Development of Two-Layer Hybrid Scaffolds Based on Oxidized Polyvinyl Alcohol and Bioactivated Chitosan Sponges for Tissue Engineering Purposes
Abstract
1. Introduction
2. Results
2.1. Mechanical Analysis of Chitosan-Based Sponges
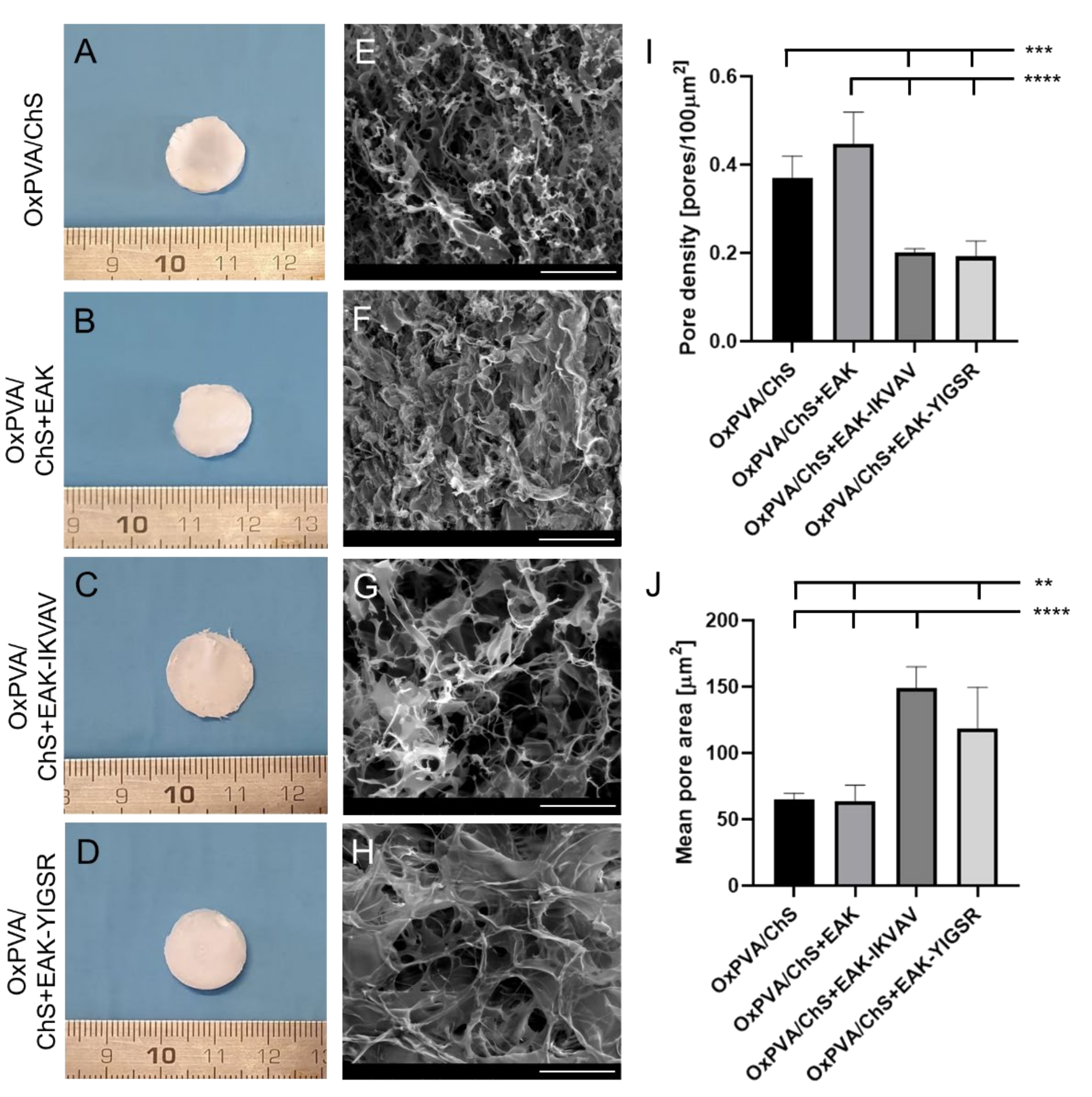
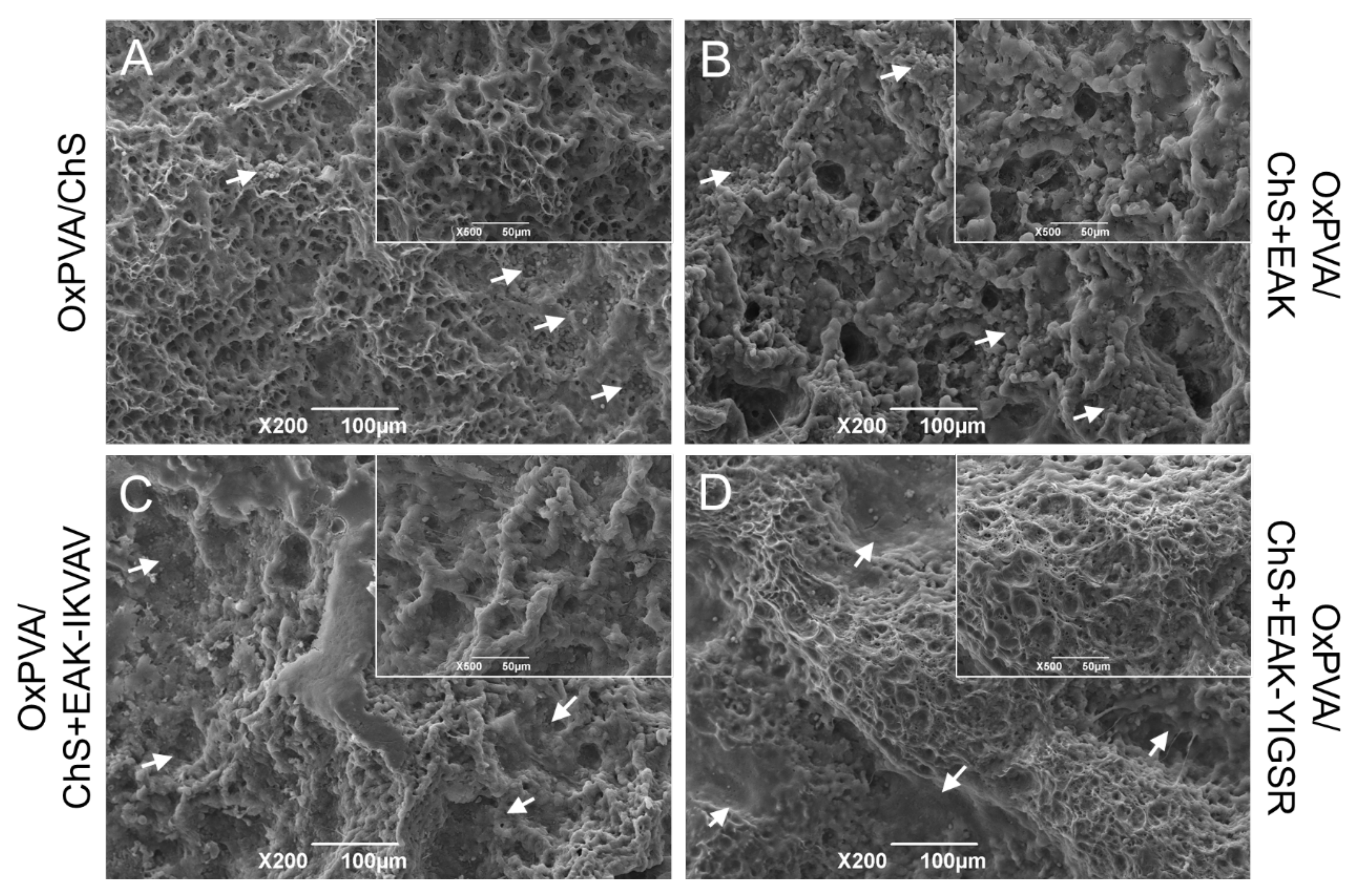
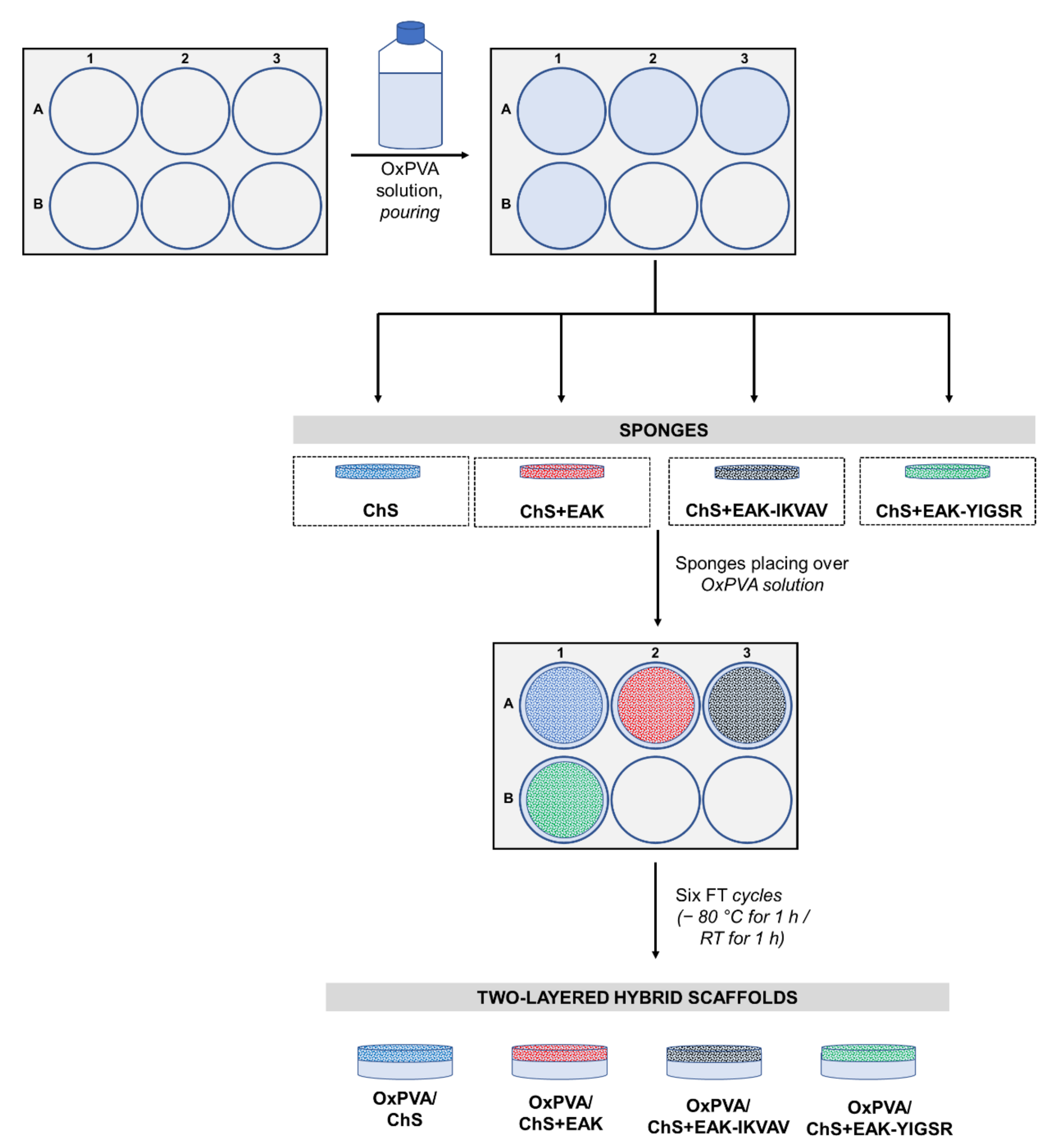
2.2. Fabrication and Morphology of OxPVA/ChS Hybrid Scaffolds
2.3. Bioactive Potential of the Hybrid Scaffolds
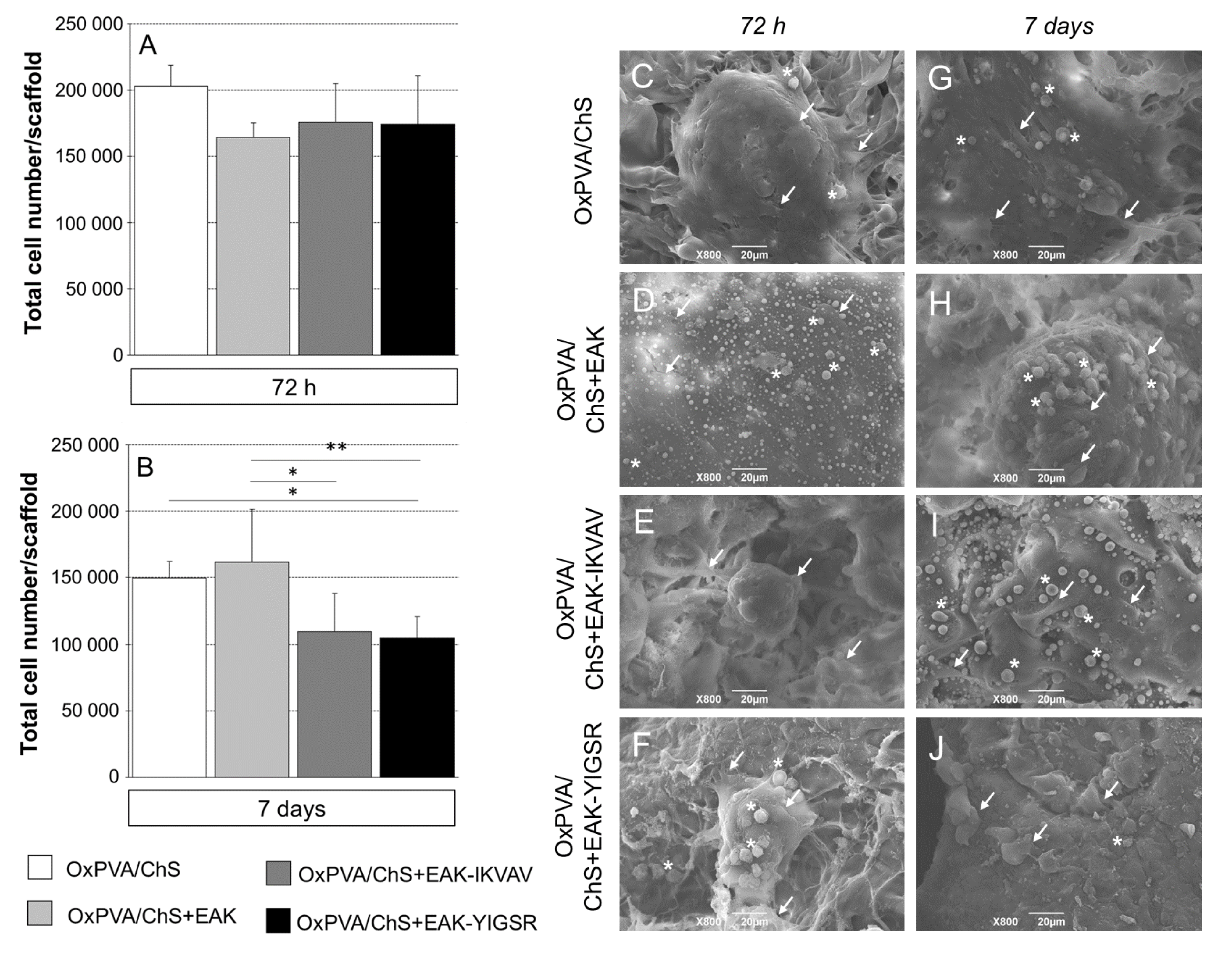
2.4. SEM Analysis of Cells Distribution on OxPVA/Chitosan-Based Scaffolds
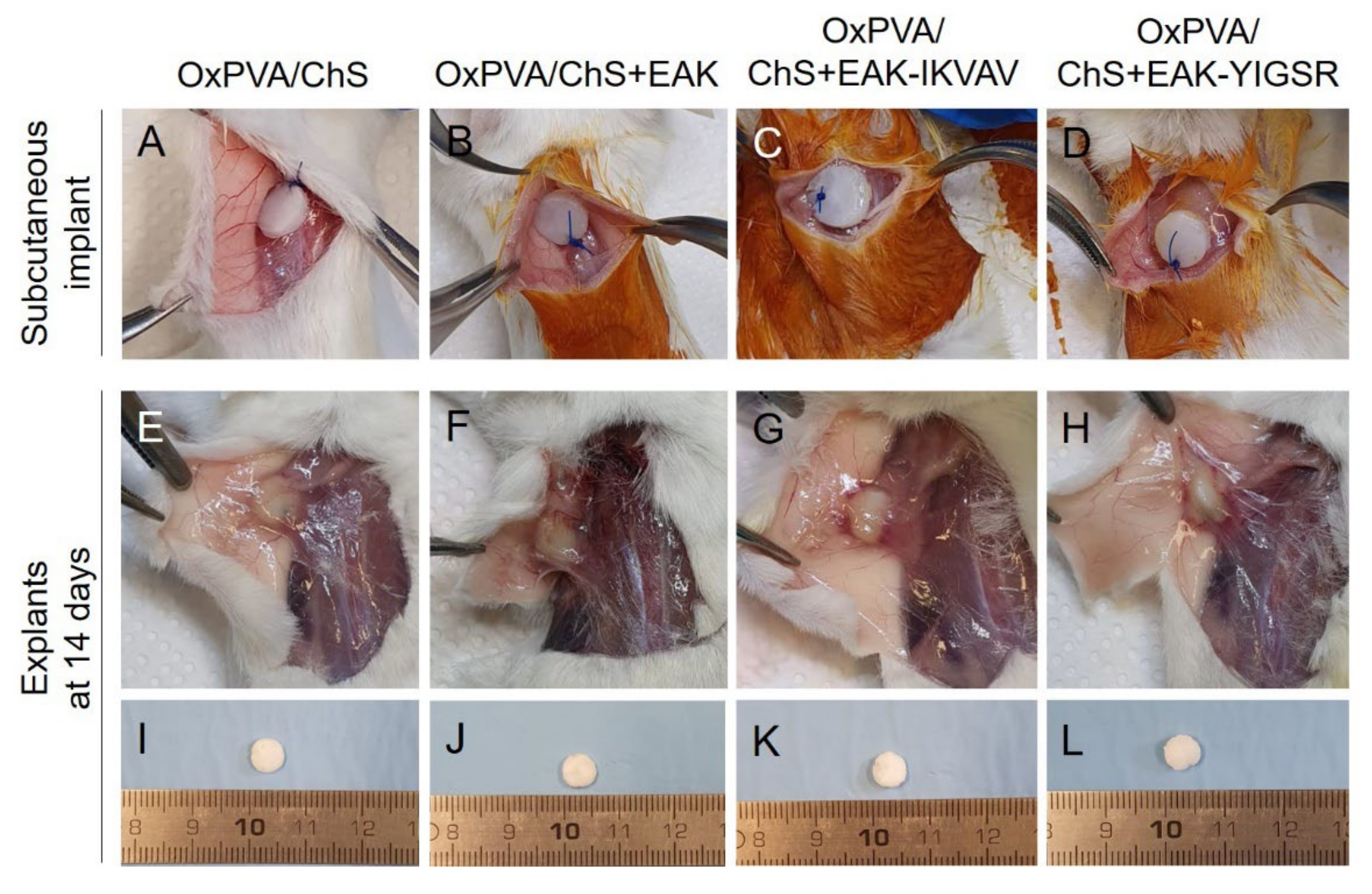
2.5. In Vivo Biocompatibility of OxPVA/Chitosan-Based Hybrid Scaffolds
3. Discussion
4. Materials and Methods
4.1. Development and Analysis of Chitosan-Based Sponges
4.1.1. Peptides’ Synthesis and Purification
4.1.2. Set-Up of the Chitosan-Based Sponges
4.1.3. Mechanical Analysis of the Chitosan-Based Sponges
4.2. Fabrication and Analysis of the Hybrid Scaffolds
4.2.1. OxPVA Solution Preparation
4.2.2. Hybrid Scaffolds Set-Up
4.2.3. Scaffolds Ultrastructure
4.3. Bioactivity of the Hybrid Scaffolds: SH-SY5Y Cell Seeding and Proliferation Assessment
4.4. In Vivo Biocompatibility Study
4.4.1. Subcutaneous Implant
4.4.2. Histological Analyses
4.4.3. Immunohistochemical Investigation
4.4.4. Ultrastructural Evaluation by SEM
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Escobar, A.; Reis, R.L.; Oliveira, J.M. Nanoparticles for neurotrophic factor delivery in nerve guidance conduits for peripheral nerve repair. Nanomedicine 2022, 17, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Arslantunali, D.; Dursun, T.; Yucel, D.; Hasirci, N.; Hasirci, V. Peripheral nerve conduits: Technology update. Med. Devices 2014, 7, 405–424. [Google Scholar]
- De Stefano, P.; Federici, A.S.; Draghi, L. In Vitro Models for the Development of Peripheral Nerve Conduits, Part I: Design of a Fibrin Gel-Based Non-Contact Test. Polymers 2021, 13, 3573. [Google Scholar] [CrossRef]
- Parker, B.J.; Rhodes, D.I.; O’Brien, C.M.; Rodda, A.E.; Cameron, N.R. Nerve guidance conduit development for primary treatment of peripheral nerve transection injuries: A commercial perspective. Acta Biomater. 2021, 135, 64–86. [Google Scholar] [CrossRef]
- Fornasari, B.E.; Carta, G.; Gambarotta, G.; Raimondo, S. Natural-Based Biomaterials for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2020, 8, 554257. [Google Scholar] [CrossRef]
- Alarcón Apablaza, J.; Lezcano, M.F.; Godoy Sánchez, K.; Oporto, G.H.; Dias, F.J. Optimal Morphometric Characteristics of a Tubular Polymeric Scaffold to Promote Peripheral Nerve Regeneration: A Scoping Review. Polymers 2022, 14, 397. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, B.; Zarrintaj, P.; Oftadeh, M.O.; Keramati, F.; Fouladiha, H.; Sohrabi-Jahromi, S.; Ziraksaz, Z. Tissue engineering; strategies, tissues, and biomaterials. Biotechnol. Genet. Eng. Rev. 2017, 33, 144–172. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Dalzoppo, D.; Lora, S.; Sartore, L.; Folin, M.; Parnigotto, P.P.; Grandi, C. Tailored PVA/ECM scaffolds for cartilage regeneration. Biomed. Res. Int. 2014, 2014, 762189. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Radossi, P.; Rajendran, S.; Dalzoppo, D.; Bortolami, M.; Bagno, A.; Grandi, F.; Gamba, P.G.; Parnigotto, P.P.; et al. Autologous chondrocytes as a novel source for neo-chondrogenesis in haemophiliacs. Cell Tissue Res. 2016, 366, 51–61. [Google Scholar] [CrossRef]
