Photochemical Synthesis of Silver Hydrosol Stabilized by Carbonate Ions and Study of Its Bactericidal Impact on Escherichia coli: Direct and Indirect Effects
Abstract
1. Introduction
2. Results
2.1. Characterization of Silver Nanoparticles
2.2. Stability of Silver Hydrosol in Culture Media
2.3. Antibacterial (Toxic) Action of Silver on the Bacterial Cells of Escherichia coli
2.3.1. Inhibition of Growth of Escherichia coli Bacterial Cells
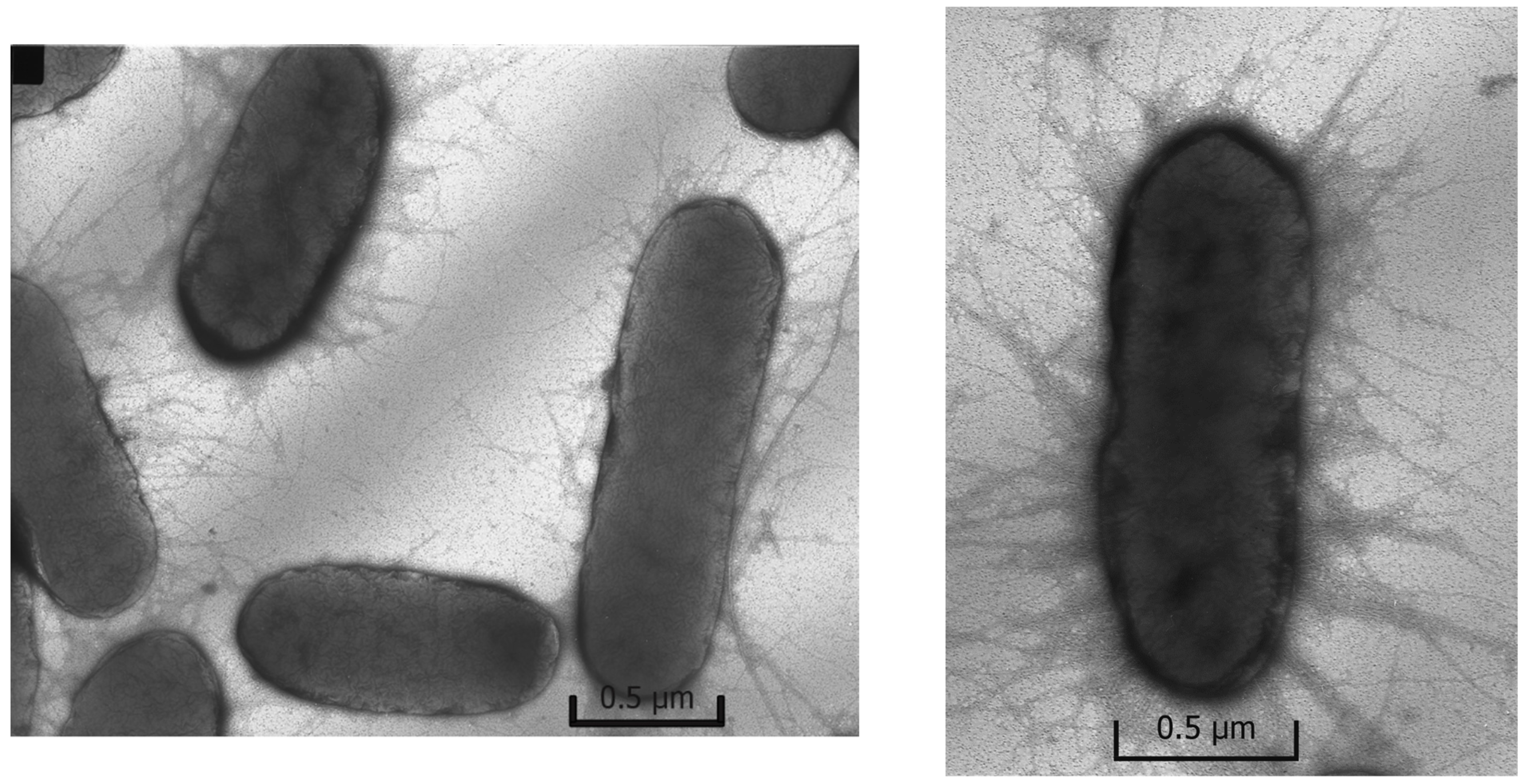
2.3.2. Determination of Morphology of the Escherichia coli Cells
Control
Ions
Nanoparticles
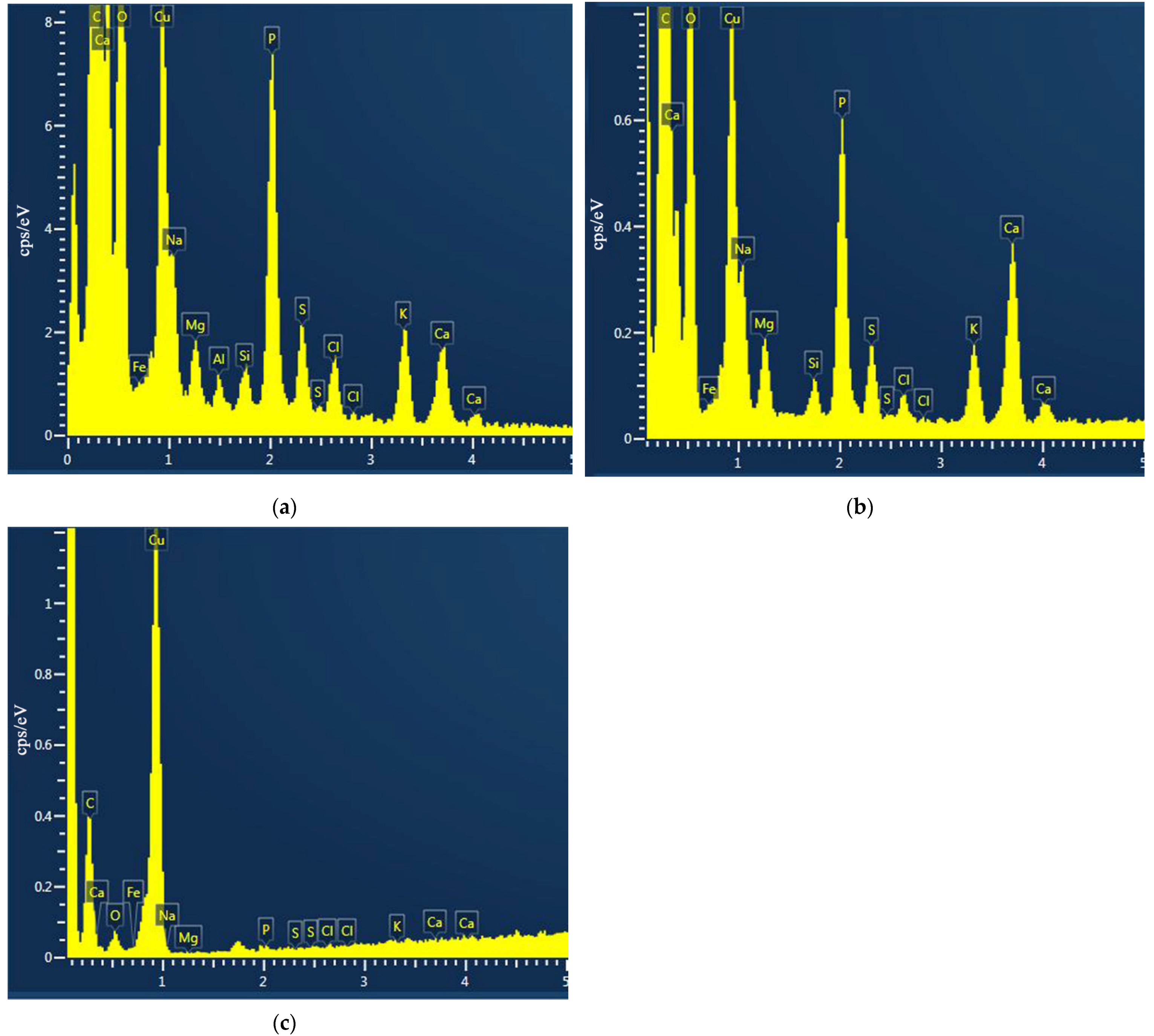
2.3.3. Change in the Cytoplasm Composition
2.4. Direct (Contact) and Indirect Mechanism of Action of Silver Nanoparticles on Escherichia coli
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Silver Nanoparticles
3.3. Physico-Chemical Characterization of Silver Nanoparticles
3.4. Determination of Dissolved Silver Ions
3.5. Bacterial Strains and Cultivation of Bacteria
3.6. Inhibition of the Bacterial Cell Growth
3.7. Effect of AgNPs on the E. coli Investigated by TEM and EDS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gunawan, C.; Marquis, C.P.; Amal, R.; Sotiriou, G.A.; Rice, S.A.; Harry, E.J. Widespread and Indiscriminate Nanosilver Use: Genuine Potential for Microbial Resistance. ACS Nano 2017, 11, 3438–3445. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.C.; Nowack, B. Exposure Modeling of Engineered Nanoparticles in the Environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef] [PubMed]
- Mafuné, F.; Kohno, J.; Takeda, Y.; Kondow, T.; Sawabe, H. Structure and Stability of Silver Nanoparticles in Aqueous Solution Produced by Laser Ablation. J. Phys. Chem. B 2000, 104, 8333–8337. [Google Scholar] [CrossRef]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic Gold Nanoparticles: Synthesis, Properties, Applications, and Toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef]
- Sidorowicz, A.; Szymański, T.; Rybka, J.D. Photodegradation of Biohazardous Dye Brilliant Blue R Using Organometallic Silver Nanoparticles Synthesized through a Green Chemistry Method. Biology 2021, 10, 784. [Google Scholar] [CrossRef]
- Maqbool, Q.; Barucca, G.; Sabbatini, S.; Parlapiano, M.; Ruello, M.L.; Tittarelli, F. Transformation of industrial and organic waste into titanium doped activated carbon—Cellulose nanocomposite for rapid removal of organic pollutants. J. Hazard. Mater. 2022, 423, 126958. [Google Scholar] [CrossRef]
- Abramenko, N.B.; Demidova, T.B.; Abkhalimov, E.V.; Ershov, B.G.; Krysanov, E.Y.; Kustov, L.M. Ecotoxicity of different-shaped silver nanoparticles: Case of zebrafish embryos. J. Hazard. Mater. 2018, 347, 89–94. [Google Scholar] [CrossRef]
