Single-Cell Transcriptomic Profiling in Inherited Retinal Degeneration Reveals Distinct Metabolic Pathways in Rod and Cone Photoreceptors
Abstract
1. Introduction
2. Results
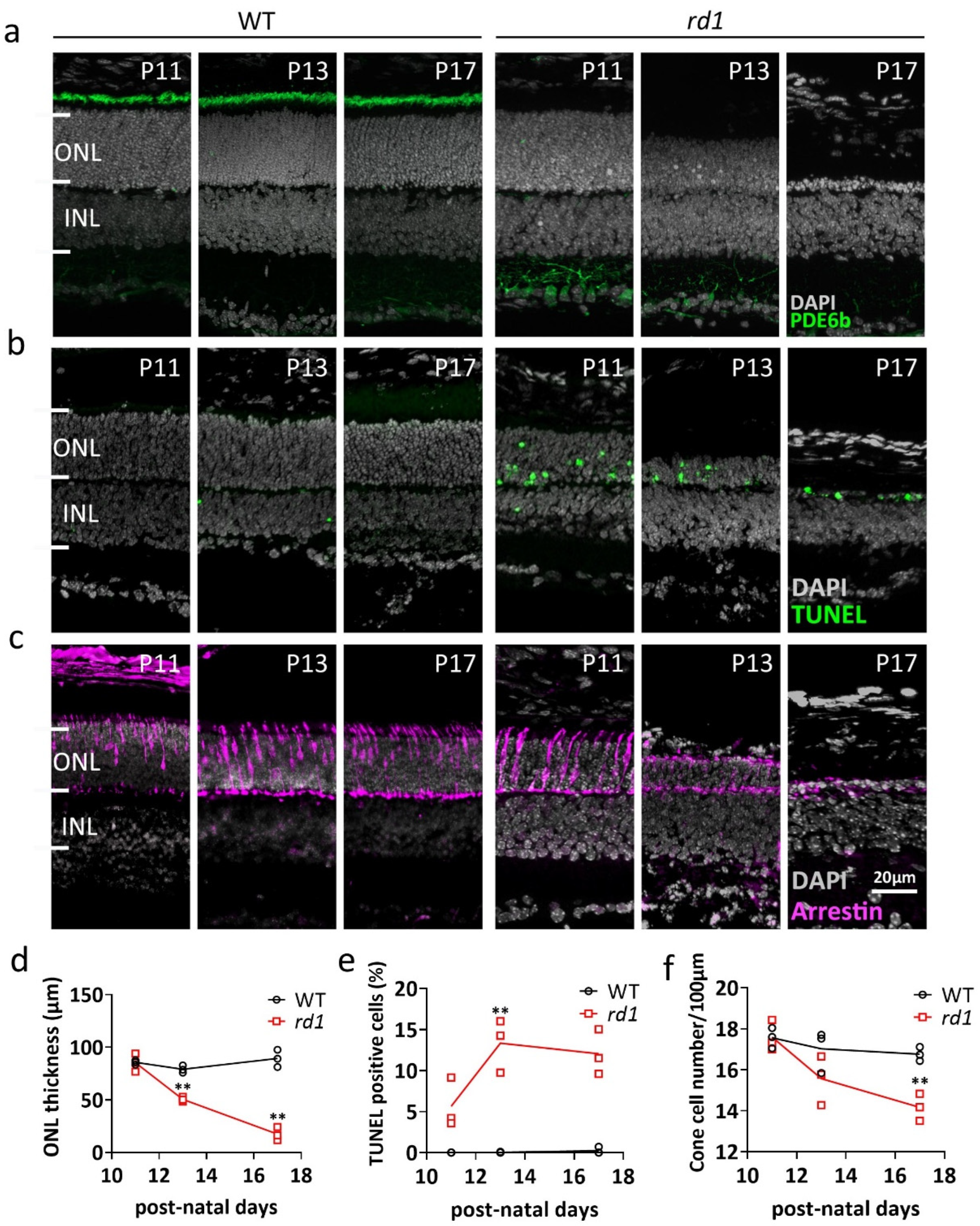
2.1. Morphologic Changes in rd1 Retina
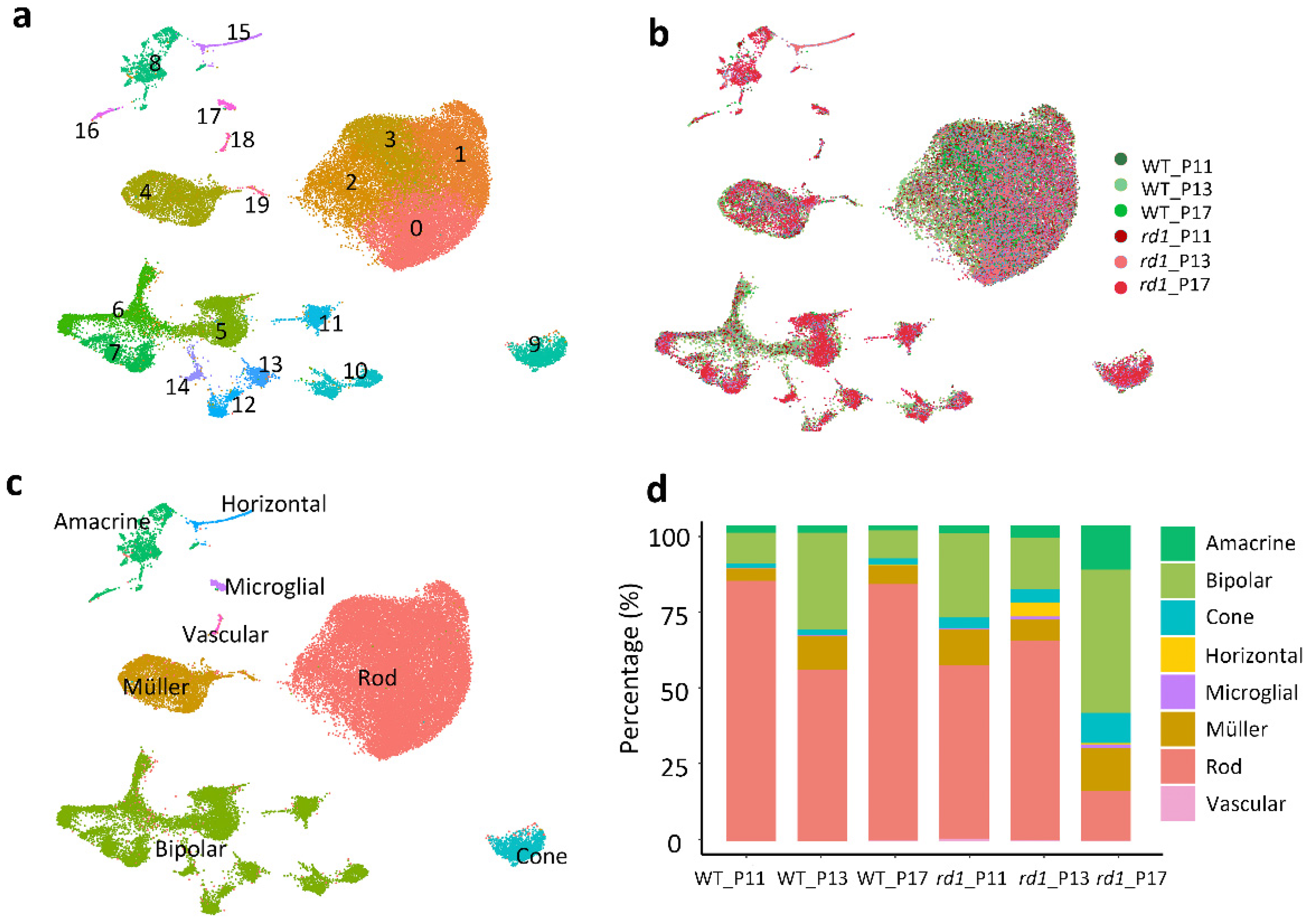
2.2. Single-Cell RNA Sequencing Analysis Yields 20 Clusters Corresponding to Eight Retinal Cell Types
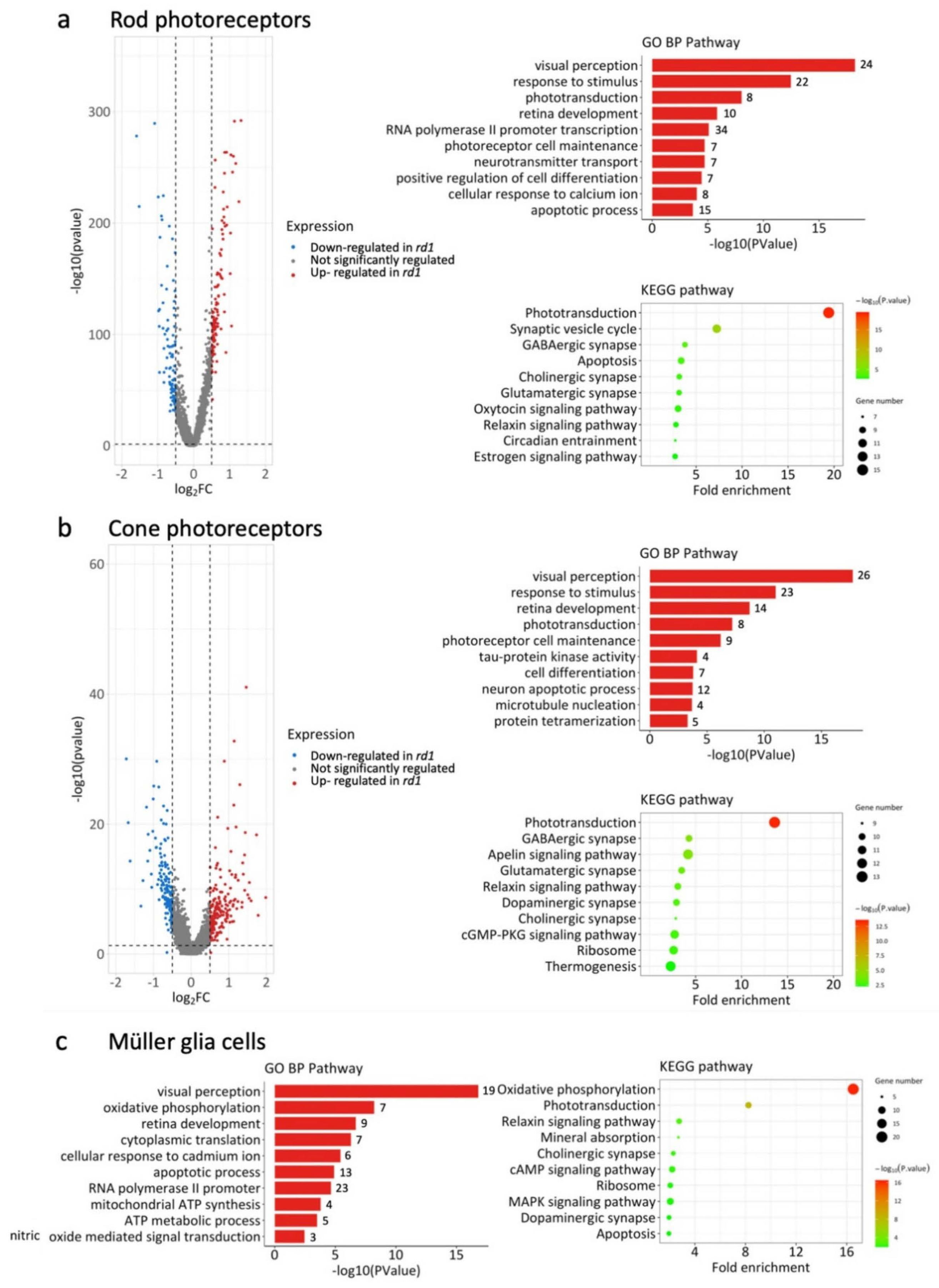
2.3. Differential Gene Expression Analysis in WT and rd1 Retinal Cell Types
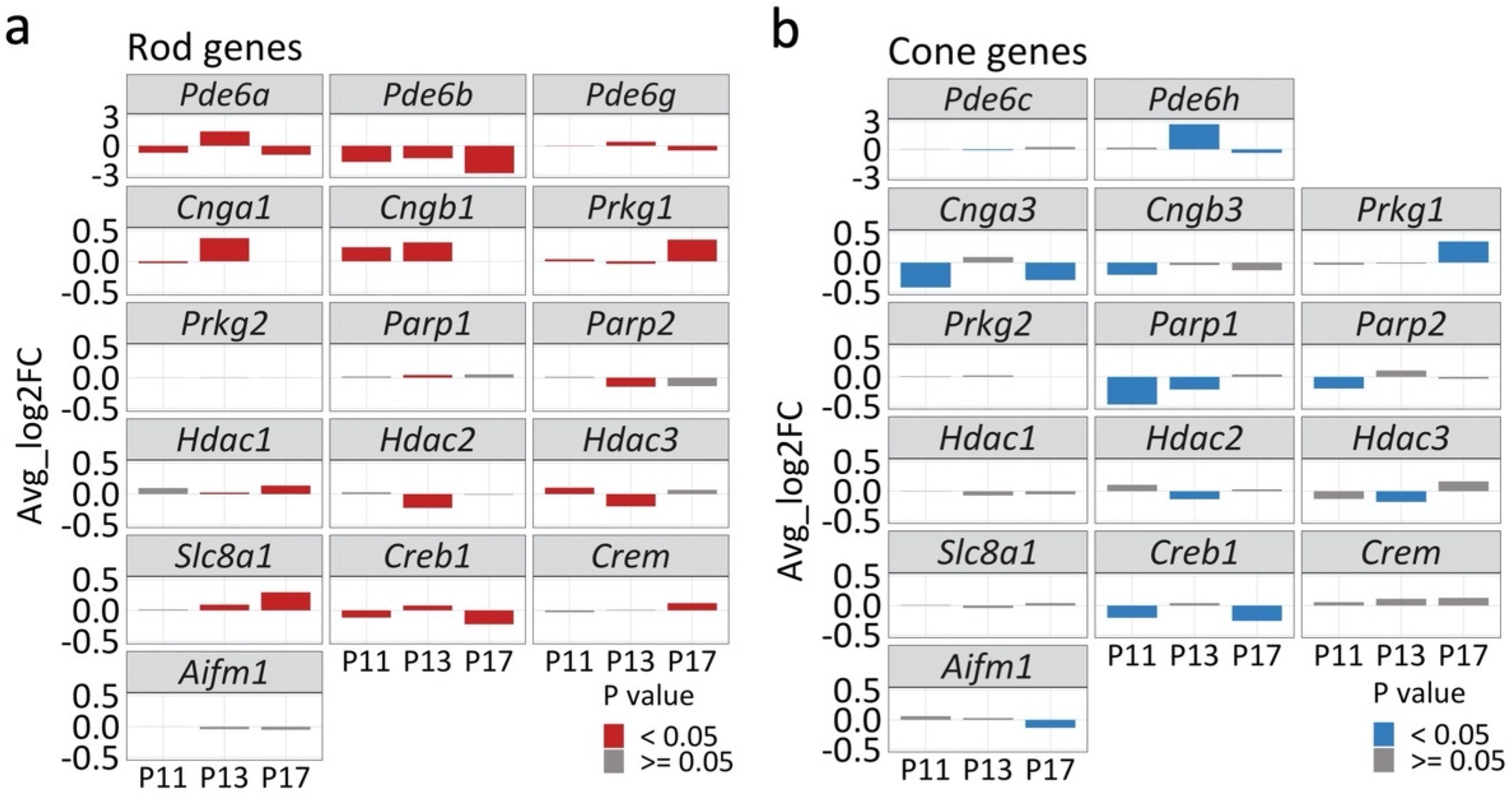
2.4. Transcriptional Changes Related to Cell Death in rd1 Mutant Retina
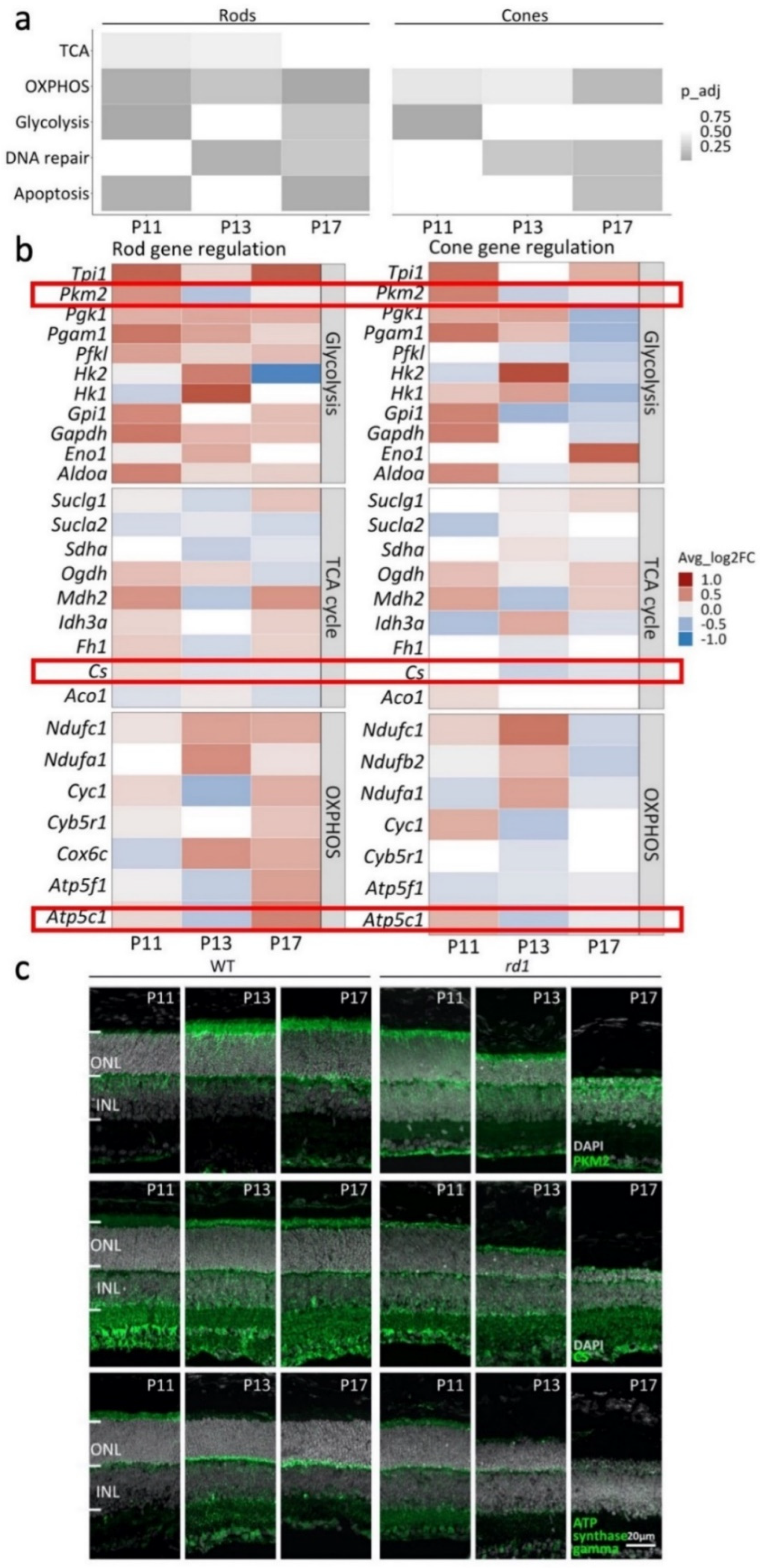
2.5. Photoreceptors in rd1 Mutant Retina Undergo a Metabolic Switch
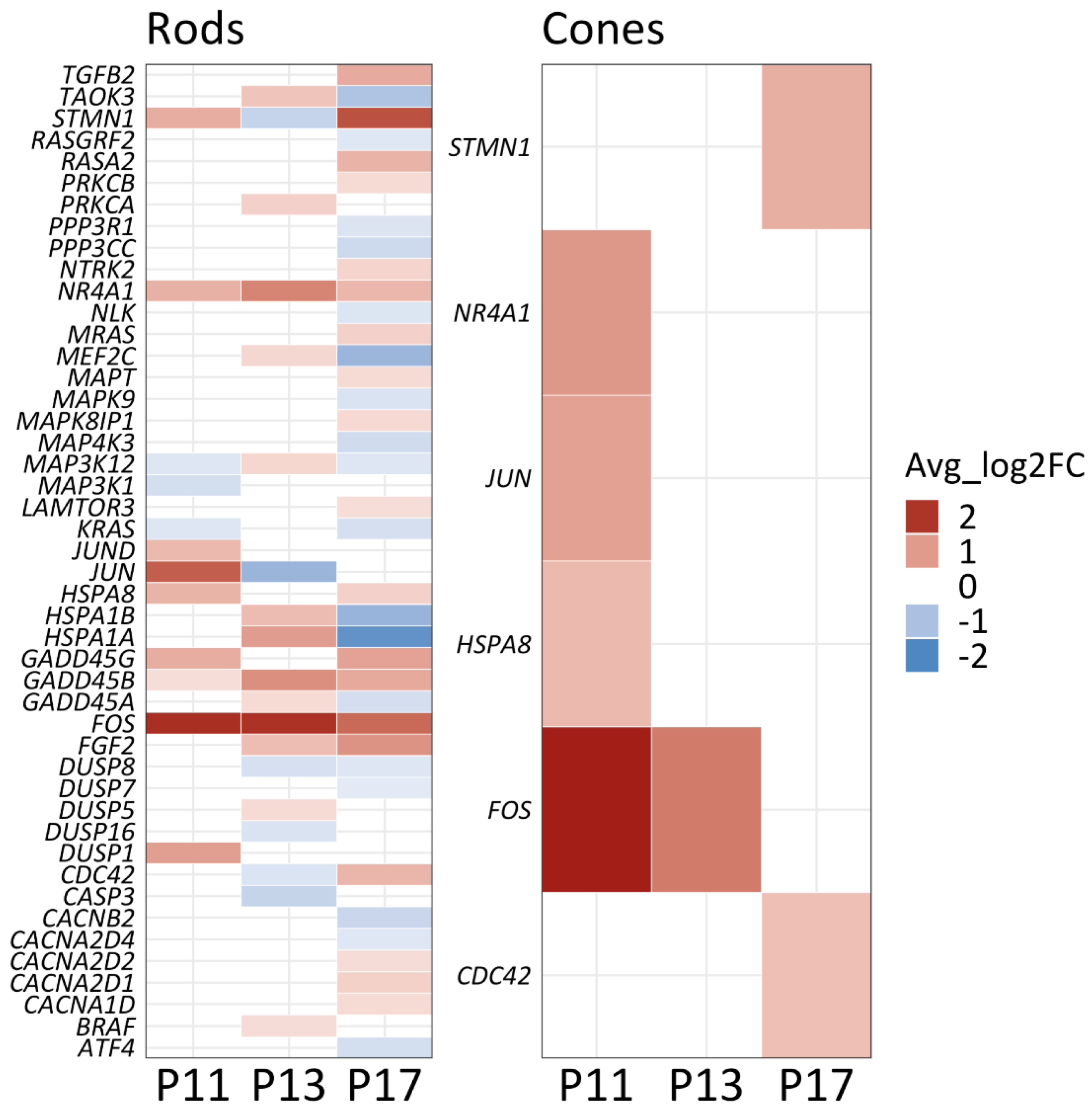
2.6. The MAPK Signaling Pathway Coordinates Energy Metabolism and Cell Death
3. Discussion
3.1. cGMP- and Ca2+- Signaling in rd1 Retinal Degeneration
3.2. Metabolic Responses of Degeneration Retina
3.3. MAPK Pathway Regulates Crosstalk between Ca2+ and Energy Metabolism
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Pagon, R.A. Retinitis pigmentosa. Surv. Ophthalmol. 1988, 33, 137–177. [Google Scholar] [CrossRef]
- García Bohórquez, B.; Aller, E.; Rodríguez Muñoz, A.; Jaijo, T.; García García, G.; Millán, J.M. Updating the Genetic Landscape of Inherited Retinal Dystrophies. Front. Cell Dev. Biol. 2021, 9, 645600. [Google Scholar] [CrossRef]
- Chang, B.; Hawes, N.L.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Heckenlively, J.R. Retinal degeneration mutants in the mouse. Vis. Res. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Bowes, C.; Li, T.; Danciger, M.; Baxter, L.C.; Applebury, M.L.; Farber, D.B. Retinal degeneration in the rd mouse is caused by a defect in the β subunit of rod cGMP-phosphodiesterase. Nature 1990, 347, 677–680. [Google Scholar] [CrossRef]
