Effect of Subthalamic Stimulation and Electrode Implantation in the Striatal Microenvironment in a Parkinson’s Disease Rat Model
Abstract
1. Introduction
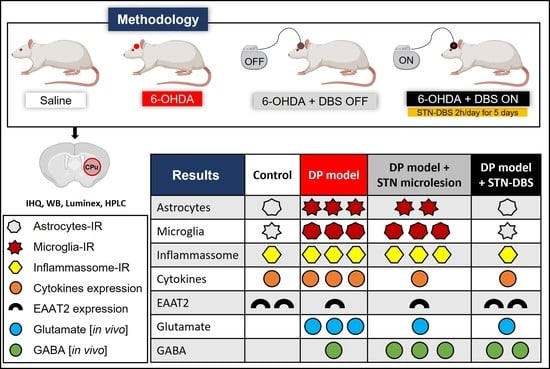
2. Results
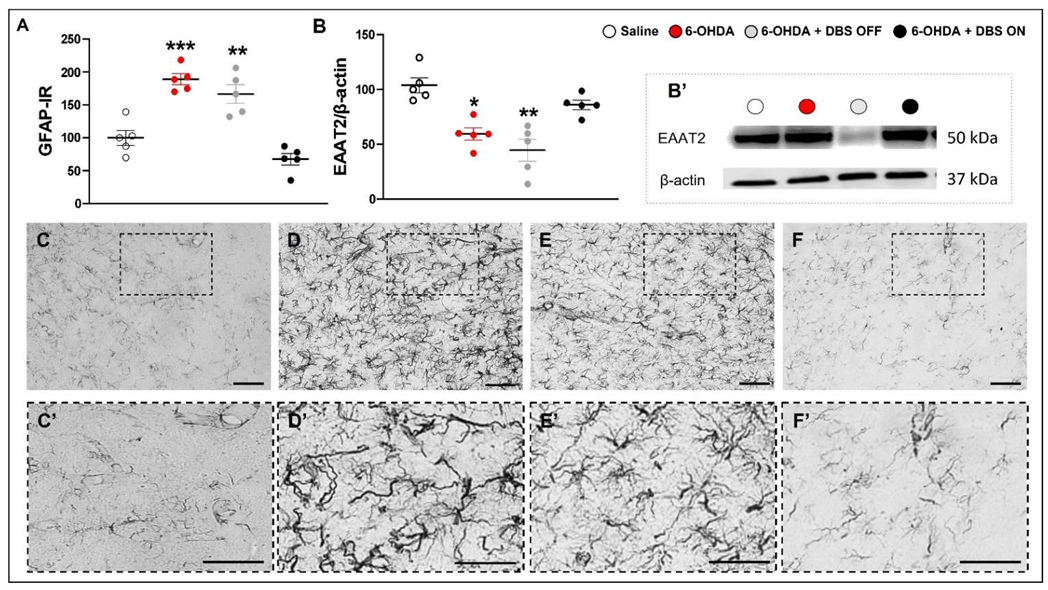
2.1. STN-DBS Alleviates Motor Impairment and Modulates Astrocytes Morphology in the Striatum
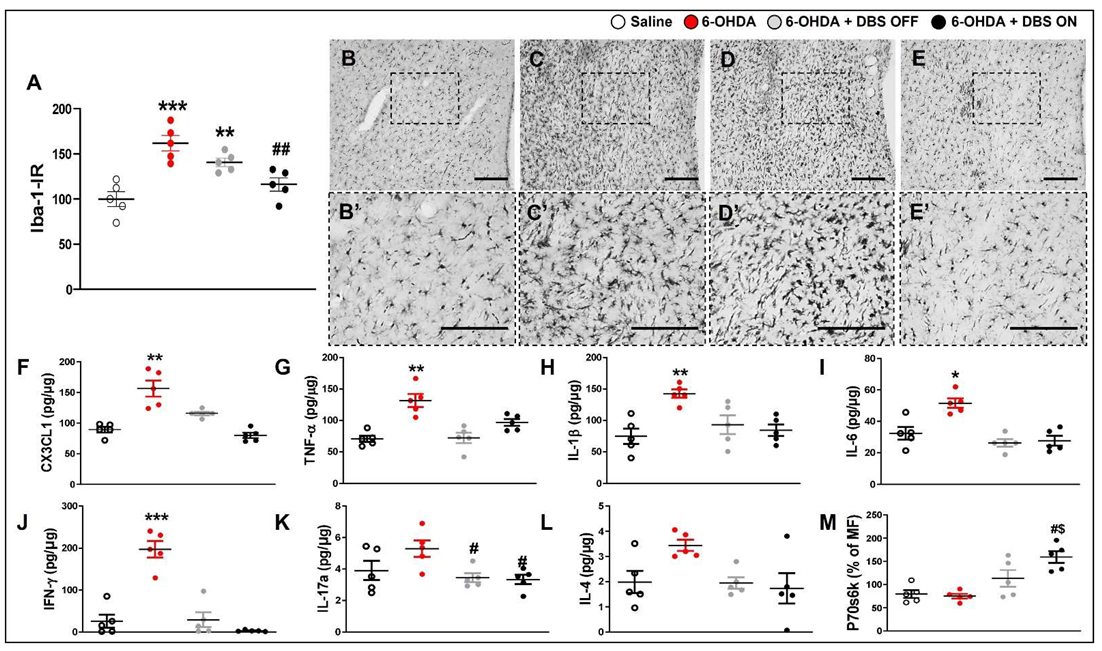
2.2. Subthalamic Microlesion and Stimulation Inhibit 6-OHDA-Induced Striatal Cytokines while Only STN-DBS Regulates Glutamate Reuptake and Neuronal Autophagy without Interfering with Microglial Immunoreactivity
2.3. Subthalamic Microlesion and Stimulation Inhibit the Relative Concentration of 6-OHDA-Induced Glutamate
3. Discussion
4. Materials and Methods
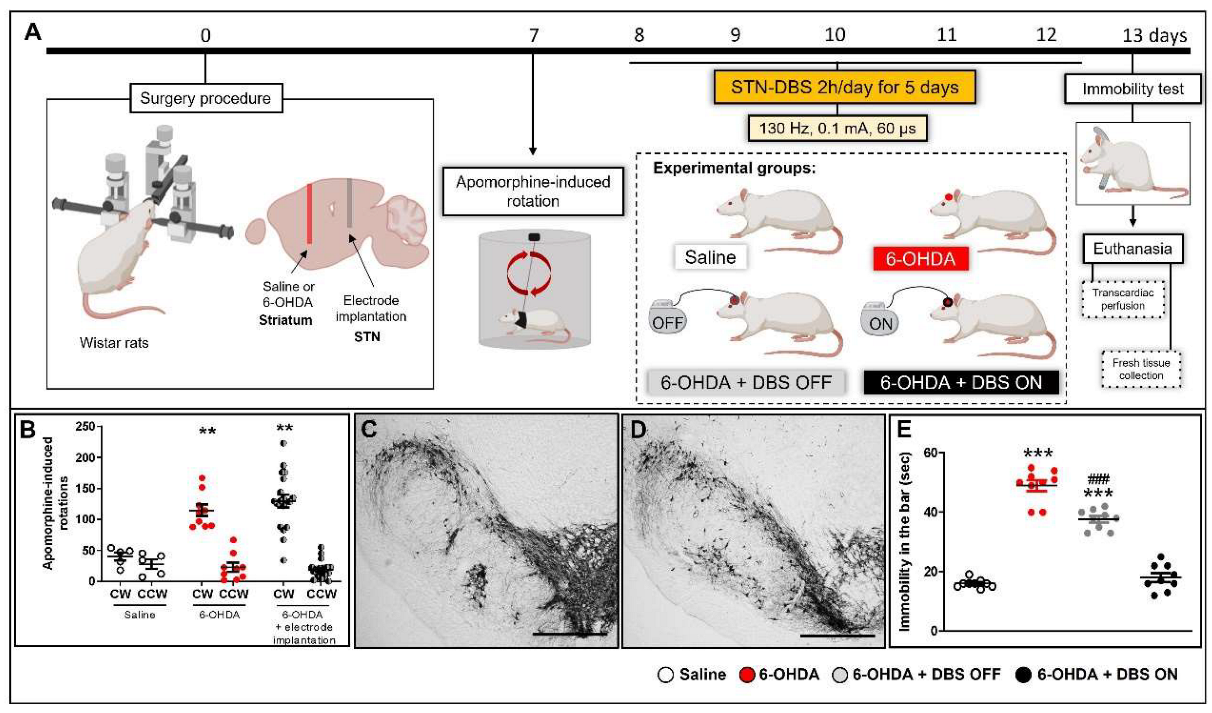
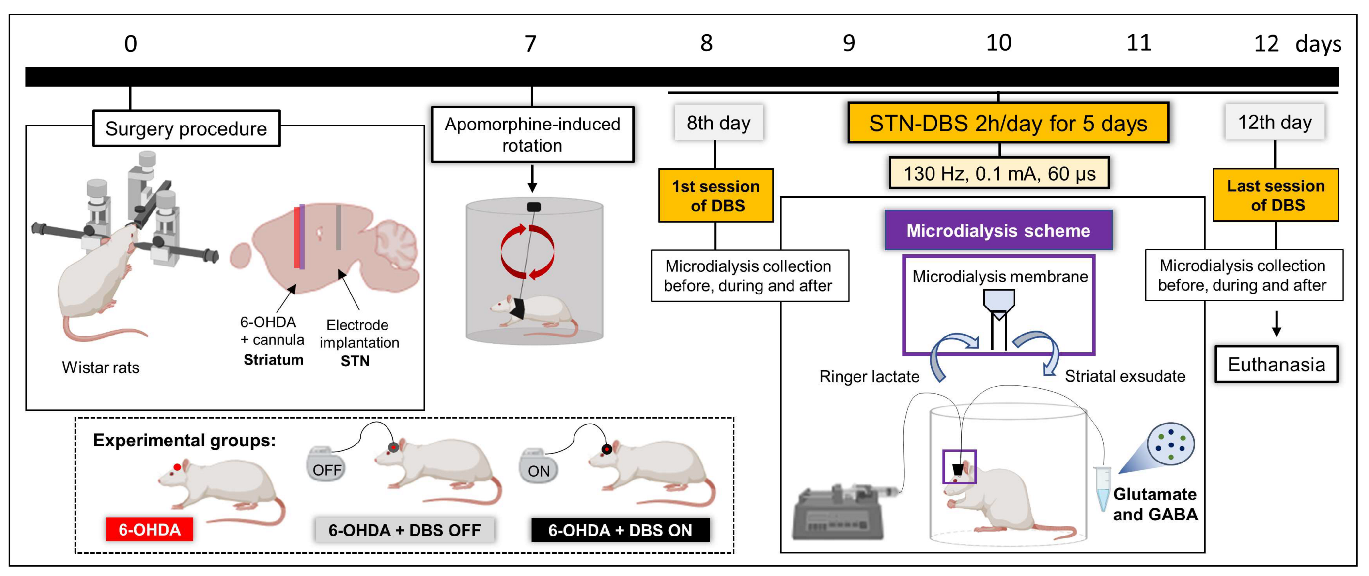
4.1. Experimental Design
4.2. Animals
4.3. Surgical Procedure for PD Model Induction
4.4. Surgical Procedure for Electrode Implantation
4.5. Surgical Procedure for Microdialysis Cannula Implantation
4.6. Evaluation of Behavioral Immobility
4.7. Evaluation of the Apomorphine-Induced Rotational Behavior
4.8. DBS Protocol
4.9. Microdialysis Procedure
4.10. Immunohistochemistry
4.11. Western Blotting
4.12. Luminex
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hornykiewicz, O.; Kish, S.J. Biochemical pathophysiology of Parkinson’s disease. Adv. Neurol. 1987, 45, 19–34. [Google Scholar] [PubMed]
- McGeer, P.L.; McGeer, P.L. Glial reactions in Parkinson’s disease. Mov. Disord. 2007, 23, 474–483. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Yasojima, K.; McGeer, E.G. Inflammation in Parkinson’s disease. Adv. Neurol. 2001, 86. [Google Scholar]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Bartels, A.; Willemsen, A.; Doorduin, J.; de Vries, E.; Dierckx, R.; Leenders, K. [11C]-PK11195 PET: Quantification of neuroinflammation and a monitor of anti-inflammatory treatment in Parkinson’s disease? Park. Relat. Disord. 2010, 16, 57–59. [Google Scholar] [CrossRef]
- Gerhard, A.; Pavese, N.; Hotton, G.; Turkheimer, F.; Es, M.; Hammers, A.; Eggert, K.; Oertel, W.; Banati, R.B.; Brooks, D.J. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 2006, 21, 404–412. [Google Scholar] [CrossRef]
- Kofler, J.; Wiley, C.A. Microglia. Toxicol. Pathol. 2010, 39, 103–114. [Google Scholar] [CrossRef]