- Raza, M.; Kanwal, Z.; Rauf, A.; Sabri, A.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Silver nanoparticles: Partial oxidation and antibacterial activities. JBIC J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Borowik, A.; Butowska, K.; Konkel, K.; Banasiuk, R.; Derewonko, N.; Wyrzykowski, D.; Davydenko, M.; Cherepanov, V.; Styopkin, V.; Prylutskyy, Y.; et al. The Impact of Surface Functionalization on the Biophysical Properties of Silver Nanoparticles. Nanomaterials 2019, 9, 973. [Google Scholar] [CrossRef]
- Rogowska, A.; Rafińska, K.; Pomastowski, P.; Walczak, J.; Railean-Plugaru, V.; Buszewska-Forajta, M.; Buszewski, B. Silver nanoparticles functionalized with ampicillin. Electrophoresis 2017, 38, 2757–2764. [Google Scholar] [CrossRef]
- Sooresh, A.; Kwon, H.; Taylor, R.; Pietrantonio, P.; Pine, M.; Sayes, C.M. Surface Functionalization of Silver Nanoparticles: Novel Applications for Insect Vector Control. ACS Appl. Mater. Interfaces 2011, 3, 3779–3787. [Google Scholar] [CrossRef]
- Di Giulio, M.; Di Bartolomeo, S.; Di Campli, E.; Sancilio, S.; Marsich, E.; Travan, A.; Cataldi, A.; Cellini, L. The Effect of a Silver Nanoparticle Polysaccharide System on Streptococcal and Saliva-Derived Biofilms. Int. J. Mol. Sci. 2013, 14, 13615–13625. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Proteomic Analysis of the Mode of Antibacterial Action of Silver Nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef]
- Vishnupriya, S.; Chaudhari, K.; Jagannathan, R.; Pradeep, T. Single-Cell Investigations of Silver Nanoparticle-Bacteria Interactions. Part. Part. Syst. Charact. 2013, 30, 1056–1062. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, H.-S.; Ryu, D.-S.; Choi, S.-J.; Lee, D.-S. Antibacterial Activity of Silver-nanoparticles Against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Abkhalimov, E.V.; Ershov, V.A.; Ershov, B.G. “Pure” silver hydrosol: Nanoparticles and stabilizing carbonate ions. J. Nanopart. Res. 2019, 21, 93. [Google Scholar] [CrossRef]
- Ershov, V.; Tarasova, N.; Ershov, B. Evolution of Electronic State and Properties of Silver Nanoparticles during Their Formation in Aqueous Solution. Int. J. Mol. Sci. 2021, 22, 10673. [Google Scholar] [CrossRef] [PubMed]
- Ershov, V.A.; Tarasova, N.P.; Ershov, B.G. Electronic State of Silver Nanoparticles during Their Photochemical Formation in a Deaerated Aqueous Solution. Dokl. Chem. 2020, 495, 171–174. [Google Scholar] [CrossRef]
- Abkhalimov, E.V.; Ershov, V.A.; Ershov, B.G. An aqueous colloidal silver solution stabilized with carbonate ions. Colloid J. 2017, 79. [Google Scholar] [CrossRef]
- Wardman, P. Reduction Potentials of One-Electron Couples Involving Free Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef]
- Kerker, M. The Scattering of Light and Other Electromagnetic Radiation, 1st ed.; Loebl, E.M., Ed.; Academic Press: New York, NY, USA, 1969; ISBN 9780124045507. [Google Scholar]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 1995; Volume 25, ISBN 978-3-642-08191-0. [Google Scholar]
