Enhanced Antimicrobial Activity of Biocompatible Bacterial Cellulose Films via Dual Synergistic Action of Curcumin and Triangular Silver Nanoplates
Abstract
1. Introduction
2. Results
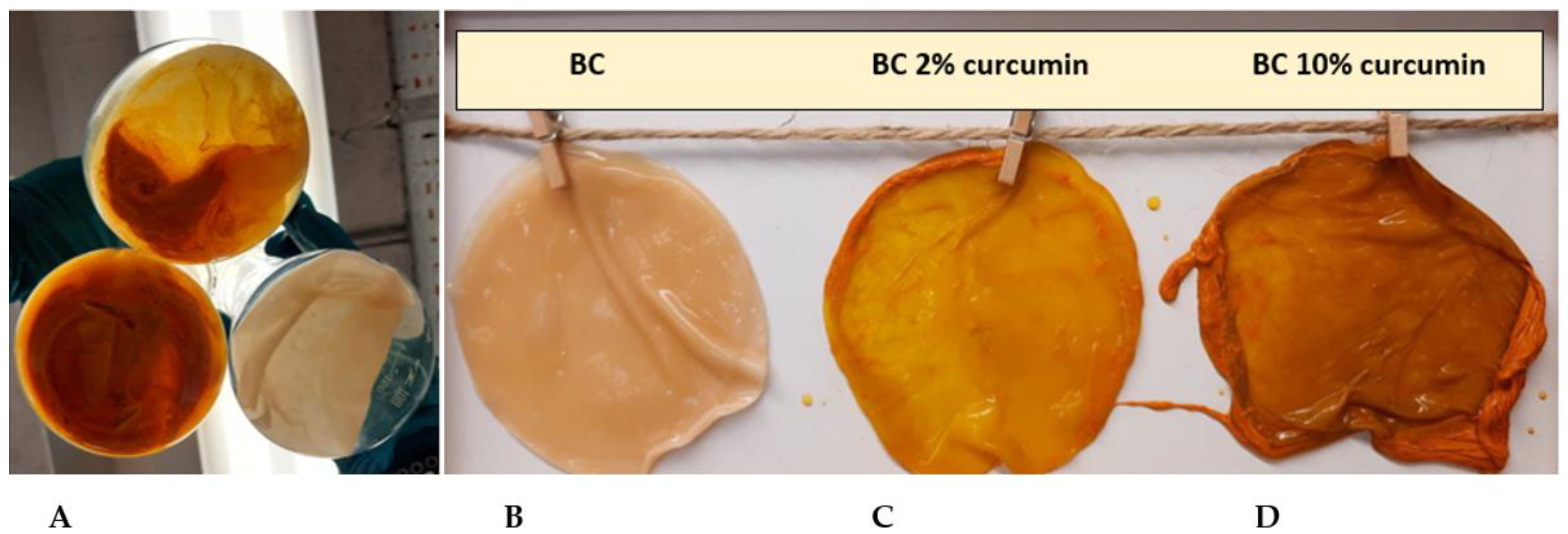
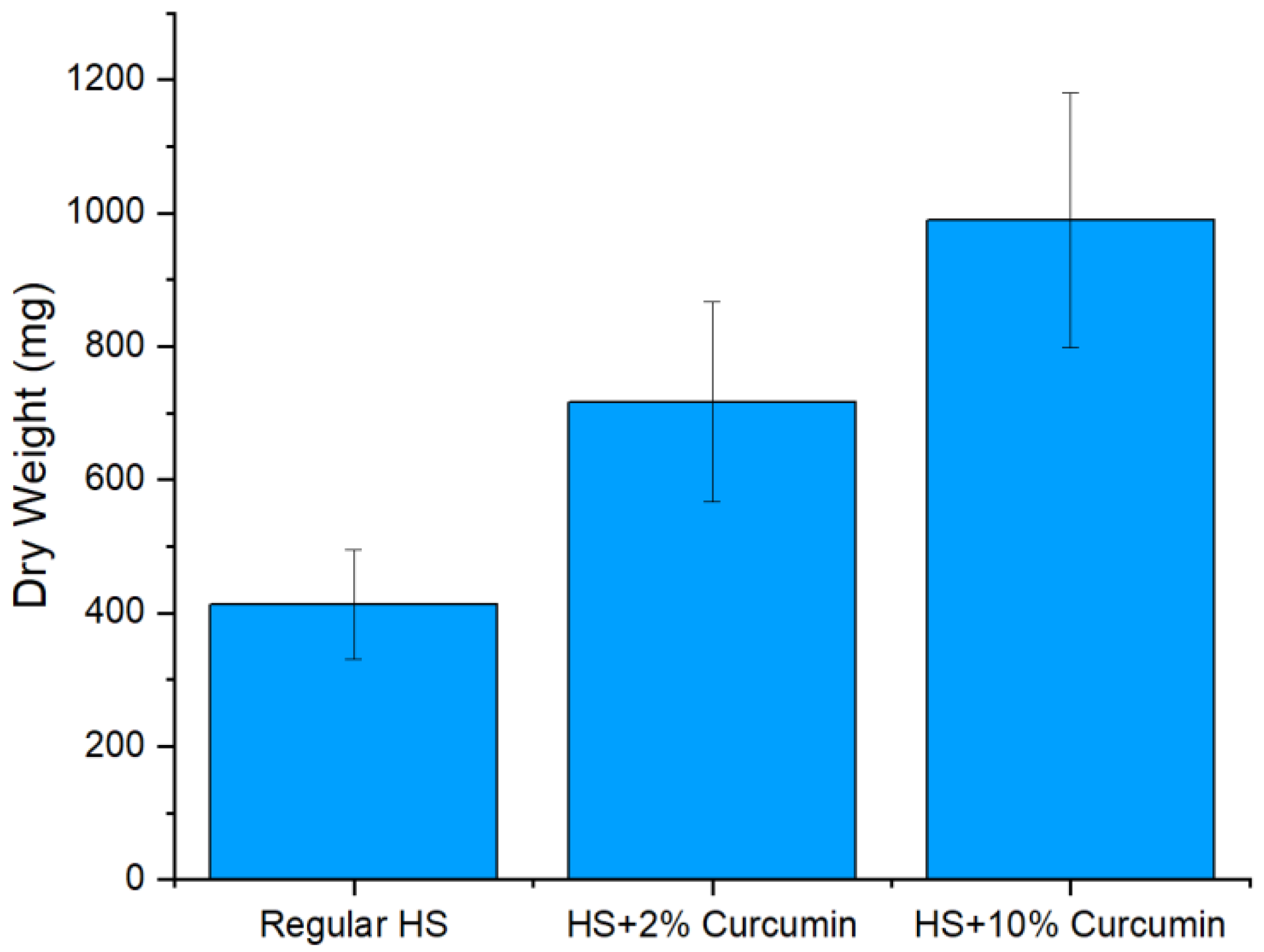
2.1. Production of BC
2.2. Characterization of Produced BC Films
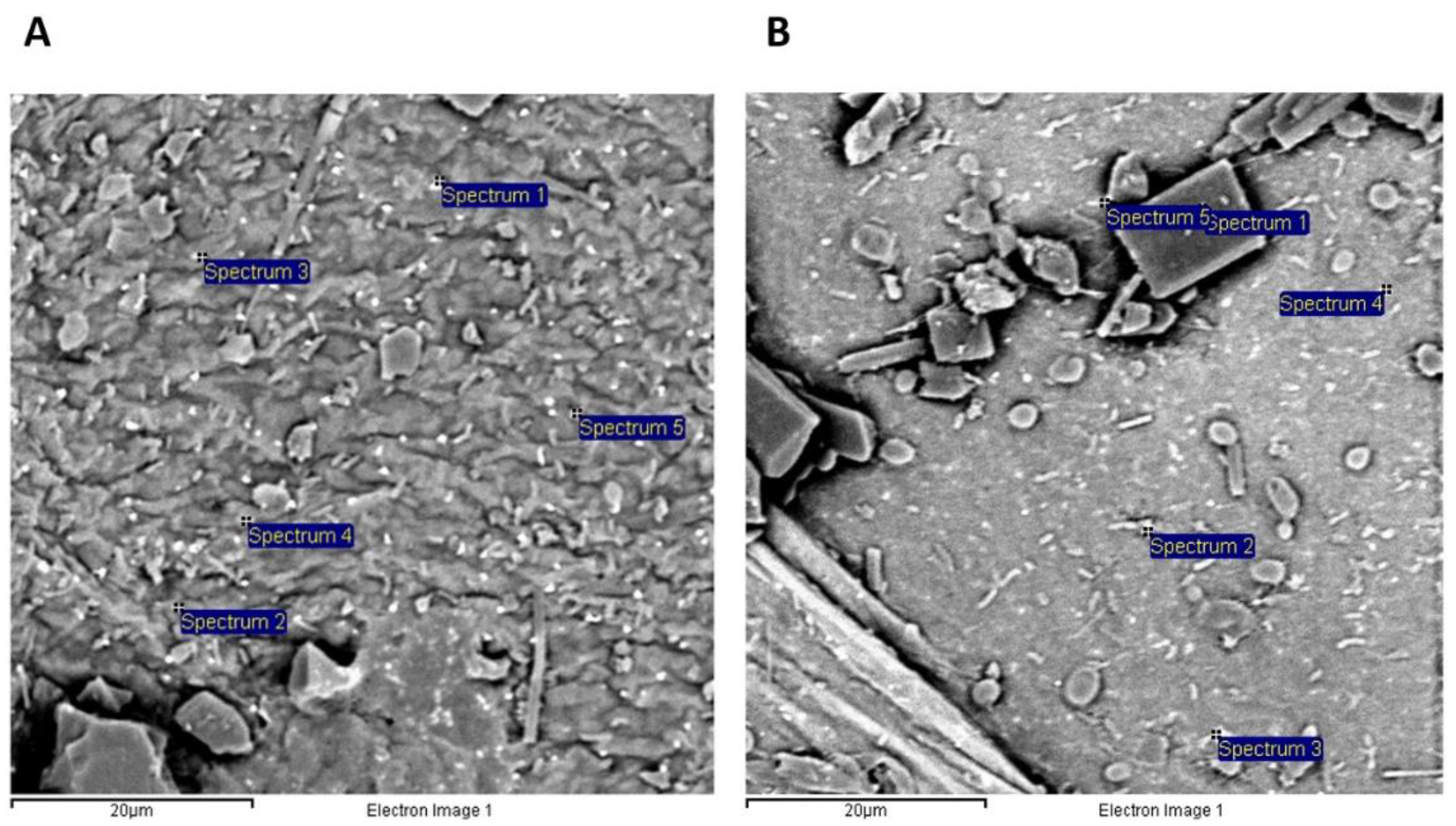
2.2.1. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS)
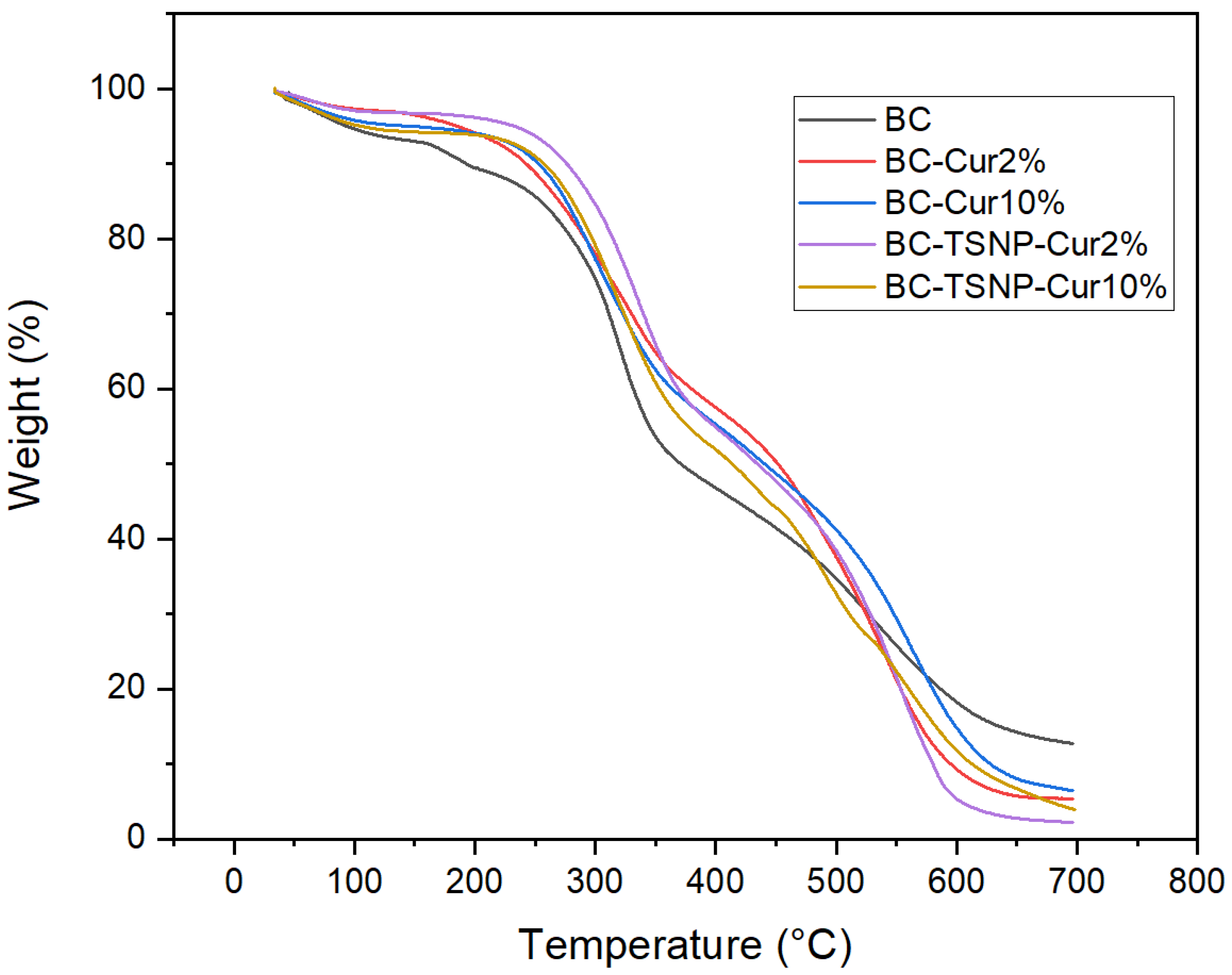
2.2.2. Thermal Gravimetric Analysis (TGA)
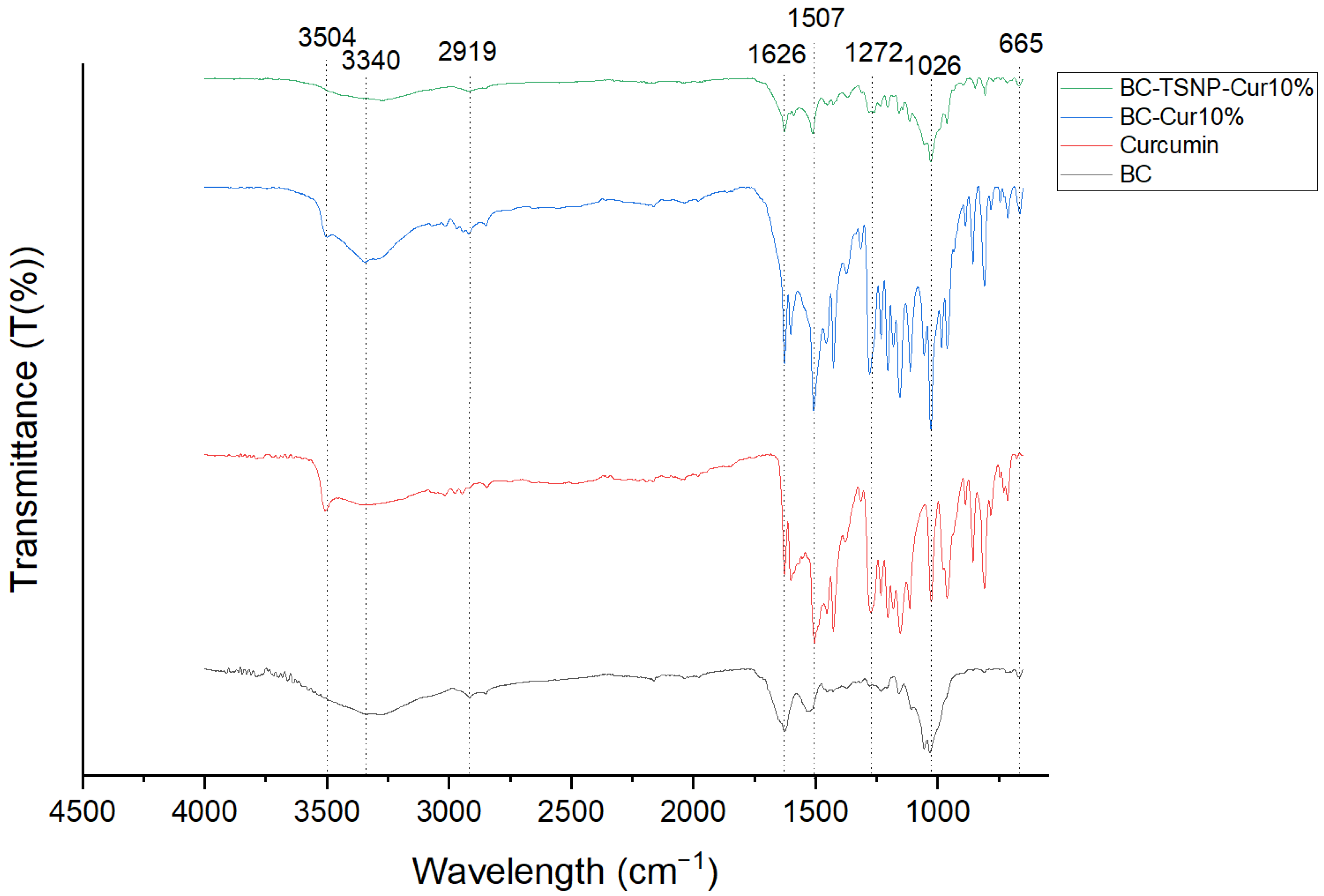
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.4. Weathering Testing
2.3. Biological Evaluations
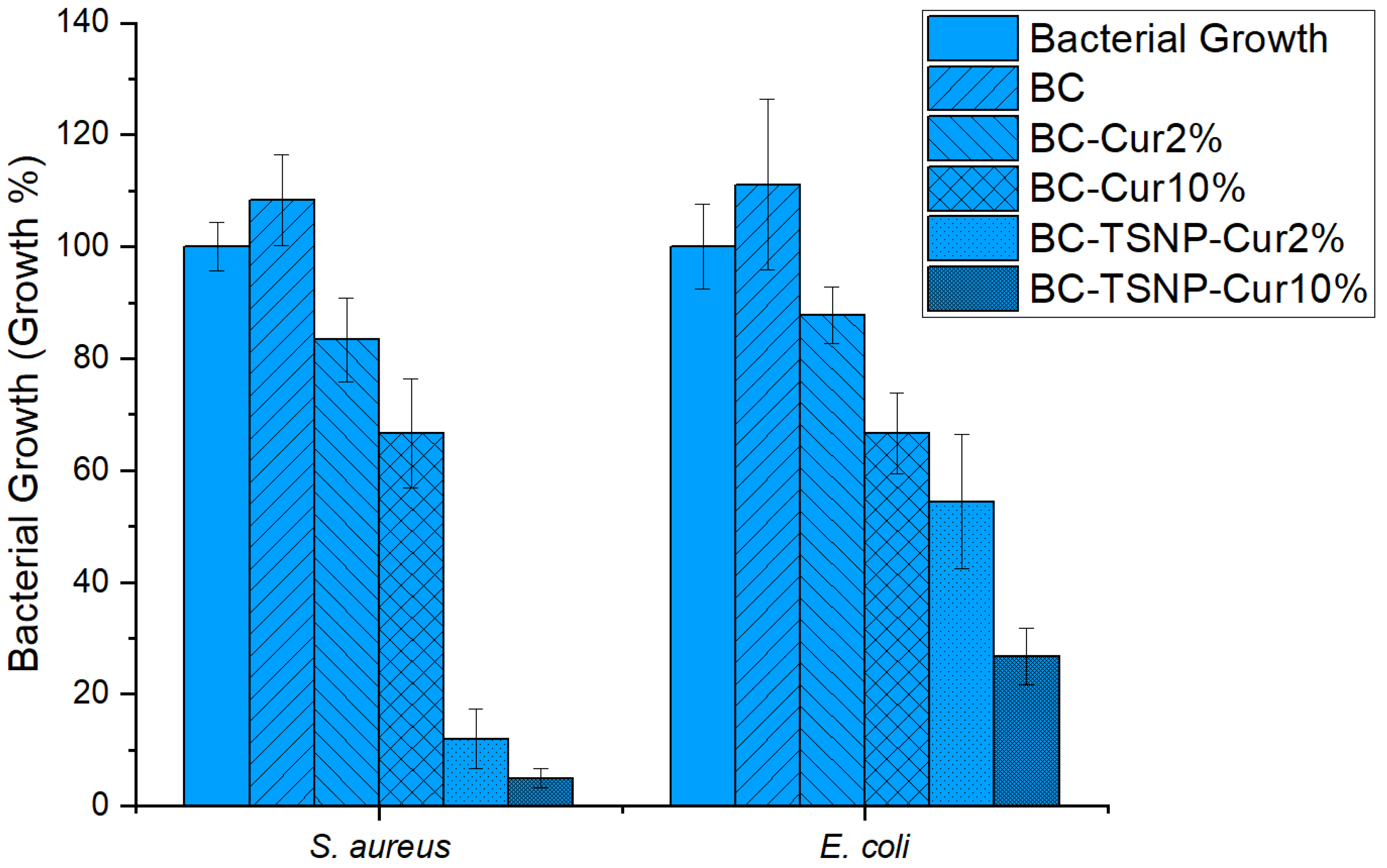
2.3.1. Antimicrobial Activity
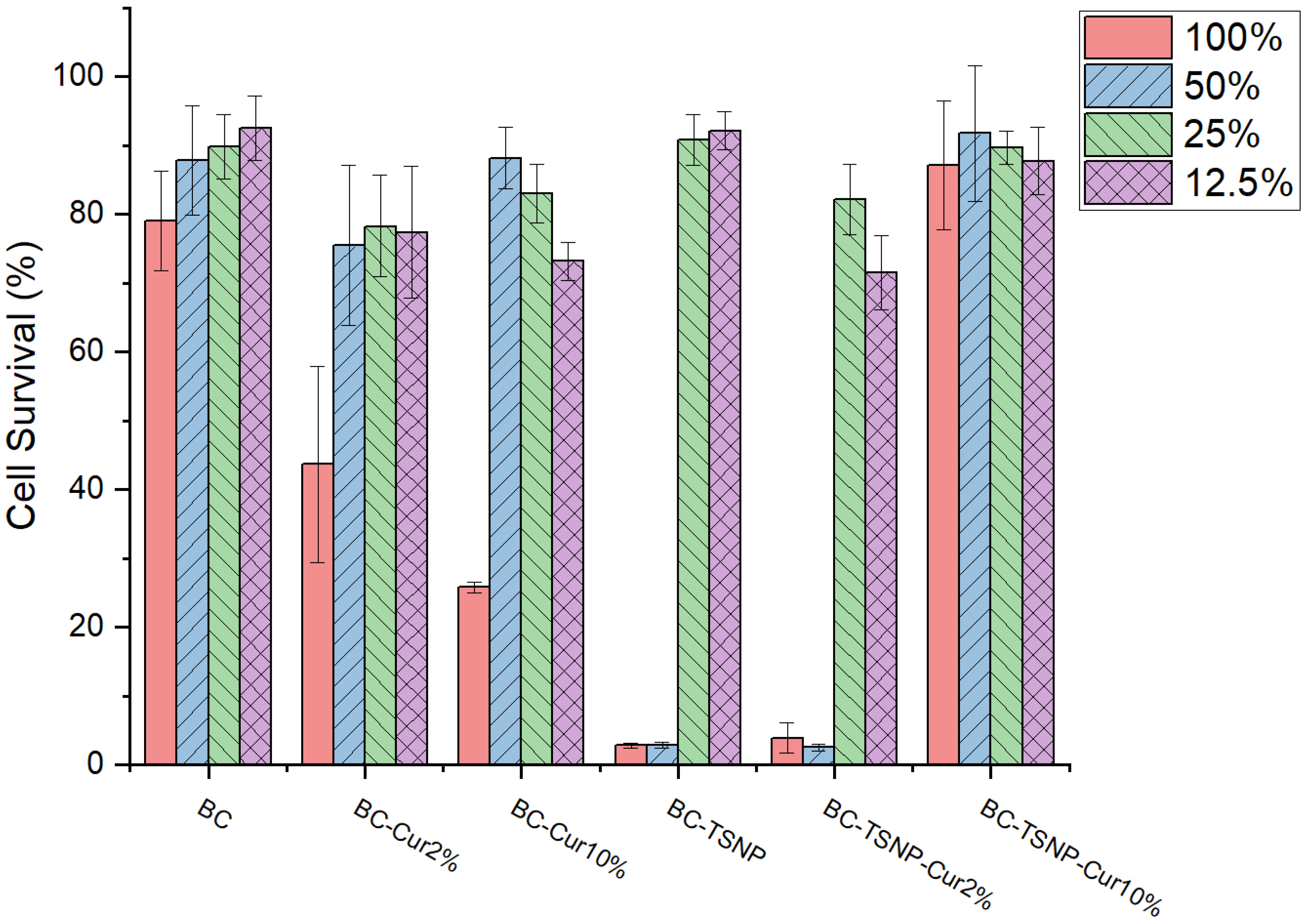
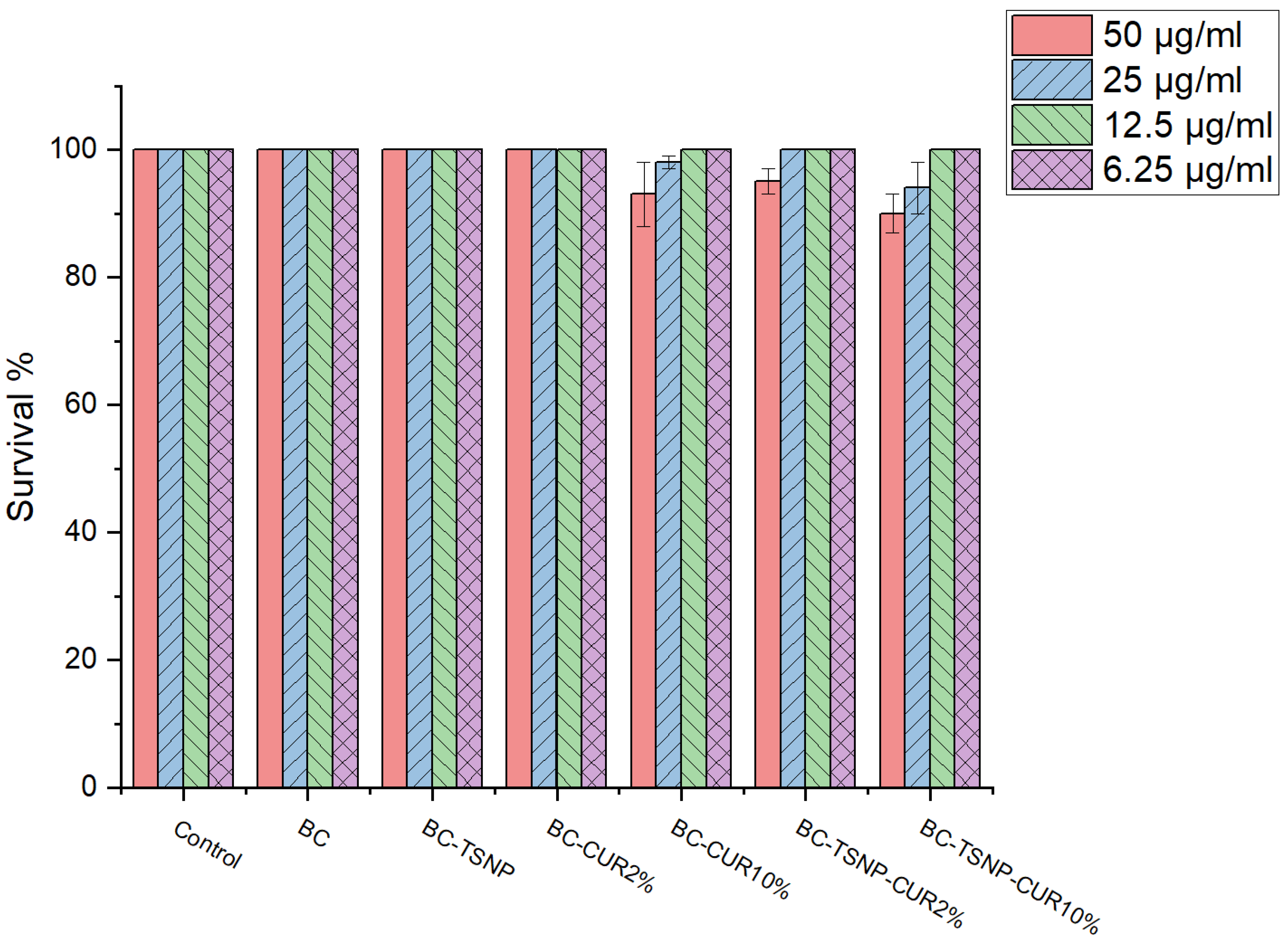
2.3.2. Cytotoxicity Assay
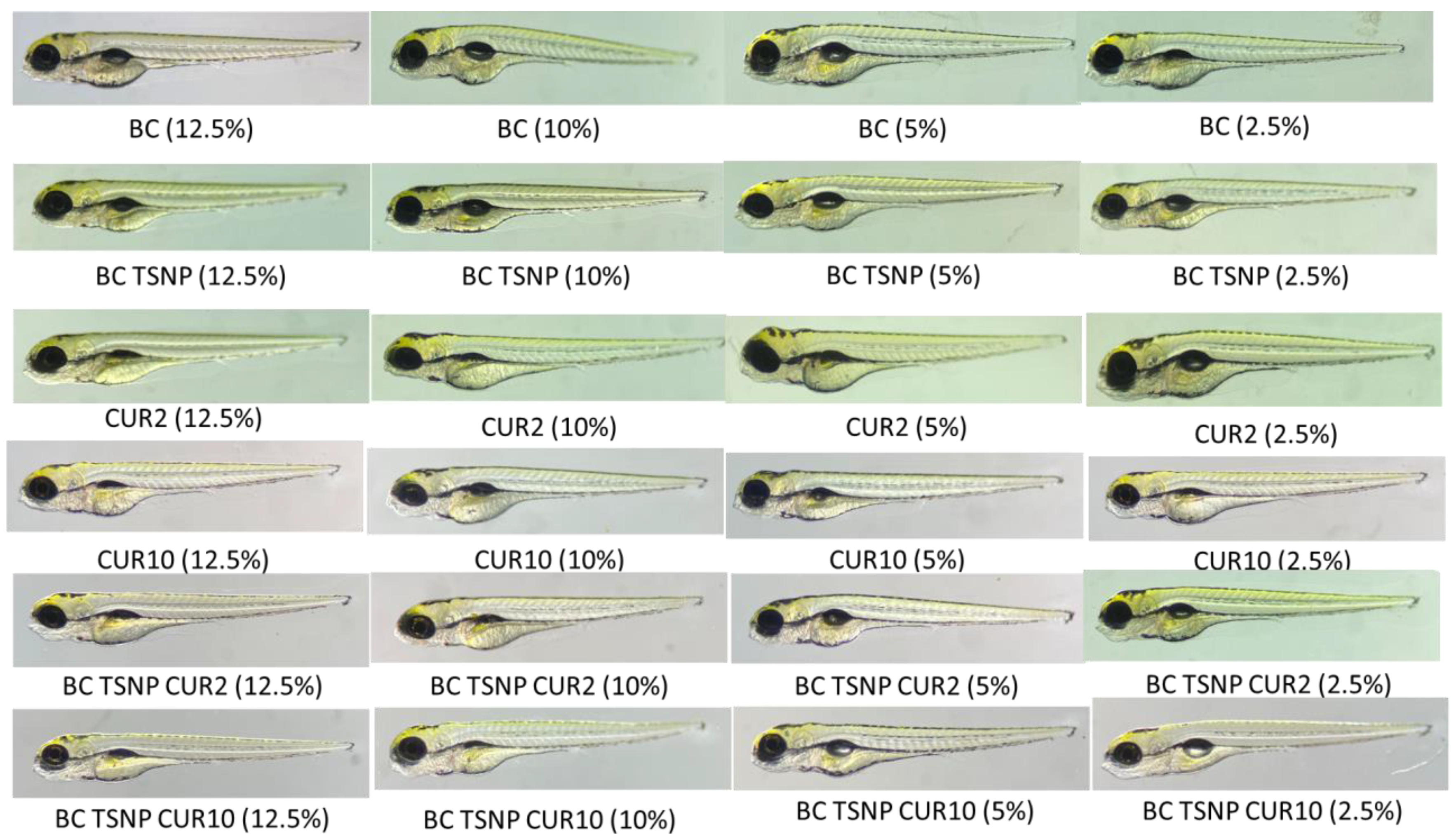
2.3.3. Danio Rerio Embryotoxicity Assay
2.3.4. Caenorhabditis elegans Survival Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of TSNPs
4.3. Production of BC Films Using Curcumin as a Supplement
Estimation of Curcumin Absorption from Media
4.4. Preparation of TSNP Incorporated BC Films
4.5. Characterisation of Derived BC Films
4.5.1. Morphological Characterization and Estimation of TSNP Absorption
4.5.2. Thermogravimetric Analysis (TGA)
4.5.3. Fourier Transforms Infrared (FTIR) Spectroscopy
4.5.4. Evaluation of BC Films Stability via Weathering Tests
4.5.5. Biological Evaluations
Antibacterial Activity of BC Films
Cytotoxicity Assay
Danio rerio Embryotoxicity Assay
Caenorhabditis elegans Survival Assay
4.6. Statistical Analysys
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gorgieva, S.; Trček, J. Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef]
- Silva, S.S.; Fernandes, E.M.; Pina, S.; Silva-Correia, J.; Vieira, S.; Oliveira, J.M.; Reis, R.L. 2.11 Polymers of Biological Origin. Compr. Biomater. II 2017, 2, 228–252. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Bacterial Nanocellulose: Present Status, Biomedical Applications and Future Perspectives. Mater. Sci. Eng. C 2019, 104, 109963. [Google Scholar] [CrossRef]
- de Amorim, J.D.P.; da Silva Junior, C.J.G.; de Medeiros, A.D.M.; do Nascimento, H.A.; Sarubbo, M.; de Medeiros, T.P.M.; Costa, A.F.d.S.; Sarubbo, L.A. Bacterial Cellulose as a Versatile Biomaterial for Wound Dressing Application. Molecules 2022, 27, 5580. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Gonzales, K.N.; Sari, R.M.; Gea, S. Bacterial Cellulose-based Biosensors. Med. Devices Sens. 2020, 3, e10102. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef]