- Grandi, F.; Stocco, E.; Barbon, S.; Rambaldo, A.; Contran, M.; Fascetti Leon, F.; Gamba, P.; Parnigotto, P.P.; Macchi, V.; De Caro, R.; et al. Composite Scaffolds Based on Intestinal Extracellular Matrices and Oxidized Polyvinyl Alcohol: A Preliminary Study for a New Regenerative Approach in Short Bowel Syndrome. Biomed. Res. Int. 2018, 2018, 7824757. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Grandi, F.; Gamba, P.G.; Borgio, L.; Del Gaudio, C.; Dalzoppo, D.; Lora, S.; Rajendran, S.; Porzionato, A.; et al. Partially oxidized polyvinyl alcohol as a promising material for tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Barbon, S.; Stocco, E.; Dalzoppo, D.; Todros, S.; Canale, A.; Boscolo-Berto, R.; Pavan, P.; Macchi, V.; Grandi, C.; De Caro, R. Halogen-Mediated Partial Oxidation of Polyvinyl Alcohol for Tissue Engineering Purposes. Int. J. Mol. Sci. 2020, 21, 801. [Google Scholar] [CrossRef] [PubMed]
- Todros, S.; Barbon, S.; Stocco, E.; Favaron, M.; Macchi, V.; De Caro, R.; Porzionato, A.; Pavan, P.G. Time-dependent mechanical behavior of partially oxidized polyvinyl alcohol hydrogels for tissue engineering. J. Mech. Behav. Biomed. Mater. 2022, 125, 104966. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Lora, L.; Grandi, F.; Sartore, L.; Tiengo, C.; Petrelli, L.; Dalzoppo, D.; Parnigotto, P.P.; Macchi, V.; et al. Partially oxidized polyvinyl alcohol conduitfor peripheral nerve regeneration. Sci. Rep. 2018, 8, 604. [Google Scholar] [CrossRef] [PubMed]
- Stocco, E.; Barbon, S.; Macchi, V.; Tiengo, C.; Petrelli, L.; Rambaldo, A.; Borean, A.; Capelli, S.; Filippi, A.; Romanato, F.; et al. New bioresorbable wraps based on oxidized polyvinyl alcohol and leukocyte-fibrin-platelet membrane to support peripheral nerve neurorrhaphy: Preclinical comparison versus NeuraWrap. Sci. Rep. 2019, 9, 17193. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Barbon, S.; Stocco, E.; Dalzoppo, D.; Contran, M.; De Rose, E.; Parnigotto, P.P.; Macchi, V.; Grandi, C.; De Caro, R. Development of Oxidized Polyvinyl Alcohol-Based Nerve Conduits Coupled with the Ciliary Neurotrophic Factor. Materials 2019, 12, 1996. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Lamanna, A.; De Rose, E.; Zamuner, A.; Sandrin, D.; Marsotto, M.; Auditore, A.; Messina, G.M.L.; Licciardello, A.; et al. Bioactivated Oxidized Polyvinyl Alcohol towards Next-Generation Nerve Conduits Development. Polymers 2021, 13, 3372. [Google Scholar] [CrossRef]
