A New Player in the Hippocampus: A Review on VGLUT3+ Neurons and Their Role in the Regulation of Hippocampal Activity and Behaviour
Abstract
1. Introduction
2. Characterisation of VGLUT3
2.1. Anatomical Distribution of VGLUT3 in the Central Nervous System
2.2. Glutamate as a Secondary Neurotransmitter in VGLUT3+ Neurons
2.3. Electrophysiological Characteristics of VGLUT3
3. Implications of VGLUT3 in Physiology
4. Characteristics of the VGLUT3 KO Mice
5. The Hippocampus
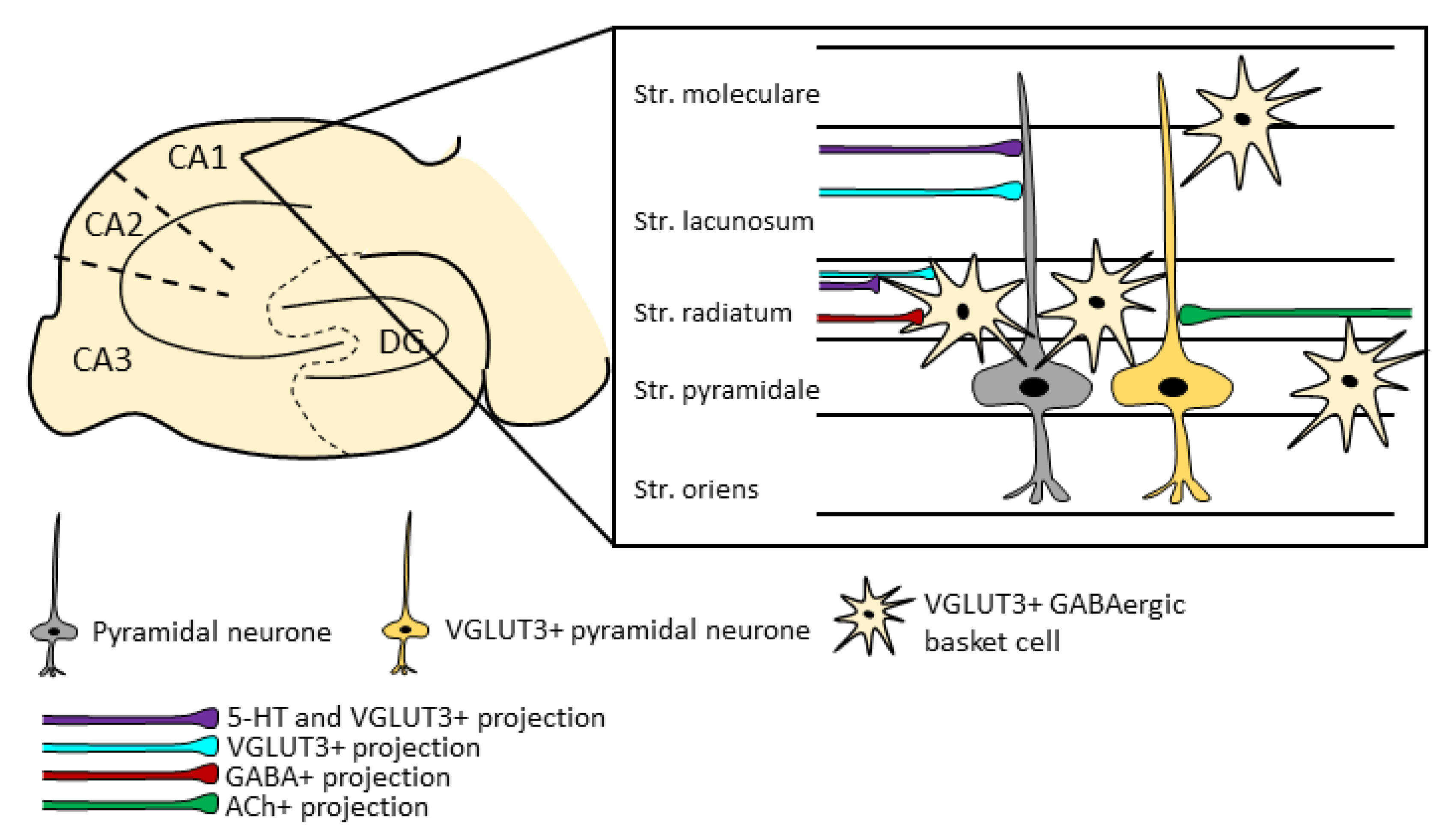
5.1. Characteristics of the VGLUT3+ Neurons in the Hippocampus
5.2. Hippocampal VGLUT3+ Projections
5.3. Role of the Hippocampal VGLUT3 Positivity
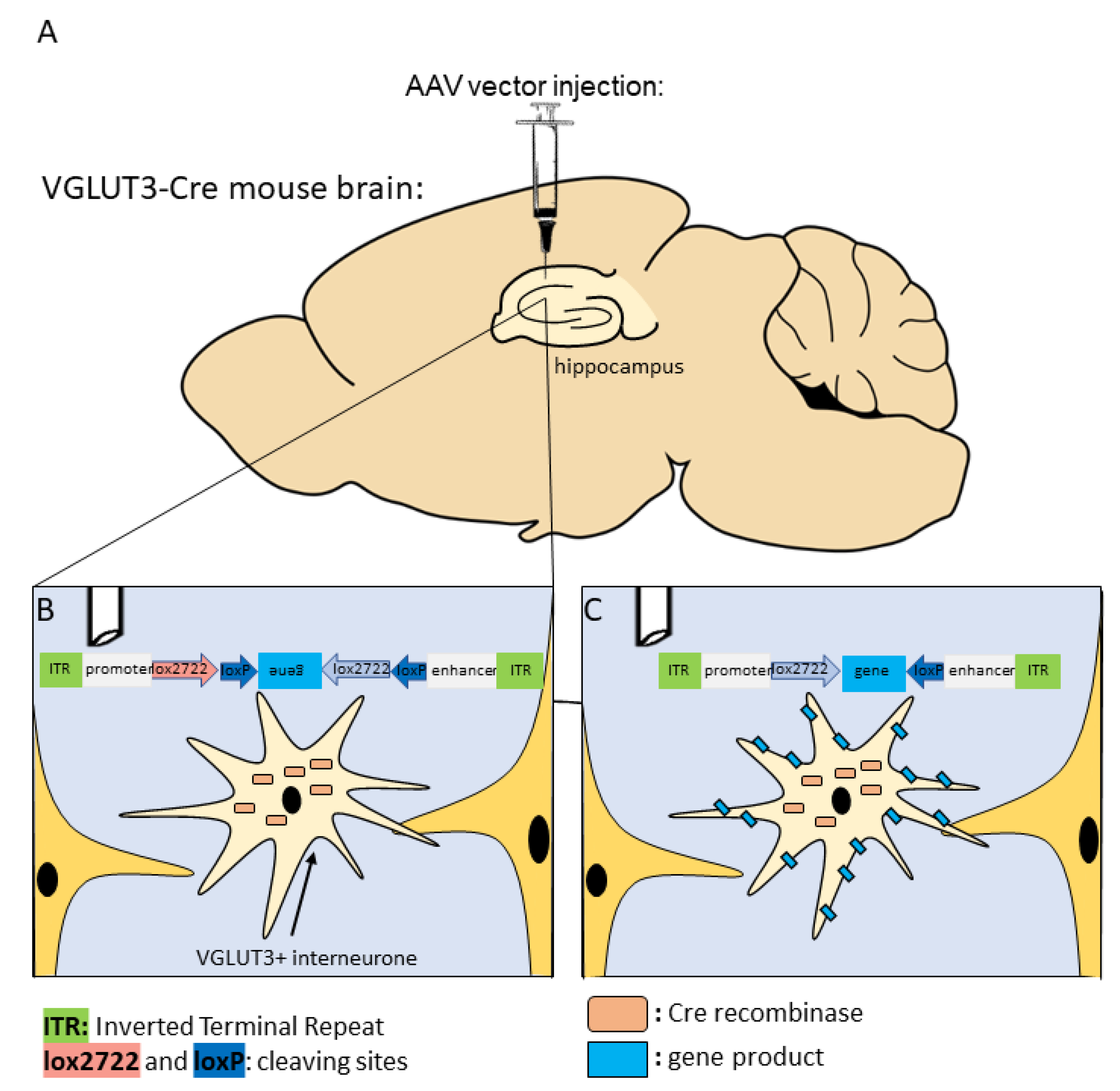
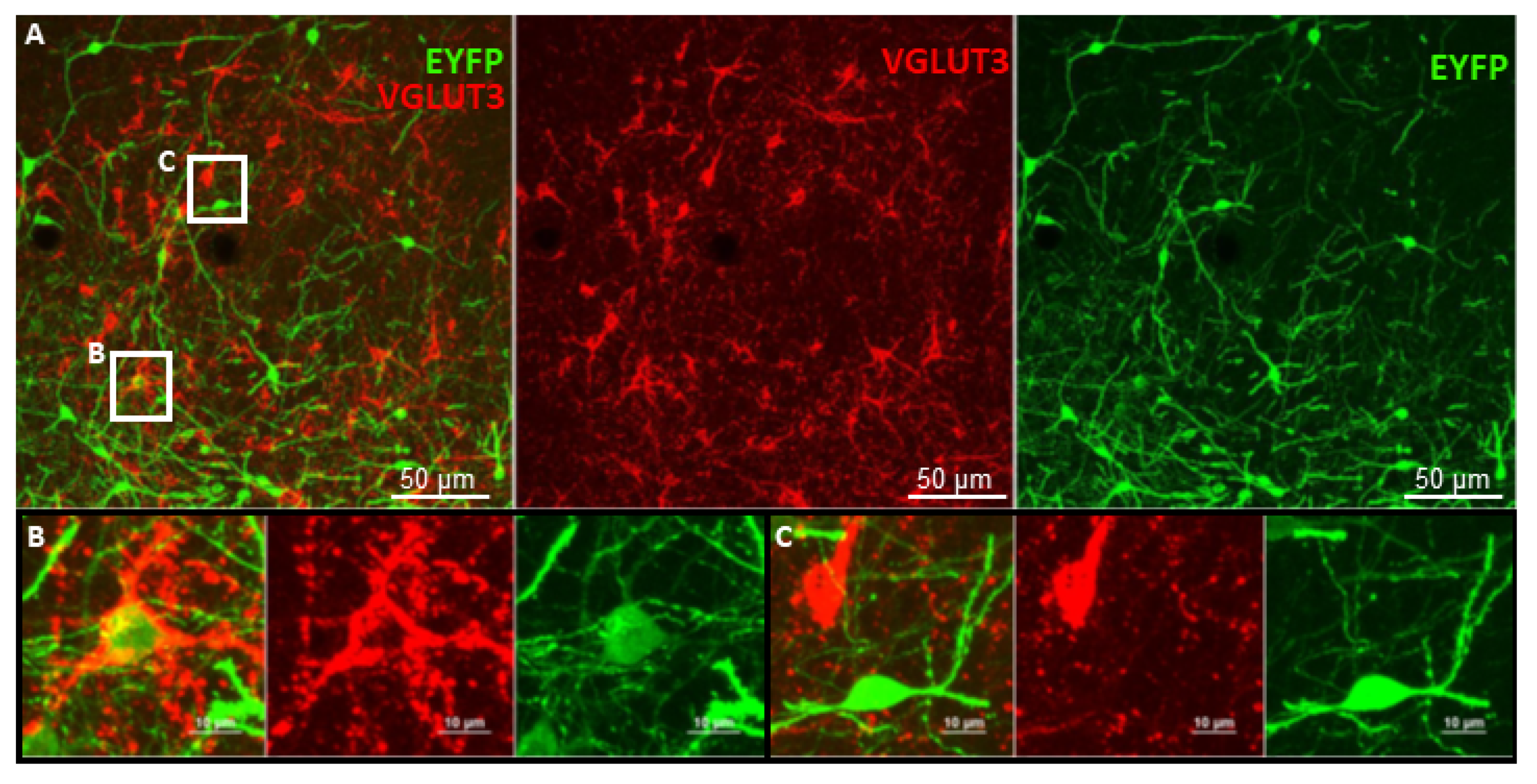
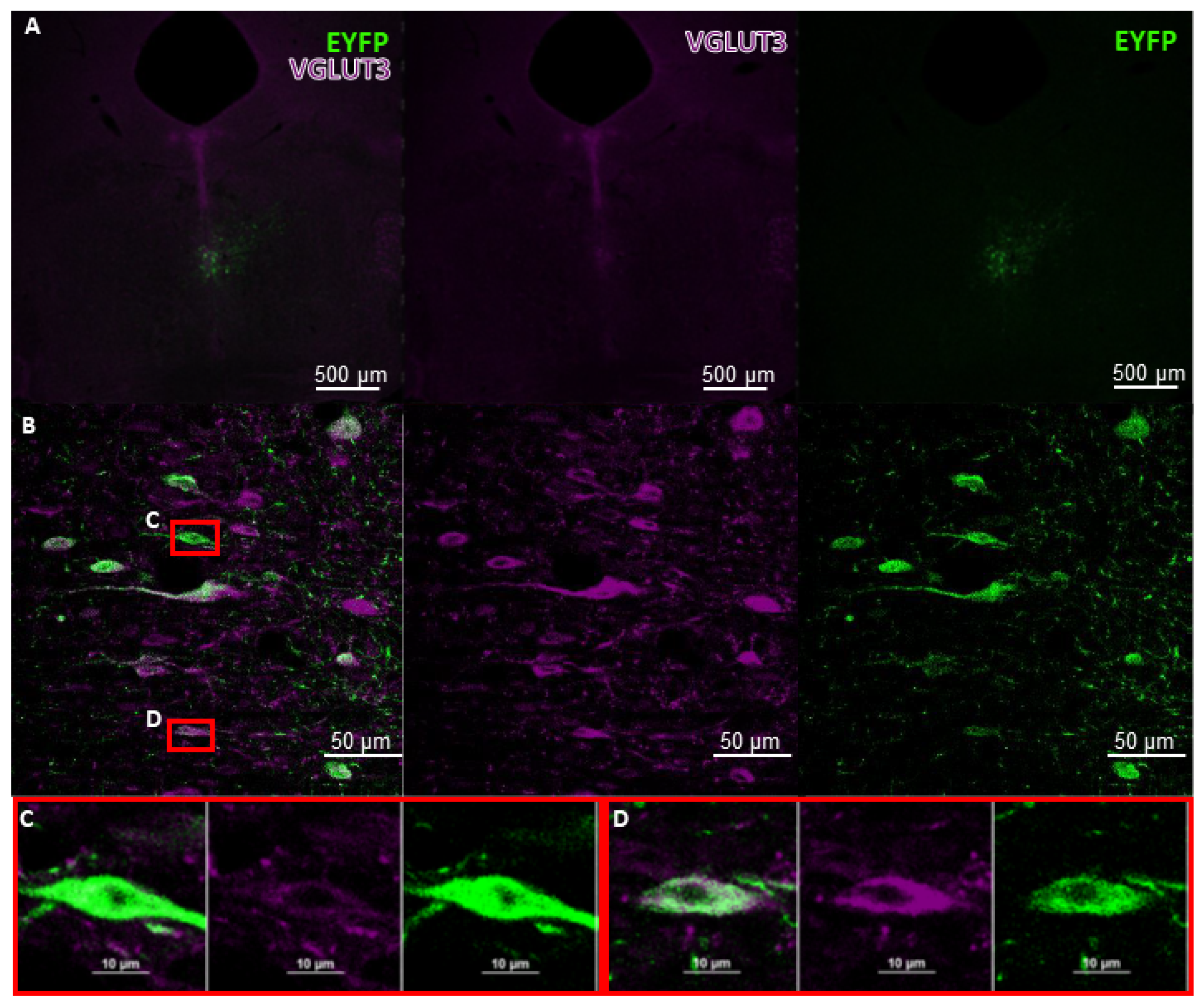
6. Future Perspectives for Selective Hippocampal VGLUT3+ Manipulations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burnstock, G. Do some nerve cells release more than one transmitter? Neuroscience 1976, 1, 239–248. [Google Scholar] [CrossRef]
- Takamori, S.; Rhee, J.S.; Rosenmund, C.; Jahn, R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature 2000, 407, 189–194. [Google Scholar] [CrossRef]
