Two-Dimensional Interfacial Exchange Diffusion Has the Potential to Augment Spatiotemporal Precision of Ca2+ Signaling
Abstract
1. Introduction
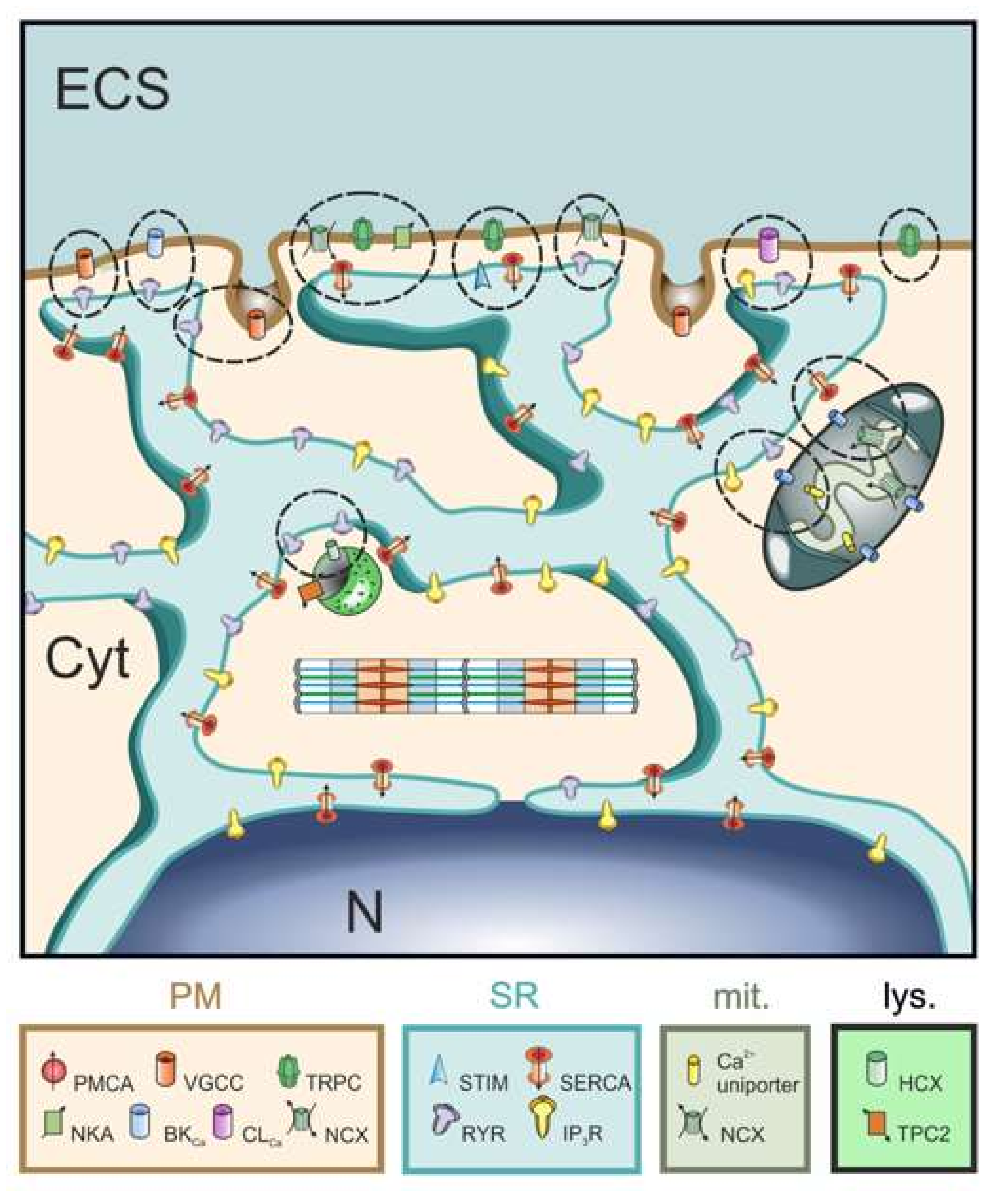
2. Background
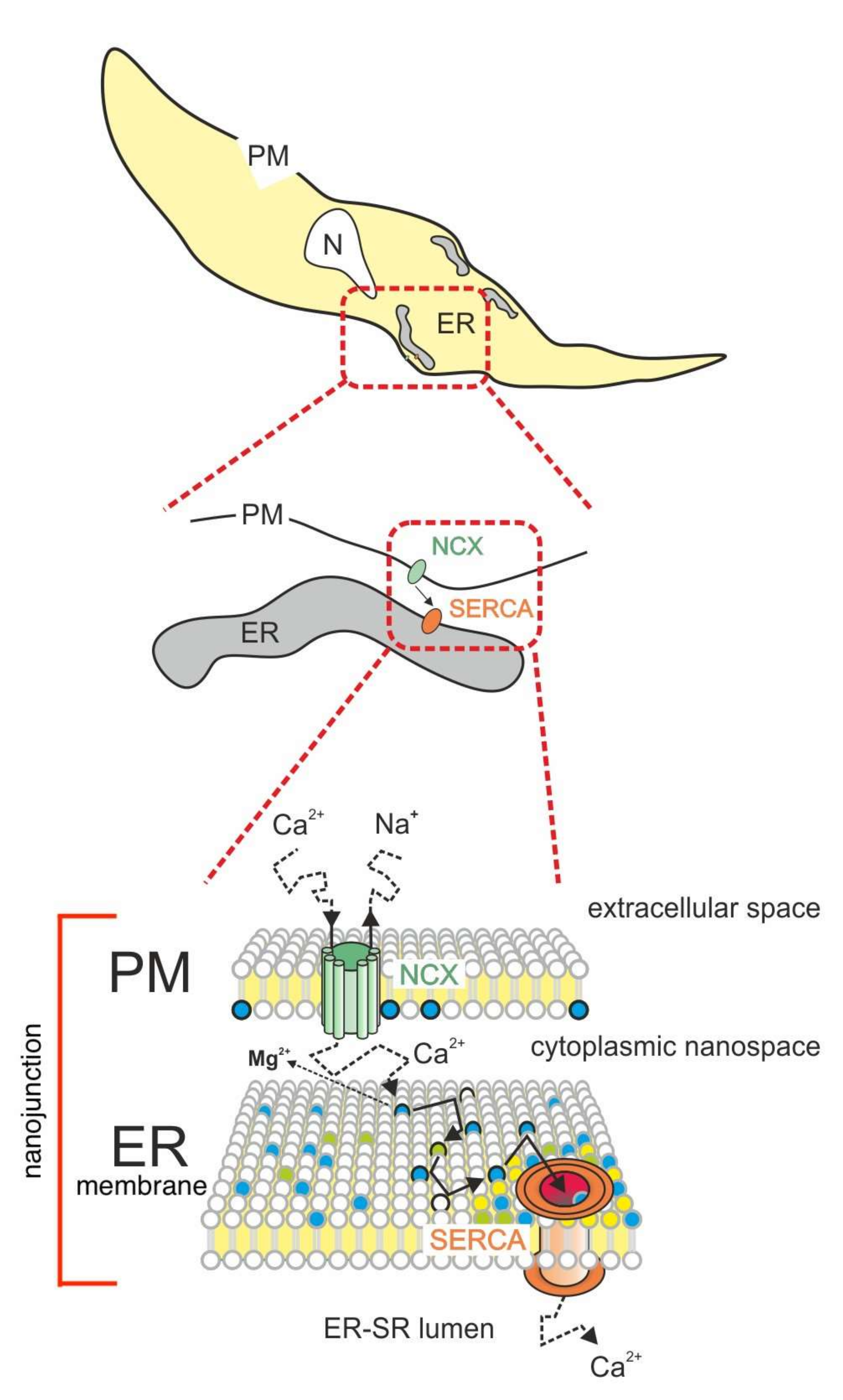
3. Model Description
4. Discussion
4.1. Rationale of the Proposed Model
4.2. Implications for Human Physiopathology
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ebashi, S.; Lipmann, F. Adenosine triphosphate-linked concentration of calcium ions in a par-ticulate fraction of rabbit muscle. J. Cell Biol. 1962, 14, 389–400. [Google Scholar] [CrossRef]
- Van Breemen, C. Blockade of Membrane Calcium Fluxes by Lanthanum in Relation to Vascular Smooth Muscle Contractility. Arch. Int. Physiol. Biochim. 1969, 77, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, C.; Fameli, N.; Evans, A.M. Pan-junctional sarcoplasmic reticulum in vascular smooth muscle: Nanospace Ca2+ transport for site- and function-specific Ca2+ signaling. J. Physiol. 2013, 591, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qian, T.; He, R.; Wan, C.; Liu, Y.; Yu, H. Endoplasmic Reticulum–Plasma Membrane Contact Sites: Regulators, Mechanisms, and Physiological Functions. Front. Cell Dev. Biol. 2021, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Muallem, S.; Chung, W.Y.; Jha, A.; Ahuja, M. Lipids at membrane contact sites: Cell signaling and ion transport. EMBO Rep. 2017, 18, 1893–1904. [Google Scholar] [CrossRef]
- Lee, C.-H.; Kuo, K.-H.; Dai, J.; Leo, J.M.; Seow, C.Y.; van Breemen, C. Calyculin-A disrupts subplasmalemmal junction and recurring Ca2+ waves in vascular smooth muscle. Cell Calcium 2005, 37, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Fameli, N.; Van Breemen, C.; Kuo, K.-H. A quantitative model for linking Na+/Ca2+ exchanger to SERCA during refilling of the sarcoplasmic reticulum to sustain [Ca2+] oscillations in vascular smooth muscle. Cell Calcium 2007, 42, 565–575. [Google Scholar] [CrossRef][Green Version]