- Kuzma, A.; Weis, M.; Flickyngerova, S.; Jakabovic, J.; Satka, A.; Dobrocka, E.; Chlpik, J.; Cirak, J.; Donoval, M.; Telek, P.; et al. Influence of surface oxidation on plasmon resonance in monolayer of gold and silver nanoparticles. J. Appl. Phys. 2012, 112, 103531. [Google Scholar] [CrossRef]
- Hedberg, J.; Blomberg, E.; Odnevall Wallinder, I. In the Search for Nanospecific Effects of Dissolution of Metallic Nanoparticles at Freshwater-Like Conditions: A Critical Review. Environ. Sci. Technol. 2019, 53, 4030–4044. [Google Scholar] [CrossRef]
- Liu, J.; Hurt, R.H. Ion Release Kinetics and Particle Persistence in Aqueous Nano-Silver Colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef]
- Ma, R.; Levard, C.; Marinakos, S.M.; Cheng, Y.; Liu, J.; Michel, F.M.; Brown, G.E.; Lowry, G.V. Size-Controlled Dissolution of Organic-Coated Silver Nanoparticles. Environ. Sci. Technol. 2012, 46, 752–759. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Ranville, J.F.; Bednar, A.; Kazor, K.; Hering, A.S.; Higgins, C.P. Tracking dissolution of silver nanoparticles at environmentally relevant concentrations in laboratory, natural, and processed waters using single particle ICP-MS (spICP-MS). Environ. Sci. Nano 2014, 1, 248–259. [Google Scholar] [CrossRef]
- Peretyazhko, T.S.; Zhang, Q.; Colvin, V.L. Size-Controlled Dissolution of Silver Nanoparticles at Neutral and Acidic pH Conditions: Kinetics and Size Changes. Environ. Sci. Technol. 2014, 48, 11954–11961. [Google Scholar] [CrossRef]
- Vanysek, P. Electrochemical series. In Handbook of Chemistry and Physics; Hayen, W.M., Bruno, T.J., Lide, D.R., Eds.; CRC-Press: Boca Raton, FL, USA, 2014; pp. 5-80–5-90. ISBN 978-1-4822-0868-9. [Google Scholar]
- Sotiriou, G.A.; Meyer, A.; Knijnenburg, J.T.N.; Panke, S.; Pratsinis, S.E. Quantifying the Origin of Released Ag+ Ions from Nanosilver. Langmuir 2012, 28, 15929–15936. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chemie Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Garner, K.L.; Suh, S.; Keller, A.A. Assessing the Risk of Engineered Nanomaterials in the Environment: Development and Application of the nanoFate Model. Environ. Sci. Technol. 2017, 51, 5541–5551. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.F.; Sørensen, S.N.; Skjolding, L.M.; Hartmann, N.B.; Baun, A. Revising REACH guidance on information requirements and chemical safety assessment for engineered nanomaterials for aquatic ecotoxicity endpoints: Recommendations from the EnvNano project. Environ. Sci. Eur. 2017, 29, 14. [Google Scholar] [CrossRef] [PubMed]
- Klaessig, F.C. Dissolution as a paradigm in regulating nanomaterials. Environ. Sci. Nano 2018, 5, 1070–1077. [Google Scholar] [CrossRef]
- Dale, A.L.; Casman, E.A.; Lowry, G.V.; Lead, J.R.; Viparelli, E.; Baalousha, M. Modeling Nanomaterial Environmental Fate in Aquatic Systems. Environ. Sci. Technol. 2015, 49, 2587–2593. [Google Scholar] [CrossRef]
