Brassinosteroids Mitigate Cadmium Effects in Arabidopsis Root System without Any Cooperation with Nitric Oxide
Abstract
:1. Introduction
2. Results
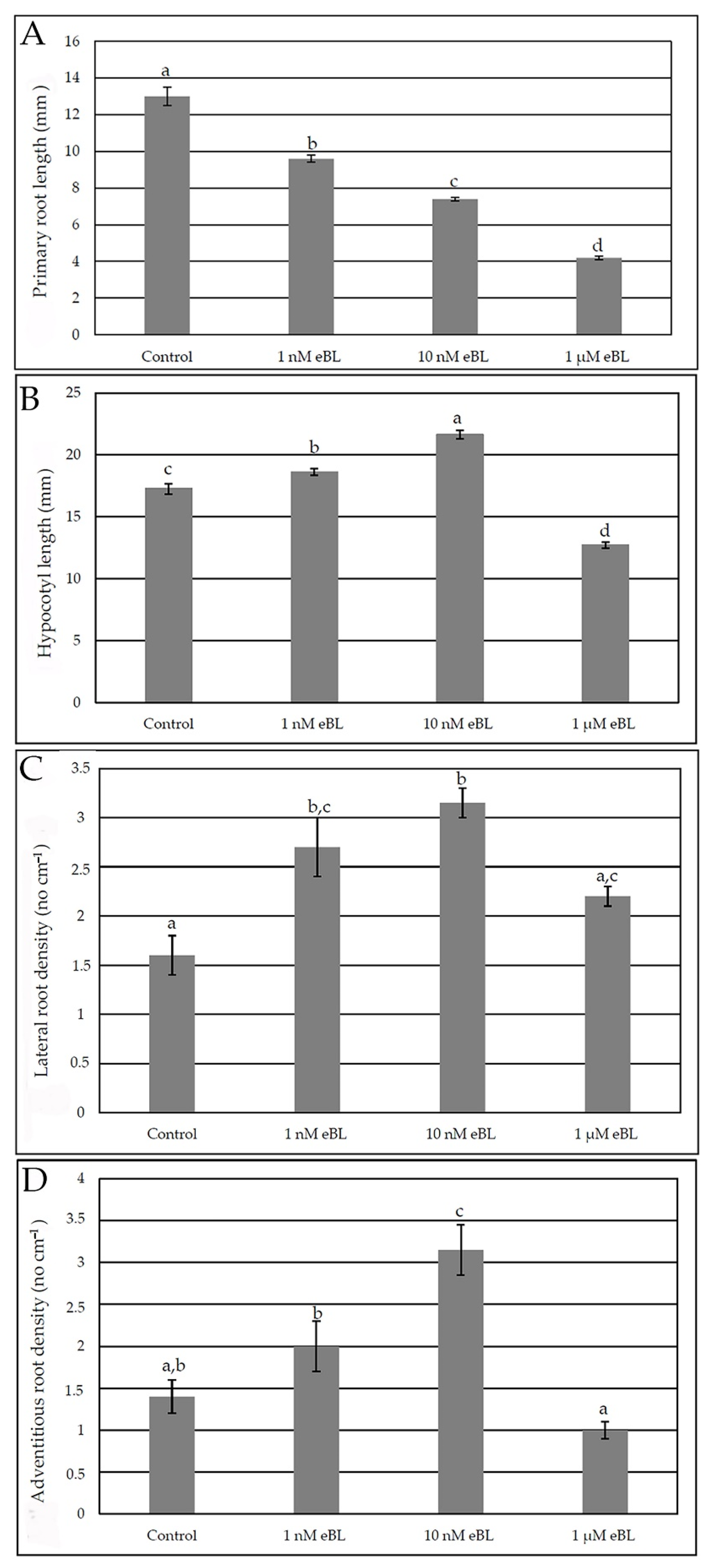
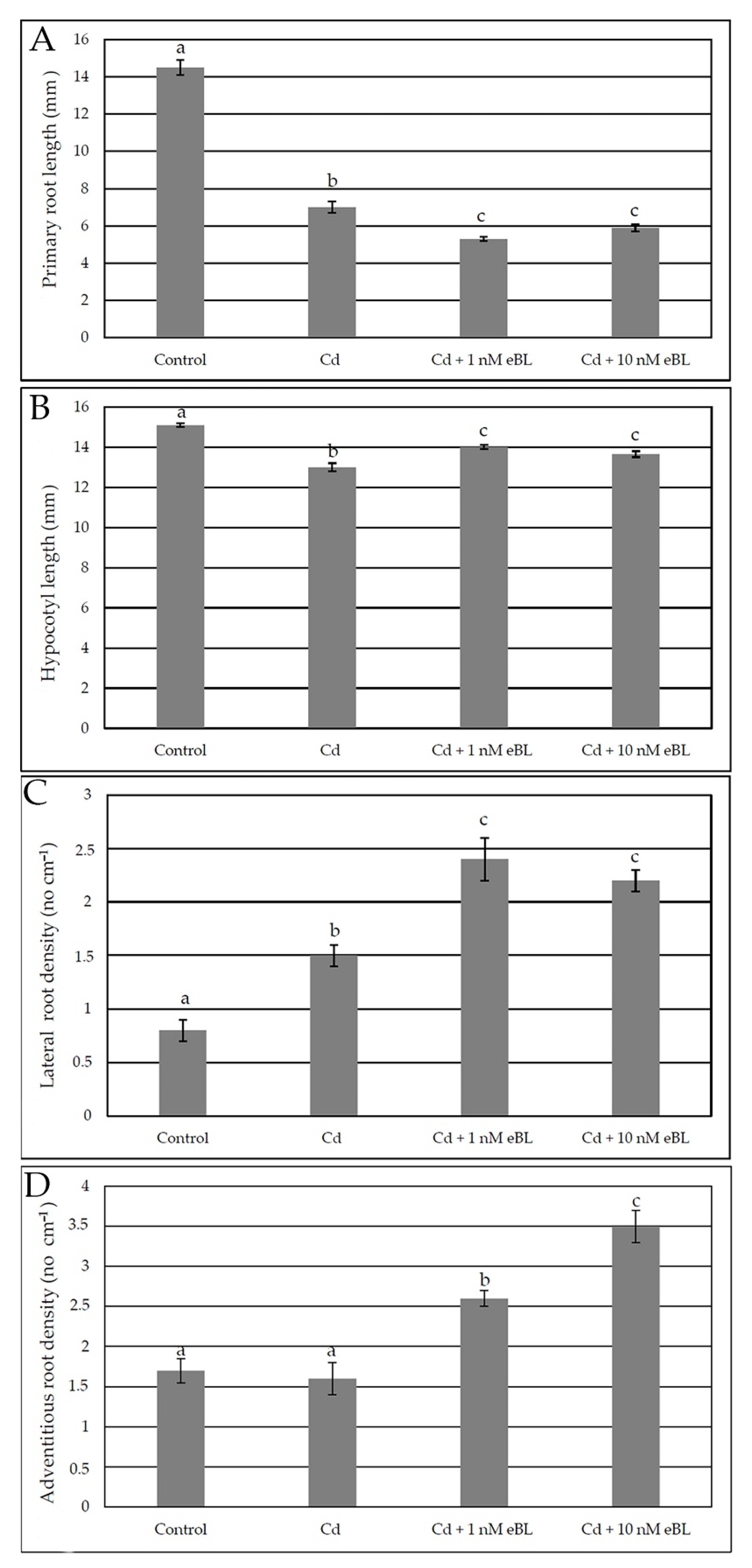
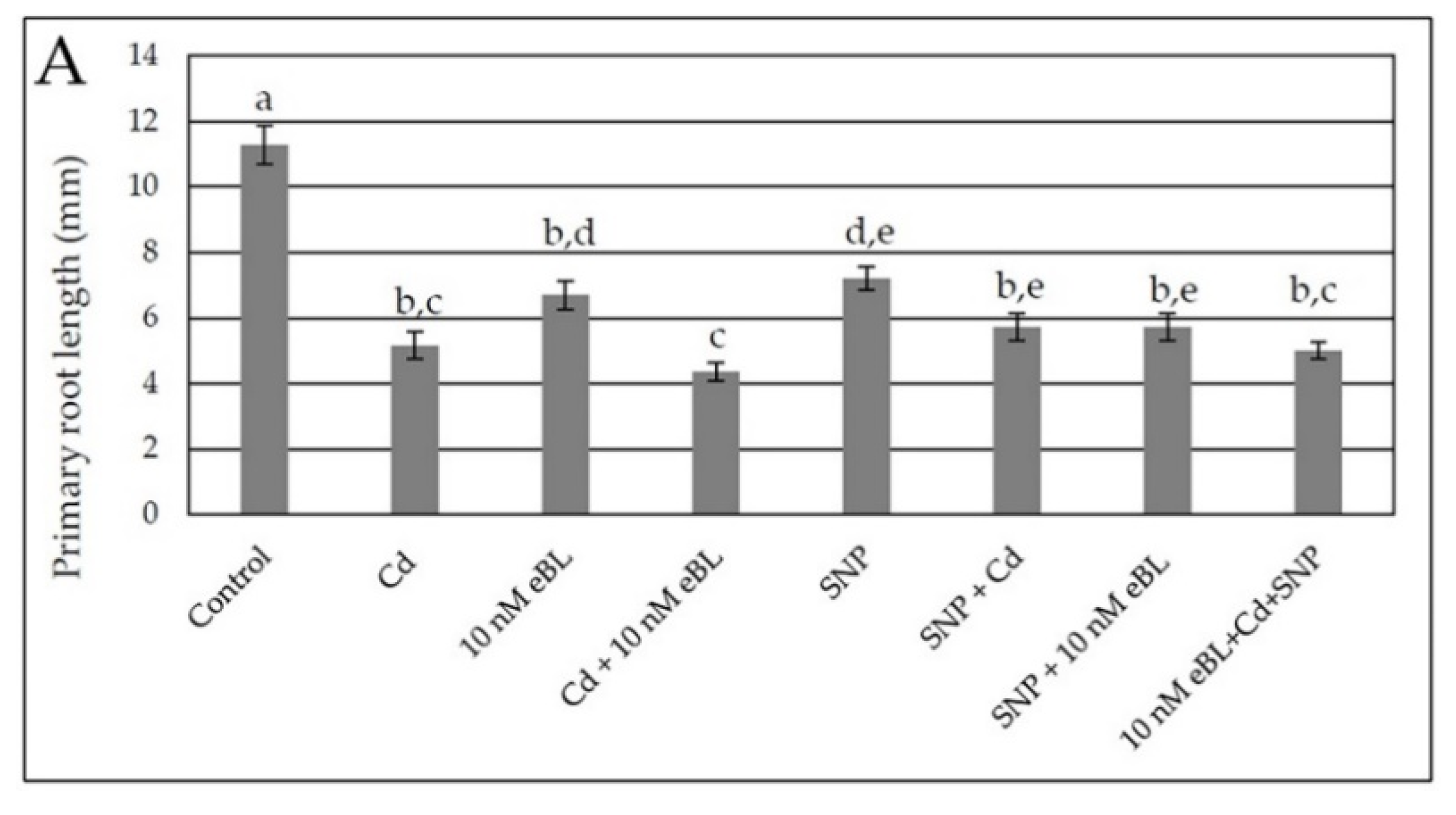
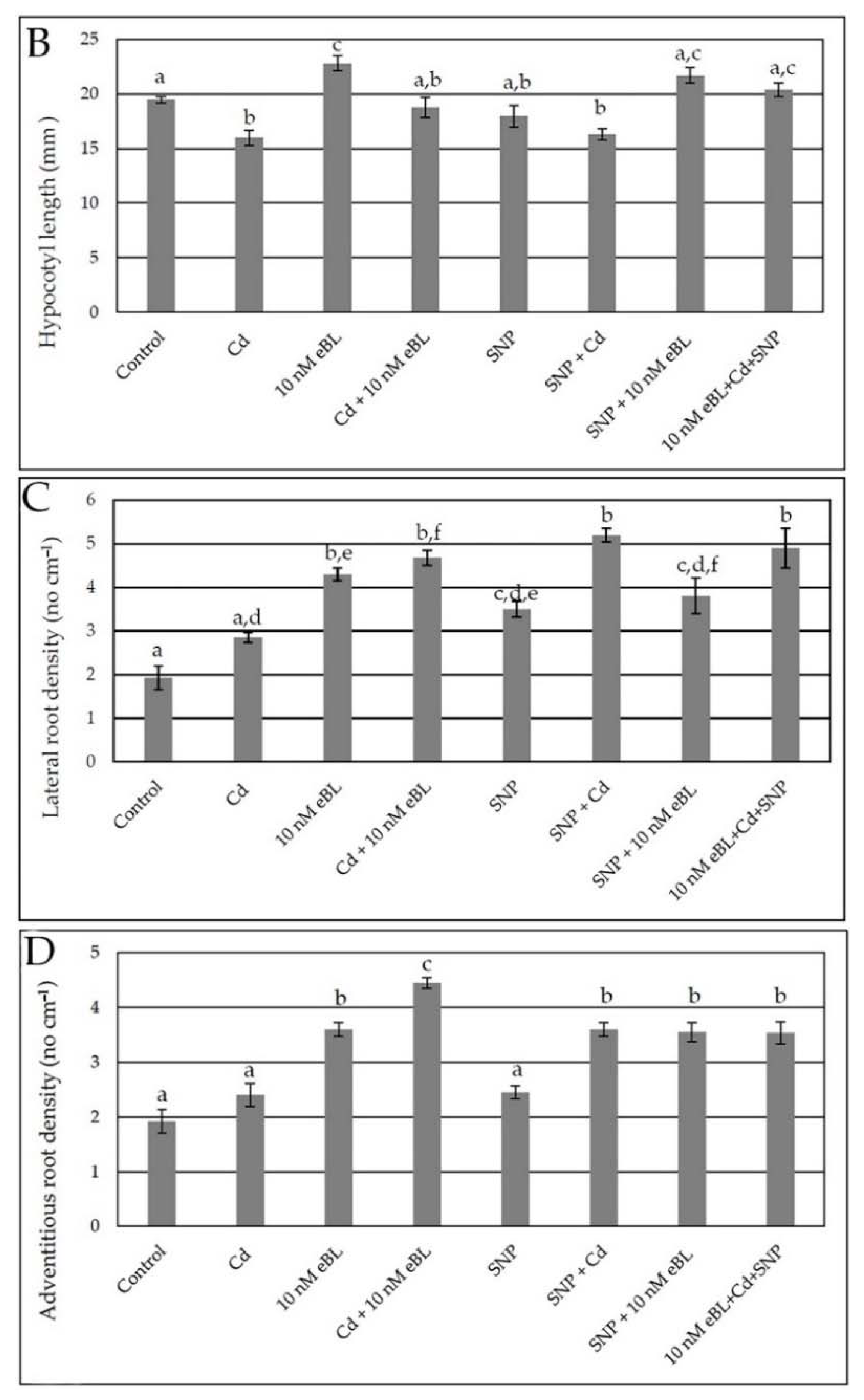
2.1. Epibrassinolide Enhances Both Lateral and Adventitious Rooting When Applied at Low Concentration, Even in the Presence of Cadmium
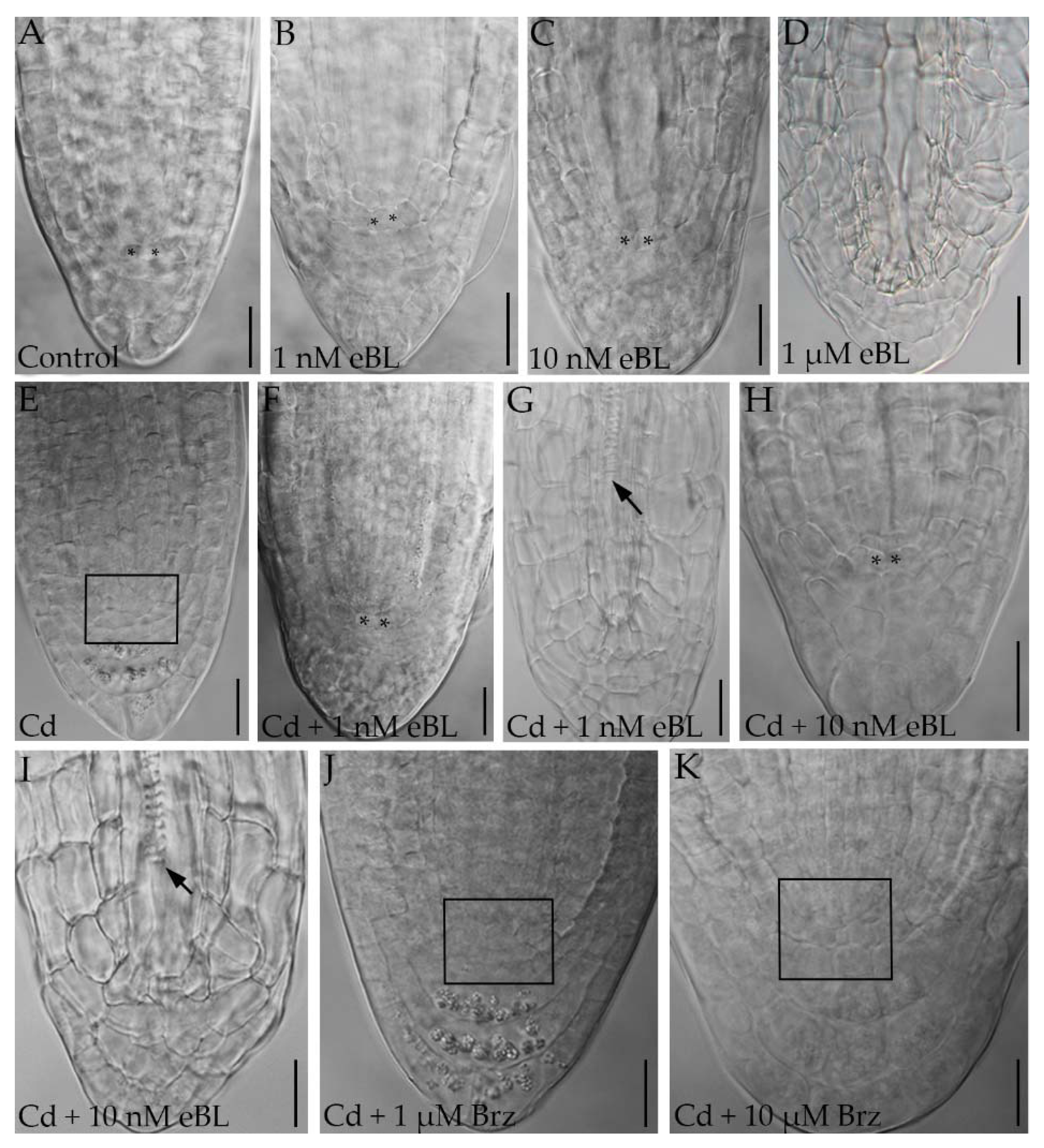
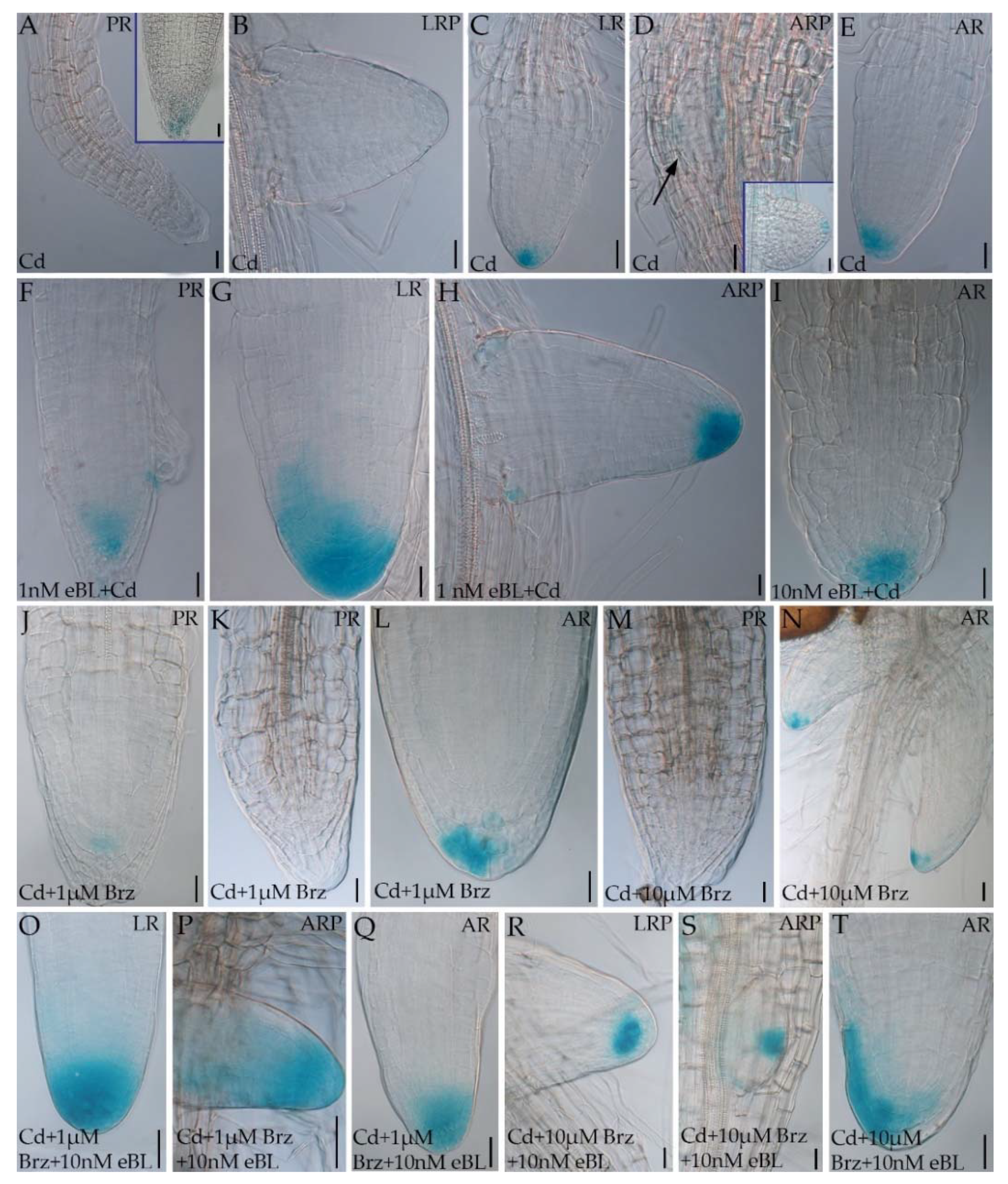
2.2. Epibrassinolide Induces Regular QC Formation in the Root Apices of Cadmium-Cultured Seedlings
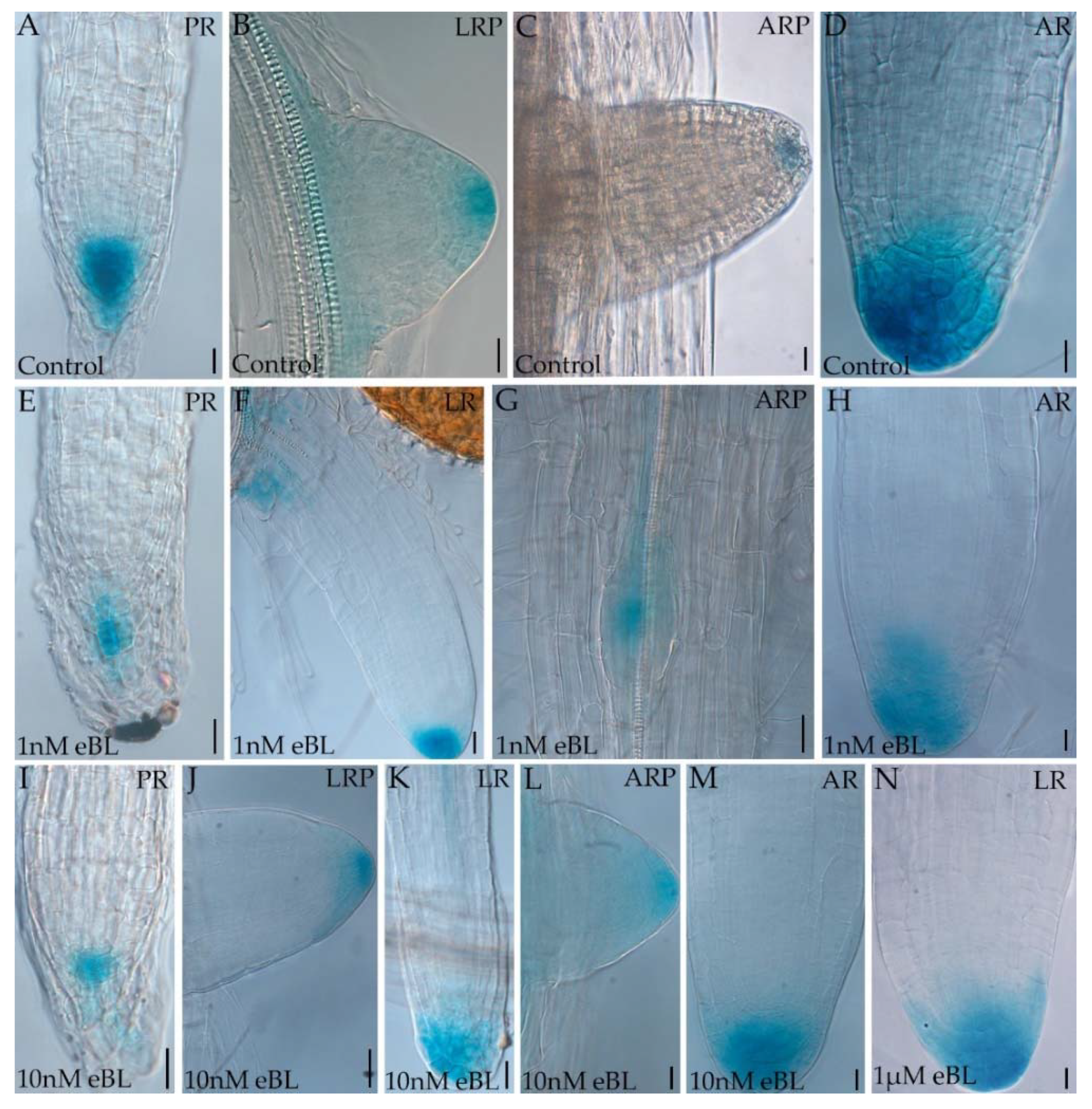
2.3. Epibrassinolide Enhances the Auxin Signal in the Root Apices of Cadmium-Cultured Seedlings
2.4. Exogenous Nitric Oxide Enhances Lateral and Adventitious Root Formation in the Presence of Cd without Any Synergism with eBL
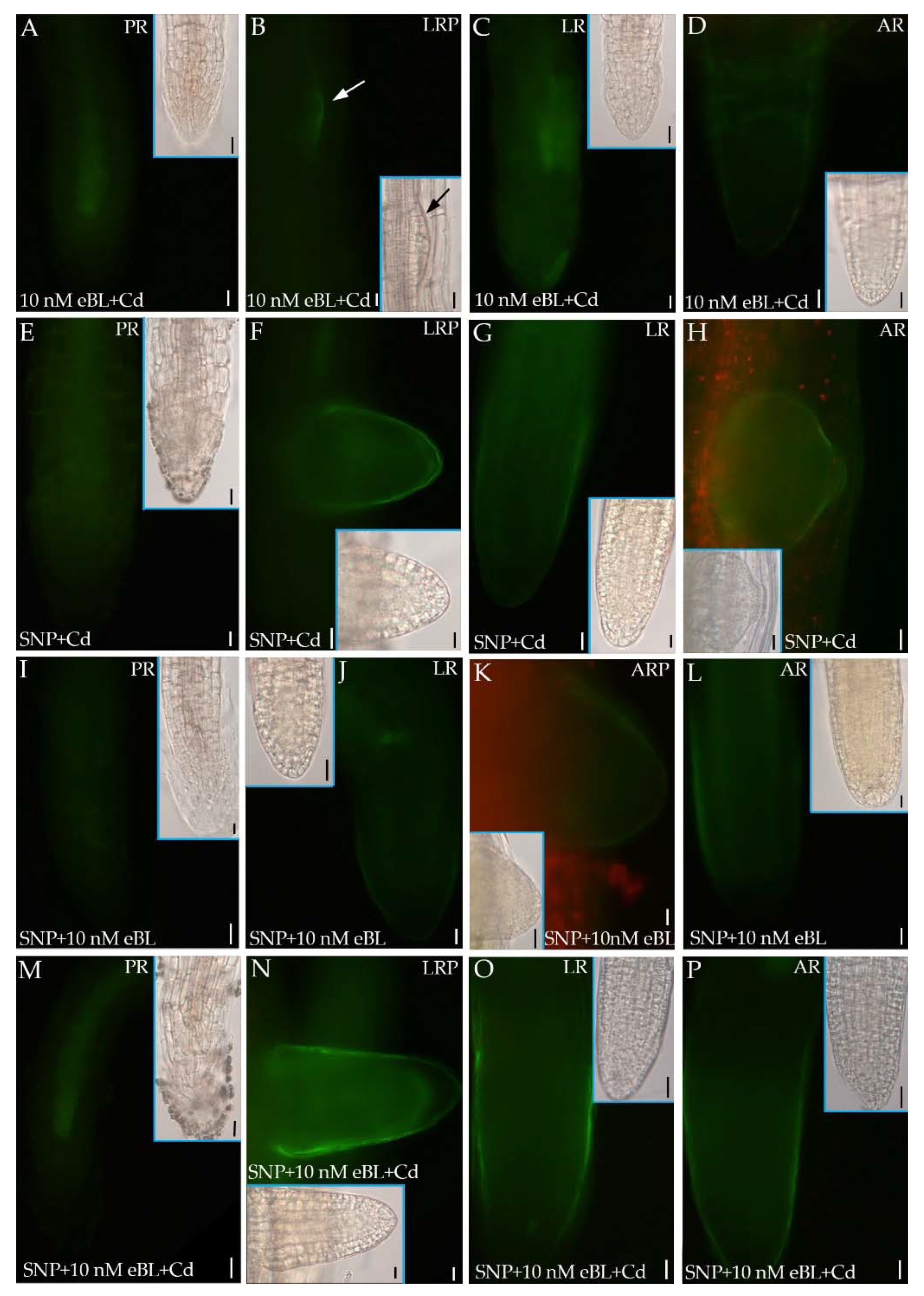
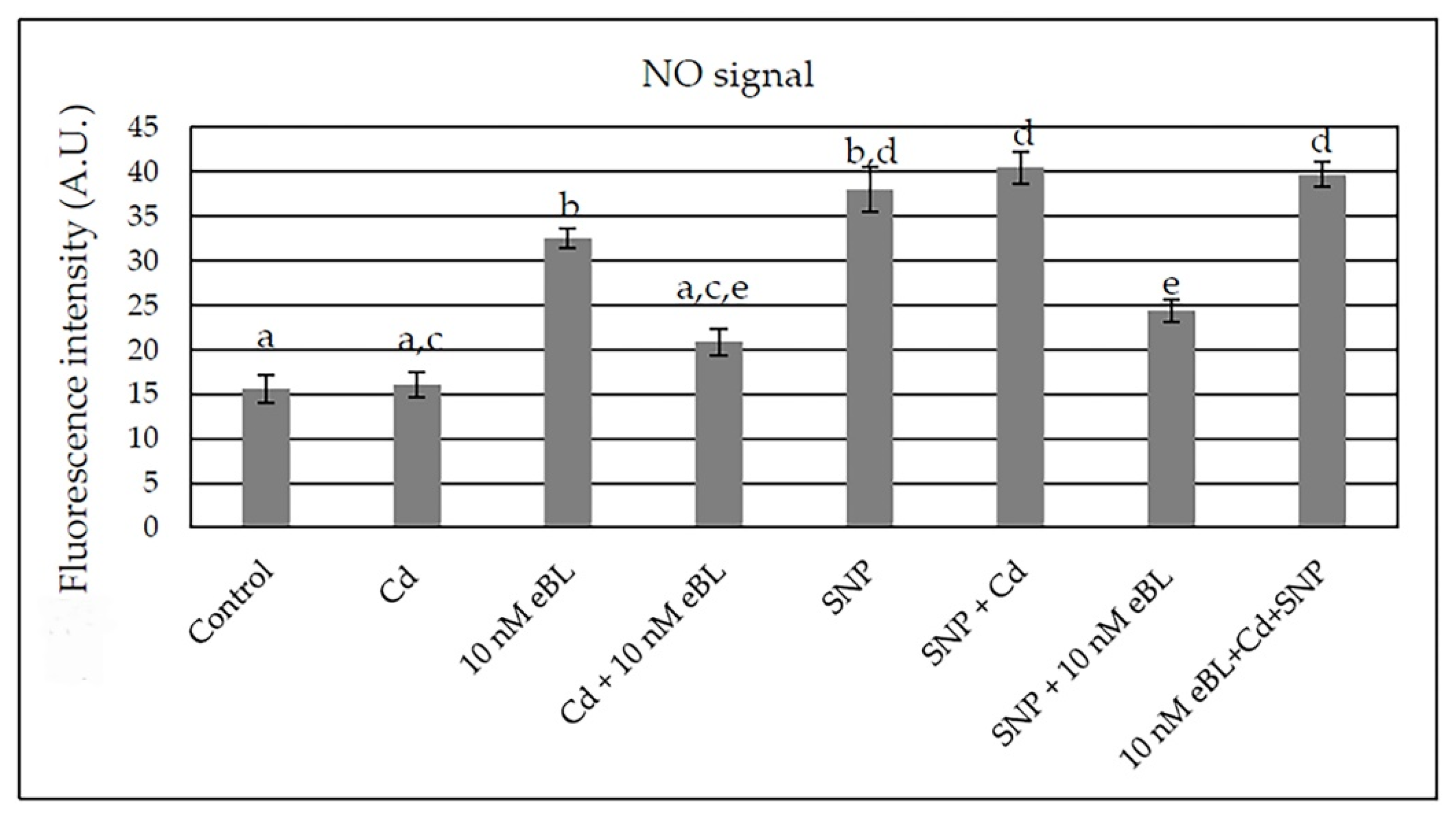
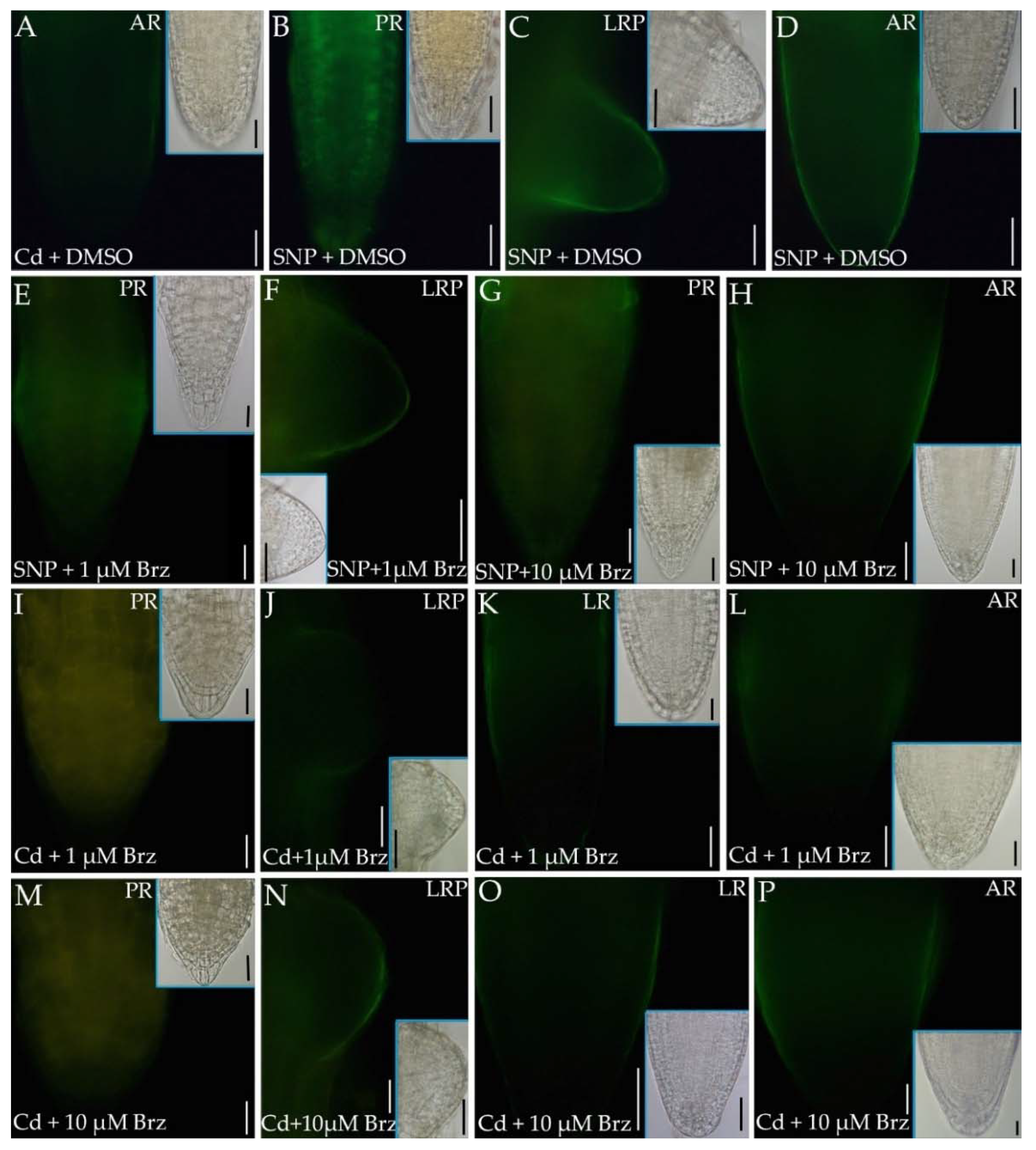
2.5. Epibrassinolide Increases NO Signal but Not in Cd Presence
2.6. The Inhibition of BR Synthesis Does Not Change the NO Signal Caused by Cd
3. Discussion
Brassinosteroids Protect the Root System against Cadmium-Stress Increasing Lateral and Adventitious Root Formation without Any Cooperation by NO
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. GUS Detection
4.3. Rooting in Response to Exogenous NO and NO Detection in Root Apices
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kagale, S.; Divi, U.K.; Krochko, J.E.; Keller, W.A.; Krishna, P. Brassinosteroid Confers Tolerance in Arabidopsis Thaliana and Brassica Napus to a Range of Abiotic Stresses. Planta 2007, 225, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [Green Version]
- Fàbregas, N.; Li, N.; Boeren, S.; Nash, T.E.; Goshe, M.B.; Clouse, S.D.; de Vries, S.; Caño-Delgado, A.I. The BRASSINOSTEROID INSENSITIVE1–LIKE3 Signalosome Complex Regulates Arabidopsis Root Development. Plant Cell 2013, 25, 3377–3388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betti, C.; Della Rovere, F.; Piacentini, D.; Fattorini, L.; Falasca, G.; Altamura, M.M. Jasmonates, Ethylene and Brassinosteroids Control Adventitious and Lateral Rooting as Stress Avoidance Responses to Heavy Metals and Metalloids. Biomolecules 2021, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Müssig, C.; Shin, G.