- Robinson, M.B. Acute regulation of sodium-dependent glutamate transporters: A focus on constitutive and regulated trafficking. Neurotransm. Transp. 2006, 175, 251–275. [Google Scholar] [CrossRef]
- Preobraschenski, J.; Zander, J.F.; Suzuki, T.; Ahnert-Hilger, G.; Jahn, R. Vesicular glutamate transporters use flexible anion and cation binding sites for efficient accumulation of neurotransmitter. Neuron 2014, 84, 1287–1301. [Google Scholar] [CrossRef]
- Fremeau, R.T., Jr.; Burman, J.; Qureshi, T.; Tran, C.H.; Proctor, J.; Johnson, J.; Zhang, H.; Sulzer, D.; Copenhagen, D.R.; Storm-Mathisen, J.; et al. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc. Natl. Acad. Sci. USA 2002, 99, 14488–14493. [Google Scholar] [CrossRef]
- Schafer, M.K.; Varoqui, H.; Defamie, N.; Weihe, E.; Erickson, J.D. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J. Biol. Chem. 2002, 277, 50734–50748. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, C.; Kuner, T. Heterogeneity of glutamatergic synapses: Cellular mechanisms and network consequences. Physiol. Rev. 2022, 102, 269–318. [Google Scholar] [CrossRef] [PubMed]