- Saijo, K.; Winner, B.; Carson, C.T.; Collier, J.G.; Boyer, L.; Rosenfeld, M.G.; Gage, F.H.; Glass, C.K. A Nurr1/CoREST Pathway in Microglia and Astrocytes Protects Dopaminergic Neurons from Inflammation-Induced Death. Cell 2009, 137, 47–59. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Godoy, M.C.P.; Tarelli, R.; Chertoff, M.; Depino, A.M.; Pitossi, F.J. Progressive neurodegeneration and motor disabilities induced by chronic expression of IL-1β in the substantia nigra. Neurobiol. Dis. 2006, 24, 183–193. [Google Scholar] [CrossRef]
- McCoy, M.K.; Martinez, T.N.; Ruhn, K.A.; Szymkowski, D.; Smith, C.G.; Botterman, B.R.; Tansey, K.E.; Tansey, M.G. Blocking Soluble Tumor Necrosis Factor Signaling with Dominant-Negative Tumor Necrosis Factor Inhibitor Attenuates Loss of Dopaminergic Neurons in Models of Parkinson’s Disease. J. Neurosci. 2006, 26, 9365–9375. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Pei, Z.; Miller, D.S.; Wu, X.; Block, M.L.; Wilson, B.; Zhang, W.; Zhou, Y.; Hong, J.-S.; et al. Aggregated α-synuclein activates microglia: A process leading to disease progression in Parkinson’s disease. FASEB J. 2005, 19, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Pelvig, D.P.; Pakkenberg, H.; Stark, A.K.; Pakkenberg, B. Neocortical glial cell numbers in human brains. Neurobiol. Aging 2008, 29, 1754–1762. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Ventura, R.; Harris, K. Three-Dimensional Relationships between Hippocampal Synapses and Astrocytes. J. Neurosci. 1999, 19, 6897–6906. [Google Scholar] [CrossRef] [PubMed]
- Fenoy, A.J.; Goetz, L.; Chabardes, S.; Xia, Y. Deep brain stimulation: Are astrocytes a key driver behind the scene? CNS Neurosci. Ther. 2014, 20, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.A. New roles for astrocytes: Regulation of synaptic transmission. Trends Neurosci. 2003, 26, 536–542. [Google Scholar] [CrossRef]
- Kimelberg, H.K.; Nedergaard, M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics 2010, 7, 338–353. [Google Scholar] [CrossRef]
- Lehre, K.P.; Danbolt, N.C. The Number of Glutamate Transporter Subtype Molecules at Glutamatergic Synapses: Chemical and Stereological Quantification in Young Adult Rat Brain. J. Neurosci. 1998, 18, 8751–8757. [Google Scholar] [CrossRef]
- Eulenburg, V.; Gomeza, J. Neurotransmitter transporters expressed in glial cells as regulators of synapse function. Brain Res. Rev. 2010, 63, 103–112. [Google Scholar] [CrossRef]