-H.; Altmann, T. Brassinosteroids Promote Root Growth in Arabidopsis. Plant Physiol. 2003, 133, 1261–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vardhini, B.V.; Anuradha, S.; Rao, S.S.R. Brassinosteroids—New Class of Plant Hormone with Potential to Improve Crop Productivity. Indian J. Plant Physiol. 2006, 11, 12. [Google Scholar]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Biju, S.; Bhardwaj, R. 24-Epibrassinolide; an Active Brassinolide and Its Role in Salt Stress Tolerance in Plants: A Review. Plant Physiol. Biochem. 2018, 130, 69–79. [Google Scholar] [CrossRef]
- Kim, T.-W.; Lee, S.M.; Joo, S.-H.; Yun, H.S.; Lee, Y.; Kaufman, P.B.; Kirakosyan, A.; Kim, S.-H.; Nam, K.H.; Lee, J.S.; et al. Elongation and Gravitropic Responses of Arabidopsis Roots Are Regulated by Brassinolide and IAA. Plant Cell Environ. 2007, 30, 679–689. [Google Scholar] [CrossRef]
- Bao, F.; Shen, J.; Brady, S.R.; Muday, G.K.; Asami, T.; Yang, Z. Brassinosteroids Interact with Auxin to Promote Lateral Root Development in Arabidopsis. Plant Physiol. 2004, 134, 1624–1631. [Google Scholar] [CrossRef] [Green Version]
- Asami, T.; Min, Y.K.; Nagata, N.; Yamagishi, K.; Takatsuto, S.; Fujioka, S.; Murofushi, N.; Yamaguchi, I.; Yoshida, S. Characterization of Brassinazole, a Triazole-Type Brassinosteroid Biosynthesis Inhibitor1. Plant Physiol. 2000, 123, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Basit, F.; Liu, J.; An, J.; Chen, M.; He, C.; Zhu, X.; Li, Z.; Hu, J.; Guan, Y. Brassinosteroids as a Multidimensional Regulator of Plant Physiological and Molecular Responses under Various Environmental Stresses. Environ. Sci. Pollut. Res. 2021, 28, 44768–44779. [Google Scholar] [CrossRef] [PubMed]
- Kothari, A.; Lachowiec, J. Roles of Brassinosteroids in Mitigating Heat Stress Damage in Cereal Crops. Int. J. Mol. Sci. 2021, 22, 2706. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sohail, H.; Nawaz, M.A.; Liu, C.; Yang, P. Physiological and Proteomic Analyses Reveals That Brassinosteroids Application Improves the Chilling Stress Tolerance of Pepper Seedlings. Plant Growth Regul. 2021. [Google Scholar] [CrossRef]
- Falasca, G.; Altamura, M.M. Histological Analysis of Adventitious Rooting in Arabidopsis thaliana (L.) Heynh Seedlings. Plant Biosyst. 2003, 137, 265–273. [Google Scholar] [CrossRef]