- Zhang, C.; Salick, M.R.; Cordie, T.M.; Ellingham, T.; Dan, Y.; Turng, L.S. Incorporation of Poly(Ethylene Glycol) Grafted Cellulose Nanocrystals in Poly(Lactic Acid) Electrospun Nanocomposite Fibers as Potential Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2015, 49, 463–471. [Google Scholar] [CrossRef]
- Zander, N.E.; Dong, H.; Steele, J.; Grant, J.T. Metal Cation Cross-Linked Nanocellulose Hydrogels as Tissue Engineering Substrates. ACS Appl. Mater. Interfaces 2014, 6, 18502–18510. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial Cellulose Production, Properties and Applications with Different Culture Methods—A Review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef]
- Jacek, P.; Dourado, F.; Gama, M.; Bielecki, S. Molecular Aspects of Bacterial Nanocellulose Biosynthesis. Microb. Biotechnol. 2019, 12, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Bagewadi, Z.K.; Bhavikatti, J.S.; Muddapur, U.M.; Yaraguppi, D.A.; Mulla, S.I. Statistical Optimization and Characterization of Bacterial Cellulose Produced by Isolated Thermophilic Bacillus Licheniformis Strain ZBT2. Carbohydr. Res. 2020, 491, 107979. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Myung, H.; Govarthanan, M.; Yi, Y.J.; Seo, S.K.; Cho, K.M.; Lovanh, N.; Oh, B.T. Production and Characterization of Bacterial Cellulose by Leifsonia Sp. CBNU-EW3 Isolated from the Earthworm, Eisenia Fetida. Biotechnol. Bioprocess Eng. 2015, 20, 410–416. [Google Scholar] [CrossRef]
- Khan, H.; Kadam, A.; Dutt, D. Studies on Bacterial Cellulose Produced by a Novel Strain of Lactobacillus Genus. Carbohydr. Polym. 2020, 229, 115513. [Google Scholar] [CrossRef] [PubMed]
- Tanskul, S.; Amornthatree, K.; Jaturonlak, N. A New Cellulose-Producing Bacterium, Rhodococcus sp. MI 2: Screening and Optimization of Culture Conditions. Carbohydr. Polym. 2013, 92, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Arrebola, E.; Carrión, V.J.; Gutiérrez-Barranquero, J.A.; Pérez-García, A.; Rodríguez-Palenzuela, P.; Cazorla, F.M.; de Vicente, A. Cellulose Production in Pseudomonas Syringae Pv. Syringae: A Compromise between Epiphytic and Pathogenic Lifestyles. FEMS Microbiol. Ecol. 2015, 91, fiv071. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Saxena, I.; Wang, X.; Kader, A.; Bokranz, W.; Simm, R.; Nobles, D.; Chromek, M.; Brauner, A.; Brown, R.M.; et al. Characterization of Cellulose Production in Escherichia Coli Nissle 1917 and Its Biological Consequences. Environ. Microbiol. 2009, 11, 1105–1116. [Google Scholar] [CrossRef]
- Cannon, R.E.; Anderson, S.M. Biogenesis of Bacterial Cellulose. Crit. Rev. Microbiol. 1991, 17, 435–447. [Google Scholar] [CrossRef]
- Hungund, B.S.; Gupta, S.G. Production of Bacterial Cellulose from Enterobacter Amnigenus GH-1 Isolated from Rotten Apple. World J. Microbiol. Biotechnol. 2010, 26, 1823–1828. [Google Scholar] [CrossRef]