- Anderson, C.M.; Swanson, R.A. Astrocyte Glutamate Transport: Review of Properties, Regulation, and Physiological Functions. Glia 2000, 32, 1–14. [Google Scholar] [CrossRef]
- Rose, C.R.; Ziemens, D.; Untiet, V.; Fahlke, C. Molecular and cellular physiology of sodium-dependent glutamate transporters. Brain Res. Bull. 2018, 136, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, J.D.; Dykes-Hoberg, M.; Pardo, C.A.; Bristol, L.A.; Jin, L.; Kuncl, R.W.; Kanai, Y.; Hediger, M.A.; Wang, Y.; Schielke, J.P.; et al. Knockout of Glutamate Transporters Reveals a Major Role for Astroglial Transport in Excitotoxicity and Clearance of Glutamate. Neuron 1996, 16, 675–686. [Google Scholar] [CrossRef]
- Chen, Y.; Vartiainen, N.E.; Ying, W.; Chan, P.H.; Koistinaho, J.; Swanson, R.A. Astrocytes protect neurons from nitric oxide toxicity by a glutathione-dependent mechanism. J. Neurochem. 2001, 77, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S. Parkinson Disease, the Effect of Levodopa, and the ELLDOPA Trial. Arch. Neurol. 1999, 56, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Rascol, O.; Brooks, D.J.; Korczyn, A.D.; De Deyn, P.P.; Clarke, C.E.; Lang, A.E. A Five-Year Study of the Incidence of Dyskinesia in Patients with Early Parkinson’s Disease Who Were Treated with Ropinirole or Levodopa. N. Engl. J. Med. 2000, 342, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Bezard, E.; Brotchie, J.; Gross, C.E. Pathophysiology of levodopa-induced dyskinesia: Potential for new therapies. Nat. Rev. Neurosci. 2001, 2, 577–588. [Google Scholar] [CrossRef]
- Benabid, A.L. Deep brain stimulation for Parkinson’s disease. Curr. Opin. Neurobiol. 2003, 13, 696–706. [Google Scholar] [CrossRef]
- Krack, P.; Batir, A.; Van Blercom, N.; Chabardes, S.; Fraix, V.; Ardouin, C.; Koudsie, A.; Limousin, P.D.; Benazzouz, A.; LeBas, J.F.; et al. Five-Year Follow-up of Bilateral Stimulation of the Subthalamic Nucleus in Advanced Parkinson’s Disease. New Engl. J. Med. 2003, 349, 1925–1934. [Google Scholar] [CrossRef]
- Kumar, R.; Lozano, A.; Kim, Y.J.; Hutchison, W.D.; Sime, E.; Halket, E.; Lang, A. Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Neurology 1998, 51, 850–855. [Google Scholar] [CrossRef]