- Pritchard, H.; Griffin, C.; Yamasaki, E.; Thakore, P.; Lane, C.; Greenstein, A.; Earley, S. Nanoscale coupling of junctophilin-2 and ryanodine receptors regulates vascular smooth muscle cell contractility. Proc. Natl. Acad. Sci. USA 2019, 116, 21874–21881. [Google Scholar] [CrossRef]
- Lee, C.-H.; Rahimian, R.; Szado, T.; Sandhu, J.; Poburko, D.; Behra, T.; Chan, L.; Van Breemen, C. Sequential opening of IP3-sensitive Ca2+channels and SOC during α-adrenergic activation of rabbit vena cava. Am. J. Physiol. Circ. Physiol. 2002, 282, H1768–H1777. [Google Scholar] [CrossRef]
- Jay, E.E.; Mallinson, P.M.; Fong, S.K.; Metcalfe, B.L.; Grimes, R.W. Divalent cation diffusion in calcium fluorapatite. J. Mater. Sci. 2011, 46, 7459–7465. [Google Scholar] [CrossRef][Green Version]
- Van Breemen, D.; Van Breemen, C. Calcium Exchange Diffusion in a Porous Phospholipid Ion-exchange Membrane. Nature 1969, 223, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.M.; Agin, D.P.; Pawlowski, R. Phospholipid-Cholesterol Membrane Model. J. Gen. Physiol. 1962, 45, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- van Breemen, C. Permselectivity of a porous phospholipid-cholesterol artificial membrane. Calcium and lanthanum effects. Biochem. Biophys. Res. Commun. 1968, 32, 977–983. [Google Scholar] [CrossRef]
- McLaughlin, S.; Brown, J. Diffusion of calcium ions in retinal rods. A theoretical calculation. J. Gen. Physiol. 1981, 77, 475–487. [Google Scholar] [CrossRef]
- Wang, D.; Schwartz, D.K. Non-Brownian Interfacial Diffusion: Flying, Hopping, and Crawling. J. Phys. Chem. C 2020, 124. [Google Scholar] [CrossRef]
- Han, K.; Gericke, A.; Pastor, R.W. Characterization of Specific Ion Effects on PI(4,5)P2 Clustering: Molecular Dynamics Simulations and Graph-Theoretic Analysis. J. Phys. Chem. B 2020, 124, 1183–1196. [Google Scholar] [CrossRef]
- Bradley, R.P.; Slochower, D.R.; Janmey, P.A.; Radhakrishnan, R. Divalent cations bind to phosphoinositides to induce ion and isomer specific propensities for nano-cluster initiation in bilayer membranes. R. Soc. Open Sci. 2020, 7, 192208. [Google Scholar] [CrossRef]
