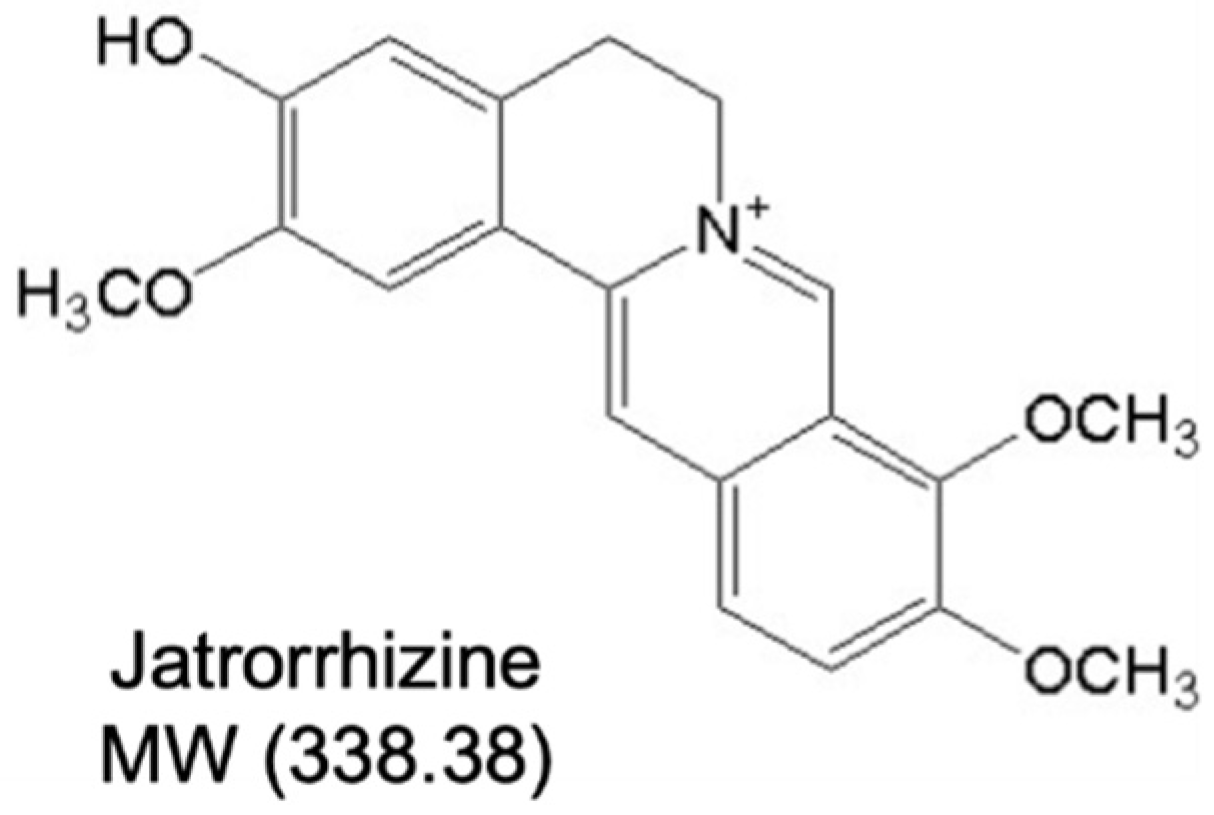
Jatrorrhizine Improves Endothelial Function in Diabetes and Obesity through Suppression of Endoplasmic Reticulum Stress
Abstract
1. Introduction
2. Results
2.1. Jatrorrhizine Improves Vascular Functions in Diabetes
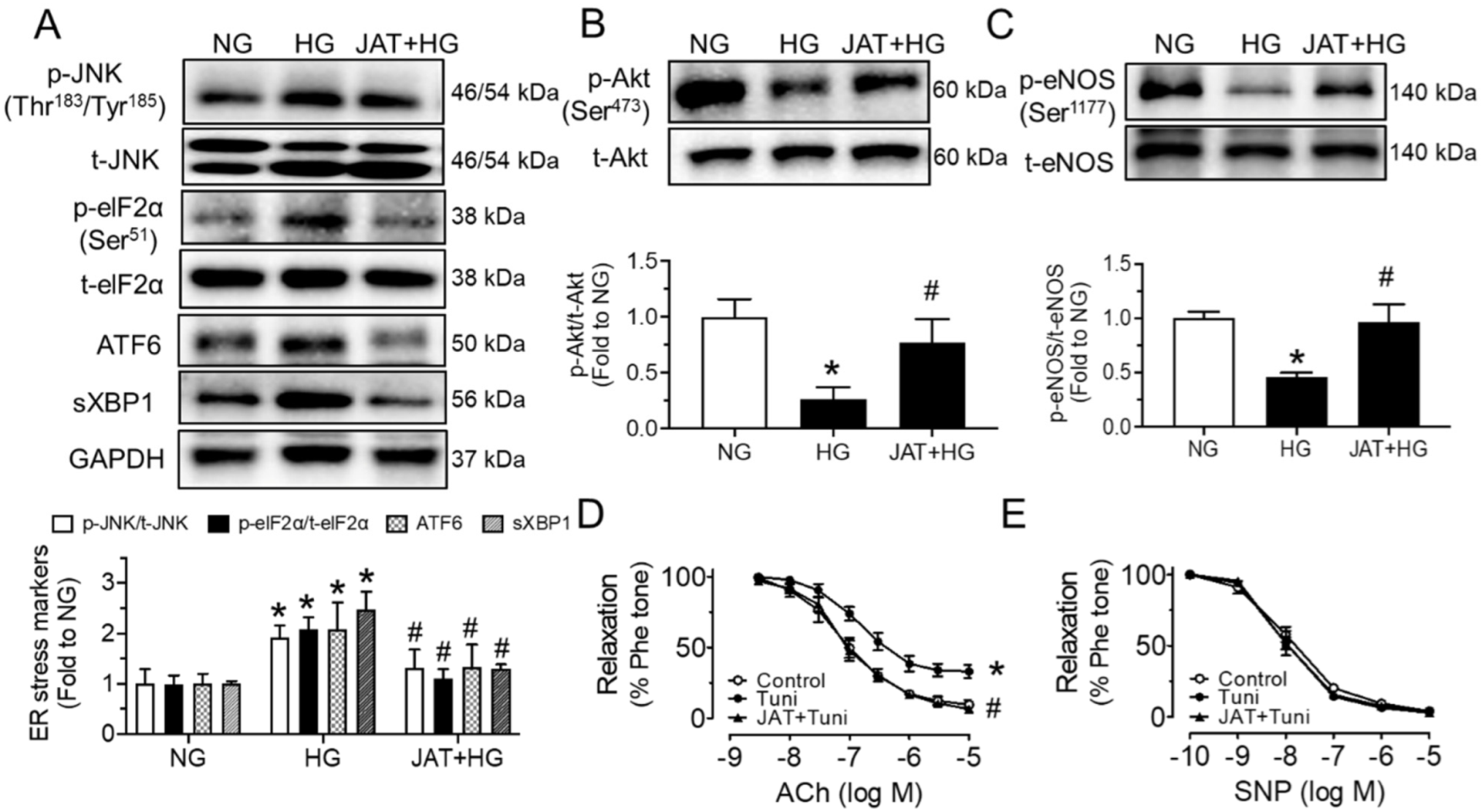
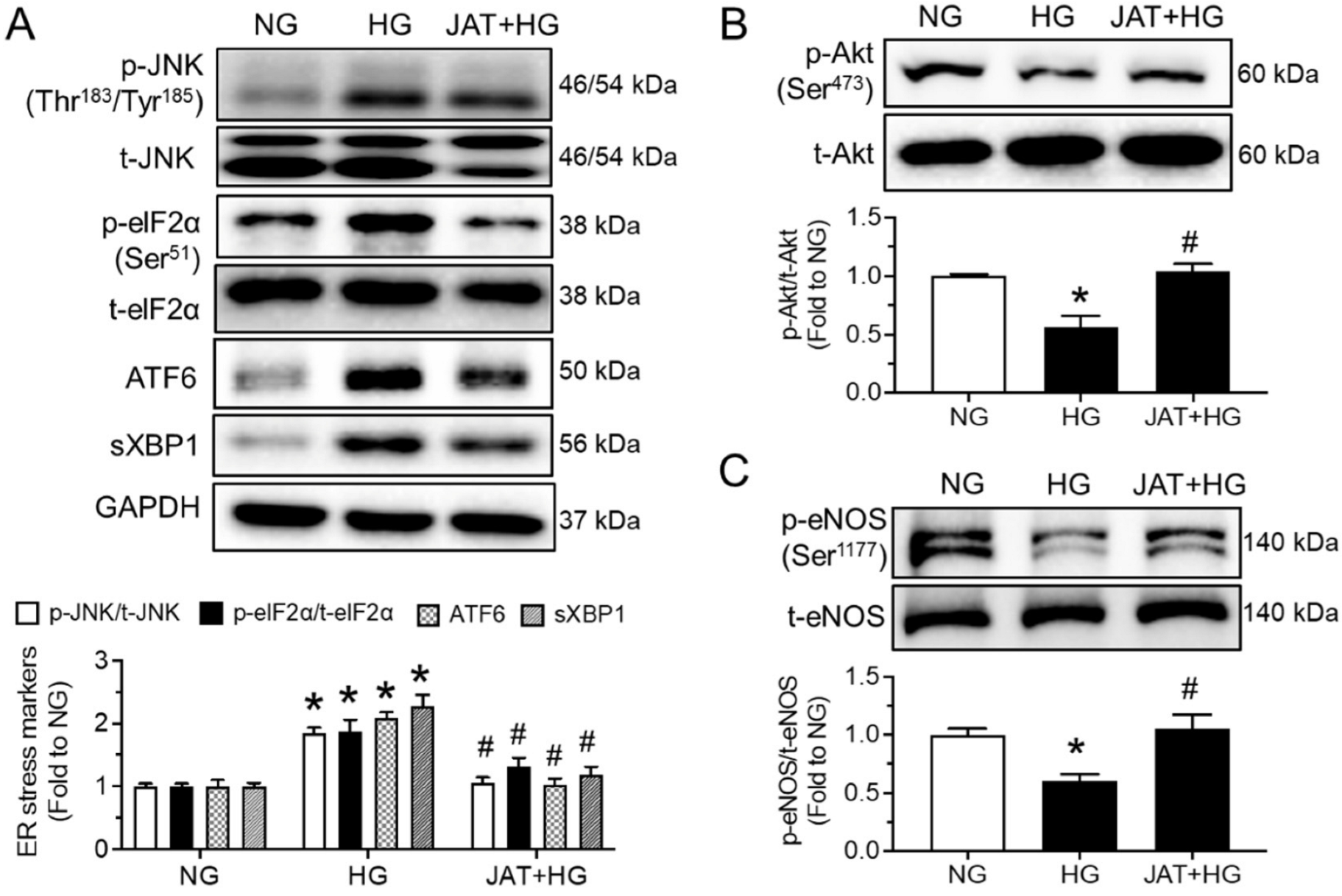
2.2. Jatrorrhizine Increases Akt/eNOS Phosphorylation and Alleviates ER Stress
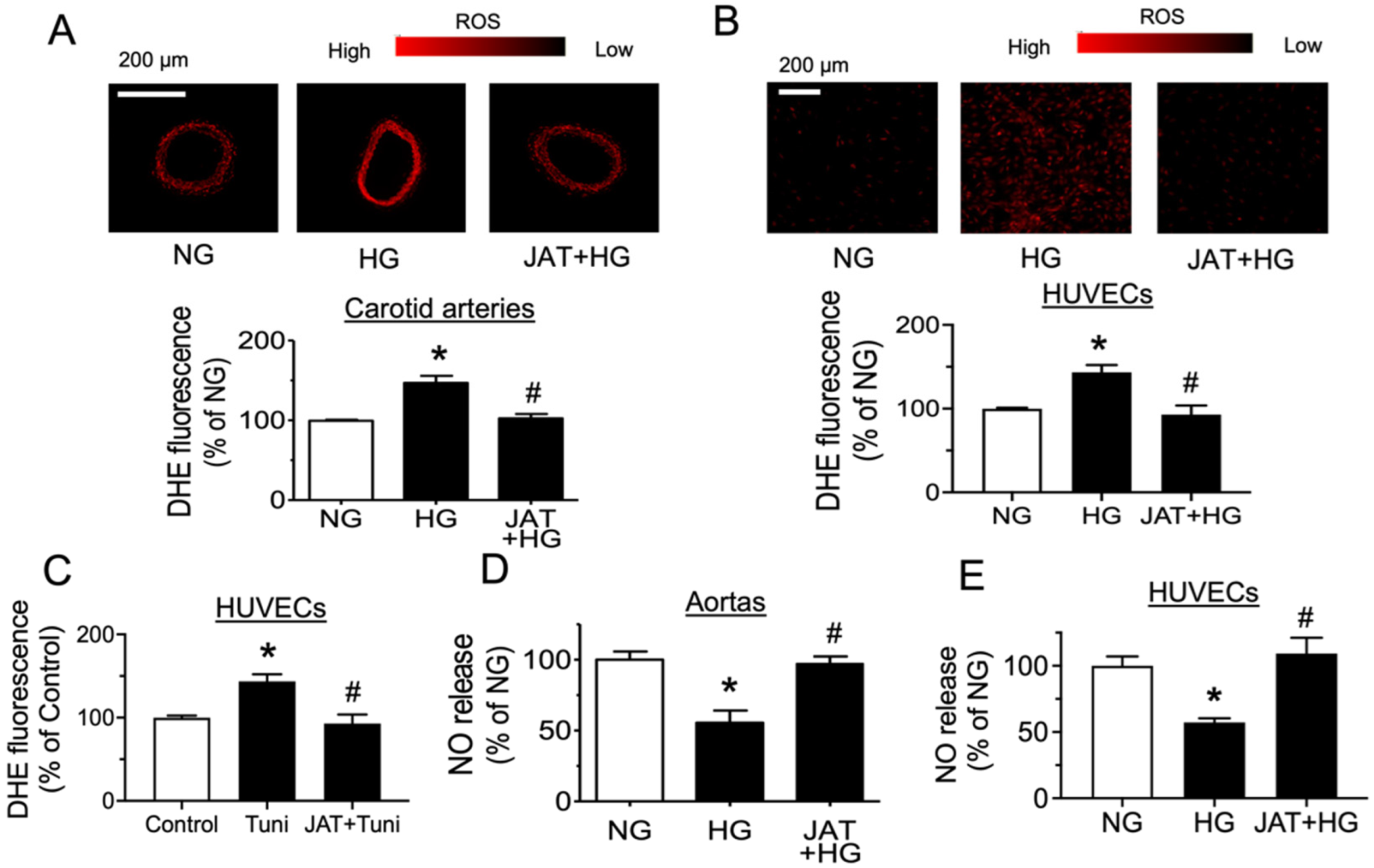
2.3. Jatrorrhizine Suppresses Oxidative Stress and Increases NO Bioavailability
2.4. Chronic Jatrorrhizine Treatment Attenuates Endothelial Function in Diabetic and Obese Mice
2.5. Jatrorrhizine Improves Liver and Plasma Lipid Profile in DIO Mice
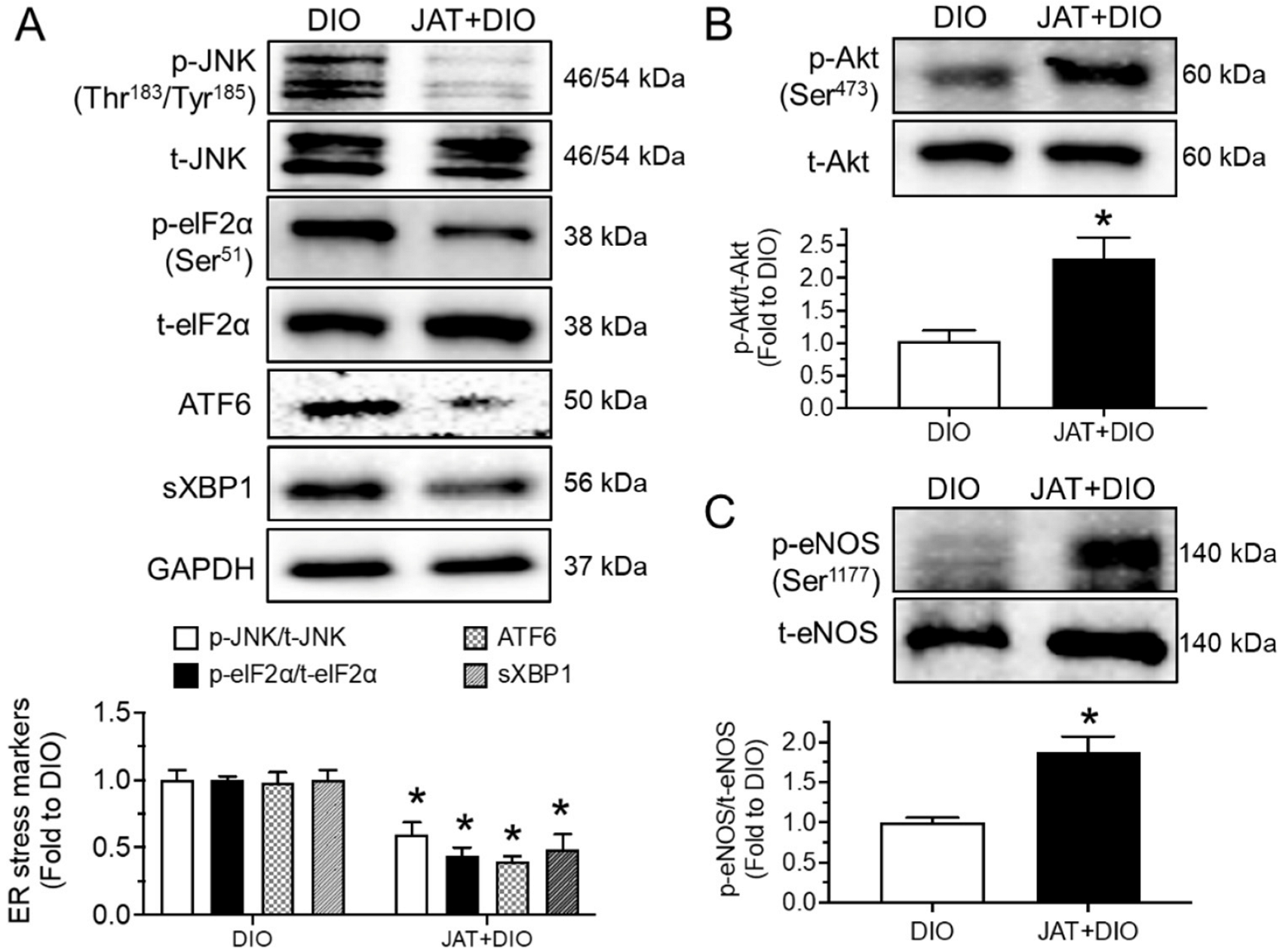
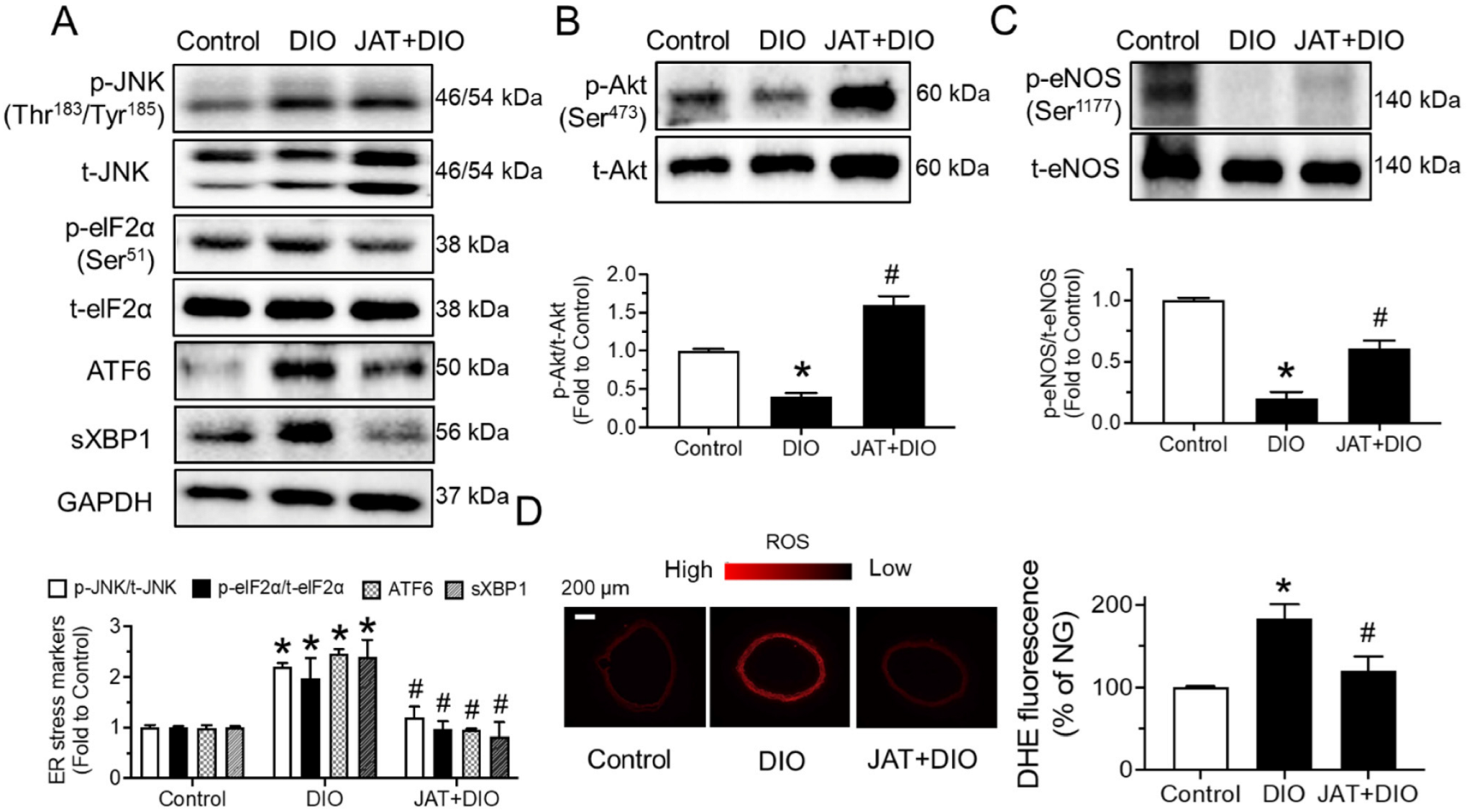
2.6. Jatrorrhizine Treatment Activates Akt/eNOS Pathway and Suppresses ER Stress and Oxidative Stress in Aortas of DIO Mice
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Blood Pressure Measurement
4.3. Blood Glucose Measurement
4.4. Determination of Plasma Lipid Profile
4.5. Hematoxylin and Eosin Staining and Oil Red O Staining
4.6. Culture of Human Umbilical Cord Vein Endothelial Cells (HUVECs)
4.7. Ex Vivo Culture of Mouse Aortas
4.8. Isometric Force Measurement in Wire Myograph
4.9. Western Blotting Assay
4.10. Determination of ROS by Dihydroethidium (DHE) Staining