- Römling, U.; Lünsdorf, H. Characterization of Cellulose Produced by Salmonella Enterica Serovar Typhimurium. Cellulose 2004, 11, 413–418. [Google Scholar] [CrossRef]
- Aswini, K.; Gopal, N.O.; Uthandi, S. Optimized Culture Conditions for Bacterial Cellulose Production by Acetobacter Senegalensis MA1. BMC Biotechnol. 2020, 20, 46. [Google Scholar] [CrossRef]
- Ullah, M.W.; Ul-Islam, M.; Khan, S.; Kim, Y.; Park, J.K. Innovative Production of Bio-Cellulose Using a Cell-Free System Derived from a Single Cell Line. Carbohydr. Polym. 2015, 132, 286–294. [Google Scholar] [CrossRef]
- Żywicka, A.; Junka, A.F.; Szymczyk, P.; Chodaczek, G.; Grzesiak, J.; Sedghizadeh, P.P.; Fijałkowski, K. Bacterial Cellulose Yield Increased over 500% by Supplementation of Medium with Vegetable Oil. Carbohydr. Polym. 2018, 199, 294–303. [Google Scholar] [CrossRef]
- Comini, S.; Scutera, S.; Sparti, R.; Banche, G.; Coppola, B.; Bertea, C.M.; Bianco, G.; Gatti, N.; Cuffini, A.M.; Palmero, P.; et al. Combination of Poly (ε-Caprolactone) Biomaterials and Essential Oils to Achieve Anti-Bacterial and Osteo-Proliferative Properties for 3D-Scaffolds in Regenerative Medicine. Pharmaceutics 2022, 14, 1873. [Google Scholar] [CrossRef]
- Lemnaru, G.M.; Truşcă, R.D.; Ilie, C.I.; Ţiplea, R.E.; Ficai, D.; Oprea, O.; Stoica-Guzun, A.; Ficai, A.; Diţu, L.M. Antibacterial Activity of Bacterial Cellulose Loaded with Bacitracin and Amoxicillin: In Vitro Studies. Molecules 2020, 25, 4069. [Google Scholar] [CrossRef]
- Pal, S.; Nisi, R.; Stoppa, M.; Licciulli, A. Silver-Functionalized Bacterial Cellulose as Antibacterial Membrane for Wound-Healing Applications. ACS Omega 2017, 2, 3632–3639. [Google Scholar] [CrossRef] [PubMed]
- Miyashiro, D.; Hamano, R.; Umemura, K. A Review of Applications Using Mixed Materials of Cellulose, Nanocellulose and Carbon Nanotubes. Nanomaterials 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Bodea, I.M.; Cătunescu, G.M.; Pop, C.R.; Fiț, N.I.; David, A.P.; Dudescu, M.C.; Stănilă, A.; Rotar, A.M.; Beteg, F.I. Antimicrobial Properties of Bacterial Cellulose Films Enriched with Bioactive Herbal Extracts Obtained by Microwave-Assisted Extraction. Polymers 2022, 14, 1435. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, S.; Luo, J.; Wang, X. Latest Advances on Bacterial Cellulose-Based Antibacterial Materials as Wound Dressings. Front. Bioeng. Biotechnol. 2020, 8, 593768. [Google Scholar] [CrossRef]
- Bush, K. Synergistic Antibiotic Combinations. Top. Med. Chem. 2017, 25, 169–244. [Google Scholar] [CrossRef]