- Yang, X.; Gondikas, A.P.; Marinakos, S.M.; Auffan, M.; Liu, J.; Hsu-Kim, H.; Meyer, J.N. Mechanism of Silver Nanoparticle Toxicity Is Dependent on Dissolved Silver and Surface Coating in Caenorhabditis elegans. Environ. Sci. Technol. 2012, 46, 1119–1127. [Google Scholar] [CrossRef]
- Xiu, Z.; Zhang, Q.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Quinteros, M.A.; Cano Aristizábal, V.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. Vitr. 2016, 36, 216–223. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Deng, K.K.; Kim, N.-J.; Ross, L.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef] [PubMed]
- Alshareef, A.; Laird, K.; Cross, R.B.M. Shape-dependent antibacterial activity of silver nanoparticles on Escherichia coli and Enterococcus faecium bacterium. Appl. Surf. Sci. 2017, 424, 310–315. [Google Scholar] [CrossRef]
- Martínez-Castañón, G.A.; Niño-Martínez, N.; Martínez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanopart. Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Gouyau, J.; Duval, R.E.; Boudier, A.; Lamouroux, E. Investigation of Nanoparticle Metallic Core Antibacterial Activity: Gold and Silver Nanoparticles against Escherichia coli and Staphylococcus aureus. Int. J. Mol. Sci. 2021, 22, 1905. [Google Scholar] [CrossRef]
- Long, Y.-M.; Hu, L.-G.; Yan, X.-T.; Zhao, X.-C.; Zhou, Q.-F.; Cai, Y.; Jiang, G.-B. Surface ligand controls silver ion release of nanosilver and its antibacterial activity against Escherichia coli. Int. J. Nanomed. 2017, 12, 3193–3206. [Google Scholar] [CrossRef]
- Jain, J.; Arora, S.; Rajwade, J.M.; Omray, P.; Khandelwal, S.; Paknikar, K.M. Silver Nanoparticles in Therapeutics: Development of an Antimicrobial Gel Formulation for Topical Use. Mol. Pharm. 2009, 6, 1388–1401. [Google Scholar] [CrossRef]
- Kim, S.W.; Baek, Y.-W.; An, Y.-J. Assay-dependent effect of silver nanoparticles to Escherichia coli and Bacillus subtilis. Appl. Microbiol. Biotechnol. 2011, 92, 1045–1052. [Google Scholar] [CrossRef]
- Suresh, A.K.; Doktycz, M.J.; Wang, W.; Moon, J.-W.; Gu, B.; Meyer, H.M.; Hensley, D.K.; Allison, D.P.; Phelps, T.J.; Pelletier, D.A. Monodispersed biocompatible silver sulfide nanoparticles: Facile extracellular biosynthesis using the γ-proteobacterium, Shewanella oneidensis. Acta Biomater. 2011, 7, 4253–4258. [Google Scholar] [CrossRef]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2, 6981. [Google Scholar] [CrossRef]
