Exosomal and Plasma Non-Coding RNA Signature Associated with Urinary Albumin Excretion in Hypertension
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Study Patients
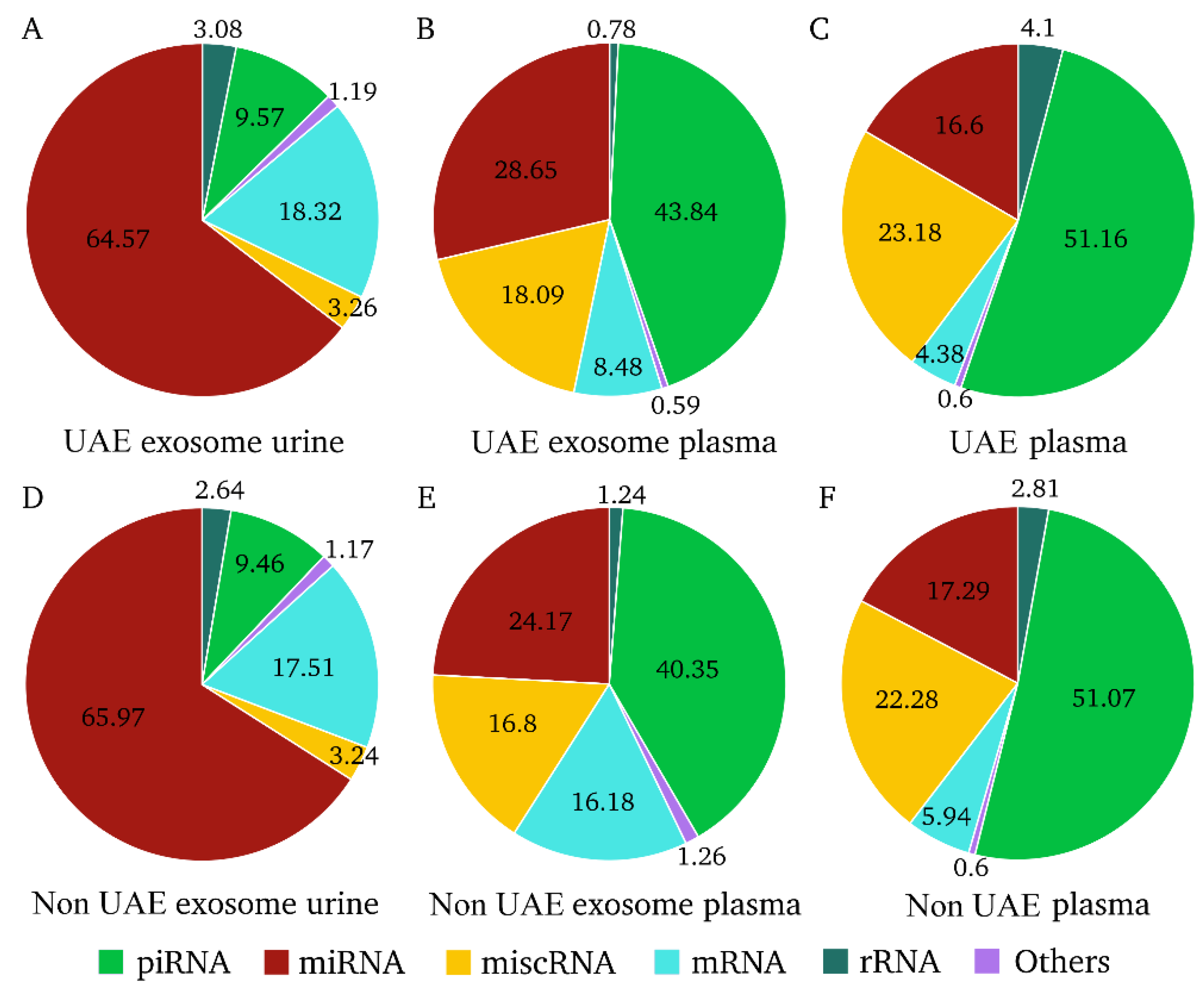
2.2. Proportions of RNA Types in Each Biological Fraction and Patient Groups
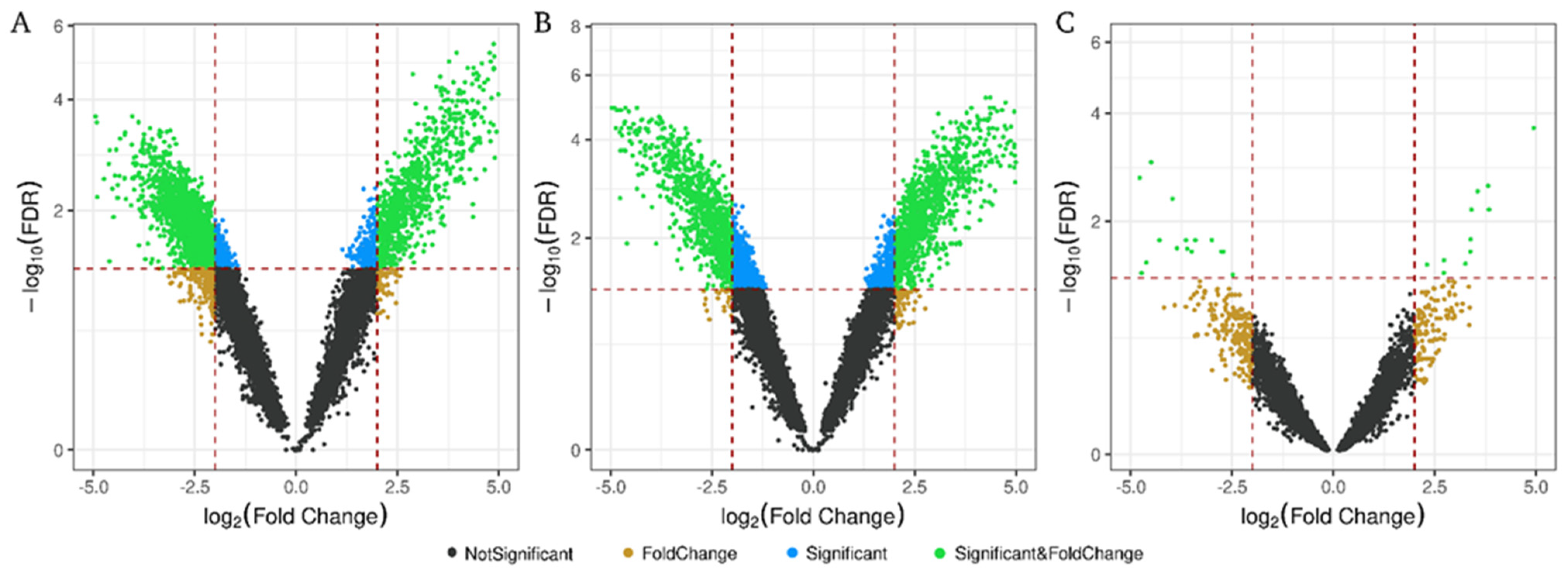
2.3. Differentially Expressed RNAs in Microalbuminuria in Each Biological Fraction
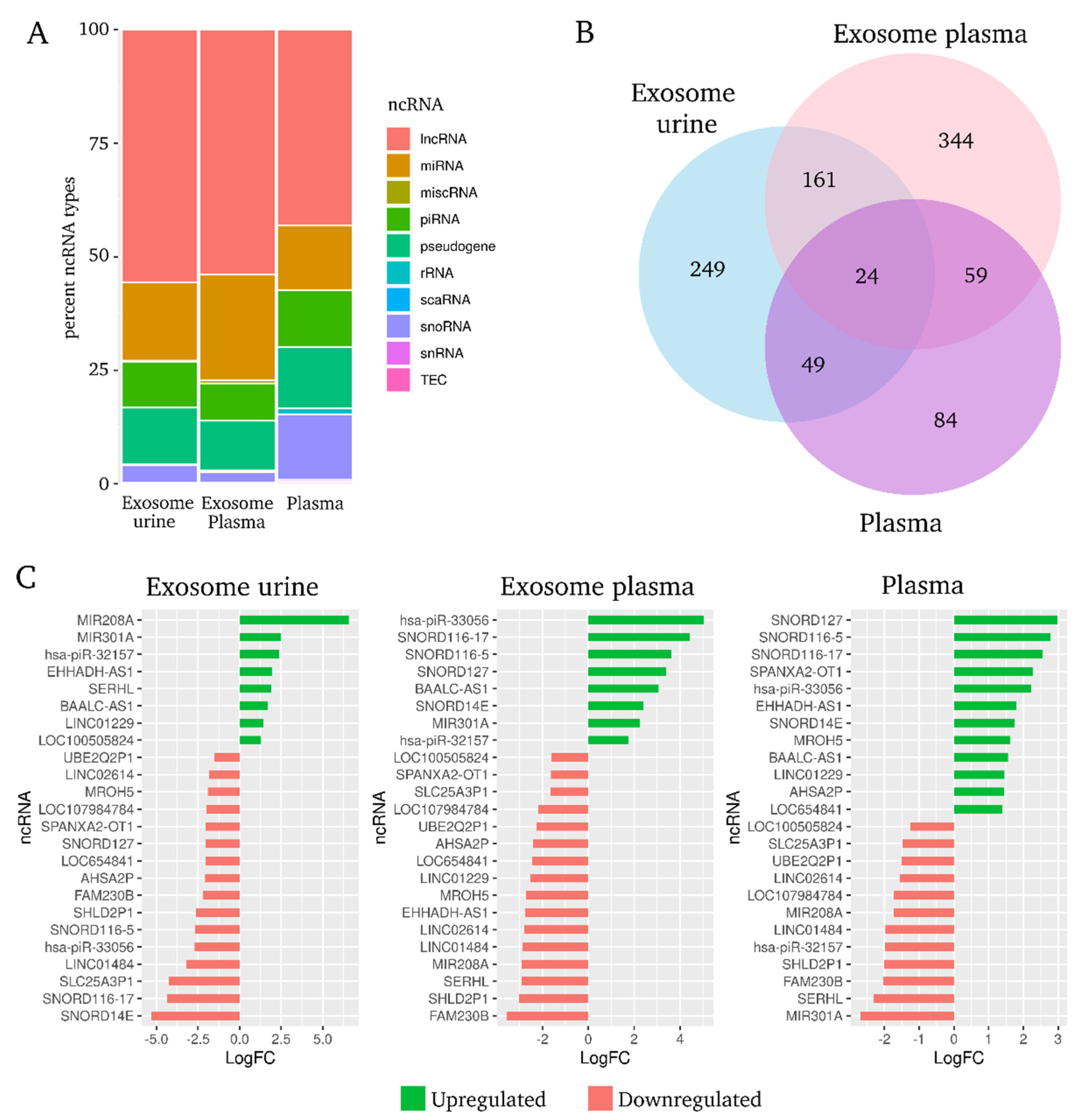
2.4. Differentially Expressed Non-Coding RNAs by Origin
2.5. Common Differentially Expressed lncRNA–miRNA–mRNA Network from Hypertensive Patients with Urinary Albumin Excretion
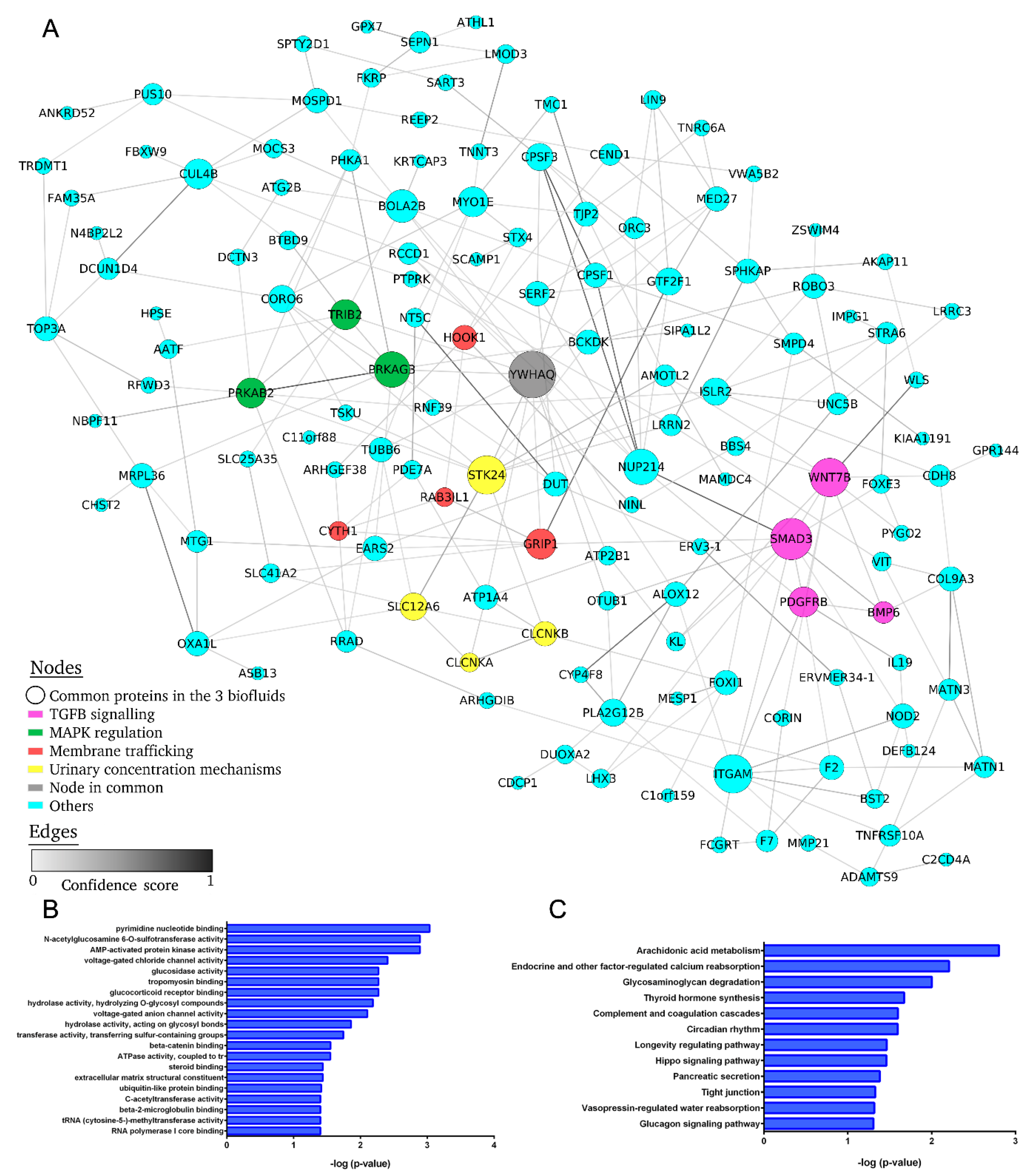
2.6. Protein–Protein Interaction Network of Differentially Expressed mRNA in Common to All Biofluids Associated with Albuminuria
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Biological Samples
4.3. Exosome Isolation and Characterization
4.4. RNA Extraction, Small RNA Library Preparation and Next-Generation Sequencing
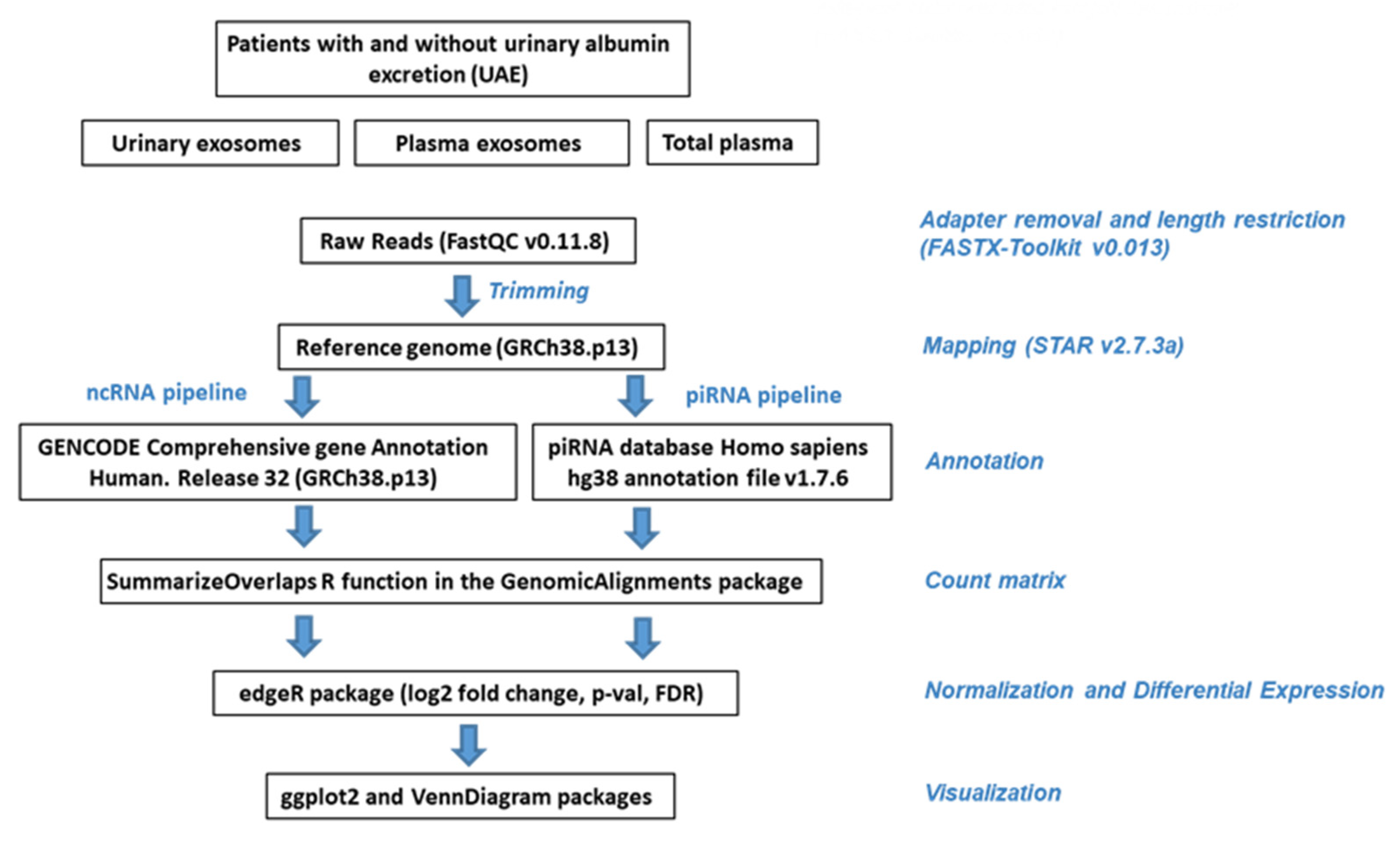
4.5. Small RNA Sequencing Data Analysis
4.6. Preprocessing, Annotation and Normalization
4.7. Statistical Analysis
4.8. Non-Coding RNA Target Predictions
4.9. Molecular Pathways Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [Green Version]
- Mennuni, S.; Rubattu, S.; Pierelli, G.; Tocci, G.; Fofi, C.; Volpe, M. Hypertension and kidneys: Unraveling complex molecular mechanisms underlying hypertensive renal damage. J. Hum. Hypertens. 2014, 28, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Mann, J.F.; Yi, Q.; Zinman, B.; Dinneen, S.F.; Hoogwerf, B.; Halle, J.P.; Young, J.; Rashkow, A.; Joyce, C.; et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001, 286, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Pascual, J.M.; Rodilla, E.; Costa, J.A.; Garcia-Escrich, M.; Gonzalez, C.; Redon, J. Prognostic value of microalbuminuria during antihypertensive treatment in essential hypertension. Hypertension 2014, 64, 1228–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.D. Non-coding RNA: A new frontier in regulatory biology. Natl. Sci. Rev. 2014, 1, 190–204. [Google Scholar] [CrossRef] [Green Version]