- Melcrova, A.; Pokorna, S.; Pullanchery, S.; Kohagen, M.; Jurkiewicz, P.; Hof, M.; Jungwirth, P.; Cremer, P.S.; Cwiklik, L. The complex nature of calcium cation interactions with phospholipid bilayers. Sci. Rep. 2016, 6, 38035. [Google Scholar] [CrossRef]
- Wen, Y.; Vogt, V.M.; Feigenson, G.W. Multivalent Cation-Bridged PI(4,5)P2 Clusters Form at Very Low Concentrations. Biophys. J. 2018, 114, 2630–2639. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Iwasawa, K.; Kalay, Z.; Tsunoyama, T.-A.; Watanabe, Y.; Umemura, Y.M.; Murakoshi, H.; Suzuki, K.G.N.; Nemoto, Y.L.; Morone, N.; et al. Confined diffusion of transmembrane proteins and lipids induced by the same actin meshwork lining the plasma membrane. Mol. Biol. Cell 2016, 27, 1101–1119. [Google Scholar] [CrossRef]
- Sarmento, M.J.; Coutinho, A.; Fedorov, A.; Prieto, M.; Fernandes, F. Ca2+ induces PI(4,5)P2 clusters on lipid bilayers at physiological PI(4,5)P2 and Ca2+ concentrations. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Drachmann, N.D.; Olesen, C.; Møller, J.V.; Guo, Z.; Nissen, P.; Bublitz, M. Comparing crystal structures of Ca2+-ATPase in the presence of different lipids. FEBS J. 2014, 281, 4249–4262. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, E.; Julius, D.; Cheng, Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 2016, 534, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Conrard, L.; Tyteca, D. Regulation of Membrane Calcium Transport Proteins by the Surrounding Lipid Environment. Biomolecules 2019, 9, 513. [Google Scholar] [CrossRef]
- Poveda, J.; Giudici, A.; Renart, M.; Molina, M.; Montoya, E.; Fernández-Carvajal, A.; Fernández-Ballester, G.; Encinar, J.; González-Ros, J. Lipid modulation of ion channels through specific binding sites. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 1560–1567. [Google Scholar] [CrossRef]
- Lichtenegger, M.; Tiapko, O.; Svobodova, B.; Stockner, T.; Glasnov, T.; Schreibmayer, W.; Platzer, D.; De La Cruz, G.G.; Krenn, S.; Schober, R.; et al. An optically controlled probe identifies lipid-gating fenestrations within the TRPC3 channel. Nat. Chem. Biol. 2018, 14, 396–404. [Google Scholar] [CrossRef]
