Erythroid Differentiation Regulator 1 Strengthens TCR Signaling by Enhancing PLCγ1 Signal Transduction Pathway
Abstract
:1. Introduction
2. Results
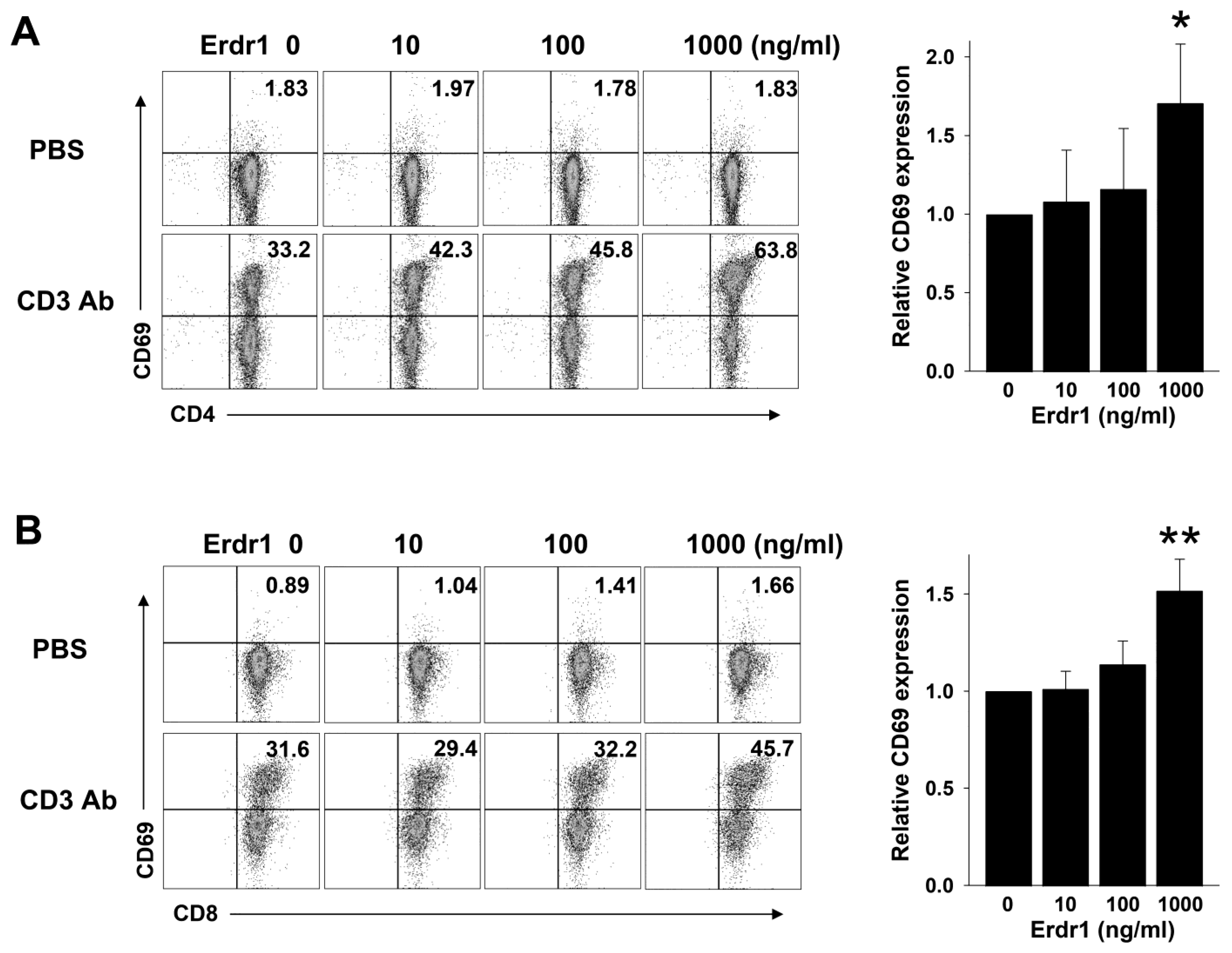
2.1. Erdr1 Enhances Activation of T Cells in the Presence of TCR Stimulation
2.2. TCR-Mediated Proliferation of CD4 T Cells Is Promoted by Erdr1
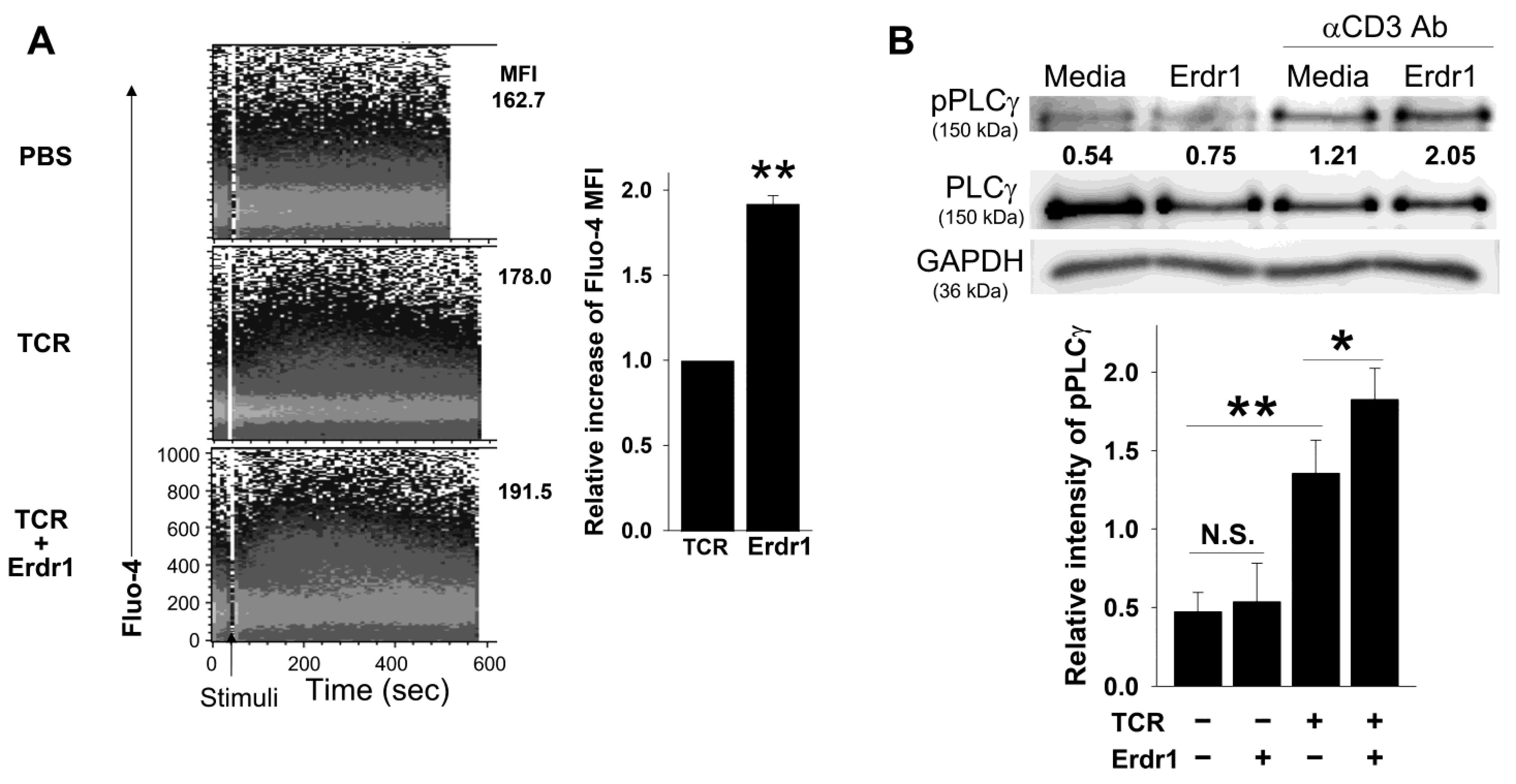
2.3. Erdr1 Strengthens TCR-Mediated Ca2+ Influx and PLCγ1 Phosphorylation in CD4 T Cells
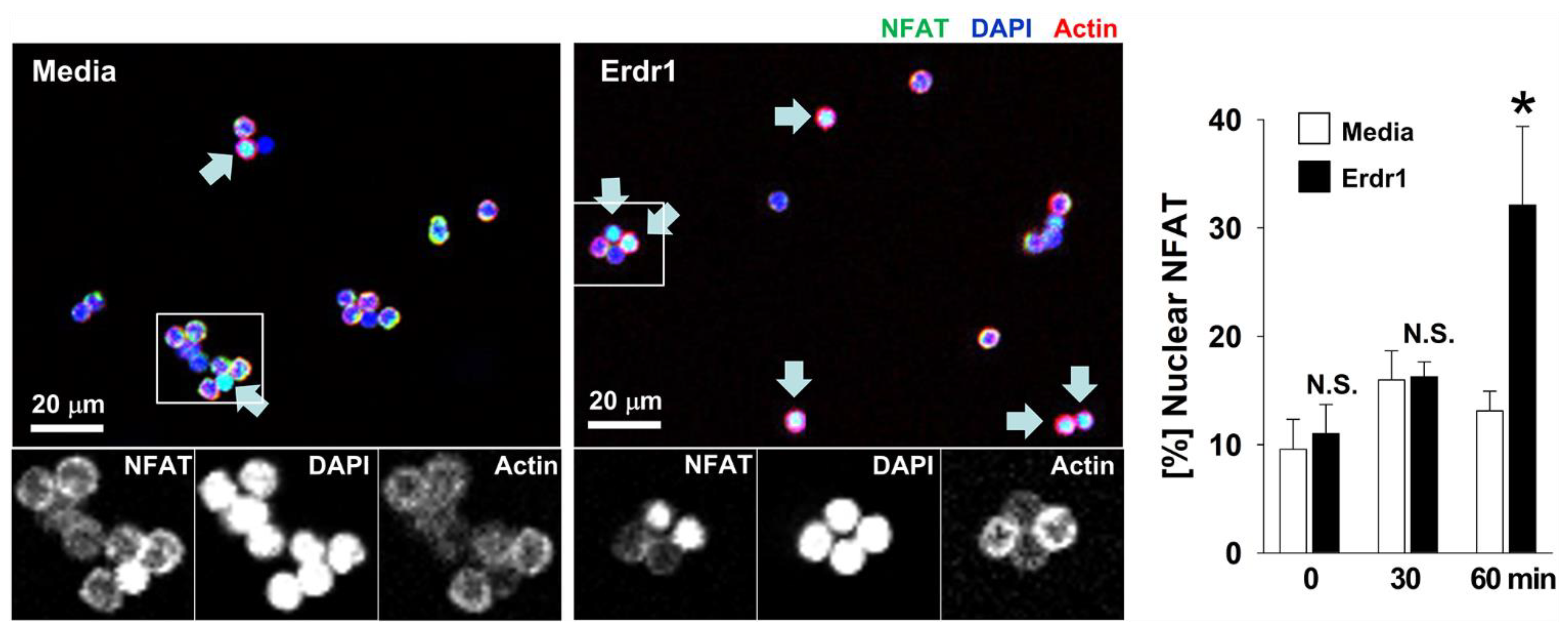
2.4. Erdr1 Increases Translocation of NFAT1 into Nuclei in CD4 T Cells with TCR Stimulation
3. Discussion
4. Materials and Methods
4.1. Mice and Cells
4.2. Antibodies and Recombinant Erdr1
4.3. T Cell Stimulation, Proliferation, and Ca2+ Influx Assay
4.4. Flow Cytometry
4.5. Western Blot Analysis
4.6. NFAT Translocation
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hawse, W.F.; Boggess, W.C.; Morel, P.A. TCR signal strength regulates Akt substrate specificity to in-duce alternate murine Th and T regulatory cell differentiation programs. J. Immunol. 2017, 199, 589–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmud, S.A.; Manlove, L.S.; Schmitz, H.M.; Xing, Y.; Wang, Y.; Owen, D.L.; Schenkel, J.M.; Boomer, J.S.; Green, J.M.; Yagita, H.; et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat. Immunol. 2014, 15, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Li, M.O.; Rudensky, A.Y. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol. 2016, 16, 220–233. [Google Scholar] [CrossRef] [Green Version]
- Snook, J.P.; Kim, C.; Williams, M.A. TCR signal strength controls the differentiation of CD4+ effector and memory T cells. Sci. Immunol. 2018, 3, eaas9103. [Google Scholar] [CrossRef] [Green Version]
- Starr, T.K.; Jameson, S.C.; Hogquist, K.A. Positive and Negative Selection of T Cells. Annu. Rev. Immunol. 2003, 21, 139–176. [Google Scholar] [CrossRef]