- Motte, H.; Vanneste, S.; Beeckman, T. Molecular and Environmental Regulation of Root Development. Annu. Rev. Plant Biol. 2019, 70, 465–488. [Google Scholar] [CrossRef] [Green Version]
- Petricka, J.J.; Winter, C.M.; Benfey, P.N. Control of Arabidopsis Root Development. Annu. Rev. Plant Biol. 2012, 63, 563–590. [Google Scholar] [CrossRef] [Green Version]
- Della Rovere, F.; Fattorini, L.; D’Angeli, S.; Veloccia, A.; Falasca, G.; Altamura, M.M. Auxin and Cytokinin Control Formation of the Quiescent Centre in the Adventitious Root Apex of Arabidopsis. Ann. Bot. 2013, 112, 1395–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besson-Bard, A.; Gravot, A.; Richaud, P.; Auroy, P.; Duc, C.; Gaymard, F.; Taconnat, L.; Renou, J.-P.; Pugin, A.; Wendehenne, D. Nitric Oxide Contributes to Cadmium Toxicity in Arabidopsis by Promoting Cadmium Accumulation in Roots and by Up-Regulating Genes Related to Iron Uptake. Plant Physiol. 2009, 149, 1302–1315. [Google Scholar] [CrossRef] [Green Version]
- Fattorini, L.; Ronzan, M.; Piacentini, D.; Della Rovere, F.; De Virgilio, C.; Sofo, A.; Altamura, M.M.; Falasca, G. Cadmium and Arsenic Affect Quiescent Centre Formation and Maintenance in Arabidopsis Thaliana Post-Embryonic Roots Disrupting Auxin Biosynthesis and Transport. Environ. Exp. Bot. 2017, 144, 37–48. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Zheng, L.; Wang, Y.; Luo, L.; Ma, M.; Zhang, C.; Han, Y.; Beeckman, T.; Xu, G.; et al. Cadmium Stress Suppresses Lateral Root Formation by Interfering with the Root Clock. Plant Cell Environ. 2019, 42, 3182–3196. [Google Scholar] [CrossRef]
- Roychoudhry, S.; Kepinski, S. Auxin in Root Development. Cold Spring Harb. Perspect. Biol. 2021, a039933. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.; Pacenza, M.; Forgione, I.; Lamerton, L.R.; Greco, M.; Chiappetta, A.; Bitonti, M.B. In Arabidopsis Thaliana Cadmium Impact on the Growth of Primary Root by Altering SCR Expression and Auxin-Cytokinin Cross-Talk. Front. Plant Sci. 2017, 8, 1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, L.; Talarico, E.; Madeo, M.L.; Muto, A.; Minervino, M.; Araniti, F.; Bitonti, M.B.; Chiappetta, A. Cadmium Affects Cell Niches Maintenance in Arabidopsis Thaliana Post-Embryonic Shoot and Root Apical Meristem by Altering the Expression of WUS/WOX Homolog Genes and Cytokinin Accumulation. Plant Physiol. Biochem. 2021, 167, 785–794. [Google Scholar] [CrossRef]