- Arango-Gonzalez, B.; Trifunović, D.; Sahaboglu, A.; Kranz, K.; Michalakis, S.; Farinelli, P.; Koch, S.; Koch, F.; Cottet, S.; Janssen-Bienhold, U.; et al. Identification of a common non-apoptotic cell death mechanism in hereditary retinal degeneration. PLoS ONE 2014, 9, e112142. [Google Scholar] [CrossRef]
- Ames, A., 3rd; Li, Y.Y.; Heher, E.C.; Kimble, C.R. Energy metabolism of rabbit retina as related to function: High cost of Na+ transport. J. Neurosci. 1992, 12, 840–853. [Google Scholar] [CrossRef]
- Wong-Riley, M.T. Energy metabolism of the visual system. Eye Brain 2010, 2, 99–116. [Google Scholar] [CrossRef]
- Guerini, D.; Coletto, L.; Carafoli, E. Exporting calcium from cells. Cell Calcium 2005, 38, 281–289. [Google Scholar] [CrossRef]
- Schreiber, V.; Dantzer, F.; Ame, J.C.; de Murcia, G. Poly(ADP-ribose): Novel functions for an old molecule. Nat. Rev. Mol. Cell Biol. 2006, 7, 517–528. [Google Scholar] [CrossRef]
- Sancho-Pelluz, J.; Arango-Gonzalez, B.; Kustermann, S.; Romero, F.J.; van Veen, T.; Zrenner, E.; Ekström, P.; Paquet-Durand, F. Photoreceptor cell death mechanisms in inherited retinal degeneration. Mol. Neurobiol. 2008, 38, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Lavail, M.M.; Matthes, M.T.; Yasumura, D.; Steinberg, R.H. Variability in Rate of Cone Degeneration in the Retinal Degeneration (rd/rd) Mouse. Exp. Eye Res. 1997, 65, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Paquet-Durand, F.; Hauck, S.M.; Van Veen, T.; Ueffing, M.; Ekström, P. PKG activity causes photoreceptor cell death in two retinitis pigmentosa models. J. Neurochem. 2009, 108, 796–810. [Google Scholar] [CrossRef]