- Poongodi, R.; Chen, Y.L.; Yang, T.H.; Huang, Y.H.; Yang, K.D.; Lin, H.C.; Cheng, J.K. Bio-Scaffolds as Cell or Exosome Carriers for Nerve Injury Repair. Int. J. Mol. Sci. 2021, 22, 13347. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Gong, J.; Yang, L.; Niu, C.; Ni, X.; Wang, Y.; Peng, S.; Gu, X.; Sun, C.; et al. Chitosan degradation products facilitate peripheral nerve regeneration by improving macrophage-constructed microenvironments. Biomaterials 2017, 134, 64–77. [Google Scholar] [CrossRef]
- Brun, P.; Zamuner, A.; Peretti, A.; Conti, J.; Messina, G.M.L.; Marletta, G.; Dettin, M. 3D Synthetic Peptide-based Architectures for the Engineering of the Enteric Nervous System. Sci. Rep. 2019, 9, 5583. [Google Scholar] [CrossRef]
- Yan, Y.; Yao, R.; Zhao, J.; Chen, K.; Duan, L.; Wang, T.; Zhang, S.; Guan, J.; Zheng, Z.; Wang, X.; et al. Implantable nerve guidance conduits: Material combinations, multi-functional strategies and advanced engineering innovations. Bioact. Mater. 2021, 11, 57–76. [Google Scholar] [CrossRef]
- Gonzalez-Perez, F.; Cobianchi, S.; Geuna, S.; Barwig, C.; Freier, T.; Udina, E.; Navarro, X. Tubulization with chitosan guides for the repair of long gap peripheral nerve injury in the rat. Microsurgery 2015, 35, 300–308. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, D.; Liu, D.; Su, J.; Jin, Y.; Wang, D.; Han, B.; Jiang, Z.; Liu, B. Applications of Chitosan and its Derivatives in Skin and Soft Tissue Diseases. Front. Bioeng. Biotechnol. 2022, 10, 894667. [Google Scholar] [CrossRef]
- Böcker, A.; Aman, M.; Kneser, U.; Harhaus, L.; Siemers, F.; Stang, F. Closing the Gap: Bridging Peripheral Sensory Nerve Defects with a Chitosan-Based Conduit a Randomized Prospective Clinical Trial. J. Pers. Med. 2022, 12, 900. [Google Scholar] [CrossRef]
- Deng, P.; Chen, F.; Zhang, H.; Chen, Y.; Zhou, J. Multifunctional Double-Layer Composite Hydrogel Conduit Based on Chitosan for Peripheral Nerve Repairing. Adv. Healthc. Mater. 2022, 11, e2200115. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, W.; Zhang, Q.; Wu, P.; Xiao, A.; Zhao, Y.; Zhou, Y.; Wang, Q.; Chen, Y.; Tong, Z. IL-17F depletion accelerates chitosan conduit guided peripheral nerve regeneration. Acta Neuropathol. Commun. 2021, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Boecker, A.; Daeschler, S.C.; Kneser, U.; Harhaus, L. Relevance and Recent Developments of Chitosan in Peripheral Nerve Surgery. Front. Cell NeuroSci. 2019, 13, 104. [Google Scholar] [CrossRef]
- Tangpasuthadol, V.; Pongchaisirikul, N.; Hoven, V.P. Surface modification of chitosan films. Effects of hydrophobicity on protein adsorption. Carbohydr. Res. 2003, 338, 937–942. [Google Scholar] [CrossRef]
- Matsuda, A.; Kobayashi, H.; Itoh, S.; Kataoka, K.; Tanaka, J. Immobilization of laminin peptide in molecularly aligned chitosan by covalent bonding. Biomaterials 2005, 26, 2273–2279. [Google Scholar] [CrossRef] [PubMed]
- Hoven, V.P.; Tangpasuthadol, V.; Angkitpaiboon, Y.; Vallapa, N.; Kiatkamjornwong, S. Surface-charged chitosan: Preparation and protein adsorption. Carbohydr. Polym. 2007, 68, 44–53. [Google Scholar] [CrossRef]