- Hogquist, K.; Jameson, S. The self-obsession of T cells: How TCR signaling thresholds affect fate ’decisions’ and effector function. Nat. Immunol. 2014, 15, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Macian, F. NFAT proteins: Key regulators of T-cell development and function. Nat. Rev. Immunol. 2005, 5, 472–484. [Google Scholar] [CrossRef]
- Hayden-Martinez, K.; Kane, L.P.; Hedrick, S.M. Effects of a constitutively active form of calcineurin on T cell activation and thymic selection. J. Immunol. 2000, 165, 3713–3721. [Google Scholar] [CrossRef] [Green Version]
- Feske, S.; Giltnane, J.; Dolmetsch, R.; Staudt, L.M.; Rao, A. Gene regulation mediated by calcium signals in T lymphocytes. Nat. Immunol. 2001, 2, 316–324. [Google Scholar] [CrossRef]
- Wells, A.D.; Liu, Q.H.; Hondowicz, B.; Zhang, J.; Turka, L.A.; Freedman, B.D. Regulation of T cell activation and tolerance by phospholipase C gamma-1-dependent integrin avidity modulation. J. Immunol. 2003, 170, 4127–4133. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kim, K.E.; Jung, H.Y.; Jo, H.; Jeong, S.W.; Lee, J.; Kim, C.H.; Kim, H.; Cho, D.; Park, H.J. Recombinant erythroid differentiation regulator 1 inhibits both inflammation and angiogenesis in a mouse model of rosacea. Exp. Dermatol. 2015, 24, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Kim, S.; Park, S.; Houh, Y.; Yang, Y.; Park, S.B.; Kim, S.; Kim, D.; Hur, D.Y.; Kim, S.; et al. Therapeutic effect of erythroid differentiation regulator 1 (Erdr1) on collagen-induced arthritis in DBA/1J mouse. Oncotarget 2016, 7, 76354–76361. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.E.; Houh, Y.; Lee, J.; Cho, D.; Park, H.J. Downregulation of Erythroid Differentiation Regulator 1 (Erdr1) plays a critical role in psoriasis pathogenesis. Exp. Dermatol. 2016, 25, 570–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Lee, S.; Park, S.; Kim, K.E.; Park, H.J.; Cho, D. Erythroid differentiation regulator 1 ameliorates collagen-induced arthritis via activation of regulatory T cells. Int. J. Mol. Sci. 2020, 21, 9555. [Google Scholar] [CrossRef] [PubMed]