- Farber, D.B.; Lolley, R.N. Cyclic guanosine monophosphate: Elevation in degenerating photoreceptor cells of the C3H mouse retina. Science 1974, 186, 449–451. [Google Scholar] [CrossRef]
- Kaupp, U.B.; Seifert, R. Cyclic nucleotide-gated ion channels. Physiol. Rev. 2002, 82, 769–824. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H.; Busch, J.L.; Corbin, J.D.; Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef] [PubMed]
- Paquet-Durand, F.; Sancho-Pelluz, J.; Sahaboglu, A.; Farinelli, P.; Azadi, S.; Veen, T.v.; Romero, J.; Ekstrom, P. Interplay Between HDAC And PARP Activity During rd1 Mouse Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4088. [Google Scholar]
- Lonze, B.E.; Ginty, D.D. Function and regulation of CREB family transcription factors in the nervous system. Neuron 2002, 35, 605–623. [Google Scholar] [CrossRef]
- Lamas, M.; Monaco, L.; Zazopoulos, E.; Lalli, E.; Tamai, K.; Penna, L.; Mazzucchelli, C.; Nantel, F.; Foulkes, N.S.; Sassone-Corsi, P. CREM: A master-switch in the transcriptional response to cAMP. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 561–567. [Google Scholar]
- Reierson, G.W.; Guo, S.; Mastronardi, C.; Licinio, J.; Wong, M.L. cGMP Signaling, Phosphodiesterases and Major Depressive Disorder. Curr. Neuropharmacol. 2011, 9, 715–727. [Google Scholar] [CrossRef]
- Sevrioukova, I.F. Apoptosis-inducing factor: Structure, function, and redox regulation. Antioxid. Redox Signal. 2011, 14, 2545–2579. [Google Scholar] [CrossRef]
- Hurley, J.B. Retina Metabolism and Metabolism in the Pigmented Epithelium: A Busy Intersection. Annu. Rev. Vis. Sci. 2021, 7, 665–692. [Google Scholar] [CrossRef]