- Consortium, E.P. The ENCODE (ENCyclopedia Of DNA Elements) Project. Science 2004, 306, 636–640. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and their Integrated Networks. J. Integr. Bioinform. 2019, 16. [Google Scholar] [CrossRef]
- Beermann, J.; Piccoli, M.T.; Viereck, J.; Thum, T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol. Rev. 2016, 96, 1297–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T. Small non-coding RNAs as epigenetic regulators. In Nutritional Epigenomics; Ferguson, B.S., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 14, pp. 37–47. [Google Scholar]
- Lopez, J.P.; Diallo, A.; Cruceanu, C.; Fiori, L.M.; Laboissiere, S.; Guillet, I.; Fontaine, J.; Ragoussis, J.; Benes, V.; Turecki, G.; et al. Biomarker discovery: Quantification of microRNAs and other small non-coding RNAs using next generation sequencing. BMC Med. Genom. 2015, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.L.J.; Zhu, L. Cancer and non-coding RNAs. In Nutritional Epigenomics; Ferguson, B.S., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 14, pp. 119–132. [Google Scholar]
- Moreno, J.A.; Hamza, E.; Guerrero-Hue, M.; Rayego-Mateos, S.; Garcia-Caballero, C.; Vallejo-Mudarra, M.; Metzinger, L.; Metzinger-Le Meuth, V. Non-Coding RNAs in Kidney Diseases: The Long and Short of Them. Int. J. Mol. Sci. 2021, 22, 6077. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, K.; Karolina, D.S.; Sepramaniam, S.; Armugam, A.; Wintour, E.M.; Bertram, J.F.; Jeyaseelan, K. Role of microRNAs in kidney homeostasis and disease. Kidney Int. 2012, 81, 617–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzen, J.M.; Thum, T. Circulating and urinary microRNAs in kidney disease. Clin. J. Am. Soc. Nephrol. 2012, 7, 1528–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, K.; Yokoi, H.; Mori, K.; Kasahara, M.; Kuwabara, T.; Imamaki, H.; Ishii, A.; Mori, K.P.; Kato, Y.; Ohno, S.; et al. MicroRNA-26a inhibits TGF-beta-induced extracellular matrix protein expression in podocytes by targeting CTGF and is downregulated in diabetic nephropathy. Diabetologia 2015, 58, 2169–2180. [Google Scholar] [CrossRef] [Green Version]