4.11. Determination of NO Generation
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Bakker, W.; Eringa, E.C.; Sipkema, P.; van Hinsbergh, V.W. Endothelial dysfunction and diabetes: Roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res. 2009, 335, 165–189. [Google Scholar] [CrossRef] [PubMed]
- Poredos, P.; Poredos, A.V.; Gregoric, I. Endothelial Dysfunction and Its Clinical Implications. Angiology 2021, 72, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M. Endothelial Dysfunction and Vascular Pathology. Bull. Mem. Acad. R Med. Belg. 2006, 161, 529–537. [Google Scholar]
- Tan, Y.; Cheong, M.S.; Cheang, W.S. Roles of Reactive Oxygen Species in Vascular Complications of Diabetes: Therapeutic Properties of Medicinal Plants and Food. Oxygen 2022, 2, 246–268. [Google Scholar] [CrossRef]
- Cheang, W.S.; Wong, W.T.; Tian, X.Y.; Yang, Q.; Lee, H.K.; He, G.W.; Yao, X.; Huang, Y. Endothelial nitric oxide synthase enhancer reduces oxidative stress and restores endothelial function in db/db mice. Cardiovasc. Res. 2011, 92, 267–275. [Google Scholar] [CrossRef]
- Tian, X.Y.; Wong, W.T.; Wang, N.; Lu, Y.; Cheang, W.S.; Liu, J.; Liu, L.; Liu, Y.; Lee, S.S.; Chen, Z.Y.; et al. PPARdelta activation protects endothelial function in diabetic mice. Diabetes 2012, 61, 3285–3293. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Q.; Gao, A.; Chen, L.; Li, L. Endoplasmic reticulum stress and focused drug discovery in cardiovascular disease. Clin. Chim. Acta 2020, 504, 125–137. [Google Scholar] [CrossRef]