- Aït-Ali, N.; Fridlich, R.; Millet-Puel, G.; Clérin, E.; Delalande, F.; Jaillard, C.; Blond, F.; Perrocheau, L.; Reichman, S.; Byrne, L.C. Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell 2015, 161, 817–832. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Capaldi, R.A.; Aggeler, R.; Turina, P.; Wilkens, S. Coupling between catalytic sites and the proton channel in F1F0-type ATPases. Trends. Biochem. Sci. 1994, 19, 284–289. [Google Scholar] [CrossRef]
- Scimeca, J.-C.; Servant, M.J.; Dyer, J.-O.; Meloche, S. Essential role of calcium in the regulation of MAP kinase phosphatase-1 expression. Oncogene 1997, 15, 717–725. [Google Scholar] [CrossRef]
- Papa, S.; Choy, P.M.; Bubici, C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene 2019, 38, 2223–2240. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Chen, W.; Cobb, M.H. Differential regulation and properties of MAPKs. Oncogene 2007, 26, 3100–3112. [Google Scholar] [CrossRef]
- Yan, J.; Günter, A.; Das, S.; Mühlfriedel, R.; Michalakis, S.; Jiao, K.; Seeliger, M.W.; Paquet-Durand, F.J.B. Inherited Retinal Degeneration: PARP-Dependent Activation of Calpain Requires CNG Channel Activity. Biomolecules 2022, 12, 455. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Popp, V.; Power, M.; Groeneveld, K.; Yan, J.; Melle, C.; Rogerson, L.; Achury, M.; Schwede, F.; Strasser, T.; et al. Redefining the role of Ca2+-permeable channels in photoreceptor degeneration using diltiazem. Cell Death Dis. 2022, 13, 47. [Google Scholar] [CrossRef]
- Power, M.; Das, S.; Schütze, K.; Marigo, V.; Ekström, P.; Paquet-Durand, F. Cellular mechanisms of hereditary photoreceptor degeneration-Focus on cGMP. Prog. Retin. Eye Res. 2020, 74, 100772. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tsang, S.H.; Chen, J. Two pathways of rod photoreceptor cell death induced by elevated cGMP. Hum. Mol. Genet. 2017, 26, 2299–2306. [Google Scholar] [CrossRef]