- Weston, M.C.; Nehring, R.B.; Wojcik, S.M.; Rosenmund, C. Interplay between VGLUT isoforms and endophilin A1 regulates neurotransmitter release and short-term plasticity. Neuron 2011, 69, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Fremeau, R.T., Jr.; Voglmaier, S.; Seal, R.P.; Edwards, R.H. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004, 27, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Hioki, H.; Fujiyama, F.; Taki, K.; Tomioka, R.; Furuta, T.; Tamamaki, N.; Kaneko, T. Differential distribution of vesicular glutamate transporters in the rat cerebellar cortex. Neuroscience 2003, 117, 1–6. [Google Scholar] [CrossRef]
- Vigneault, E.; Poirel, O.; Riad, M.; Prud’homme, J.; Dumas, S.; Turecki, G.; Fasano, C.; Mechawar, N.; El Mestikawy, S. Distribution of vesicular glutamate transporters in the human brain. Front. Neuroanat. 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Fremeau, R.T., Jr.; Troyer, M.D.; Pahner, I.; Nygaard, G.O.; Tran, C.H.; Reimer, R.J.; Bellocchio, E.E.; Fortin, D.; Storm-Mathisen, J.; Edwards, R.H. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron 2001, 31, 247–260. [Google Scholar] [CrossRef]