- Dörmer, P.; Spitzer, E.; Frankenberger, M.; Kremmer, E. Erythroid differentiation regulator (EDR), a novel, highly conserved factor: I. Induction of haemoglobin synthesis in erythroleukaemic cells. Cytokine 2004, 26, 231–242. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, S.; Jung, S.J.; Park, S.; Kim, K.E.; Kim, T.S.; Park, H.J.; Cho, D. Erythroid differentiation regulator 1 strengthens TCR signaling in thymocytes by modulating calcium flux. Cell. Immunol. 2019, 336, 28–33. [Google Scholar] [CrossRef]
- Bird, J.J.; Brown, D.R.; Mullen, A.C.; Moskowitz, N.H.; Mahowald, A.M.; Sider, J.R.; Gajewski, T.F.; Wang, C.-R.; Reiner, S.L. Helper T Cell Differentiation is Controlled by the Cell Cycle. Immunity 1998, 9, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Putney, J.W.; Tomita, T. Phospholipase C signaling and calcium influx. Adv. Biol. Regul. 2012, 52, 152–164. [Google Scholar] [CrossRef] [Green Version]
- Rudensky, A.Y.; Gavin, M.; Zheng, Y. FOXP3 and NFAT: Partners in Tolerance. Cell 2006, 126, 253–256. [Google Scholar] [CrossRef] [Green Version]
- Martinez, G.J.; Hu, J.K.; Pereira, R.M.; Crampton, J.S.; Togher, S.; Bild, N.; Crotty, S.; Rao, A. Cutting Edge: NFAT Transcription Factors Promote the Generation of Follicular Helper T Cells in Response to Acute Viral Infection. J. Immunol. 2016, 196, 2015–2019. [Google Scholar] [CrossRef] [Green Version]
- Masuda, T.; Maeda, K.; Sato, W.; Kosugi, T.; Sato, Y.; Kojima, H.; Kato, N.; Ishimoto, T.; Tsuboi, N.; Uchimura, K.; et al. Growth Factor Midkine Promotes T-Cell Activation through Nuclear Factor of Activated T Cells Signaling and Th1 Cell Differentiation in Lupus Nephritis. Am. J. Pathol. 2017, 187, 740–751. [Google Scholar] [CrossRef] [Green Version]