- Li, P.; Zhao, C.; Zhang, Y.; Wang, X.; Wang, X.; Wang, J.; Wang, F.; Bi, Y. Calcium Alleviates Cadmium-Induced Inhibition on Root Growth by Maintaining Auxin Homeostasis in Arabidopsis Seedlings. Protoplasma 2016, 253, 185–200. [Google Scholar] [CrossRef]
- Mazzoni-Putman, S.M.; Brumos, J.; Zhao, C.; Alonso, J.M.; Stepanova, A.N. Auxin Interactions with Other Hormones in Plant Development. Cold Spring Harb. Perspect. Biol. 2021, 13, a039990. [Google Scholar] [CrossRef] [PubMed]
- Ackerman-Lavert, M.; Fridman, Y.; Matosevich, R.; Khandal, H.; Friedlander-Shani, L.; Vragović, K.; Ben El, R.; Horev, G.; Tarkowská, D.; Efroni, I.; et al. Auxin Requirements for a Meristematic State in Roots Depend on a Dual Brassinosteroid Function. Curr. Biol. 2021, 31, 4462–4472.e6. [Google Scholar] [CrossRef]
- Wei, Z.; Li, J. Brassinosteroids Regulate Root Growth, Development, and Symbiosis. Mol. Plant 2016, 9, 86–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verstraeten, I.; Schotte, S.; Geelen, D. Hypocotyl Adventitious Root Organogenesis Differs from Lateral Root Development. Front. Plant Sci. 2014, 5, 495. [Google Scholar] [CrossRef] [Green Version]
- Piacentini, D.; Della Rovere, F.; Sofo, A.; Fattorini, L.; Falasca, G.; Altamura, M.M. Nitric Oxide Cooperates With Auxin to Mitigate the Alterations in the Root System Caused by Cadmium and Arsenic. Front. Plant Sci. 2020, 11, 1182. [Google Scholar] [CrossRef]
- Alam, P.; Balawi, T.A.; Ashraf, M.; Ahmad, P. 24-Epibrassinolide (EBR) Reduces Oxidative Stress Damage Induced by Cadmium Toxicity by Restricting Cd Uptake and Modulating Some Key Antioxidant Enzymes in Maize Plants. Pak. J. Bot. 2021, 53, 59–66. [Google Scholar] [CrossRef]
- Kosakivska, I.V.; Vedenicheva, N.P.; Babenko, L.M.; Voytenko, L.V.; Romanenko, K.O.; Vasyuk, V.A. Exogenous Phytohormones in the Regulation of Growth and Development of Cereals under Abiotic Stresses. Mol. Biol. Rep. 2022, 49, 617–628. [Google Scholar] [CrossRef]
- Ageeva-Kieferle, A.; Georgii, E.; Winkler, B.; Ghirardo, A.; Albert, A.; Hüther, P.; Mengel, A.; Becker, C.; Schnitzler, J.-P.; Durner, J.; et al. Nitric Oxide Coordinates Growth, Development, and Stress Response via Histone Modification and Gene Expression. Plant Physiol. 2021, 187, 336–360. [Google Scholar] [CrossRef]