- Cheang, W.S.; Wong, W.T.; Zhao, L.; Xu, J.; Wang, L.; Lau, C.W.; Chen, Z.Y.; Ma, R.C.; Xu, A.; Wang, N.; et al. PPARdelta Is Required for Exercise to Attenuate Endoplasmic Reticulum Stress and Endothelial Dysfunction in Diabetic Mice. Diabetes 2017, 66, 519–528. [Google Scholar] [CrossRef]
- Zhou, Y.; Murugan, D.D.; Khan, H.; Huang, Y.; Cheang, W.S. Roles and Therapeutic Implications of Endoplasmic Reticulum Stress and Oxidative Stress in Cardiovascular Diseases. Antioxidants 2021, 10, 1167. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Fang, S.; Liu, X.; Li, J.; Wang, X.; Cui, J.; Chen, T.; Li, Z.; Yang, F.; Tian, J.; et al. Omentin-1 protects against high glucose-induced endothelial dysfunction via the AMPK/PPARdelta signaling pathway. Biochem. Pharmacol. 2020, 174, 113830. [Google Scholar] [CrossRef] [PubMed]
- Beriault, D.R.; Werstuck, G.H. The role of glucosamine-induced ER stress in diabetic atherogenesis. Exp. Diabetes Res. 2012, 2012, 187018. [Google Scholar] [CrossRef]
- Yan, T.; Liang, C.; Fan, H.; Zhou, W.; Huang, L.; Qi, S.; Wang, W.; Ma, P. KAP1 silencing relieves OxLDL-induced vascular endothelial dysfunction by down-regulating LOX-1. Biosci. Rep. 2020, 40, BSR20200821. [Google Scholar] [CrossRef] [PubMed]