- Hartl, F.A.; Beck-Garcìa, E.; Woessner, N.M.; Flachsmann, L.J.; Cárdenas, R.M.V.; Brandl, S.M.; Taromi, S.; Fiala, G.J.; Morath, A.; Mishra, P.; et al. Noncanonical binding of Lck to CD3ε promotes TCR signaling and CAR function. Nat. Immunol. 2020, 21, 902–913. [Google Scholar] [CrossRef]
- He, L.; Gu, W.; Wang, M.; Chang, X.; Sun, X.; Zhang, Y.; Lin, X.; Yan, C.; Fan, W.; Su, P.; et al. Extracellular matrix protein 1 promotes follicular helper T cell differentiation and antibody production. Proc. Natl. Acad. Sci. USA 2018, 115, 8621–8626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodygin, D.; Odoardi, F.; Schläger, C.; Körner, H.; Kitz, A.; Nosov, M.; van den Brandt, J.; Reichardt, H.M.; Haberl, M.; Flügel, A. A combination of fluorescent NFAT and H2B sensors uncovers dynamics of T cell activation in real time during CNS autoimmunity. Nat. Med. 2013, 19, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Cocco, L.; Follo, M.Y.; Manzoli, L.; Suh, P.-G. Phosphoinositide-specific phospholipase C in health and disease. J. Lipid Res. 2015, 56, 1853–1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, H.J.; Sie, C.; Schumann, E.; Witte, A.-K.; Dressel, R.; Brandt, J.V.D.; Reichardt, H.M. The Insulin Receptor Plays a Critical Role in T Cell Function and Adaptive Immunity. J. Immunol. 2017, 198, 1910–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccaluga, P.P.; Rossi, M.; Agostinelli, C.; Ricci, F.; Gazzola, A.; Righi, S.; Fuligni, F.; Laginestra, M.A.; Mancini, M.; Sapienza, M.R.; et al. Platelet-derived growth factor alpha mediates the proliferation of peripheral T-cell lymphoma cells via an autocrine regulatory pathway. Leukemia 2014, 28, 1687–1697. [Google Scholar] [CrossRef]