- Gracia, T.; Wang, X.; Su, Y.; Norgett, E.E.; Williams, T.L.; Moreno, P.; Micklem, G.; Karet Frankl, F.E. Urinary Exosomes Contain MicroRNAs Capable of Paracrine Modulation of Tubular Transporters in Kidney. Sci. Rep. 2017, 7, 40601. [Google Scholar] [CrossRef] [Green Version]
- Ignarski, M.; Islam, R.; Muller, R.U. Long Non-Coding RNAs in Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3276. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Ma, Y.; Wu, S.; Zhuang, Y.; Liu, X.; Lv, L.; Zhang, G. Construction and analysis of a lncRNAmiRNAmRNA network based on competitive endogenous RNA reveals functional lncRNAs in diabetic cardiomyopathy. Mol. Med. Rep. 2019, 20, 1393–1403. [Google Scholar] [CrossRef]
- Zou, J.B.; Chai, H.B.; Zhang, X.F.; Guo, D.Y.; Tai, J.; Wang, Y.; Liang, Y.L.; Wang, F.; Cheng, J.X.; Wang, J.; et al. Reconstruction of the lncRNA-miRNA-mRNA network based on competitive endogenous RNA reveal functional lncRNAs in Cerebral Infarction. Sci. Rep. 2019, 9, 12176. [Google Scholar] [CrossRef]
- Charles, S.; Natarajan, J. Integrated regulatory network based on lncRNA-miRNA-mRNA-TF reveals key genes and sub-networks associated with dilated cardiomyopathy. Comput. Biol. Chem. 2021, 92, 107500. [Google Scholar] [CrossRef]
- Rayford, K.J.; Cooley, A.; Rumph, J.T.; Arun, A.; Rachakonda, G.; Villalta, F.; Lima, M.F.; Pratap, S.; Misra, S.; Nde, P.N. piRNAs as Modulators of Disease Pathogenesis. Int. J. Mol. Sci. 2021, 22, 2373. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.W.; Siomi, M.C.; Siomi, H. PIWI-Interacting RNA: Its Biogenesis and Functions. Ann. Rev. Biochem. 2015, 84, 405–433. [Google Scholar] [CrossRef]
- Preethi Krishnan, S.D. piRNAs in the Pathophysiology of Disease and Potential Clinical Applications. In AGO-Driven Non-Coding RNAs; Mallick, B., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 335–356. [Google Scholar]