- Radwan, E.; Bakr, M.H.; Taha, S.; Sayed, S.A.; Farrag, A.A.; Ali, M. Inhibition of endoplasmic reticulum stress ameliorates cardiovascular injury in a rat model of metabolic syndrome. J. Mol. Cell. Cardiol. 2020, 143, 15–25. [Google Scholar] [CrossRef]
- Cheang, W.S.; Tian, X.Y.; Wong, W.T.; Lau, C.W.; Lee, S.S.; Chen, Z.Y.; Yao, X.; Wang, N.; Huang, Y. Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5’ adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor delta pathway. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 830–836. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Lou, G.H.; Zeng, H.R.; Hu, J.; Huang, Q.W.; Peng, W.; Yang, X.B. Coptidis Rhizoma: A comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. Pharm. Biol. 2019, 57, 193–225. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Huang, Z.; Yu, X.; Zhang, X.; Dou, D.; Huang, Y. Berberine improves endothelial function by inhibiting endoplasmic reticulum stress in the carotid arteries of spontaneously hypertensive rats. Biochem. Biophys. Res. Commun. 2015, 458, 796–801. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, C.; Zhang, X.; Vong, C.T.; Wang, Y.; Cheang, W.S. Coptisine Attenuates Diabetes-Associated Endothelial Dysfunction through Inhibition of Endoplasmic Reticulum Stress and Oxidative Stress. Molecules 2021, 26, 4210. [Google Scholar] [CrossRef]