- Cai, R.; Liu, X.; Zhang, R.; Hofmann, L.; Zheng, W.; Amin, R.; Wang, L.; Hu, Q.; Peng, J.-B.; Michalak, M.; et al. Autoinhibition of TRPV6 Channel and Regulation by PIP2. Iscience 2020, 23, 101444. [Google Scholar] [CrossRef] [PubMed]
- Barth, D.; Lückhoff, A.; Kühn, F. Species-Specific Regulation of TRPM2 by PI(4,5)P2 via the Membrane Interfacial Cavity. Int. J. Mol. Sci. 2021, 22, 4637. [Google Scholar] [CrossRef]
- Espinoza-Fonseca, L.M. Probing the effects of nonannular lipid binding on the stability of the calcium pump SERCA. Sci. Rep. 2019, 9, 3349. [Google Scholar] [CrossRef]
- Toyoshima, C.; Nakasako, M.; Nomura, H.; Ogawa, H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 2000, 405, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Bi, Y.; Yang, W.; Guo, X.; Jiang, Y.; Wan, C.; Li, L.; Bai, Y.; Guo, J.; Wang, Y.; et al. Ca2+ regulates T-cell receptor activation by modulating the charge property of lipids. Nat. Cell Biol. 2012, 493, 111–115. [Google Scholar] [CrossRef]
- Yoshida, T.; Taga, K.; Okabayashi, H.; Kamaya, H.; Issaku, U. Changes in surface capacitance and conductance parallel to phospholipid membranes associated with phase transition: Effects of halothane. Biochim. Biophys. Acta (BBA) Biomembr. 1989, 984, 253–256. [Google Scholar] [CrossRef]
- Navarro-Dorado, J.; García-Alonso, M.; Van Breemen, C.; Tejerina, T.; Fameli, N. Calcium oscillations in human mesenteric vascular smooth muscle. Biochem. Biophys. Res. Commun. 2014, 445, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.M.; Syyong, H.; Navarro-Dorado, J.; Redondo, S.; Alonso, M.; van Breemen, C.; Tejerina, T. A comparative study of α-adrenergic receptor mediated Ca2+ signals and contraction in intact human and mouse vascular smooth muscle. Eur. J. Pharmacol. 2010, 629, 82–88. [Google Scholar] [CrossRef]
- Piccialli, I.; Ciccone, R.; Secondo, A.; Boscia, F.; Tedeschi, V.; de Rosa, V.; Cepparulo, P.; Annunziato, L.; Pannaccione, A. The Na+/Ca2+ Exchanger 3 Is Functionally Coupled With the NaV1.6 Voltage-Gated Channel and Promotes an Endoplasmic Reticulum Ca2+ Refilling in a Transgenic Model of Alzheimer’s Disease. Front. Pharmacol. 2021, 12, 3582. [Google Scholar] [CrossRef]