- Yang, J.; Yan, J.; Liu, B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front. Immunol. 2018, 9, 978. [Google Scholar] [CrossRef] [Green Version]
- Smith, X.; Schneider, H.; Köhler, K.; Liu, H.; Lu, Y.; Rudd, C.E. The Chemokine CXCL12 Generates Costimulatory Signals in T Cells to Enhance Phosphorylation and Clustering of the Adaptor Protein SLP-76. Sci. Signal. 2013, 6, ra65. [Google Scholar] [CrossRef] [PubMed]
- Gaud, G.; Lesourne, R.; Love, P.E. Regulatory mechanisms in T cell receptor signalling. Nat. Rev. Immunol. 2018, 18, 485–497. [Google Scholar] [CrossRef]
- Szabo, M.; Czompoly, T.; Kvell, K.; Talaber, G.; Bartis, D.; Nemeth, P.; Berki, T.; Boldizsar, F. Fine-tuning of proximal TCR sig-naling by ZAP-70 tyrosine residues in Jurkat cells. Int. Immunol. 2012, 24, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Balagopalan, L.; Kortum, R.L.; Coussens, N.P.; Barr, V.A.; Samelson, L.E. The Linker for Activation of T Cells (LAT) Signaling Hub: From Signaling Complexes to Microclusters. J. Biol. Chem. 2015, 290, 26422–26429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harder, T. The T Cell Plasma Membrane Lipid Bilayer Stages TCR-proximal Signaling Events. Front. Immunol. 2012, 3, 50. [Google Scholar] [CrossRef] [Green Version]
- Dinic, J.; Riehl, A.; Adler, J.; Parmryd, I. The T cell receptor resides in ordered plasma membrane nanodomains that aggregate upon patching of the receptor. Sci. Rep. 2015, 5, 10082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Lee, A.; Cho, D.; Kim, T.S. AIMP1 regulates TCR signaling and induces differentiation of regulatory T cells by interfering with lipid raft association. Biochem. Biophys. Res. Commun. 2019, 514, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.H.; Kim, D.G.; Ahn, Y.S. Microarray Analysis of Differentially Expressed Genes in the Brains of Tubby Mice. Korean J. Physiol. Pharmacol. 