- Paquet-Durand, F.; Beck, S.; Michalakis, S.; Goldmann, T.; Huber, G.; Mühlfriedel, R.; Trifunović, D.; Fischer, M.D.; Fahl, E.; Duetsch, G.; et al. A key role for cyclic nucleotide gated (CNG) channels in cGMP-related retinitis pigmentosa. Hum. Mol. Genet. 2011, 20, 941–947. [Google Scholar] [CrossRef]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef]
- Khananshvili, D. Sodium-calcium exchangers (NCX): Molecular hallmarks underlying the tissue-specific and systemic functions. Pflügers Arch.-Eur. J. Physiol. 2014, 466, 43–60. [Google Scholar] [CrossRef]
- Khananshvili, D. The SLC8 gene family of sodium–calcium exchangers (NCX)–Structure, function, and regulation in health and disease. Mol. Asp. Med. 2013, 34, 220–235. [Google Scholar] [CrossRef]
- Okawa, H.; Sampath, A.P.; Laughlin, S.B.; Fain, G.L. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr. Biol. 2008, 18, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Mencl, S.; Trifunović, D.; Zrenner, E.; Paquet-Durand, F. PKG-Dependent Cell Death in 661W Cone Photoreceptor-Like Cell Cultures (Experimental Study). In Retinal Degenerative Diseases; Springer: Cham, Switzerland, 2018; pp. 511–517. [Google Scholar]
- Narayan, D.S.; Ao, J.; Wood, J.P.M.; Casson, R.J.; Chidlow, G. Spatio-temporal characterization of S- and M/L-cone degeneration in the Rd1 mouse model of retinitis pigmentosa. BMC Neurosci. 2019, 20, 46. [Google Scholar] [CrossRef]
- Sancho-Pelluz, J.; Paquet-Durand, F. HDAC Inhibition Prevents Rd1 Mouse Photoreceptor Degeneration. In Retinal Degenerative Diseases; Springer: Boston, MA, USA, 2012; pp. 107–113. [Google Scholar]