- Kam, L.; Shain, W.; Turner, J.N.; Bizios, R. Selective adhesion of astrocytes to surfaces modified with immobilized peptides. Biomaterials 2002, 23, 511–515. [Google Scholar] [CrossRef]
- Hozumi, K.; Nomizu, M. Mixed Peptide-Conjugated Chitosan Matrices as Multi-Receptor Targeted Cell-Adhesive Scaffolds. Int. J. Mol. Sci. 2018, 19, 2713. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.H.; Wang, D.M.; Hsieh, H.J.; Liu, H.C.; Hsien, T.Y.; Lai, J.Y.; Hou, L.T. Preparation and characterization of RGD-immobilized chitosan scaffolds. Biomaterials 2005, 26, 3197–3206. [Google Scholar] [CrossRef] [PubMed]
- Tiğli, R.S.; Gümüşderelioğlu, M. Evaluation of RGD- or EGF-immobilized chitosan scaffolds for chondrogenic activity. Int. J. Biol. Macromol. 2008, 43, 121–128. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.; Lee, Y.C. Thiolation of chitosan. Attachment of proteins via thioether formation. Biomacromolecules 2005, 6, 880–884. [Google Scholar] [CrossRef]
- Hozumi, K.; Nomizu, M. Cell Adhesion Activity of Peptides Conjugated to Polysaccharides. Curr. Protoc. Cell Biol. 2018, 80, e53. [Google Scholar] [CrossRef]
- Wang, W.; Itoh, S.; Matsuda, A.; Aizawa, T.; Demura, M.; Ichinose, S.; Shinomiya, K.; Tanaka, J. Enhanced nerve regeneration through a bilayered chitosan tube: The effect of introduction of glycine spacer into the CYIGSR sequence. J. Biomed. Mater. Res. A 2008, 85, 919–928. [Google Scholar] [CrossRef]
- Itoh, S.; Matsuda, A.; Kobayashi, H.; Ichinose, S.; Shinomiya, K.; Tanaka, J. Effects of a laminin peptide (YIGSR) immobilized on crab-tendon chitosan tubes on nerve regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 73, 375–382. [Google Scholar] [CrossRef]
- Sabourian, P.; Tavakolian, M.; Yazdani, H.; Frounchi, M.; van de Ven, T.G.M.; Maysinger, D.; Kakkar, A. Stimuli-responsive chitosan as an advantageous platform for efficient delivery of bioactive agents. J. Control. Release 2020, 317, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, L.; An, H.; Zhang, P.; Liu, P. Repair of Peripheral Nerve Injury Using Hydrogels Based on Self-Assembled Peptides. Gels 2021, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.; Marchesan, S. Self-Assembled Peptide Nanostructures for ECM Biomimicry. Nanomaterials 2022, 12, 2147. [Google Scholar] [CrossRef] [PubMed]
- Zamuner, A.; Cavo, M.; Scaglione, S.; Messina, G.M.L.; Russo, T.; Gloria, A.; Marletta, G.; Dettin, M. Design of Decorated Self-Assembling Peptide Hydrogels as Architecture for Mesenchymal Stem Cells. Materials 2016, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Park, C.B. High stability of self-assembled peptide nanowires against thermal, chemical, and proteolytic attacks. Biotechnol. Bioeng. 2010, 105, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Conconi, M.T.; Ghezzo, F.; Dettin, M.; Urbani, L.; Grandi, C.; Guidolin, D.; Nico, B.; Di Bello, C.; Ribatti, D.; Parnigotto, P.P. Effects on in vitro and in vivo angiogenesis induced by small peptides carrying adhesion sequences. J. Pept. Sci. 2010, 16, 349–357. [Google Scholar] [CrossRef]