- Krce, L.; Šprung, M.; Maravić, A.; Umek, P.; Salamon, K.; Krstulović, N.; Aviani, I. Bacteria Exposed to Silver Nanoparticles Synthesized by Laser Ablation in Water: Modelling E. coli Growth and Inactivation. Materials 2020, 13, 653. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, K.-J.; Lee, D.G. A novel mechanism for the antibacterial effect of silver nanoparticles on Escherichia coli. BioMetals 2014, 27, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.-L.; Capjak, I.; Vrček, I.V.; Bondarenko, O.M.; Kurvet, I.; Vija, H.; Ivask, A.; Kasemets, K.; Kahru, A. Antimicrobial potency of differently coated 10 and 50 nm silver nanoparticles against clinically relevant bacteria Escherichia coli and Staphylococcus aureus. Colloids Surf. B Biointerfaces 2018, 170, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Imamura, R.; Brown, G.A.; Krishnamurthi, V.R.; Niyonshuti, I.I.; Marcelle, T.; Mathurin, L.E.; Chen, J.; Wang, Y. An experiment-based model quantifying antimicrobial activity of silver nanoparticles on Escherichia coli. RSC Adv. 2017, 7, 56173–56182. [Google Scholar] [CrossRef]
- Méndez-Pfeiffer, P.A.; Soto Urzúa, L.; Sánchez-Mora, E.; González, A.L.; Romo-Herrera, J.M.; Gervacio Arciniega, J.J.; Martínez Morales, L.J. Damage on Escherichia coli and Staphylococcus aureus using white light photoactivation of Au and Ag nanoparticles. J. Appl. Phys. 2019, 125, 213102. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-dependent antimicrobial activities of silver nanoparticles. Int. J. Nanomed. 2019, 14, 2773–2780. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Quinteros, M.A.; Aiassa Martínez, I.M.; Dalmasso, P.R.; Páez, P.L. Silver Nanoparticles: Biosynthesis Using an ATCC Reference Strain of Pseudomonas aeruginosa and Activity as Broad Spectrum Clinical Antibacterial Agents. Int. J. Biomater. 2016, 2016, 5971047. [Google Scholar] [CrossRef]
- Gambino, M.; Cappitelli, F. Mini-review: Biofilm responses to oxidative stress. Biofouling 2016, 32, 167–178. [Google Scholar] [CrossRef]
- Quinteros, M.A.; Viviana, C.A.; Onnainty, R.; Mary, V.S.; Theumer, M.G.; Granero, G.E.; Paraje, M.G.; Páez, P.L. Biosynthesized silver nanoparticles: Decoding their mechanism of action in Staphylococcus aureus and Escherichia coli. Int. J. Biochem. Cell Biol. 2018, 104, 87–93. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. Silver in Healthcare: Its Antimicrobial Efficacy and Safety in Use; Royal Society of Chemistry: London, UK, 2010; ISBN 9781849731799. [Google Scholar]
- Nowack, B.; Krug, H.F.; Height, M. 120 Years of Nanosilver History: Implications for Policy Makers. Environ. Sci. Technol. 2011, 45, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.M.; Badireddy, A.R.; Ryde, I.T.; Seidler, F.J.; Slotkin, T.A. Silver Nanoparticles Compromise Neurodevelopment in PC12 Cells: Critical Contributions of Silver Ion, Particle Size, Coating, and Composition. Environ. Health Perspect. 2011, 119, 37–44. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Weinstein, J.; Bielski, B.H.J. Kinetics of the interaction of perhydroxyl and superoxide radicals with hydrogen peroxide. The Haber-Weiss reaction. J. Am. Chem. Soc. 1979, 101, 58–62. [Google Scholar] [CrossRef]