- Benabid, A.L.; Chabardes, S.; Mitrofanis, J.; Pollak, P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 2009, 8, 67–81. [Google Scholar] [CrossRef]
- Pereira, J.L.; Furie, S.; Sharim, J.; Yazdi, D.; DeSalles, A.A.; Pouratian, N. Lateralization of the subthalamic nucleus with age in Parkinson’s disease. Basal Ganglia 2016, 6, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Dafsari, H.S.; Reddy, P.; Herchenbach, C.; Wawro, S.; Petry-Schmelzer, J.N.; Visser-Vandewalle, V.; Rizos, A.; Silverdale, M.; Ashkan, K.; Samuel, M.; et al. Beneficial Effects of Bilateral Subthalamic Stimulation on Non-Motor Symptoms in Parkinson’s Disease. Brain Stimul. 2015, 9, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Juri, C.; Oroz, M.C.R.; Obeso, J.A. The pathophysiological basis of sensory disturbances in Parkinson’s disease. J. Neurol. Sci. 2010, 289, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Hamani, C.; Florence, G.; Heinsen, H.; Plantinga, B.R.; Temel, Y.; Uludag, K.; Alho, E.; Teixeira, M.J.; Amaro, E.; Fonoff, E.T. Subthalamic Nucleus Deep Brain Stimulation: Basic Concepts and Novel Perspectives. eneuro 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.P.; Kikuchi, D.S.; Paschoa, A.F.N.; Kuroki, M.A.; Fonoff, E.T.; Hamani, C.; Pagano, R.L.; Hernandes, M.S. Unraveling the Role of Astrocytes in Subthalamic Nucleus Deep Brain Stimulation in a Parkinson’s Disease Rat Model. Cell. Mol. Neurobiol. 2020, 40, 939–954. [Google Scholar] [CrossRef]
- Iovino, L.; Tremblay, M.; Civiero, L. Glutamate-induced excitotoxicity in Parkinson’s disease: The role of glial cells. J. Pharmacol. Sci. 2020, 144, 151–164. [Google Scholar] [CrossRef]
- Ambrosi, G.; Cerri, S.; Blandini, F. A further update on the role of excitotoxicity in the pathogenesis of Parkinson’s disease. J. Neural Transm. 2014, 121, 849–859. [Google Scholar] [CrossRef]
- Sood, A.; Preeti, K.; Fernandes, V.; Khatri, D.K.; Singh, S.B. Glia: A major player in glutamate–GABA dysregulation-mediated neurodegeneration. J. Neurosci. Res. 2021, 99, 3148–3189. [Google Scholar] [CrossRef]
- Jech, R.; Mueller, K.; Urgošík, D.; Sieger, T.; Holiga, Š.; Růžička, F.; Dušek, P.; Havránková, P.; Vymazal, J.; Růžička, E. The Subthalamic Microlesion Story in Parkinson’s Disease: Electrode Insertion-Related Motor Improvement with Relative Cortico-Subcortical Hypoactivation in fMRI. PLoS ONE 2012, 7, e49056. [Google Scholar] [CrossRef]