- Fu, Y.; Hu, B.; Tang, Q.; Fu, Q.; Xiang, J. Hypoglycemic activity of jatrorrhizine. J. Huazhong Univ. Sci. Technol. Med. Sci. 2005, 25, 491–493. [Google Scholar] [CrossRef]
- Yang, W.; She, L.; Yu, K.; Yan, S.; Zhang, X.; Tian, X.; Ma, S.; Zhang, X. Jatrorrhizine hydrochloride attenuates hyperlipidemia in a high-fat diet-induced obesity mouse model. Mol. Med. Rep. 2016, 14, 3277–3284. [Google Scholar] [CrossRef] [PubMed]
- Han, K.A.; Patel, Y.; Lteif, A.A.; Chisholm, R.; Mather, K.J. Contributions of dysglycaemia, obesity, and insulin resistance to impaired endothelium-dependent vasodilation in humans. Diabetes Metab. Res. Rev. 2011, 27, 354–361. [Google Scholar] [CrossRef] [PubMed]
- El-Remessy, A.B.; Abou-Mohamed, G.; Caldwell, R.W.; Caldwell, R.B. High glucose-induced tyrosine nitration in endothelial cells: Role of eNOS uncoupling and aldose reductase activation. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3135–3143. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Munzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.P.; Hsu, S.C.; Li, D.E.; Chen, K.H.; Kuo, C.Y.; Hung, L.M. Resveratrol Mitigates High-Fat Diet-Induced Vascular Dysfunction by Activating the Akt/eNOS/NO and Sirt1/ER Pathway. J. Cardiovasc. Pharmacol. 2018, 72, 231–241. [Google Scholar] [CrossRef]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, M.; Wang, S.; Liang, B.; Zhao, Z.; Liu, C.; Wu, M.; Choi, H.C.; Lyons, T.J.; Zou, M.H. Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes 2010, 59, 1386–1396. [Google Scholar] [CrossRef]
