021, 64, 26–41. [Google Scholar] [CrossRef]
- Khan, A.; Ding, Z.; Ishaq, M.; Bacha, A.S.; Khan, I.; Hanif, A.; Li, W.; Guo, X. Understanding the effects of gut microbiota dysbiosis on nonalcoholic fatty liver disease and the possible probiotics role: Recent updates. Int. J. Biol. Sci. 2021, 17, 818. [Google Scholar] [CrossRef]
- Vu, V.; Muthuramalingam, K.; Singh, V.; Hyun, C.; Kim, Y.M.; Unno, T.; Cho, M. Effects of β-glucan, probiotics, and synbiotics on obesity-associated colitis and hepatic manifestations in C57BL/6J mice. Eur. J. Nutr. 2022, 61, 793–807. [Google Scholar] [CrossRef]
- Jiang, S.; Yan, F.-F.; Hu, J.-Y.; Mohammed, A.; Cheng, H.-W. Bacillus subtilis-based probiotic improves skeletal health and immunity in broiler chickens exposed to heat stress. Animals 2021, 11, 1494. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.J.; Guo, J.; Wang, Q.; Wang, L.; Wang, Y.; Zhang, F.; Huang, W.-J.; Zhang, W.; Liu, W.J.; Wang, Y. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 577–598. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Khedmatgozar, H.; Nachvak, S.M.; Abdollahzad, H.; Moradinazar, M.; Sadeghpour Tabaei, A. The effects of co-administration of probiotics and prebiotics on chronic inflammation, and depression symptoms in patients with coronary artery diseases: A randomized clinical trial. Nutr. Neurosci. 2022, 25, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, J.; Yi, S.; Li, X.; Guo, Z.; Zhou, X.; Mu, J.; Yi, R. Lactobacillus plantarum CQPC02 prevents obesity in mice through the PPAR-α signaling pathway. Biomolecules 2019, 9, 407. [Google Scholar]
- Guida, B.; Germanò, R.; Trio, R.; Russo, D.; Memoli, B.; Grumetto, L.; Barbato, F.; Cataldi, M. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: A randomized clinical trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1043–1049. [Google Scholar] [CrossRef]
- Lutgendorff, F.; Trulsson, L.M.; van Minnen, L.P.; Rijkers, G.T.; Timmerman, H.M.; Franzén, L.E.; Gooszen, H.G.; Akkermans, L.M.; Soderholm, J.D.; Sandstrom, P.A. Probiotics enhance pancreatic glutathione biosynthesis and reduce oxidative stress in experimental acute pancreatitis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2008, 295, G1111–G1121. [Google Scholar] [CrossRef]
- Ghoniem, G.A.A.; Shalaby, M.T.; Elias, A.Z. Biological Effect of Green Tea on Hypercholesterolemia Level in Rats. J. Food Dairy Sci. 2018, 2018, 7–12. [Google Scholar] [CrossRef]
- Li, Y.; Peng, B.; Li, Y.; Huang, A.; Peng, Y.; Yu, Q.; Li, Y. MiR-203a-3p/153-3p improves cognitive impairments induced by ischemia/reperfusion via blockade of SRC-mediated MAPK signaling pathway in ischemic stroke. Chem.-Biol. Interact. 2022, 358, 109900. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Lin, L.-Z.; Chen, P.; Harnly, J.M. New phenolic components and chromatographic profiles of green and fermented teas. J. Agric. Food Chem. 2008, 56, 8130–8140. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.J.; de Camargo, A.C.; Shahidi, F. Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities. J. Funct. Foods 2017, 35, 622–634. [Google Scholar] [CrossRef]
- Kulczyński, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michałowska, A. The chemical composition and nutritional value of chia seeds—Current state of knowledge. Nutrients 2019, 11, 1242. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Velázquez, O.A.; Mondor, M.; Segura-Campos, M.R.; del Carmen Quintal-Bojórquez, N.; Hernández-Álvarez, A.J. Bioactive Phytochemicals from Chia Seed (Salvia hispanica) Oil Processing By-Products. In Bioactive Phytochemicals from Vegetable Oil and Oilseed Processing By-Products; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–25. [Google Scholar]
- Martínez-Cruz, O.; Paredes-López, O. Phytochemical profile and nutraceutical potential of chia seeds (Salvia hispanica L.) by ultra high performance liquid chromatography. J. Chromatogr. A 2014, 1346, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Aaradhya, M.; Kodikonda, M.; Naik, P.R. Antihyperglycemic, antihyperlipidemic and antioxidant activity of phenolic rich extract of Brassica oleraceae var gongylodes on streptozotocin induced Wistar rats. Springerplus 2015, 4, 212. [Google Scholar] [CrossRef]