- Piacentini, D.; Ronzan, M.; Fattorini, L.; Della Rovere, F.; Massimi, L.; Altamura, M.M.; Falasca, G. Nitric Oxide Alleviates Cadmium- but Not Arsenic-Induced Damages in Rice Roots. Plant Physiol. Biochem. 2020, 151, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Correa-Aragunde, N.; París, R.; Foresi, N.; Terrile, C.; Casalongué, C.; Lamattina, L. The Auxin-Nitric Oxide Highway: A Right Direction in Determining the Plant Root System. In Gasotransmitters in Plants: The Rise of a New Paradigm in Cell Signaling; Lamattina, L., García-Mata, C., Eds.; Springer International Publishing: Cham, Switherland, 2016; pp. 117–136. ISBN 978-3-319-40713-5. [Google Scholar]
- Yuan, H.-M.; Huang, X. Inhibition of Root Meristem Growth by Cadmium Involves Nitric Oxide-Mediated Repression of Auxin Accumulation and Signalling in Arabidopsis. Plant Cell Environ. 2016, 39, 120–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between Plant Hormones and Heavy Metals Responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Liu, L.; Zhao, Q.; Zhang, Z.; Li, Q.; Lin, B.; Wu, Y.; Tang, S.; Xie, Q. Arabidopsis Ubiquitin Conjugase UBC32 Is an ERAD Component That Functions in Brassinosteroid-Mediated Salt Stress Tolerance. Plant Cell 2012, 24, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.-J.; Wang, Y.-J.; Zhou, Y.-H.; Tao, Y.; Mao, W.-H.; Shi, K.; Asami, T.; Chen, Z.; Yu, J.-Q. Reactive Oxygen Species Are Involved in Brassinosteroid-Induced Stress Tolerance in Cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.; Zhang, J.; Zhang, J.; Ye, N.; Zhang, H.; Tan, M.; Jiang, M. Nitric Oxide Mediates Brassinosteroid-Induced ABA Biosynthesis Involved in Oxidative Stress Tolerance in Maize Leaves. Plant Cell Physiol. 2011, 52, 181–192. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Liao, W.; Hu, L.; Dawuda, M.M.; Jin, X.; Tang, Z.; Yang, J.; Yu, J. Nitric Oxide Is Involved in the Brassinolide-Induced Adventitious Root Development in Cucumber. BMC Plant Biol. 2020, 20, 102. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, P.; Zanella, L.; Proia, A.; De Paolis, A.; Falasca, G.; Altamura, M.M.; Sanità di Toppi, L.; Costantino, P.; Cardarelli, M. Cadmium Tolerance and Phytochelatin Content of Arabidopsis Seedlings Over-Expressing the Phytochelatin Synthase Gene AtPCS1. J. Exp. Bot. 2011, 62, 5509–5519. [Google Scholar] [CrossRef] [Green Version]