- Guo, C.; Cheng, F.; Liang, G.; Zhang, S.; Duan, S.; Fu, Y.; Marchetti, F.; Zhang, Z.; Du, M. Multimodal Antibacterial Platform Constructed by the Schottky Junction of Curcumin-Based Bio Metal—Organic Frameworks and Ti3C2Tx MXene Nanosheets for Ef Fi Cient Wound Healing. Adv. NanoBiomed Res. 2022, 2, 2200064. [Google Scholar] [CrossRef]
- Praditya, D.; Kirchhoff, L.; Brüning, J.; Rachmawati, H.; Steinmann, J.; Steinmann, E. Anti-Infective Properties of the Golden Spice Curcumin. Front. Microbiol. 2019, 10, 912. [Google Scholar] [CrossRef]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. Biomed. Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Chiaoprakobkij, N.; Suwanmajo, T.; Sanchavanakit, N. Curcumin-Loaded Bacterial Cellulose/Alginate/Gelatin. Mol. Impr. Sens. 2020, 25, 3800. [Google Scholar] [CrossRef]
- Gupta, A.; Keddie, D.J.; Kannappan, V.; Gibson, H.; Khalil, I.R.; Kowalczuk, M.; Martin, C.; Shuai, X.; Radecka, I. Production and Characterisation of Bacterial Cellulose Hydrogels Loaded with Curcumin Encapsulated in Cyclodextrins as Wound Dressings. Eur. Polym. J. 2019, 118, 437–450. [Google Scholar] [CrossRef]
- Khamrai, M.; Banerjee, S.L.; Paul, S.; Samanta, S.; Kundu, P.P. Curcumin Entrapped Gelatin/Ionically Modified Bacterial Cellulose Based Self-Healable Hydrogel Film: An Eco-Friendly Sustainable Synthesis Method of Wound Healing Patch. Int. J. Biol. Macromol. 2019, 122, 940–953. [Google Scholar] [CrossRef]
- Sajjad, W.; He, F.; Ullah, M.W.; Ikram, M.; Shah, S.M.; Khan, R.; Khan, T.; Khalid, A.; Yang, G.; Wahid, F. Fabrication of Bacterial Cellulose-Curcumin Nanocomposite as a Novel Dressing for Partial Thickness Skin Burn. Front. Bioeng. Biotechnol. 2020, 8, 553037. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.G.; Lee, B.T. Curcumin Incorporation into an Oxidized Cellulose Nanofiber-Polyvinyl Alcohol Hydrogel System Promotes Wound Healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Subtaweesin, C.; Woraharn, W.; Taokaew, S.; Chiaoprakobkij, N.; Sereemaspun, A.; Phisalaphong, M. Characteristics of Curcumin-Loaded Bacterial Cellulose Films and Anticancer Properties against Malignant Melanoma Skin Cancer Cells. Appl. Sci. 2018, 8, 1188. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Jiang, Q.; Yu, D.; Xu, Y.; Wang, B.; Xia, W. Development and Properties of Bacterial Cellulose, Curcumin, and Chitosan Composite Biodegradable Films for Active Packaging Materials. Carbohydr. Polym. 2021, 260, 117778. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Barud, H.S.; Regiani, T.; Marques, R.F.C.; Lustri, W.R.; Messaddeq, Y.; Ribeiro, S.J.L. Antimicrobial Bacterial Cellulose-Silver Nanoparticles Composite Membranes. J. Nanomater. 2011, 2011, 721631. [Google Scholar] [CrossRef]