- Herzog, E.; Gilchrist, J.; Gras, C.; Muzerelle, A.; Ravassard, P.; Giros, B.; Gaspar, P.; El Mestikawy, S. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience 2004, 123, 983–1002. [Google Scholar] [CrossRef] [PubMed]
- Szonyi, A.; Zicho, K.; Barth, A.M.; Gonczi, R.T.; Schlingloff, D.; Torok, B.; Sipos, E.; Major, A.; Bardoczi, Z.; Sos, K.E.; et al. Median raphe controls acquisition of negative experience in the mouse. Science 2019, 366, 6469:eaay8746. [Google Scholar] [CrossRef]
- Bai, L.; Xu, H.; Collins, J.F.; Ghishan, F.K. Molecular and functional analysis of a novel neuronal vesicular glutamate transporter. J. Biol. Chem. 2001, 276, 36764–36769. [Google Scholar] [CrossRef] [PubMed]
- Gras, C.; Herzog, E.; Bellenchi, G.C.; Bernard, V.; Ravassard, P.; Pohl, M.; Gasnier, B.; Giros, B.; El Mestikawy, S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J. Neurosci. 2002, 22, 5442–5451. [Google Scholar] [CrossRef]
- Takamori, S.; Malherbe, P.; Broger, C.; Jahn, R. Molecular cloning and functional characterization of human vesicular glutamate transporter 3. EMBO Rep. 2002, 3, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Harkany, T.; Hartig, W.; Berghuis, P.; Dobszay, M.B.; Zilberter, Y.; Edwards, R.H.; Mackie, K.; Ernfors, P. Complementary distribution of type 1 cannabinoid receptors and vesicular glutamate transporter 3 in basal forebrain suggests input-specific retrograde signalling by cholinergic neurons. Eur. J. Neurosci. 2003, 18, 1979–1992. [Google Scholar] [CrossRef] [PubMed]
- Hioki, H.; Nakamura, H.; Ma, Y.F.; Konno, M.; Hayakawa, T.; Nakamura, K.C.; Fujiyama, F.; Kaneko, T. Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J. Comp. Neurol. 2010, 518, 668–686. [Google Scholar] [CrossRef]
- Hioki, H.; Fujiyama, F.; Nakamura, K.; Wu, S.X.; Matsuda, W.; Kaneko, T. Chemically specific circuit composed of vesicular glutamate transporter 3- and preprotachykinin B-producing interneurons in the rat neocortex. Cereb. Cortex. 2004, 14, 1266–1275. [Google Scholar] [CrossRef]
- Kudo, T.; Uchigashima, M.; Miyazaki, T.; Konno, K.; Yamasaki, M.; Yanagawa, Y.; Minami, M.; Watanabe, M. Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. J. Neurosci. 2012, 32, 18035–18046. [Google Scholar] [CrossRef]