2009, 13, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, F.; Wang, L.; Qiu, S.; Yao, Y.; Yan, C.; Xiong, X.; Chen, X.; Ji, Q.; Cao, J.; et al. NAD+ supplement potentiates tumor-killing func-tion by rescuing defective TUB-mediated NAMPT transcription in tumor-infiltrated T cells. Cell. Rep. 2021, 36, 109516. [Google Scholar] [CrossRef]
- Jung, M.K.; Park, Y.; Song, S.B.; Cheon, S.Y.; Park, S.; Houh, Y.; Ha, S.; Kim, H.J.; Park, J.M.; Kim, T.S.; et al. Erythroid differentiation regulator 1, an inter-leukin 18-regulated gene, acts as a metastasis suppressor in melanoma. J. Investig. Dermatol. 2013, 131, 2096–2104. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.S.; Park, D.; Lee, S.; Park, S.; Kim, K.E.; Kim, T.S.; Park, H.J.; Cho, D. Erythroid Differentiation Regulator 1 Strengthens TCR Signaling by Enhancing PLCγ1 Signal Transduction Pathway. Int. J. Mol. Sci. 2022, 23, 844. https://doi.org/10.3390/ijms23020844
Kim MS, Park D, Lee S, Park S, Kim KE, Kim TS, Park HJ, Cho D. Erythroid Differentiation Regulator 1 Strengthens TCR Signaling by Enhancing PLCγ1 Signal Transduction Pathway. International Journal of Molecular Sciences. 2022; 23(2):844. https://doi.org/10.3390/ijms23020844
Chicago/Turabian StyleKim, Myun Soo, Dongmin Park, Sora Lee, Sunyoung Park, Kyung Eun Kim, Tae Sung Kim, Hyun Jeong Park, and Daeho Cho. 2022. "Erythroid Differentiation Regulator 1 Strengthens TCR Signaling by Enhancing PLCγ1 Signal Transduction Pathway" International Journal of Molecular Sciences 23, no. 2: 844. https://doi.org/10.3390/ijms23020844
APA StyleKim, M. S., Park, D., Lee, S., Park, S., Kim, K. E., Kim, T. S., Park, H. J., & Cho, D. (2022). Erythroid Differentiation Regulator 1 Strengthens TCR Signaling by Enhancing PLCγ1 Signal Transduction Pathway. International Journal of Molecular Sciences, 23(2), 844. https://doi.org/10.3390/ijms23020844