- Fadakar Sarkandi, A.; Montazer, M.; Harifi, T.; Mahmoudi Rad, M. Innovative Preparation of Bacterial Cellulose/Silver Nanocomposite Hydrogels: In Situ Green Synthesis, Characterization, and Antibacterial Properties. J. Appl. Polym. Sci. 2021, 138, 49824. [Google Scholar] [CrossRef]
- Stanisławska, A. Bacterial Nanocellulose as a Microbiological Derived Nanomaterial. Adv. Mater. Sci. 2016, 16, 67. [Google Scholar] [CrossRef]
- Kalantari, K.; Afifi, A.M.; Moniri, M.; Moghaddam, A.B.; Kalantari, A.; Izadiyan, Z. Autoclave-Assisted Synthesis of AgNPs in Z. Officinale Extract and Assessment of Their Cytotoxicity, Antibacterial and Antioxidant Activities. IET Nanobiotechnology 2019, 13, 262–268. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Wen, X.; Lin, Q.; Chen, X.; Wu, Z. Silver Nanoparticle/Bacterial Cellulose Gel Membranes for Antibacterial Wound Dressing: Investigation in Vitro and in Vivo. Biomed. Mater. 2014, 9, 035005. [Google Scholar] [CrossRef]
- Nicomrat, D. Silver Nanoparticles Impregnated Biocellulose Produced by Sweet Glutinous Rice Fermentation with the Genus Acetobacter. E3S Web Conf. 2020, 141, 03003. [Google Scholar] [CrossRef]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of Silver Nanoparticles into Bacterial Cellulose for Antimicrobial Wound Dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Pourali, P.; Yahyaei, B.; Ajoudanifar, H.; Taheri, R.; Alavi, H.; Hoseini, A. Impregnation of the Bacterial Cellulose Membrane with Biologically Produced Silver Nanoparticles. Curr. Microbiol. 2014, 69, 785–793. [Google Scholar] [CrossRef]
- Wang, C.; Liu, W.; Cao, H.; Jia, L.; Liu, P. Cellulose Nanofibers Aerogels Functionalized with AgO: Preparation, Characterization and Antibacterial Activity. Int. J. Biol. Macromol. 2022, 194, 58–65. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, S.; Wang, J.; Gan, D.; Gama, M.; Yang, Z.; Zhu, Y.; Yao, F.; Luo, H. Scalable Synthesis of Robust and Stretchable Composite Wound Dressings by Dispersing Silver Nanowires in Continuous Bacterial Cellulose. Compos. Part B Eng. 2020, 199, 108259. [Google Scholar] [CrossRef]
- Lu, W.; Yao, K.; Wang, J.; Yuan, J. Ionic Liquids-Water Interfacial Preparation of Triangular Ag Nanoplates and Their Shape-Dependent Antibacterial Activity. J. Colloid Interface Sci. 2015, 437, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle: A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Van Dong, P.; Ha, C.H.; Binh, L.T.; Kasbohm, J. Chemical Synthesis and Antibacterial Activity of Novel-Shaped Silver Nanoparticles. Int. Nano Lett. 2012, 2, 9. [Google Scholar] [CrossRef]