- Sancho-Pelluz, J.; Alavi, M.V.; Sahaboglu, A.; Kustermann, S.; Farinelli, P.; Azadi, S.; van Veen, T.; Romero, F.J.; Paquet-Durand, F.; Ekström, P. Excessive HDAC activation is critical for neurodegeneration in the rd1 mouse. Cell Death Dis. 2010, 1, e24. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, L.; Ahel, I. PARPs in genome stability and signal transduction: Implications for cancer therapy. Biochem. Soc. Trans. 2018, 46, 1681–1695. [Google Scholar] [CrossRef]
- Cohen, M.S.; Chang, P. Insights into the biogenesis, function, and regulation of ADP-ribosylation. Nat. Chem. Biol. 2018, 14, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.L. PARPs and ADP-ribosylation: 50 years… and counting. Mol. Cell 2015, 58, 902–910. [Google Scholar] [CrossRef]
- Ceccacci, E.; Minucci, S. Inhibition of histone deacetylases in cancer therapy: Lessons from leukaemia. Br. J. Cancer 2016, 114, 605–611. [Google Scholar] [CrossRef]
- Ekström, P.A.; Ueffing, M.; Zrenner, E.; Paquet-Durand, F. Novel in situ activity assays for the quantitative molecular analysis of neurodegenerative processes in the retina. Curr. Med. Chem. 2014, 21, 3478–3493. [Google Scholar] [CrossRef]
- Bai, P. Biology of Poly(ADP-Ribose) Polymerases: The Factotums of Cell Maintenance. Mol. Cell 2015, 58, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD(+) metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. The Metabolism of Carcinoma Cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Dayton, T.L.; Jacks, T.; Vander Heiden, M.G. PKM2, cancer metabolism, and the road ahead. EMBO Rep. 2016, 17, 1721–1730. [Google Scholar] [CrossRef]
- Morrison, D.K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Schubert, T.; Paquet-Durand, F.; Tanimoto, N.; Chang, L.; Koeppen, K.; Ott, T.; Griesbeck, O.; Seeliger, M.W.; Euler, T.; et al. Light-Driven Calcium Signals in Mouse Cone Photoreceptors. J. Neurosci. 2012, 32, 6981–6994. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Trifunović, D.; Schubert, T.; Euler, T.; Paquet-Durand, F. Calcium dynamics change in degenerating cone photoreceptors. Hum. Mol. Genet. 2016, 25, 3729–3740. [Google Scholar] [CrossRef]