- Jiang, K.; Feldman, L.J. Regulation of Root Apical Meristem Development. Annu. Rev. Cell Dev. Biol. 2005, 21, 485–509. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, C.; Willemsen, V.; Hendriks, G.; Weisbeek, P.; Scheres, B. Short-Range Control of Cell Differentiation in the Arabidopsis Root Meristem. Nature 1997, 390, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Marcos, M.; Sanz, L.; Lewis, D.R.; Muday, G.K.; Lorenzo, O. Nitric Oxide Causes Root Apical Meristem Defects and Growth Inhibition While Reducing PIN-FORMED 1 (PIN1)-Dependent Acropetal Auxin Transport. Proc. Natl. Acad. Sci. USA 2011, 108, 18506–18511. [Google Scholar] [CrossRef] [Green Version]
- Bentur, O.S.; Chernichovski, T.; Ingbir, M.; Weinstein, T.; Schwartz, I.F. Dimethyl Sulfoxide Attenuates Nitric Oxide Generation via Modulation of Cationic Amino Acid Transporter-1 in Human Umbilical Vein Endothelial Cells. Cryobiology 2016, 73, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Fridman, Y.; Strauss, S.; Horev, G.; Ackerman-Lavert, M.; Reiner-Benaim, A.; Lane, B.; Smith, R.S.; Savaldi-Goldstein, S. The Root Meristem Is Shaped by Brassinosteroid Control of Cell Geometry. Nat. Plants 2021, 7, 1475–1484. [Google Scholar] [CrossRef]
- Li, T.; Kang, X.; Lei, W.; Yao, X.; Zou, L.; Zhang, D.; Lin, H. SHY2 as a Node in the Regulation of Root Meristem Development by Auxin, Brassinosteroids, and Cytokinin. J. Integr. Plant Biol. 2020, 62, 1500–1517. [Google Scholar] [CrossRef]
- Xuan, W.; Beeckman, T. Plant Signaling: Interplay of Brassinosteroids and Auxin in Root Meristems. Curr. Biol. 2021, 31, R1392–R1395. [Google Scholar] [CrossRef]
- Zhou, X.; Joshi, S.; Khare, T.; Patil, S.; Shang, J.; Kumar, V. Nitric Oxide, Crosstalk with Stress Regulators and Plant Abiotic Stress Tolerance. Plant Cell Rep. 2021, 40, 1395–1414. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Basalah, M.O. Role of Nitric Oxide in Tolerance of Plants to Abiotic Stress. Protoplasma 2011, 248, 447–455. [Google Scholar] [CrossRef]
- Fancy, N.N.; Bahlmann, A.-K.; Loake, G.J. Nitric Oxide Function in Plant Abiotic Stress. Plant Cell Environ. 2017, 40, 462–472. [Google Scholar] [CrossRef]