- Vo, Q.K.; Phung, D.D.; Nguyen, Q.N.V.; Thi, H.H.; Thi, N.H.N.; Thi, P.P.N.; Bach, L.G.; Tan, L. Van Controlled Synthesis of Triangular Silver Nanoplates by Gelatin-Chitosan Mixture and the Influence of Their Shape on Antibacterial Activity. Processes 2019, 7, 873. [Google Scholar] [CrossRef]
- Akrami-Hasan-Kohal, M.; Tayebi, L.; Ghorbani, M. Curcumin-Loaded Naturally-Based Nanofibers as Active Wound Dressing Mats: Morphology, Drug Release, Cell Proliferation, and Cell Adhesion Studies. New J. Chem. 2020, 44, 10343–10351. [Google Scholar] [CrossRef]
- Miñano, J.; Puiggalí, J.; Franco, L. Effect of Curcumin on Thermal Degradation of Poly(Glycolic Acid) and Poly(ε-Caprolactone) Blends. Thermochim. Acta 2020, 693, 178764. [Google Scholar] [CrossRef]
- Gea, S.; Reynolds, C.T.; Roohpour, N.; Wirjosentono, B.; Soykeabkaew, N.; Bilotti, E.; Peijs, T. Investigation into the Structural, Morphological, Mechanical and Thermal Behaviour of Bacterial Cellulose after a Two-Step Purification Process. Bioresour. Technol. 2011, 102, 9105–9110. [Google Scholar] [CrossRef]
- Wang, S.S.; Han, Y.H.; Chen, J.L.; Zhang, D.C.; Shi, X.X.; Ye, Y.X.; Chen, D.L.; Li, M. Insights into Bacterial Cellulose Biosynthesis from Different Carbon Sources and the Associated Biochemical Transformation Pathways in Komagataeibacter sp. W1. Polymers 2018, 10, 963. [Google Scholar] [CrossRef]
- Chen, X.; Zou, L.Q.; Niu, J.; Liu, W.; Peng, S.F.; Liu, C.M. The Stability, Sustained Release and Cellular Antioxidant Activity of Curcumin Nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef]
- Ismail, E.H.; Sabry, D.Y.; Mahdy, H.; Khalil, M.M.H. Synthesis and Characterization of Some Ternary Metal Complexes of Curcumin with 1,10-Phenanthroline and Their Anticancer Applications. J. Sci. Res. 2014, 6, 509–519. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Wiryani, A.S.; Rusli, A.; Purnamasari, A.; Abdullah, A.G.; Ana; Widiaty, I.; Hurriyati, R. Extraction of Curcumin Pigment from Indonesian Local Turmeric with Its Infrared Spectra and Thermal Decomposition Properties. Mater. Sci. Eng. 2017, 755, 012136. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Natarajan, R.K.; Natarajan, S.; Rathikha, R. Structural Investigation on Curcumin. Asian J. Chem. 2008, 20, 2903–2913. [Google Scholar]
- Wang, S.S.; Han, Y.H.; Ye, Y.X.; Shi, X.X.; Xiang, P.; Chen, D.L.; Li, M. Physicochemical Characterization of High-Quality Bacterial Cellulose Produced by Komagataeibacter sp. Strain W1 and Identification of the Associated Genes in Bacterial Cellulose Production. RSC Adv. 2017, 7, 45145–45155. [Google Scholar] [CrossRef]
- Andreani, T.; Nogueira, V.; Pinto, V.V.; Ferreira, M.J.; Rasteiro, M.G.; Silva, A.M.; Pereira, R.; Pereira, C.M. Influence of the Stabilizers on the Toxicity of Metallic Nanomaterials in Aquatic Organisms and Human Cell Lines. Sci. Total Environ. 2017, 607–608, 1264–1277. [Google Scholar] [CrossRef]