- Dong, Y.; Tu, R.; Liu, H.; Qing, G. Regulation of cancer cell metabolism: Oncogenic MYC in the driver’s seat. Signal Transduct. Target. Ther. 2020, 5, 124. [Google Scholar] [CrossRef]
- Sanyal, S.; Bal, A.K. Comparative light and electron microscopic study of retinal histogenesis in normal and rd mutant mice. Z Anat Entwickl. 1973, 142, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ning, B.; Shi, T. Single-Cell RNA-Seq Technologies and Related Computational Data Analysis. Front. Genet. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Ilicic, T.; Kim, J.K.; Kolodziejczyk, A.A.; Bagger, F.O.; McCarthy, D.J.; Marioni, J.C.; Teichmann, S.A. Classification of low quality cells from single-cell RNA-seq data. Genome Biol. 2016, 17, 1–15. [Google Scholar] [CrossRef]

| Antibodies, Species | Cat. | Company, City, State and Country | Dilution | Concentration |
|---|---|---|---|---|
| anti-PDE6B, rabbit | Cat# PA1-722 | Thermo Fisher, Waltham, MA, USA | 1:300 | 3.3 μg/mL |
| anti-ATP synthase subunit gamma, rabbit | Cat# GTX114275S | GeneTex, Irving, CA, USA | 1:300 | 1.45 μg/mL |
| anti-Apoptosis-inducing factor, rabbit | Cat# A7549 | Sigma-Aldrich, St Louis, MO, USA | 1:1000 | 5–10 μg/mL |
| anti-CREB1, rabbit | Cat# 12208-1-AP | Proteintech, Chicago, IL, USA | 1:300 | 2.31 μg/mL |
| anti-CREM, mouse | Cat# sc-390426 | Santa Cruz Biotechnology, Santa Cruz, CA, USA | 1:100 | 2 μg/mL |
| anti-Citrate synthase, rabbit | Cat# GTX110624 | GeneTex, Irving, TX, USA | 1:300 | 3.1 μg/mL |