- San Cheang, W.; Yuen Ngai, C.; Yen Tam, Y.; Yu Tian, X.; Tak Wong, W.; Zhang, Y.; Wai Lau, C.; Chen, Z.Y.; Bian, Z.X.; Huang, Y.; et al. Black tea protects against hypertension-associated endothelial dysfunction through alleviation of endoplasmic reticulum stress. Sci. Rep. 2015, 5, 10340. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, J.; Cheng, B.; Lu, Q.; Chen, X. Oleanolic acid protects against oxidative stressinduced human umbilical vein endothelial cell injury by activating AKT/eNOS signaling. Mol. Med. Rep. 2018, 18, 3641–3648. [Google Scholar] [CrossRef]
- Luo, J.; Huang, L.; Wang, A.; Liu, Y.; Cai, R.; Li, W.; Zhou, M.S. Corrigendum: Resistin-Induced Endoplasmic Reticulum Stress Contributes to the Impairment of Insulin Signaling in Endothelium. Front. Pharmacol. 2018, 9, 1446. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.; Qi, J.; Jin, Y.; Tong, L. Activation of NADPH/ROS pathway contributes to angiogenesis through JNK signaling in brain endothelial cells. Microvasc. Res. 2020, 131, 104012. [Google Scholar] [CrossRef] [PubMed]