- Ha, N.M.; Tran, S.H.; Shim, Y.H.; Kang, K. Caenorhabditis Elegans as a Powerful Tool in Natural Product Bioactivity Research. Appl. Biol. Chem. 2022, 65, 18. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, L.; Zhang, L.; Shen, X.; Kong, L.; Wu, T. Research Advances on the Adverse Effects of Nanomaterials in a Model Organism, Caenorhabditis Elegans. Environ. Toxicol. Chem. 2021, 40, 2406–2424. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Ebadati, A.; Hosseini, E.S.; Vahabi, A.H.; Oshaghi, M.; Rahighi, R.; Orooji, Y.; Jahromi, M.A.M.; Varma, R.S.; Hamblin, M.R.; et al. Antibacterial, Antibiofilm, Anti-Inflammatory, and Wound Healing Effects of Nanoscale Multifunctional Cationic Alternating Copolymers. Bioorg. Chem. 2022, 119, 105550. [Google Scholar] [CrossRef]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal Activity of Curcumin I Is Associated with Damaging of Bacterial Membrane. PLoS ONE 2015, 10, 0121313. [Google Scholar] [CrossRef]
- Wu, J.Y.; Lin, C.Y.; Lin, T.W.; Ken, C.F.; Wen, Y. Der Curcumin Affects Development of Zebrafish Embryo. Biol. Pharm. Bull. 2007, 30, 1336–1339. [Google Scholar] [CrossRef]
- Gao, X.P.; Feng, F.; Zhang, X.Q.; Liu, X.X.; Wang, Y.B.; She, J.X.; He, Z.H.; He, M.F. Toxicity Assessment of 7 Anticancer Compounds in Zebrafish. Int. J. Toxicol. 2014, 33, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Lian Wu, Y.; Gong, Z.; Valiyaveettil, S. Toxicity of Silver Nanoparticles in Zebrafish Models. Nanotechnology 2008, 19, 255102. [Google Scholar] [CrossRef] [PubMed]
- Liao, V.H.C.; Yu, C.W.; Chu, Y.J.; Li, W.H.; Hsieh, Y.C.; Wang, T.T. Curcumin-Mediated Lifespan Extension in Caenorhabditis Elegans. Mech. Ageing Dev. 2011, 132, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gondikas, A.P.; Marinakos, S.M.; Auffan, M.; Liu, J.; Hsu-kim, H.; Meyer, J.N. Mechanism of Silver Nanoparticle Toxicity Is Dependent on Dissolved Silver and Surface Coating in Caenorhabditis Elegans. Environ. Sci. Technol. 2012, 46, 1119–1127. [Google Scholar] [CrossRef]
- Garcia, E.L.; Attallah, O.A.; Mojicevic, M.; Devine, D.M.; Fournet, M.B. Antimicrobial Active Bioplastics Using Triangular Silver Nanoplate Integrated Polycaprolactone and Polylactic Acid Films. Materials 2021, 14, 1132. [Google Scholar] [CrossRef]