- Bielski, B.H.J. Reevaluation of the spectral and kinetic properties of HO2 and O2-free radicals. Photochem. Photobiol. 1978, 28, 645–649. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Butler, J.; van Leeuwen, J.W. The Haber-Weiss cycle. Photochem. Photobiol. 1978, 28, 655–658. [Google Scholar] [CrossRef]
- Ferradini, C.; Foos, J.; Houee, C.; Pucheault, J. The reaction between superoxide anion and hydrogen peroxide. Photochem. Photobiol. 1978, 28, 697–700. [Google Scholar] [CrossRef]
- Schmidt, K.H. Electrical conductivity techniques for studying the kinetics of radiation-induced chemical reactions in aqueous solutions. Int. J. Radiat. Phys. Chem. 1972, 4, 439–468. [Google Scholar] [CrossRef]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique Cellular Interaction of Silver Nanoparticles: Size-Dependent Generation of Reactive Oxygen Species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, Y.; Wang, C.; Li, H.C.; Wang, T.; Liao, C.Y.; Cui, L.; Zhou, Q.F.; Yan, B.; Jiang, G.B. Impact of silver nanoparticles on human cells: Effect of particle size. Nanotoxicology 2010, 4, 319–330. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Jin, T.; Behari, J. Dose-dependent in-vivo toxicity assessment of silver nanoparticle in Wistar rats. Toxicol. Mech. Methods 2011, 21, 13–24. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Valodkar, M.; Jadeja, R.N.; Thounaojam, M.C.; Devkar, R.V.; Thakore, S. In vitro toxicity study of plant latex capped silver nanoparticles in human lung carcinoma cells. Mater. Sci. Eng. C 2011, 31, 1723–1728. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Dang, D.A.; Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch. Toxicol. 2011, 85, 743–750. [Google Scholar] [CrossRef]
- Piao, M.J.; Kim, K.C.; Choi, J.-Y.; Choi, J.; Hyun, J.W. Silver nanoparticles down-regulate Nrf2-mediated 8-oxoguanine DNA glycosylase 1 through inactivation of extracellular regulated kinase and protein kinase B in human Chang liver cells. Toxicol. Lett. 2011, 207, 143–148. [Google Scholar] [CrossRef]
- He, W.; Zhou, Y.-T.; Wamer, W.G.; Boudreau, M.D.; Yin, J.-J. Mechanisms of the pH dependent generation of hydroxyl radicals and oxygen induced by Ag nanoparticles. Biomaterials 2012, 33, 7547–7555. [Google Scholar] [CrossRef]
- Ivask, A.; ElBadawy, A.; Kaweeteerawat, C.; Boren, D.; Fischer, H.; Ji, Z.; Chang, C.H.; Liu, R.; Tolaymat, T.; Telesca, D.; et al. Toxicity Mechanisms in Escherichia coli Vary for Silver Nanoparticles and Differ from Ionic Silver. ACS Nano 2014, 8, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Leonov, K.; Astashkina, A.; Bakibayev, A. Gas Chromatographic Investigation of Oil Biodegradation Degree. Procedia Chem. 2014, 10, 504–507. [Google Scholar] [CrossRef][Green Version]
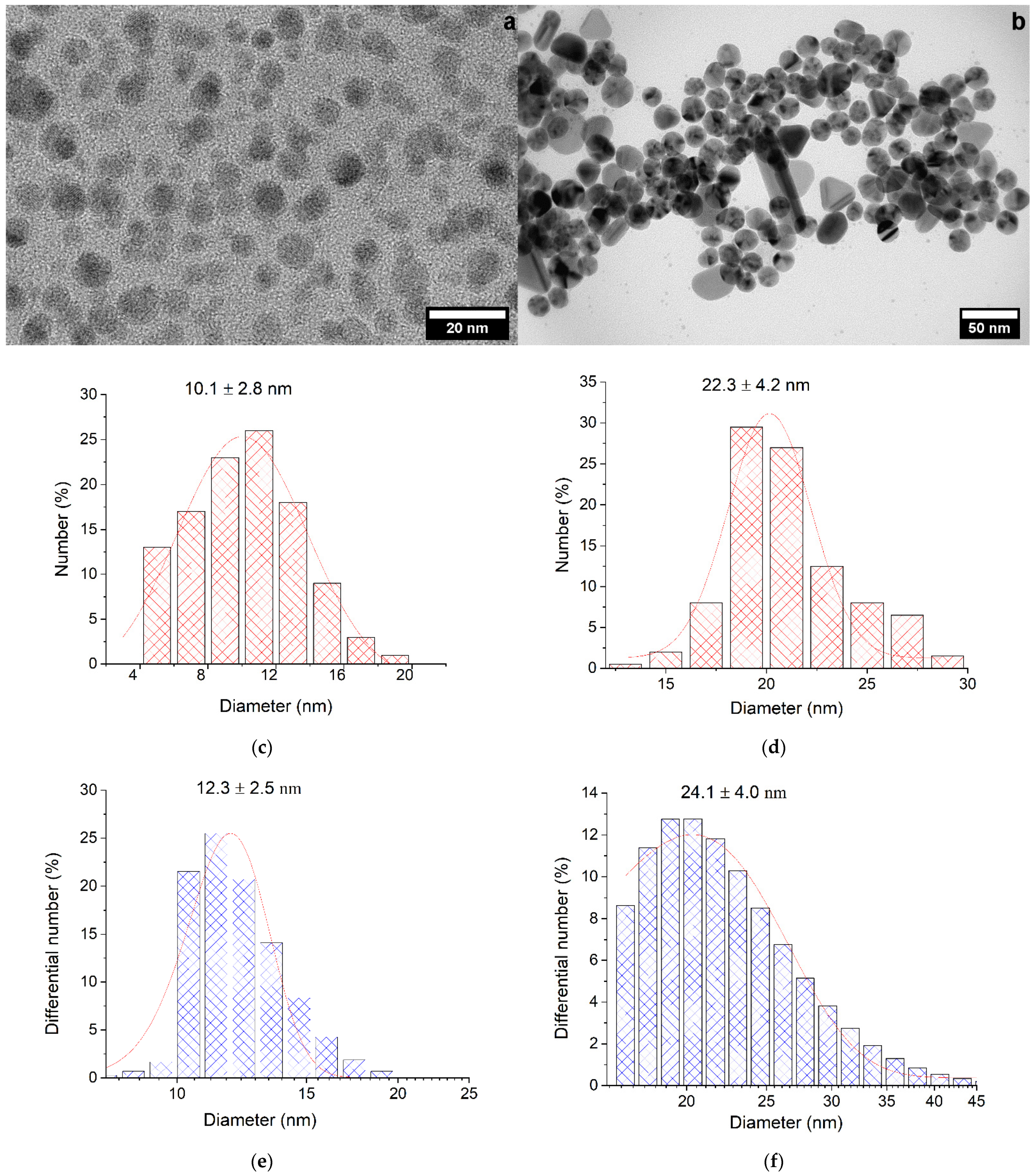
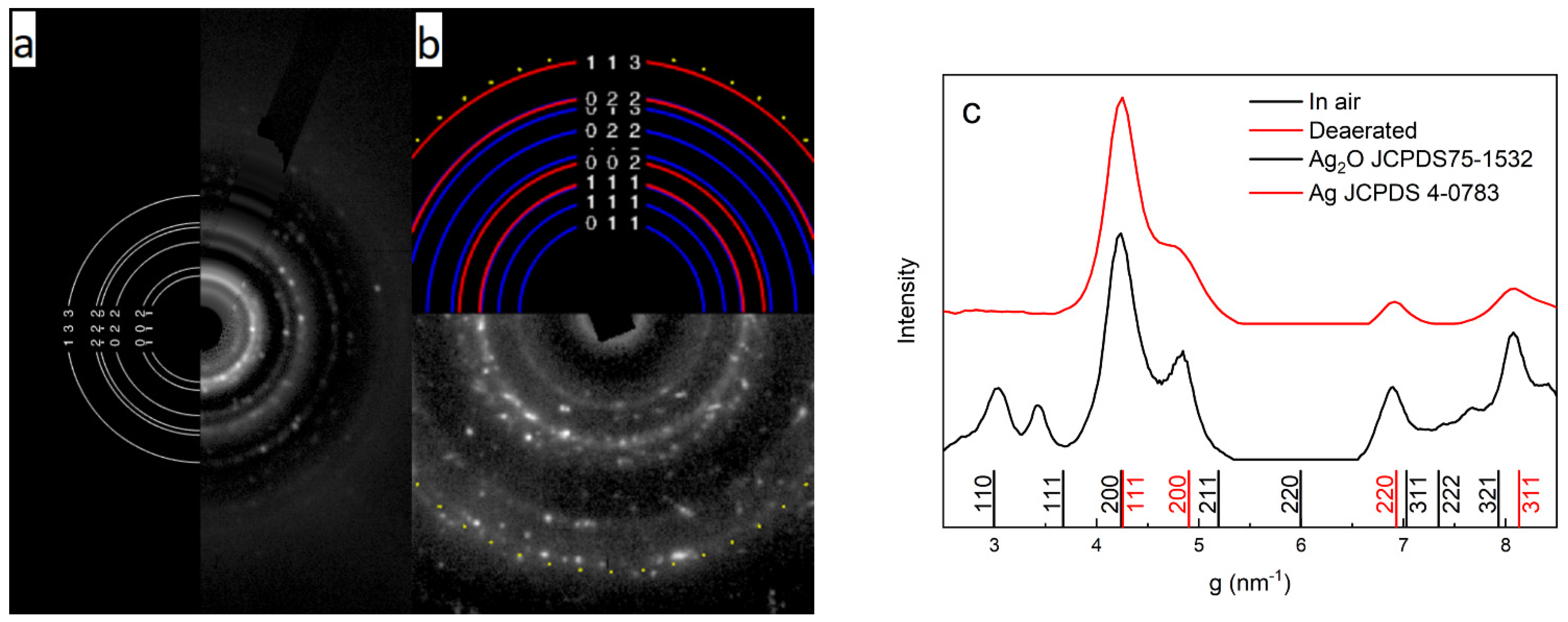

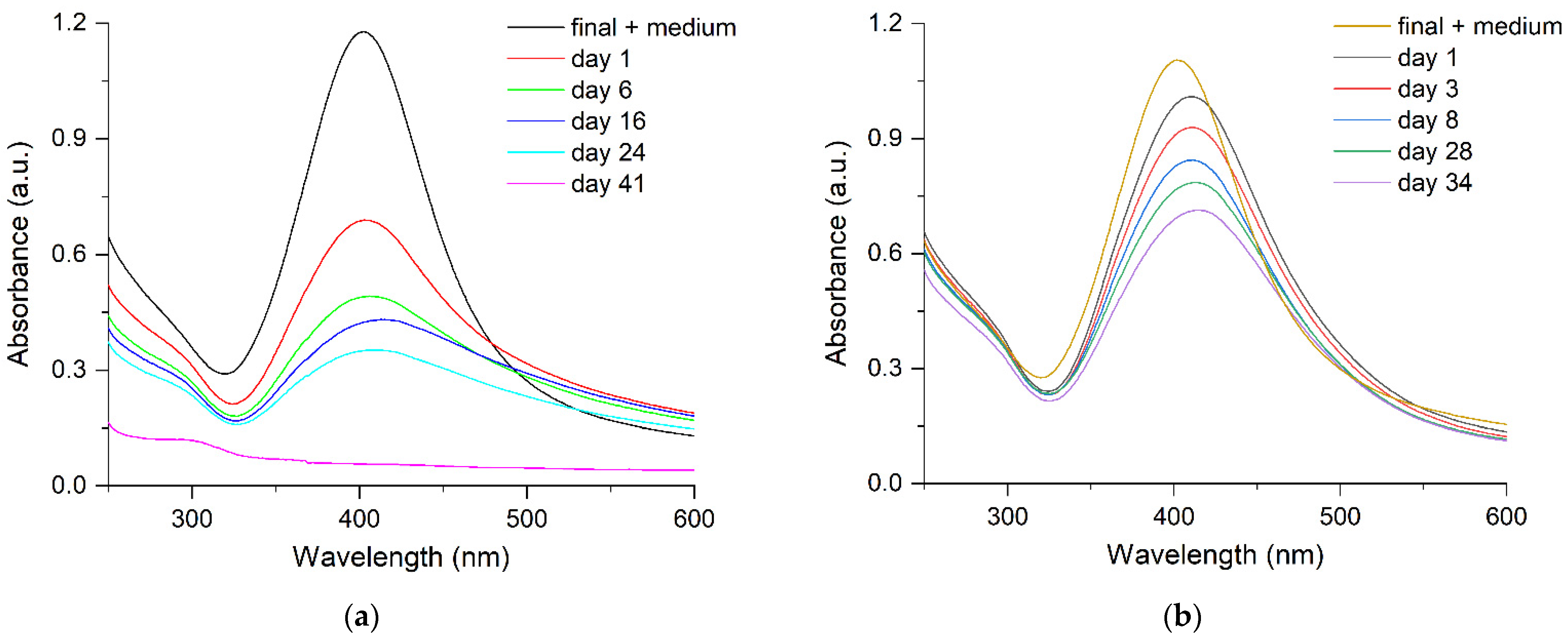

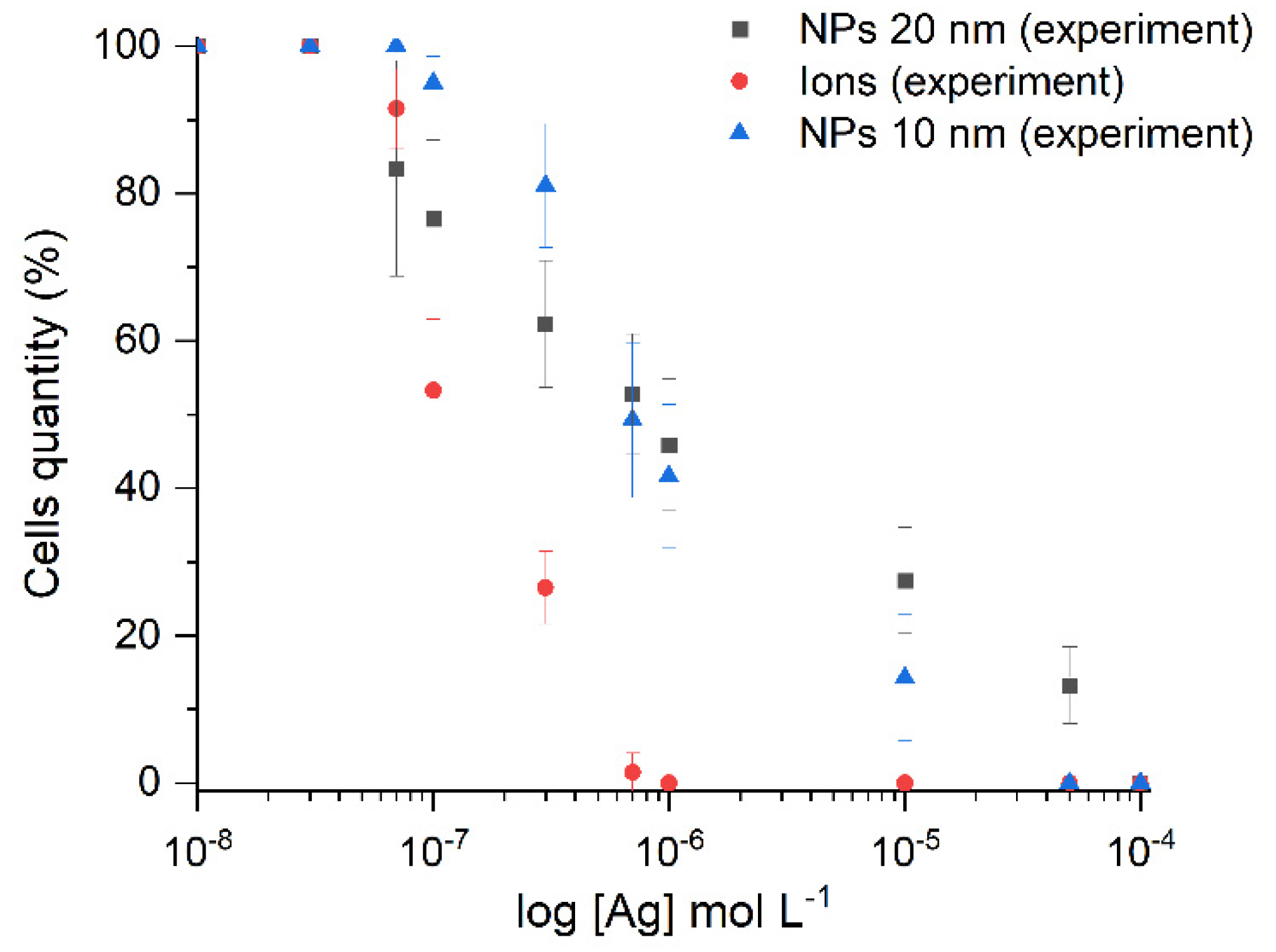