- Erdbrugger, U.; Le, T.H. Extracellular vesicles as a novel diagnostic and research tool for patients with HTN and kidney disease. Am. J. Physiol. Ren. Physiol. 2019, 317, F641–F647. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Arroyo, O.; Ortega, A.; Redon, J.; Cortes, R. Therapeutic Potential of Extracellular Vesicles in Hypertension-Associated Kidney Disease. Hypertension 2021, 77, 28–38. [Google Scholar] [CrossRef]
- Khurana, R.; Ranches, G.; Schafferer, S.; Lukasser, M.; Rudnicki, M.; Mayer, G.; Huttenhofer, A. Identification of urinary exosomal noncoding RNAs as novel biomarkers in chronic kidney disease. RNA 2017, 23, 142–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelemen, E.; Danis, J.; Goblos, A.; Bata-Csorgo, Z.; Szell, M. Exosomal long non-coding RNAs as biomarkers in human diseases. EJIFCC 2019, 30, 224–236. [Google Scholar] [PubMed]
- Zeuschner, P.; Linxweiler, J.; Junker, K. Non-coding RNAs as biomarkers in liquid biopsies with a special emphasis on extracellular vesicles in urological malignancies. Expert. Rev. Mol. Diagn. 2020, 20, 151–167. [Google Scholar] [CrossRef]
- Perez-Hernandez, J.; Riffo-Campos, A.L.; Ortega, A.; Martinez-Arroyo, O.; Perez-Gil, D.; Olivares, D.; Solaz, E.; Martinez, F.; Martinez-Hervas, S.; Chaves, F.J.; et al. Urinary- and Plasma-Derived Exosomes Reveal a Distinct MicroRNA Signature Associated With Albuminuria in Hypertension. Hypertension 2021, 77, 960–971. [Google Scholar] [CrossRef]
- Perez-Hernandez, J.; Olivares, D.; Forner, M.J.; Ortega, A.; Solaz, E.; Martinez, F.; Chaves, F.J.; Redon, J.; Cortes, R. Urinary exosome miR-146a is a potential marker of albuminuria in essential hypertension. J. Transl. Med. 2018, 16, 228. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Sun, X.; Scicluna, B.J.; Coleman, B.M.; Hill, A.F. Characterization and deep sequencing analysis of exosomal and non-exosomal miRNA in human urine. Kidney Int. 2014, 86, 433–444. [Google Scholar] [CrossRef] [Green Version]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Villarroya-Beltri, C.; Gutierrez-Vazquez, C.; Sanchez-Cabo, F.; Perez-Hernandez, D.; Vazquez, J.; Martin-Cofreces, N.; Martinez-Herrera, D.J.; Pascual-Montano, A.; Mittelbrunn, M.; Sanchez-Madrid, F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013, 4, 2980. [Google Scholar] [CrossRef] [Green Version]
- Ju, W.; Nair, V.; Smith, S.; Zhu, L.; Shedden, K.; Song, P.X.K.; Mariani, L.H.; Eichinger, F.H.; Berthier, C.C.; Randolph, A.; et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci. Transl. Med. 2015, 7, 316ra193. [Google Scholar] [CrossRef] [Green Version]
- Beckerman, P.; Qiu, C.; Park, J.; Ledo, N.; Ko, Y.A.; Park, A.D.; Han, S.Y.; Choi, P.; Palmer, M.; Susztak, K. Human Kidney Tubule-Specific Gene Expression Based Dissection of Chronic Kidney Disease Traits. EBioMedicine 2017, 24, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Usa, K.; Wang, F.; Liu, P.; Geurts, A.M.; Li, J.; Williams, A.M.; Regner, K.R.; Kong, Y.; Liu, H.; et al. MicroRNA-214-3p in the Kidney Contributes to the Development of Hypertension. J. Am. Soc. Nephrol. 2018, 29, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Ozata, D.M.; Gainetdinov, I.; Zoch, A.; O’Carroll, D.; Zamore, P.D. PIWI-interacting RNAs: Small RNAs with big functions. Nat. Rev. Genet. 2019, 20, 89–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhari, S.; Mallet, R.T.; Shotorbani, P.Y.; Tao, Y.; Ma, R. Store-operated calcium entry: Pivotal roles in renal physiology and pathophysiology. Exp. Biol. Med. 2021, 246, 305–316. [Google Scholar] [CrossRef]
- Zeng, B.; Chen, G.L.; Garcia-Vaz, E.; Bhandari, S.; Daskoulidou, N.; Berglund, L.M.; Jiang, H.; Hallett, T.; Zhou, L.P.; Huang, L.; et al. ORAI channels are critical for receptor-mediated endocytosis of albumin. Nat. Commun. 2017, 8, 1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huwiler, A.; Pfeilschifter, J. Sphingolipid signaling in renal fibrosis. Matrix Biol. 2018, 68–69, 230–247. [Google Scholar] [CrossRef]