- Molina-ramírez, C.; Enciso, C.; Torres-taborda, M.; Zuluaga, R.; Gañán, P.; Rojas, O.J.; Castro, C. Effects of Alternative Energy Sources on Bacterial Cellulose Characteristics Produced by Komagataeibacter Medellinensis. Int. J. Biol. Macromol. 2018, 117, 735–741. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, S.; Moulik, S.P. Stability of Curcumin in Different Solvent and Solution Media: UV–Visible and Steady-State Fluorescence Spectral Study. J. Photochem. Photobiol. B Biol. 2016, 158, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-Examination and Further Development of a Precise and Rapid Dye Method for Measuring Cell Growth/Cell Kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. In OECD Guidelines for the Testing of Chemicals; Section 2; OECD: Paris, France, 2013; ISBN 9789264203709. [Google Scholar]
- Stiernagle, T. Maintenance of C. Elegans. WormBook 2006, 1, 1–11. [Google Scholar] [CrossRef]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum Sensing Inhibitors Increase the Susceptibility of Bacterial Biofilms to Antibiotics in Vitro and in Vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef]



| Samples | ΔE* |
|---|---|
| BC | 2.83 |
| BC-Cur2% | 12.74 |
| BC-Cur10% | 6.40 |
| BC-TSNP-Cur2% | 4.69 |
| BC-TSNP-Cur10% | 6.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, E.L.; Mojicevic, M.; Milivojevic, D.; Aleksic, I.; Vojnovic, S.; Stevanovic, M.; Murray, J.; Attallah, O.A.; Devine, D.; Fournet, M.B. Enhanced Antimicrobial Activity of Biocompatible Bacterial Cellulose Films via Dual Synergistic Action of Curcumin and Triangular Silver Nanoplates. Int. J. Mol. Sci. 2022, 23, 12198. https://doi.org/10.3390/ijms232012198
Garcia EL, Mojicevic M, Milivojevic D, Aleksic I, Vojnovic S, Stevanovic M, Murray J, Attallah OA, Devine D, Fournet MB. Enhanced Antimicrobial Activity of Biocompatible Bacterial Cellulose Films via Dual Synergistic Action of Curcumin and Triangular Silver Nanoplates. International Journal of Molecular Sciences. 2022; 23(20):12198. https://doi.org/10.3390/ijms232012198
Chicago/Turabian StyleGarcia, Eduardo Lanzagorta, Marija Mojicevic, Dusan Milivojevic, Ivana Aleksic, Sandra Vojnovic, Milena Stevanovic, James Murray, Olivia Adly Attallah, Declan Devine, and Margaret Brennan Fournet. 2022. "Enhanced Antimicrobial Activity of Biocompatible Bacterial Cellulose Films via Dual Synergistic Action of Curcumin and Triangular Silver Nanoplates" International Journal of Molecular Sciences 23, no. 20: 12198. https://doi.org/10.3390/ijms232012198
APA StyleGarcia, E. L., Mojicevic, M., Milivojevic, D., Aleksic, I., Vojnovic, S., Stevanovic, M., Murray, J., Attallah, O. A., Devine, D., & Fournet, M. B. (2022). Enhanced Antimicrobial Activity of Biocompatible Bacterial Cellulose Films via Dual Synergistic Action of Curcumin and Triangular Silver Nanoplates. International Journal of Molecular Sciences, 23(20), 12198. https://doi.org/10.3390/ijms232012198