- Guan, T.; Li, J.; Chen, C.; Liu, Y. Self-Assembling Peptide-Based Hydrogels for Wound Tissue Repair. Adv. Sci. 2022, 9, e2104165. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, M.; Li, M.; Song, X.; Chen, D.; Zhu, M.; Zheng, H.; Chen, S. The Bridging Effect of Controlled-Release Glial Cell-Derived Neurotrophic Factor Microcapsules within Nerve Conduits on Rat Facial Nerve Regeneration. Dis. Markers 2022, 2022, 8942985. [Google Scholar] [CrossRef] [PubMed]
- Barbon, S.; Stocco, E.; Negro, A.; Dalzoppo, D.; Borgio, L.; Rajendran, S.; Grandi, F.; Porzionato, A.; Macchi, V.; De Caro, R.; et al. In vitro assessment of TAT—Ciliary Neurotrophic Factor therapeutic potential for peripheral nerve regeneration. Toxicol. Appl. Pharm. 2016, 309, 121–128. [Google Scholar] [CrossRef]
- Xie, F.; Li, Q.F.; Gu, B.; Liu, K.; Shen, G.X. In vitro and in vivo evaluation of a biodegradable chitosan-PLA composite peripheral nerve guide conduit material. Microsurgery 2008, 28, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Nawrotek, K.; Tylman, M.; Rudnicka, K.; Gatkowska, J.; Wieczorek, M. Epineurium-mimicking chitosan conduits for peripheral nervous tissue engineering. Carbohydr. Polym. 2016, 152, 119–128. [Google Scholar] [CrossRef]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar]
- Luna, S.M.; Silva, S.S.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Cell adhesion and proliferation onto chitosan-based membranes treated by plasma surface modification. J. Biomater. Appl. 2011, 26, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Ligorio, C.; Hoyland, J.A.; Saiani, A. Self-Assembling Peptide Hydrogels as Functional Tools to Tackle Intervertebral Disc Degeneration. Gels 2022, 8, 211. [Google Scholar] [CrossRef]
- Webber, M.J.; Khan, O.F.; Sydlik, S.A.; Tang, B.C.; Langer, R. A perspective on the clinical translation of scaffolds for tissue engineering. Ann. Biomed. Eng. 2015, 43, 641–656. [Google Scholar] [CrossRef]
- Anderson, J.M. Biological responses to materials. Annu. Rev. Mater. Sci. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Abd Aziz, A.J.; Baharuddin, N.A.; Somalu, M.R.; Muchtar, A. Layering Optimization of the SrFe0.9Ti0.1O3−δ-Ce0.8Sm0.2O1.9 Composite Cathode. Molecules 2022, 27, 2549. [Google Scholar] [CrossRef]
- Wang, B.; Liu, W.; Xing, D.; Li, R.; Lv, C.; Li, Y.; Yan, X.; Ke, Y.; Xu, Y.; Du, Y.; et al. Injectable nanohydroxyapatite-chitosan-gelatin micro-scaffolds induce regeneration of knee subchondral bone lesions. Sci. Rep. 2017, 7, 16709. [Google Scholar] [CrossRef] [PubMed]
- Barbon, S.; Stocco, E.; Contran, M.; Facchin, F.; Boscolo-Berto, R.; Todros, S.; Sandrin, D.; Romanato, F.; Pavan, P.; Macchi, V.; et al. Preclinical Development of Bioengineered Allografts Derived from Decellularized Human Diaphragm. Biomedicines 2022, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-6 2009; Biological Evaluation of Medical Devices—Part 6: Tests for Local Effects after Implantation. International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/obp/ui/#iso:std:iso:10993:-6:en (accessed on 22 March 2022).