- Loeffler, I.; Wolf, G. Transforming growth factor-beta and the progression of renal disease. Nephrol. Dial Transplant. 2014, 29 (Suppl. S1), i37–i45. [Google Scholar] [CrossRef] [Green Version]
- Falaleeva, M.; Surface, J.; Shen, M.; de la Grange, P.; Stamm, S. SNORD116 and SNORD115 change expression of multiple genes and modify each other’s activity. Gene 2015, 572, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Sachdev, P.; Yan, L.; Chan, J.L.; Trenkle, T.; McClelland, M.; Welsh, J.; Wang, L.H. Vav3 mediates receptor protein tyrosine kinase signaling, regulates GTPase activity, modulates cell morphology, and induces cell transformation. Mol. Cell Biol. 2000, 20, 9212–9224. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, H.L.; Liu, T.T.; Lan, H.Y. TGF-Beta as a Master Regulator of Diabetic Nephropathy. Int. J. Mol. Sci. 2021, 22, 7881. [Google Scholar] [CrossRef]
- Wang, R.; Wu, S.T.; Yang, X.; Qian, Y.; Choi, J.P.; Gao, R.; Song, S.; Wang, Y.; Zhuang, T.; Wong, J.J.; et al. Pdcd10-Stk24/25 complex controls kidney water reabsorption by regulating Aqp2 membrane targeting. JCI Insight 2021, 6, 6. [Google Scholar] [CrossRef]
- Wandinger-Ness, A.; Zerial, M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb. Perspect. Biol. 2014, 6, a022616. [Google Scholar] [CrossRef]
- Sztul, E.; Chen, P.W.; Casanova, J.E.; Cherfils, J.; Dacks, J.B.; Lambright, D.G.; Lee, F.S.; Randazzo, P.A.; Santy, L.C.; Schurmann, A.; et al. ARF GTPases and their GEFs and GAPs: Concepts and challenges. Mol. Biol. Cell 2019, 30, 1249–1271. [Google Scholar] [CrossRef] [PubMed]
- Devuyst, O.; Guggino, W.B. Chloride channels in the kidney: Lessons learned from knockout animals. Am. J. Physiol. Renal Physiol. 2002, 283, F1176–F1191. [Google Scholar] [CrossRef]
- Kuo, I.Y.; Ehrlich, B.E. Ion channels in renal disease. Chem. Rev. 2012, 112, 6353–6372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, S.P.; Zhou, H.; Setia, O.; Dardik, A.; Fernandez-Hernando, C.; Goodwin, J. Podocyte Glucocorticoid Receptors Are Essential for Glomerular Endothelial Cell Homeostasis in Diabetes Mellitus. J. Am. Heart Assoc. 2021, 10, e019437. [Google Scholar] [CrossRef] [PubMed]