- Lei, G.J.; Sun, L.; Sun, Y.; Zhu, X.F.; Li, G.X.; Zheng, S.J. Jasmonic Acid Alleviates Cadmium Toxicity in Arabidopsis via Suppression of Cadmium Uptake and Translocation. J. Integr. Plant Biol. 2020, 62, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, Y.-Y.; Wang, L.-F.; Li, Y.-H.; Li, T.-T.; Liu, W.-C. WDR5a Functions in Cadmium-Inhibited Root Meristem Growth by Regulating Nitric Oxide Accumulation in Arabidopsis. Planta 2020, 252, 78. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Barroso, J.B. Peroxynitrite (ONOO−) Is Endogenously Produced in Arabidopsis Peroxisomes and Is Overproduced under Cadmium Stress. Ann. Bot. 2014, 113, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Zabalza, A.; Corpas, F.J.; Gómez, M.; Del Río, L.A.; Sandalio, L.M. Cadmium Effect on Oxidative Metabolism of Pea (Pisum sativum L.) Roots. Imaging of Reactive Oxygen Species and Nitric Oxide Accumulation in Vivo. Plant Cell Environ. 2006, 29, 1532–1544. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, H.; Fang, X.; Zhang, Y.; Jin, C. Auxin Acts Downstream of Ethylene and Nitric Oxide to Regulate Magnesium Deficiency-Induced Root Hair Development in Arabidopsis Thaliana. Plant Cell Physiol. 2018, 59, 1452–1465. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Tanaka, K.; Nakamura, Y.; Asami, T.; Yoshida, S.; Matsuo, T.; Okamoto, S. Physiological Roles of Brassinosteroids in Early Growth of Arabidopsis: Brassinosteroids Have a Synergistic Relationship with Gibberellin as Well as Auxin in Light-Grown Hypocotyl Elongation. J. Plant Growth Regul. 2003, 22, 259–271. [Google Scholar] [CrossRef]
- Betti, C.; Vanhoutte, I.; Coutuer, S.; De Rycke, R.; Mishev, K.; Vuylsteke, M.; Aesaert, S.; Rombaut, D.; Gallardo, R.; De Smet, F.; et al. Sequence-Specific Protein Aggregation Generates Defined Protein Knockdowns in Plants. Plant Physiol. 2016, 171, 773–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willemsen, V.; Wolkenfelt, H.; de Vrieze, G.; Weisbeek, P.; Scheres, B. The HOBBIT Gene Is Required for Formation of the Root Meristem in the Arabidopsis Embryo. Development 1998, 125, 521–531. [Google Scholar] [CrossRef]
- Weigel, D.; Glazebrook, J. Arabidopsis: A Laboratory Manual; CSHL Press: New York, NY, USA, 2002; ISBN 978-0-87969-573-6. [Google Scholar]