| anti-PKM2, rabbit | Cat# 4053 | Cell Signaling Technology, Danvars, MA, USA | 1:100 | 1.2 μg/mL |
| anti-Cone arrestin, rabbit | Cat# AB15282 | Sigma-Aldrich, St Louis, MO, USA | 1:300 | 3.3 μg/mL |
| Goat anti rabbit IgG secondary antibody, Alexa FluorTM488 | Cat# A11034 | Molecular Probes, Eugene, OR, USA | 1:500 | 4 μg/mL |
| Goat anti mouse IgG secondary antibody, Alexa FluorTM488 | Cat# A11029 | Molecular Probes, Eugene, OR, USA | 1:500 | 4 μg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Dong, Y.; Yan, J.; Wang, L.; Yu, S.; Jiao, K.; Paquet-Durand, F. Single-Cell Transcriptomic Profiling in Inherited Retinal Degeneration Reveals Distinct Metabolic Pathways in Rod and Cone Photoreceptors. Int. J. Mol. Sci. 2022, 23, 12170. https://doi.org/10.3390/ijms232012170
Chen Y, Dong Y, Yan J, Wang L, Yu S, Jiao K, Paquet-Durand F. Single-Cell Transcriptomic Profiling in Inherited Retinal Degeneration Reveals Distinct Metabolic Pathways in Rod and Cone Photoreceptors. International Journal of Molecular Sciences. 2022; 23(20):12170. https://doi.org/10.3390/ijms232012170
Chicago/Turabian StyleChen, Yiyi, Yujie Dong, Jie Yan, Lan Wang, Shirley Yu, Kangwei Jiao, and François Paquet-Durand. 2022. "Single-Cell Transcriptomic Profiling in Inherited Retinal Degeneration Reveals Distinct Metabolic Pathways in Rod and Cone Photoreceptors" International Journal of Molecular Sciences 23, no. 20: 12170. https://doi.org/10.3390/ijms232012170
APA StyleChen, Y., Dong, Y., Yan, J., Wang, L., Yu, S., Jiao, K., & Paquet-Durand, F. (2022). Single-Cell Transcriptomic Profiling in Inherited Retinal Degeneration Reveals Distinct Metabolic Pathways in Rod and Cone Photoreceptors. International Journal of Molecular Sciences, 23(20), 12170. https://doi.org/10.3390/ijms232012170