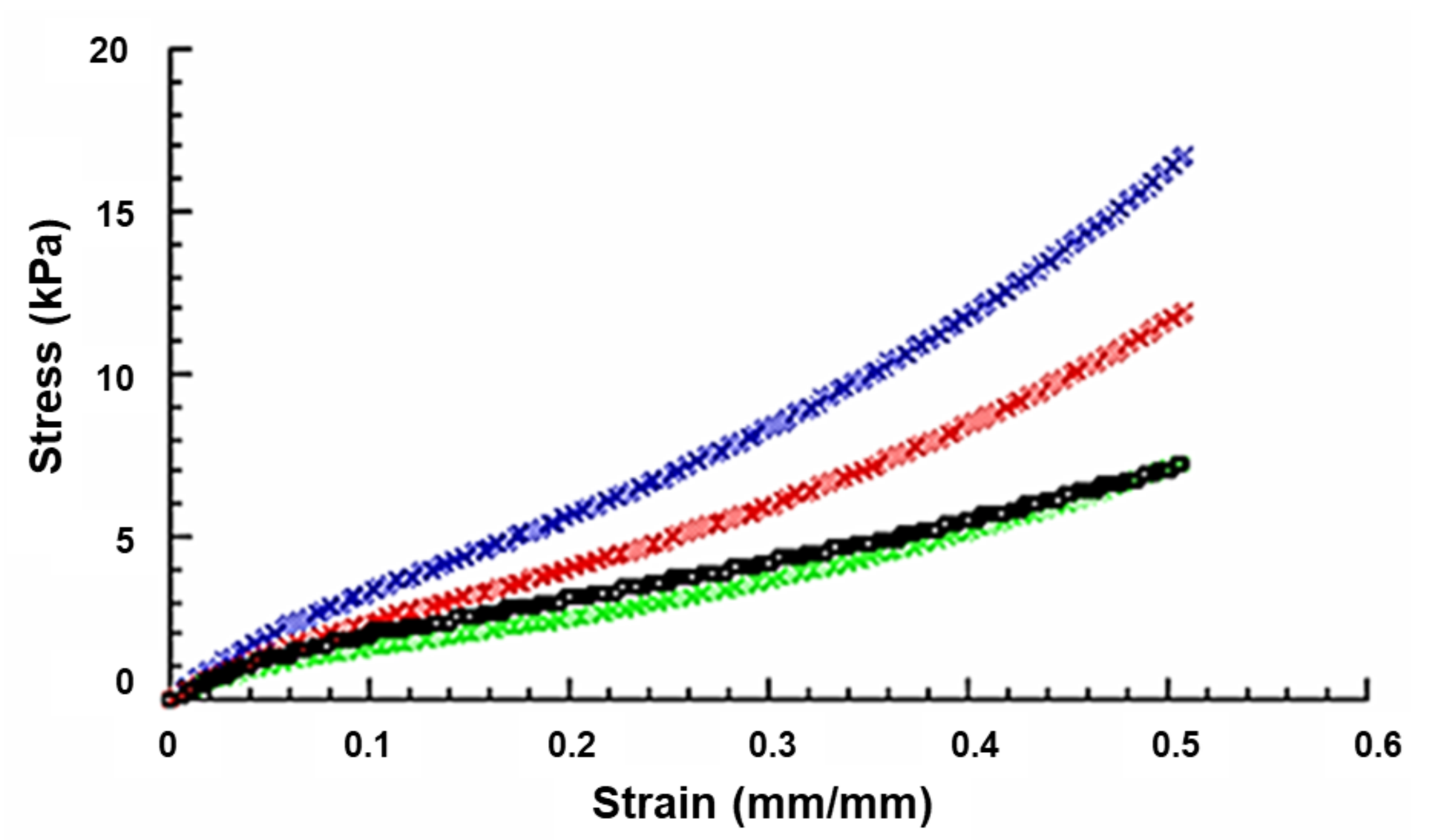
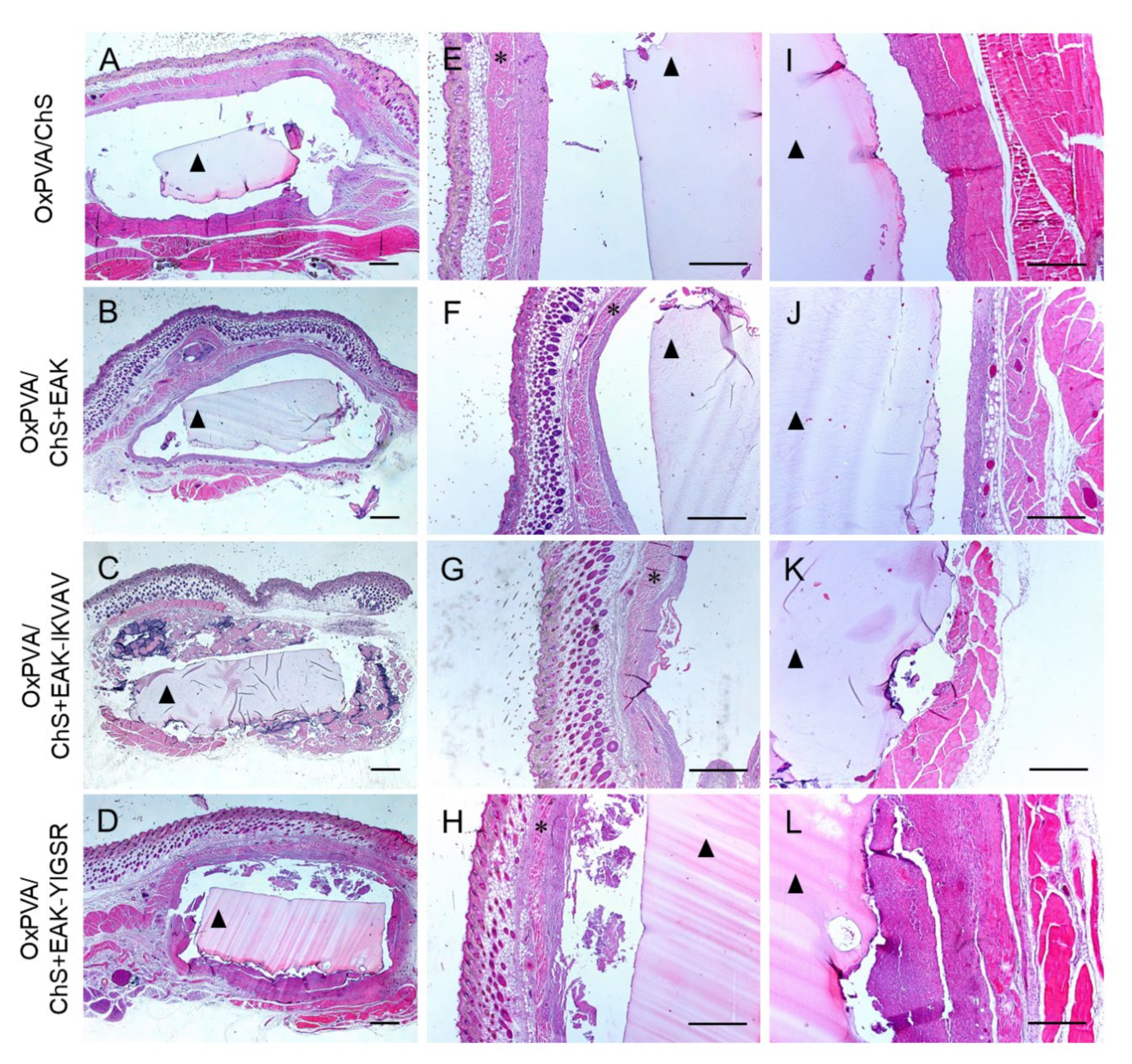
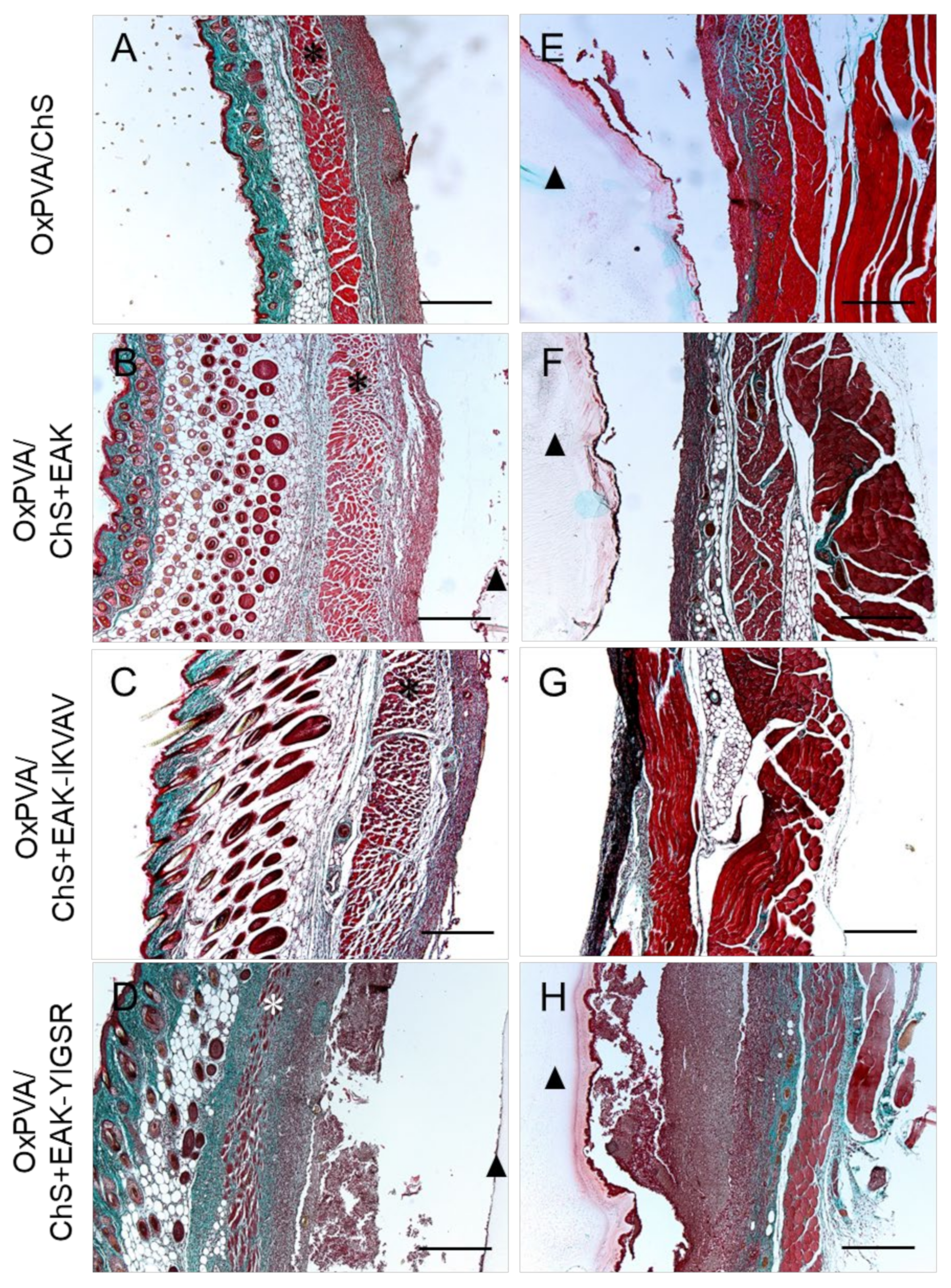
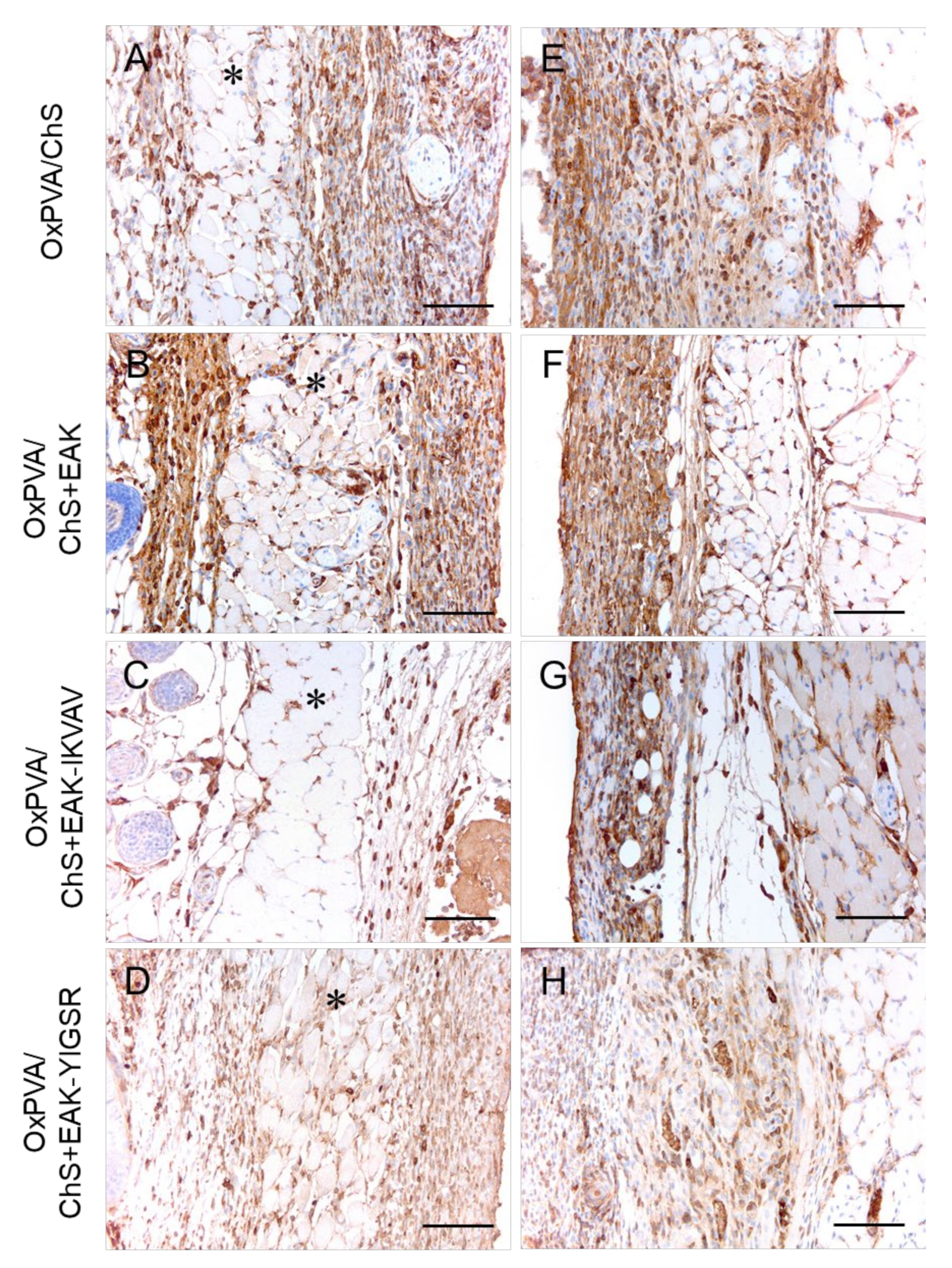
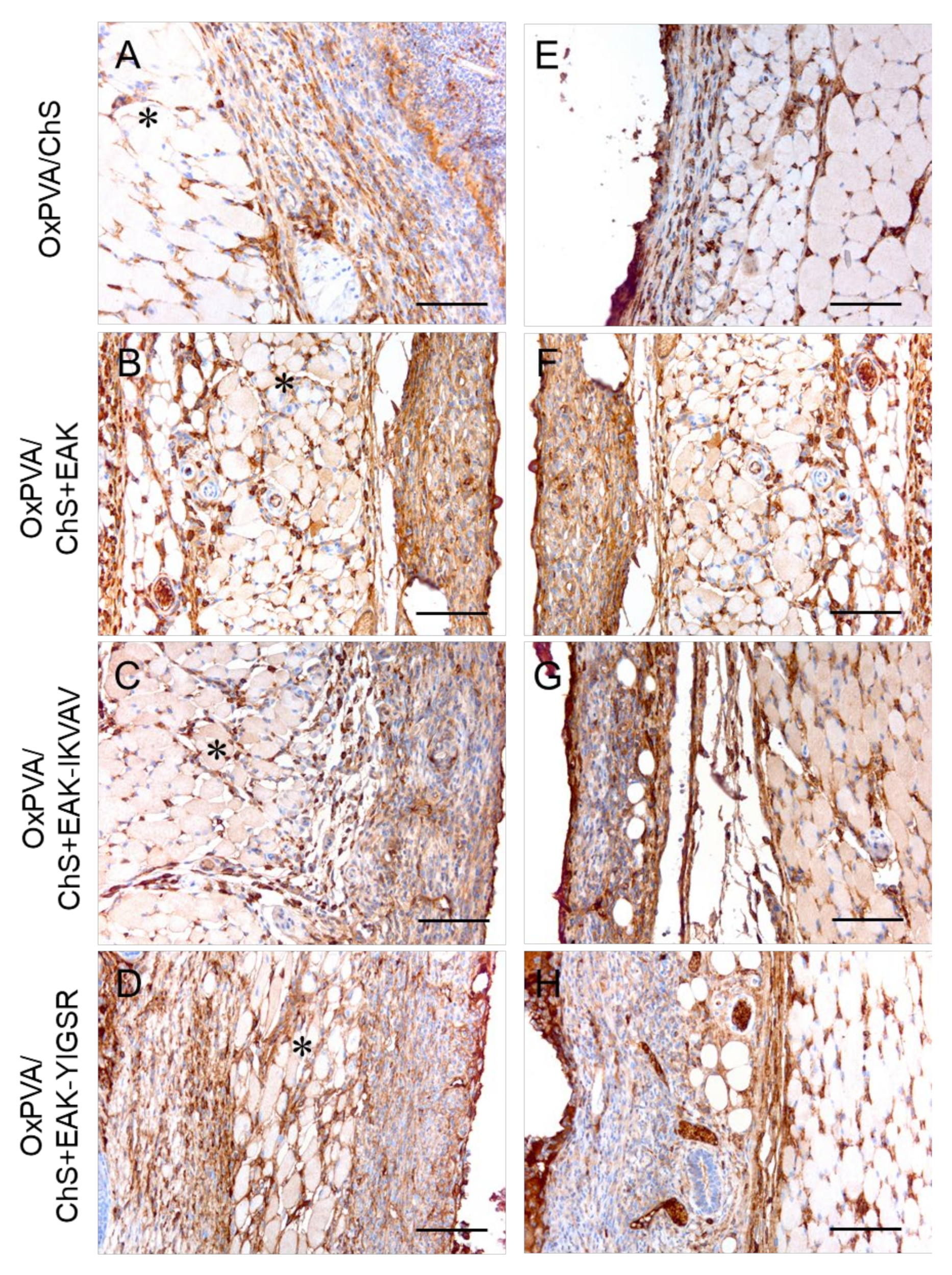
| Scaffold | E (kPa) | σmax (kPa) |
|---|---|---|
| ChS | 40.4 ± 3.1 | 16.8 ± 1.0 |
| ChS+EAK | 31.1 ± 2.1 | 11.9 ± 0.6 |
| ChS+EAK-IKVAV | 30.9 ± 1.9 | 7.3 ± 0.4 |
| ChS+EAK-YIGSR | 30.6 ± 2.0 | 7.2 ± 0.4 |
| Pairwise Comparisons | E—Remarks | σmax—Remarks |
|---|---|---|
| ChS vs. ChS+EAK | ** p < 0.01 | ** p < 0.01 |
| ChS vs. ChS+EAK-IKVAV | ** p < 0.01 | ** p < 0.01 |
| ChS vs. ChS+EAK-YIGSR | ** p < 0.01 | ** p < 0.01 |
| ChS+EAK vs. ChS+EAK-IKVAV | Not Significant | ** p < 0.01 |
| ChS+EAK vs. ChS+EAK-YIGR | Not Significant | ** p < 0.01 |