- Singh, H.P.; Kaur, S.; Batish, D.R.; Sharma, V.P.; Sharma, N.; Kohli, R.K. Nitric Oxide Alleviates Arsenic Toxicity by Reducing Oxidative Damage in the Roots of Oryza Sativa (Rice). Nitric Oxide 2009, 20, 289–297. [Google Scholar] [CrossRef]
- Della Rovere, F.; Fattorini, L.; Ronzan, M.; Falasca, G.; Altamura, M.M.; Betti, C. Jasmonic Acid Methyl Ester Induces Xylogenesis and Modulates Auxin-Induced Xylary Cell Identity with NO Involvement. Int. J. Mol. Sci. 2019, 20, 4469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ImageJ. RRID: SCR_003070. Available online: https://imagej.nih.gov/ij (accessed on 20 December 2021).
- Piacentini, D.; Corpas, F.J.; D’Angeli, S.; Altamura, M.M.; Falasca, G. Cadmium and Arsenic-Induced-Stress Differentially Modulates Arabidopsis Root Architecture, Peroxisome Distribution, Enzymatic Activities and Their Nitric Oxide Content. Plant Physiol. Biochem. 2020, 148, 312–323. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Rovere, F.; Piacentini, D.; Fattorini, L.; Girardi, N.; Bellanima, D.; Falasca, G.; Altamura, M.M.; Betti, C. Brassinosteroids Mitigate Cadmium Effects in Arabidopsis Root System without Any Cooperation with Nitric Oxide. Int. J. Mol. Sci. 2022, 23, 825. https://doi.org/10.3390/ijms23020825
Della Rovere F, Piacentini D, Fattorini L, Girardi N, Bellanima D, Falasca G, Altamura MM, Betti C. Brassinosteroids Mitigate Cadmium Effects in Arabidopsis Root System without Any Cooperation with Nitric Oxide. International Journal of Molecular Sciences. 2022; 23(2):825. https://doi.org/10.3390/ijms23020825
Chicago/Turabian StyleDella Rovere, Federica, Diego Piacentini, Laura Fattorini, Nicoletta Girardi, Dario Bellanima, Giuseppina Falasca, Maria Maddalena Altamura, and Camilla Betti. 2022. "Brassinosteroids Mitigate Cadmium Effects in Arabidopsis Root System without Any Cooperation with Nitric Oxide" International Journal of Molecular Sciences 23, no. 2: 825. https://doi.org/10.3390/ijms23020825
APA StyleDella Rovere, F., Piacentini, D., Fattorini, L., Girardi, N., Bellanima, D., Falasca, G., Altamura, M. M., & Betti, C. (2022). Brassinosteroids Mitigate Cadmium Effects in Arabidopsis Root System without Any Cooperation with Nitric Oxide. International Journal of Molecular Sciences, 23(2), 825. https://doi.org/10.3390/ijms23020825