| Nanoparticle | λmax, nm | W1/2, nm | dTEM, nm | dDLS, nm | ζ, mV | Hydrosol Stability, τ1/2, Months |
|---|---|---|---|---|---|---|
| Ag-NP | 386 | 35 | 10.1 ± 2.8 | 12.3 ± 2.5 | −68.3 | 2–3 |
| Ag-Ag2O-NP | 408 | 86 | 22.3 ± 4.2 | 24.1 ± 4.0 | −67.4 | >6 |
| Type | Size, nm | Surface Functionalization | Effect | Medium | Reference |
|---|---|---|---|---|---|
| Ag+ | - | - | MIC = 0.3 mg L−1, IC50 = 0.03 mg L−1 | Adkins M | This work |
| Ag-Ag2O nanoparticles | 22 ± 3 | Carbonate | MIC = 11 mg L−1, IC50 = 0.11 mg L−1 | Adkins M | This work |
| Ag nanoparticles | 10 ± 2 | Carbonate | MIC = 5.4 mg L−1, IC50 = 0.07 mg L−1 | Adkins M | This work |
| AgCl nanoparticles | 250 | Unfunctionalized | 0.5 mg L−1 inhibits bacterial growth by 66 ± 6% | BBL™ containing 5 g/L Gelysate™ peptone and 3 g/L beef extract, pH = 6.9 ± 0.2 | [46] |
| Ag nanoparticles | 14–16 | PVA | 0.5 mg L−1 inhibits bacterial growth by 55 ± 8% | BBL™ containing 5 g/L Gelysate™ peptone and 3 g/L beef extract, pH = 6.9 ± 0.2 | [46] |
| Ag+ | - | - | 0.5 mg L−1 inhibits bacterial growth by 100% | BBL™ containing 5 g/L Gelysate™ peptone and 3 g/L beef extract, pH = 6.9 ± 0.2 | [46] |
| Ag nanoparticles (octahedral) | 194 ± 50 | PVP | MIC = 50 mg L−1 | LB medium | [47] |
| Ag nanoparticles (spherical) | 195 ± 50 | Citrate | MIC not achieved, IC50 = 1000 mg L−1 | LB medium | [47] |
| Ag nanoparticles | 7 | Gallic acid | MIC = 6.25 mg L−1 | Mueller–Hinton broth | [48] |
| Ag nanoparticles | 29 | Gallic acid | MIC = 13.02 mg L−1 | Mueller–Hinton broth | [48] |
| Ag nanoparticles | 89 | Gallic acid | MIC = 11.79 mg L−1 | Mueller–Hinton broth | [48] |
| Ag nanoparticles | 12.2 | Citrate | MIC = 13.8 mg L−1 | Cation-adjusted Mueller–Hinton broth (CA-MHB) | [49] |
| Ag nanoparticles | 10.2 ± 2.3 | Citrate | IC50 = 5 mg L−1 MIC = 15 mg L−1 | 2 mM NaHCO3 | [50] |
| Ag nanoparticles | 10.2 ± 2.3 | Citrate | IC50 = 5 mg L−1 MIC = 15 mg L−1 | 2 mM NaHCO3 | [50] |
| Ag nanoparticles | 9.9 ± 2.0 | Mercaptopropionic sulfonic acid | MIC = 15 mg L−1 | 2 mM NaHCO3 | [50] |
| Ag nanoparticles | 16.6 (6.5–43.8) | Not reported | IC50 = 1.56 mg L−1 IC90 = 6.25 mg L−1 MIC = 12.5 mg L−1 | DMEM supplemented with l-glutamine (4 mM), penicillin (100 units/mL), streptomycin (100 μg/mL) and 10% (v/v) heat inactivated fetal bovine serum | [51] |
| Ag nanoparticles | 10 | Citrate | 30 mg L−1 inhibit bacterial growth; EC50 (CFU assay) = 3.2–4.2 mg L−1; EC50 (LTP assay, 0 h) > 0.25 mg L−1; EC50 (LTP assay, 1 h) = 0.06–0.09 mg L−1; EC50 (LTP assay, 2 h) < 0.025 mg L−1; | LB medium | [52] |
| Ag2S nanoparticles | 9 ± 3.5 | Unfunctionalized | Not toxic at 150 mg L−1 | RPMI medium supplemented with 0.2 mM l-glutamine, 100 μg mL−1 penicillin, 100 μg mL−1 streptomycin and 10% FBS | [53] |
| Ag+ | - | - | MIC = 3.5 mg L−1 for 103 cells; MBC = 3.5–5 mg L−1 | LB medium | [54] |