- Holiga, Š.; Mueller, K.; Möller, H.E.; Urgošík, D.; Růžička, E.; Schroeter, M.L.; Jech, R. Resting-state functional magnetic resonance imaging of the subthalamic microlesion and stimulation effects in Parkinson’s disease: Indications of a principal role of the brainstem. NeuroImage: Clin. 2015, 9, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Lu, Y.; Qiu, C.; Dong, W.; Xue, C.; Zhang, L.; Liu, W.; Zhang, W. Altered Spontaneous Neural Activity and Functional Connectivity in Parkinson’s Disease With Subthalamic Microlesion. Front. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
- Alvarez, L.; Macias, R.; Lopez, G.; Pavon, N.; Rodriguez-Oroz, M.C.; Juncos, J.L.; Maragoto, C.; Guridi, J.; Litvan, I.; Tolosa, E.S.; et al. Bilateral subthalamotomy in Parkinson’s disease: Initial and long-term response. Brain 2005, 128, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Benazzouz, A.; Piallat, B.; Pollak, P.; Benabid, A.-L. Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: Electrophysiological data. Neurosci. Lett. 1995, 189, 77–80. [Google Scholar] [CrossRef]
- Benabid, A.; Pollak, P.; Gross, C.; Hoffmann, D.; Benazzouz, A.; Gao, D.; Laurent, A.; Gentil, M.; Perret, J. Acute and Long-Term Effects of Subthalamic Nucleus Stimulation in Parkinson’s Disease. Ster. Funct. Neurosurg. 1994, 62, 76–84. [Google Scholar] [CrossRef]
- Campos, A.C.P.; Berzuino, M.B.; Hernandes, M.S.; Fonoff, E.T.; Pagano, R.L. Monoaminergic regulation of nociceptive circuitry in a Parkinson’s disease rat model. Exp. Neurol. 2019, 318, 12–21. [Google Scholar] [CrossRef]
- Domenici, R.A.; Campos, A.C.P.; Maciel, S.T.; Berzuino, M.B.; Hernandes, M.S.; Fonoff, E.T.; Pagano, R.L. Parkinson’s disease and pain: Modulation of nociceptive circuitry in a rat model of nigrostriatal lesion. Exp. Neurol. 2019, 315, 72–81. [Google Scholar] [CrossRef]
- Higuchi, Y.; Matsuda, S.; Serizawa, T. Gamma knife radiosurgery in movement disorders: Indications and limitations. Mov. Disord. 2016, 32, 28–35. [Google Scholar] [CrossRef]
- Weintraub, D.; Elias, W.J. The emerging role of transcranial magnetic resonance imaging-guided focused ultrasound in functional neurosurgery. Mov. Disord. 2016, 32, 20–27. [Google Scholar] [CrossRef]