- Franzini-Armstrong, C. The relationship between form and function throughout the history of excitation–contraction coupling. J. Gen. Physiol. 2018, 150, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Petersen, O.H.; Courjaret, R.; Machaca, K. Ca2+ tunnelling through the ER lumen as a mechanism for delivering Ca2+entering via store-operated Ca2+channels to specific target sites. J. Physiol. 2017, 595, 2999–3014. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Navarro-Dorado, J.; Clark, J.H.; Kinnear, N.P.; Meinke, P.; Schirmer, E.C.; Evans, A.M. The cell-wide web coordinates cellular processes by directing site-specific Ca2+ flux across cytoplasmic nanocourses. Nat. Commun. 2019, 10, 2299. [Google Scholar] [CrossRef]
- Iino, M.; Kasai, H.; Yamazawa, T. Visualization of neural control of intracellular Ca2+ concentration in single vascular smooth muscle cells in situ. EMBO J. 1994, 13, 5026–5031. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Breemen, C.; Fameli, N.; Groschner, K. Two-Dimensional Interfacial Exchange Diffusion Has the Potential to Augment Spatiotemporal Precision of Ca2+ Signaling. Int. J. Mol. Sci. 2022, 23, 850. https://doi.org/10.3390/ijms23020850
van Breemen C, Fameli N, Groschner K. Two-Dimensional Interfacial Exchange Diffusion Has the Potential to Augment Spatiotemporal Precision of Ca2+ Signaling. International Journal of Molecular Sciences. 2022; 23(2):850. https://doi.org/10.3390/ijms23020850
Chicago/Turabian Stylevan Breemen, Cornelis, Nicola Fameli, and Klaus Groschner. 2022. "Two-Dimensional Interfacial Exchange Diffusion Has the Potential to Augment Spatiotemporal Precision of Ca2+ Signaling" International Journal of Molecular Sciences 23, no. 2: 850. https://doi.org/10.3390/ijms23020850
APA Stylevan Breemen, C., Fameli, N., & Groschner, K. (2022). Two-Dimensional Interfacial Exchange Diffusion Has the Potential to Augment Spatiotemporal Precision of Ca2+ Signaling. International Journal of Molecular Sciences, 23(2), 850. https://doi.org/10.3390/ijms23020850