- Saaoud, F.; Drummer, I.V.C.; Shao, Y.; Sun, Y.; Lu, Y.; Xu, K.; Ni, D.; Jiang, X.; Wang, H.; Yang, X. Circular RNAs are a novel type of non-coding RNAs in ROS regulation, cardiovascular metabolic inflammations and cancers. Pharmacol. Ther. 2021, 220, 107715. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.; Michel, L.Y.M.; Balligand, J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Wang, X.C.; Xu, Y.M.; Luo, N.C.; Luo, S.; Hao, X.Y.; Cheng, S.Y.; Fang, J.S.; Wang, Q.; Zhang, S.J.; et al. Berberine Improves Diabetic Encephalopathy Through the SIRT1/ER Stress Pathway in db/db Mice. Rejuvenation Res. 2018, 21, 200–209. [Google Scholar] [CrossRef]
- Pang, B.; Yu, X.T.; Zhou, Q.; Zhao, T.Y.; Wang, H.; Gu, C.J.; Tong, X.L. Effect of Rhizoma coptidis (Huang Lian) on Treating Diabetes Mellitus. Evid. Based Complement. Alternat. Med. 2015, 2015, 921416. [Google Scholar] [CrossRef]
- Zheng, X.K.; Li, Y.J.; Zhang, L.; Feng, W.S.; Zhang, X. Antihyperglycemic activity of Selaginella tamariscina (Beauv.) Spring. J. Ethnopharmacol. 2011, 133, 531–537. [Google Scholar] [CrossRef]
- Wang, Y.; You, Y.; Tian, Y.; Sun, H.; Li, X.; Wang, X.; Wang, Y.; Liu, J. Pediococcus pentosaceus PP04 Ameliorates High-Fat Diet-Induced Hyperlipidemia by Regulating Lipid Metabolism in C57BL/6N Mice. J. Agric. Food Chem. 2020, 68, 15154–15163. [Google Scholar] [CrossRef]
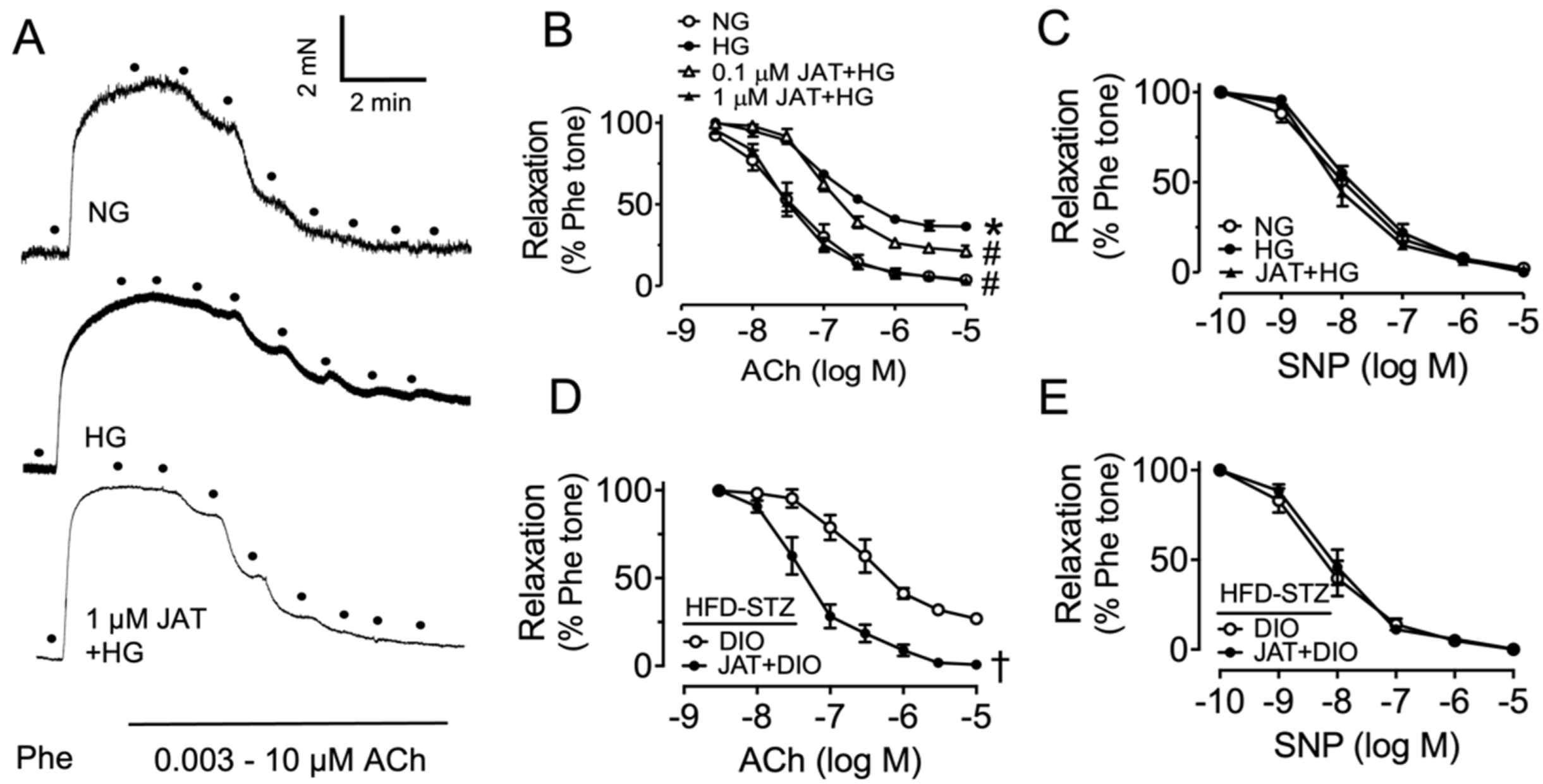


| Treatment | pD2 | Emax% |
|---|---|---|
| NG | 7.48 ± 0.12 | 95.38 ± 2.96 |
| HG | 6.97 ± 0.05 * | 65.05 ± 1.29 * |
| 0.1 µM JAT+HG | 6.97 ± 0.07 | 80.96 ± 2.08 # |