- Elias, W.J.; Lipsman, N.; Ondo, W.G.; Ghanouni, P.; Kim, Y.G.; Lee, W.; Schwartz, M.; Hynynen, K.; Lozano, A.M.; Shah, B.B.; et al. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N. Engl. J. Med. 2016, 375, 730–739. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Pekny, M.; Nilsson, M. Astrocyte activation and reactive gliosis. Glia 2005, 50, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.; Guo, H.; David, C.N.; Brickey, W.J.; Jha, S.; Ting, J.P.-Y. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J. Exp. Med. 2017, 214, 1351–1370. [Google Scholar] [CrossRef]
- Chung, E.; Chen, L.; Chan, Y.; Yung, K. Downregulation of glial glutamate transporters after dopamine denervation in the striatum of 6-hydroxydopamine-lesioned rats. J. Comp. Neurol. 2008, 511, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Meshul, C.; Emre, N.; Nakamura, C.; Allen, C.; Donohue, M.; Buckman, J. Time-dependent changes in striatal glutamate synapses following a 6-hydroxydopamine lesion. Neuroscience 1998, 88, 1–16. [Google Scholar] [CrossRef]
- Robinson, S.; Freeman, P.; Moore, C.; Touchon, J.C.; Krentz, L.; Meshul, C.K. Acute and subchronic MPTP administration differentially affects striatal glutamate synaptic function. Exp. Neurol. 2003, 180, 74–87. [Google Scholar] [CrossRef]
- Amorim, B.O.; Covolan, L.; Ferreira, E.; Brito, J.G.; Nunes, D.P.; De Morais, D.G.; Nobrega, J.N.; Rodrigues, A.M.; DeAlmeida, A.C.G.; Hamani, C. Deep brain stimulation induces antiapoptotic and anti-inflammatory effects in epileptic rats. J. Neuroinflammation 2015, 12, 162. [Google Scholar] [CrossRef]
- Wei, L.; Chen, C.; Ding, L.; Mo, M.; Zou, J.; Lu, Z.; Li, H.; Wu, H.; Dai, Y.; Xu, P.; et al. Wnt1 Promotes EAAT2 Expression and Mediates the Protective Effects of Astrocytes on Dopaminergic Cells in Parkinson’s Disease. Neural Plast. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010, 11, 682–696. [Google Scholar] [CrossRef]
- Parsons, M.P.; Raymond, L.A. Extrasynaptic NMDA Receptor Involvement in Central Nervous System Disorders. Neuron 2014, 82, 279–293. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Akwa, Y.; Di Malta, C.; Zallo, F.; Gondard, E.; Lunati, A.; Diaz-De-Grenu, L.Z.; Zampelli, A.; Boiret, A.; Santamaria, S.; Martinez-Preciado, M.; et al. Stimulation of synaptic activity promotes TFEB-mediated clearance of pathological MAPT/Tau in cellular and mouse models of tauopathies. Autophagy 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.I.; Baek, J.Y.; Chung, Y.C.; Nam, J.H.; Shin, W.; Jin, B.K. p70S6K on astrocytes protects dopamine neurons from 1-methyl-4-phenylpyridinium neurotoxicity. Glia 2021, 69, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Herrington, T.M.; Cheng, J.J.; Eskandar, E.N. Mechanisms of deep brain stimulation. J. Neurophysiol. 2016, 115, 19–38. [Google Scholar] [CrossRef]