- Tabernero, L.; Rodwell, V.W.; Stauffacher, C.V. Crystal structure of a statin bound to a class II hydroxymethylglutaryl-CoA reductase. J. Biol. Chem. 2003, 278, 19933–19938. [Google Scholar] [CrossRef]
- Wang, J.L.; Limburg, D.; Graneto, M.J.; Springer, J.; Hamper, J.R.B.; Liao, S.; Pawlitz, J.L.; Kurumbail, R.G.; Maziasz, T.; Talley, J.J. The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: The second clinical candidate having a shorter and favorable human half-life. Bioorganic Med. Chem. Lett. 2010, 20, 7159–7163. [Google Scholar] [CrossRef]



| Grade | Grade Description |
|---|---|
| 0 | No apparent injury by light microscopy |
| I | Hepatocytes swelling |
| II | Hepatocytes ballooning |
| III | Lipid droplets in hepatocytes |
| Grade | Grade Description |
|---|---|
| 0 | Normal histology |
| I | Tubular epithelial cell degeneration without significant necrosis or apoptosis |
| 2 | Tubular epithelial cell necrosis and apoptosis are less than 25% |
| No. | Compound Name | Docking Scores kcal/mol (RMSD Å) | ||
|---|---|---|---|---|
| Xanthine oxidase | HMG-CoA | COX-2 | ||
| 1 | Gallic Acid | −16.8 (1.01) | −10.6 (0.88) | −11.1 (1.15) |
| 2 | Caffeic Acid | −17.6 (0.52) | −9.8 (0.89) | −10.8 (1.19) |
| 3 | Chlorogenic Acid | −20.2 (0.88) | −13.5 (1.15) | −14.2 (1.03) |
| 4 | Daidzin7-O-Glucoside | −17.2 (1.24) | −11.1 (1.12) | −15.0 (0.95) |
| 5 | Genistein | −14.7 (0.86) | −10.9 (0.65) | −12.7 (0.75) |
| 6 | (+)-Catechin | −22.4 (0.44) | −12.8 (0.89) | −10.6 (0.72) |
| 7 | (-)-Epicatechin Gallate | −21.5 (1.04) | −17.7 (1.03) | −21.5 (1.01) |
| 8 | (+)-Gallocatechin | −24.3 (1.06) | −14.4 (1.24) | −14.5 (0.82) |
| 9 | Epigallocatechin Gallate | −16.9 (0.95) | −14.5 (0.99) | −16.9 (1.06) |
| 11 | Oxypurinol | −21.3 | - | - |
| 12 | LVA | - | −14.4 | - |
| 13 | D72 | - | - | −15.0 |
| Constituent | (%) in SBD | (%) in HFD |
|---|---|---|
| Corn starch | 56.1 | 26.1 |
| Casein | 14 | 14 |
| Sucrose | 10 | 10 |
| Corn oil | 10 | 10 |
| Cellulose | 5 | 5 |
| Minerals | 3.5 | 3.5 |
| Vitamins | 1 | 1 |
| Methionine | 0.18 | 0.18 |
| Choline chloride | 0.25 | 0.25 |
| Tert-butyl hydroquinone | 0 | 0 |
| Lard | 0 | 30 |
| Intake Type | Group | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| SBD | HFD | HFD | HFD | HFD | HFD | HFD | |
| Chia seed (%) mg/kg diet | – | – | 20 | – | – | 20 | |
| Chitosan (mg/kg diet) | – | – | – | 400 | – | 400 | |
| Green tea extract (mg/kg diet) | – | – | – | – | 200 | 200 | |
| L. plantarum (CFU) | – | – | – | – | – | 1 × 106 | 1 × 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elebeedy, D.; Ghanem, A.; Saleh, A.; Ibrahim, M.H.; Kamaly, O.A.; Abourehab, M.A.S.; Ali, M.A.; Abd El Maksoud, A.I.; El Hassab, M.A.; Eldehna, W.M. In Vivo and In Silico Investigation of the Anti-Obesity Effects of Lactiplantibacillus plantarum Combined with Chia Seeds, Green Tea, and Chitosan in Alleviating Hyperlipidemia and Inflammation. Int. J. Mol. Sci. 2022, 23, 12200. https://doi.org/10.3390/ijms232012200
Elebeedy D, Ghanem A, Saleh A, Ibrahim MH, Kamaly OA, Abourehab MAS, Ali MA, Abd El Maksoud AI, El Hassab MA, Eldehna WM. In Vivo and In Silico Investigation of the Anti-Obesity Effects of Lactiplantibacillus plantarum Combined with Chia Seeds, Green Tea, and Chitosan in Alleviating Hyperlipidemia and Inflammation. International Journal of Molecular Sciences. 2022; 23(20):12200. https://doi.org/10.3390/ijms232012200
Chicago/Turabian StyleElebeedy, Dalia, Aml Ghanem, Asmaa Saleh, Mona H. Ibrahim, Omkulthom Al Kamaly, Mohammed A. S. Abourehab, Mohamed A. Ali, Ahmed I. Abd El Maksoud, Mahmoud A. El Hassab, and Wagdy M. Eldehna. 2022. "In Vivo and In Silico Investigation of the Anti-Obesity Effects of Lactiplantibacillus plantarum Combined with Chia Seeds, Green Tea, and Chitosan in Alleviating Hyperlipidemia and Inflammation" International Journal of Molecular Sciences 23, no. 20: 12200. https://doi.org/10.3390/ijms232012200
APA StyleElebeedy, D., Ghanem, A., Saleh, A., Ibrahim, M. H., Kamaly, O. A., Abourehab, M. A. S., Ali, M. A., Abd El Maksoud, A. I., El Hassab, M. A., & Eldehna, W. M. (2022). In Vivo and In Silico Investigation of the Anti-Obesity Effects of Lactiplantibacillus plantarum Combined with Chia Seeds, Green Tea, and Chitosan in Alleviating Hyperlipidemia and Inflammation. International Journal of Molecular Sciences, 23(20), 12200. https://doi.org/10.3390/ijms232012200