| 1 µM JAT+HG | 7.57 ± 0.06 # | 95.72 ± 1.60 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wang, Y.; Vong, C.T.; Zhu, Y.; Xu, B.; Ruan, C.-C.; Wang, Y.; Cheang, W.S. Jatrorrhizine Improves Endothelial Function in Diabetes and Obesity through Suppression of Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2022, 23, 12064. https://doi.org/10.3390/ijms232012064
Zhou Y, Wang Y, Vong CT, Zhu Y, Xu B, Ruan C-C, Wang Y, Cheang WS. Jatrorrhizine Improves Endothelial Function in Diabetes and Obesity through Suppression of Endoplasmic Reticulum Stress. International Journal of Molecular Sciences. 2022; 23(20):12064. https://doi.org/10.3390/ijms232012064
Chicago/Turabian StyleZhou, Yan, Yuehan Wang, Chi Teng Vong, Yanyan Zhu, Baojun Xu, Cheng-Chao Ruan, Yitao Wang, and Wai San Cheang. 2022. "Jatrorrhizine Improves Endothelial Function in Diabetes and Obesity through Suppression of Endoplasmic Reticulum Stress" International Journal of Molecular Sciences 23, no. 20: 12064. https://doi.org/10.3390/ijms232012064
APA StyleZhou, Y., Wang, Y., Vong, C. T., Zhu, Y., Xu, B., Ruan, C.-C., Wang, Y., & Cheang, W. S. (2022). Jatrorrhizine Improves Endothelial Function in Diabetes and Obesity through Suppression of Endoplasmic Reticulum Stress. International Journal of Molecular Sciences, 23(20), 12064. https://doi.org/10.3390/ijms232012064