- Yang, J.; Hu, L.-F.; Liu, X.; Zhou, F.; Ding, J.-H.; Hu, G. Effects of iptakalim on extracellular glutamate and dopamine levels in the striatum of unilateral 6-hydroxydopamine-lesioned rats: A microdialysis study. Life Sci. 2006, 78, 1940–1944. [Google Scholar] [CrossRef]
- Centonze, D.; Gubellini, P.; Rossi, S.; Picconi, B.; Pisani, A.; Bernardi, G.; Calabresi, P.; Baunez, C. Subthalamic nucleus lesion reverses motor abnormalities and striatal glutamatergic overactivity in experimental parkinsonism. Neuroscience 2005, 133, 831–840. [Google Scholar] [CrossRef]
- Mallet, N.; Ballion, B.; Le Moine, C.; Gonon, F. Cortical Inputs and GABA Interneurons Imbalance Projection Neurons in the Striatum of Parkinsonian Rats. J. Neurosci. 2006, 26, 3875–3884. [Google Scholar] [CrossRef]
- Tritsch, N.X.; Ding, J.B.; Sabatini, B.L. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature 2012, 490, 262–266. [Google Scholar] [CrossRef]
- Obeso, J.A.; Rodriguez-Oroz, M.C.; Rodriguez, M.; Lanciego, J.L.; Artieda, J.; Gonzalo, N.; Olanow, C.W. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci. 2000, 23, S8–S19. [Google Scholar] [CrossRef]
- Tawfik, V.L.; Chang, S.-Y.; Hitti, F.L.; Roberts, D.W.; Leiter, J.C.; Jovanovic, S.; Lee, K.H. Deep Brain Stimulation Results in Local Glutamate and Adenosine Release. Neurosurgery 2010, 67, 367–375. [Google Scholar] [CrossRef]
- Lee, K.J.; Shim, I.; Sung, J.H.; Hong, J.T.; Kim, I.S.; Cho, C.B. Striatal Glutamate and GABA after High Frequency Subthalamic Stimulation in Parkinsonian Rat. J. Korean Neurosurg. Soc. 2017, 60, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Dvorzhak, A.; Gertler, C.; Harnack, D.; Grantyn, R. High Frequency Stimulation of the Subthalamic Nucleus Leads to Presynaptic GABA(B)-Dependent Depression of Subthalamo-Nigral Afferents. PLoS ONE 2013, 8, e82191. [Google Scholar] [CrossRef] [PubMed]
- Chudler, E.H.; Lu, Y. Nociceptive behavioral responses to chemical, thermal and mechanical stimulation after unilateral, intrastriatal administration of 6-hydroxydopamine. Brain Res. 2008, 1213, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: New York, NY, USA, 2005. [Google Scholar]