| Ag+ | - | - | MIC = 0.5–1 mg L−1 for 103 cells, MBC = 0.5–1.25 mg L−1 | RPMI/FCS | [54] |
| Ag nanoparticles | 75 ± 20 | PVP | MBC = 12.5–20 mg L−1 for 103 cells | RPMI/FCS | [54] |
| Ag nanoparticles | 11.3 (3–40) | Laser ablation | MIC = 110 ± 16 mg L−1 (microdillution assay); MIC = 73 ± 11 mg L−1 (optical density assay); | Nutritionally impoverished LB (5.0 g of tryptone, 2.5 g of yeast extract, and 5.0 g of NaCl per 1 L) | [55] |
| AgCl nanoparticles | 3 | Unfunctionalized | MIC = 2 mg L−1 | LB | [56] |
| Ag nanoparticles | 10.8 ± 4.2 | Bis-2-ethylhexyl sulfosuccinate (AOT) | MBC = 0.3 mg L−1 | LB (solid, with agar) | [57] |
| Ag nanoparticles | 10.8 ± 4.3 | Cetyltrimethyl-ammonium bromide (CTAB) | MBC = 0.2 mg L−1 | LB (solid, with agar) | [57] |
| Ag nanoparticles | 13.5 ± 7.1 | Poly-L-lysine (PLL) | MBC = 0.2 mg L−1 | LB (solid, with agar) | [57] |
| Ag nanoparticles | 15.2 ± 6.9 | Polysorbate 80 (Tween 80) | MBC = 0.5 mg L−1 | LB (solid, with agar) | [57] |
| Ag+ | - | - | IC50 = 7 mg L−1 12 h) MIC = 10 mg L−1 (12 h) | LB medium containing ampicillin (100 μg mL−1) | [58] |
| Ag nanoparticles | 39.5 ± 10.7 | PVP | IC50 = 4 mg L−1 12 h) MIC = 30.2 mg L−1 (12 h) | LB medium containing ampicillin (100 μg mL−1) | [59] |
| Ag nanoparticles | 4.65 ± 0.5 | Citrate | MIC = 5.59 mg L−1 | LB | [59] |
| Ag nanoparticles | 38.5 | PVP | MIC = 700 mg L−1 | DMEM containing 10% fetal calf serum and 1% penicillin G-streptomycin | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ershov, V.; Tarasova, N.; Abkhalimov, E.; Safonov, A.; Sorokin, V.; Ershov, B. Photochemical Synthesis of Silver Hydrosol Stabilized by Carbonate Ions and Study of Its Bactericidal Impact on Escherichia coli: Direct and Indirect Effects. Int. J. Mol. Sci. 2022, 23, 949. https://doi.org/10.3390/ijms23020949
Ershov V, Tarasova N, Abkhalimov E, Safonov A, Sorokin V, Ershov B. Photochemical Synthesis of Silver Hydrosol Stabilized by Carbonate Ions and Study of Its Bactericidal Impact on Escherichia coli: Direct and Indirect Effects. International Journal of Molecular Sciences. 2022; 23(2):949. https://doi.org/10.3390/ijms23020949
Chicago/Turabian StyleErshov, Vadim, Natalia Tarasova, Evgeny Abkhalimov, Alexey Safonov, Vladimir Sorokin, and Boris Ershov. 2022. "Photochemical Synthesis of Silver Hydrosol Stabilized by Carbonate Ions and Study of Its Bactericidal Impact on Escherichia coli: Direct and Indirect Effects" International Journal of Molecular Sciences 23, no. 2: 949. https://doi.org/10.3390/ijms23020949
APA StyleErshov, V., Tarasova, N., Abkhalimov, E., Safonov, A., Sorokin, V., & Ershov, B. (2022). Photochemical Synthesis of Silver Hydrosol Stabilized by Carbonate Ions and Study of Its Bactericidal Impact on Escherichia coli: Direct and Indirect Effects. International Journal of Molecular Sciences, 23(2), 949. https://doi.org/10.3390/ijms23020949