- Deckert, T.; Feldt-Rasmussen, B.; Borch-Johnsen, K.; Jensen, T.; Kofoed-Enevoldsen, A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989, 32, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Bohm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press 2014, 23, 3–16. [Google Scholar] [CrossRef]
- Olivares, D.; Perez-Hernandez, J.; Perez-Gil, D.; Chaves, F.J.; Redon, J.; Cortes, R. Optimization of small RNA library preparation protocol from human urinary exosomes. J. Transl. Med. 2020, 18, 132. [Google Scholar] [CrossRef]
- Wingett, S.W.; Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Research 2018, 7, 1338. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing, S. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, M.; Huber, W.; Pages, H.; Aboyoun, P.; Carlson, M.; Gentleman, R.; Morgan, M.T.; Carey, V.J. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 2013, 9, e1003118. [Google Scholar] [CrossRef]
- Morgan, M.P.H.; Obenchain, V.; Hayden, N. Rsamtools: Binary alignment (BAM), FASTA, Variant Call (BCF), and Tabix File Import; 2021. Available online: https://bioconductor.org/packages/Rsamtools (accessed on 15 October 2021).
- Frankish, A.; Diekhans, M.; Ferreira, A.M.; Johnson, R.; Jungreis, I.; Loveland, J.; Mudge, J.M.; Sisu, C.; Wright, J.; Armstrong, J.; et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019, 47, D766–D773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhang, P.; Lu, Y.; Li, Y.; Zheng, Y.; Kan, Y.; Chen, R.; He, S. piRBase: A comprehensive database of piRNA sequences. Nucleic Acids Res. 2019, 47, D175–D180. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, M. org.Hs.eg.db: Genome Wide Annotation for Human; R Package Version 3.8.2. 2019. Available online: https://bioconductor.org/packages/release/data/annotation/html/org.Hs.eg.db.html (accessed on 13 October 2021).