| ChS+EAK-IKVAV vs. ChS+EAK-YIGSR | Not Significant | Not Significant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stocco, E.; Barbon, S.; Zeni, E.; Cassari, L.; Zamuner, A.; Gloria, A.; Russo, T.; Boscolo-Berto, R.; Sfriso, M.M.; Macchi, V.; et al. Development of Two-Layer Hybrid Scaffolds Based on Oxidized Polyvinyl Alcohol and Bioactivated Chitosan Sponges for Tissue Engineering Purposes. Int. J. Mol. Sci. 2022, 23, 12059. https://doi.org/10.3390/ijms232012059
Stocco E, Barbon S, Zeni E, Cassari L, Zamuner A, Gloria A, Russo T, Boscolo-Berto R, Sfriso MM, Macchi V, et al. Development of Two-Layer Hybrid Scaffolds Based on Oxidized Polyvinyl Alcohol and Bioactivated Chitosan Sponges for Tissue Engineering Purposes. International Journal of Molecular Sciences. 2022; 23(20):12059. https://doi.org/10.3390/ijms232012059
Chicago/Turabian StyleStocco, Elena, Silvia Barbon, Elena Zeni, Leonardo Cassari, Annj Zamuner, Antonio Gloria, Teresa Russo, Rafael Boscolo-Berto, Maria Martina Sfriso, Veronica Macchi, and et al. 2022. "Development of Two-Layer Hybrid Scaffolds Based on Oxidized Polyvinyl Alcohol and Bioactivated Chitosan Sponges for Tissue Engineering Purposes" International Journal of Molecular Sciences 23, no. 20: 12059. https://doi.org/10.3390/ijms232012059
APA StyleStocco, E., Barbon, S., Zeni, E., Cassari, L., Zamuner, A., Gloria, A., Russo, T., Boscolo-Berto, R., Sfriso, M. M., Macchi, V., De Caro, R., Dettin, M., & Porzionato, A. (2022). Development of Two-Layer Hybrid Scaffolds Based on Oxidized Polyvinyl Alcohol and Bioactivated Chitosan Sponges for Tissue Engineering Purposes. International Journal of Molecular Sciences, 23(20), 12059. https://doi.org/10.3390/ijms232012059
