- Sanberg, P.R. Haloperidol-induced catalepsy is mediated by postsynaptic dopamine receptors. Nature 1980, 284, 472–473. [Google Scholar] [CrossRef]
- de Andrade, E.M.; Martinez, R.C.R.; Pagano, R.L.; Lopes, P.S.S.; Auada, A.V.V.; Gouveia, F.V.; Antunes, G.F.; Assis, D.V.; Lebrun, I.; Fonoff, E.T. Neurochemical effects of motor cortex stimulation in the periaqueductal gray during neuropathic pain. J. Neurosurg. 2020, 132, 239–251. [Google Scholar] [CrossRef]
- Martinez, R.C.; Hamani, C.; De Carvalho, M.C.; De Oliveira, A.R.; Alho, E.; Navarro, J.; Ghilardi, M.G.D.S.; Bor-Seng-Shu, E.; Heinsen, H.; Otoch, J.P.; et al. Intraoperative dopamine release during globus pallidus internus stimulation in Parkinson’s disease. Mov. Disord. 2013, 28, 2027–2032. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro Campos, A.C.; Martinez, R.C.R.; Auada, A.V.V.; Lebrun, I.; Fonoff, E.T.; Hamani, C.; Pagano, R.L. Effect of Subthalamic Stimulation and Electrode Implantation in the Striatal Microenvironment in a Parkinson’s Disease Rat Model. Int. J. Mol. Sci. 2022, 23, 12116. https://doi.org/10.3390/ijms232012116
Pinheiro Campos AC, Martinez RCR, Auada AVV, Lebrun I, Fonoff ET, Hamani C, Pagano RL. Effect of Subthalamic Stimulation and Electrode Implantation in the Striatal Microenvironment in a Parkinson’s Disease Rat Model. International Journal of Molecular Sciences. 2022; 23(20):12116. https://doi.org/10.3390/ijms232012116
Chicago/Turabian StylePinheiro Campos, Ana Carolina, Raquel Chacon Ruiz Martinez, Aline Vivian Vatti Auada, Ivo Lebrun, Erich Talamoni Fonoff, Clement Hamani, and Rosana Lima Pagano. 2022. "Effect of Subthalamic Stimulation and Electrode Implantation in the Striatal Microenvironment in a Parkinson’s Disease Rat Model" International Journal of Molecular Sciences 23, no. 20: 12116. https://doi.org/10.3390/ijms232012116
APA StylePinheiro Campos, A. C., Martinez, R. C. R., Auada, A. V. V., Lebrun, I., Fonoff, E. T., Hamani, C., & Pagano, R. L. (2022). Effect of Subthalamic Stimulation and Electrode Implantation in the Striatal Microenvironment in a Parkinson’s Disease Rat Model. International Journal of Molecular Sciences, 23(20), 12116. https://doi.org/10.3390/ijms232012116