- Wickham, H. ggplot2. Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Fukunaga, T.; Iwakiri, J.; Ono, Y.; Hamada, M. LncRRIsearch: A Web Server for lncRNA-RNA Interaction Prediction Integrated with Tissue-Specific Expression and Subcellular Localization Data. Front. Genet. 2019, 10, 462. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.Y.; Lin, Y.C.; Li, J.; Huang, K.Y.; Shrestha, S.; Hong, H.C.; Tang, Y.; Chen, Y.G.; Jin, C.N.; Yu, Y.; et al. miRTarBase 2020: Updates to the experimentally validated microRNA-target interaction database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]

| Variables | Albuminuria (UAE) (n = 22) | Normoalbuminuria (Non-UAE) (n = 26) |
|---|---|---|
| Age (years) | 52.2 ± 8.3 | 55.0 ± 5.3 |
| Gender (male) | 68.2% | 65.4% |
| SBP (mmHg) | 136± 15 | 136 ± 24 |
| DBP (mmHg) | 85 ± 10 | 87 ± 14 |
| PP (mmHg) | 51 ± 12 | 48 ± 17 |
| Glucose (mg/dL) | 122 ± 46 | 119 ± 41 |
| Glycated hemoglobin (%) | 6.6 ± 1.2 | 6.0 ± 0.9 |
| Total cholesterol (mg/dL) | 200 ± 34 ** | 173 ± 29 |
| LDL (mg/dL) | 128 ± 30 ** | 108 ± 25 |
| HDL (mg/dL) | 51 ± 14 | 50 ± 10 |
| Triglycerides (mg/dL) | 153 ± 78 | 127 ± 60 |
| Plasma creatinine (mg/dL) | 0.87 ± 0.30 | 0.90 ± 0.22 |
| GFR (mL/min/1.73 m2) | 96 ± 27 | 87 ± 19 |
| Body mass index (kg/m2) | 32 ± 7 | 30 ± 5 |
| Obesity grade (%) | ||
| Grade I | 29 | 20 |
| Grade II | 9 | 12 |
| Grade III | 14 | 8 |
| Diabetes (%) | 41 | 35 |
| Dyslipidemia (%) | 86 | 85 |
| Smoking (%) | 55 | 48 |
| UAE/Creatinine (mg/g) | 146.4 ± 144.3 *** | 3.1 ± 1.7 |
| Antihypertensive treatment (%) | ||
| ARB | 95 | 92 |
| CCB | 36 | 38 |
| Diuretics | 68 | 62 |
| Statins | 32 | 8 |
| RNA | Degree | Betweenness Centrality | Closeness Centrality |
|---|---|---|---|
| LINC02614 | 49 | 0.321215546 | 0.447368421 |
| hsa-miR-301a-3p | 34 | 0.223753645 | 0.354166667 |
| BAALC-AS1 | 32 | 0.127310962 | 0.392307692 |
| FAM230B | 31 | 0.136936472 | 0.375 |
| LOC100505824 | 28 | 0.128194316 | 0.387341772 |
| LINC01484 | 20 | 0.090634319 | 0.350114416 |
| LOC654841 | 14 | 0.020020697 | 0.348519362 |
| LINC01229 | 14 | 0.015796606 | 0.334792123 |
| EHHADH-AS1 | 13 | 0.036008241 | 0.34537246 |
| SPANXA2-OT1 | 9 | 0.01894646 | 0.31875 |
| LOC107984784 | 7 | 0.003982221 | 0.330453564 |
| hsa-mir-208a-5p | 6 | 0.037362168 | 0.263339071 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riffo-Campos, A.L.; Perez-Hernandez, J.; Ortega, A.; Martinez-Arroyo, O.; Flores-Chova, A.; Redon, J.; Cortes, R. Exosomal and Plasma Non-Coding RNA Signature Associated with Urinary Albumin Excretion in Hypertension. Int. J. Mol. Sci. 2022, 23, 823. https://doi.org/10.3390/ijms23020823
Riffo-Campos AL, Perez-Hernandez J, Ortega A, Martinez-Arroyo O, Flores-Chova A, Redon J, Cortes R. Exosomal and Plasma Non-Coding RNA Signature Associated with Urinary Albumin Excretion in Hypertension. International Journal of Molecular Sciences. 2022; 23(2):823. https://doi.org/10.3390/ijms23020823
Chicago/Turabian StyleRiffo-Campos, Angela L., Javier Perez-Hernandez, Ana Ortega, Olga Martinez-Arroyo, Ana Flores-Chova, Josep Redon, and Raquel Cortes. 2022. "Exosomal and Plasma Non-Coding RNA Signature Associated with Urinary Albumin Excretion in Hypertension" International Journal of Molecular Sciences 23, no. 2: 823. https://doi.org/10.3390/ijms23020823
APA StyleRiffo-Campos, A. L., Perez-Hernandez, J., Ortega, A., Martinez-Arroyo, O., Flores-Chova, A., Redon, J., & Cortes, R. (2022). Exosomal and Plasma Non-Coding RNA Signature Associated with Urinary Albumin Excretion in Hypertension. International Journal of Molecular Sciences, 23(2), 823. https://doi.org/10.3390/ijms23020823