- Stornetta, R.L.; Rosin, D.L.; Simmons, J.R.; McQuiston, T.J.; Vujovic, N.; Weston, M.C.; Guyenet, P.G. Coexpression of vesicular glutamate transporter-3 and gamma-aminobutyric acidergic markers in rat rostral medullary raphe and intermediolateral cell column. J. Comp. Neurol. 2005, 492, 477–494. [Google Scholar] [CrossRef]
- Somogyi, J.; Baude, A.; Omori, Y.; Shimizu, H.; El Mestikawy, S.; Fukaya, M.; Shigemoto, R.; Watanabe, M.; Somogyi, P. GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur. J. Neurosci. 2004, 19, 552–569. [Google Scholar] [CrossRef]
- Harkany, T.; Holmgren, C.; Hartig, W.; Qureshi, T.; Chaudhry, F.A.; Storm-Mathisen, J.; Dobszay, M.B.; Berghuis, P.; Schulte, G.; Sousa, K.M.; et al. Endocannabinoid-independent retrograde signaling at inhibitory synapses in layer 2/3 of neocortex: Involvement of vesicular glutamate transporter 3. J. Neurosci. 2004, 24, 4978–4988. [Google Scholar] [CrossRef]
- Miot, S.; Voituron, N.; Sterlin, A.; Vigneault, E.; Morel, L.; Matrot, B.; Ramanantsoa, N.; Amilhon, B.; Poirel, O.; Lepicard, E.; et al. The vesicular glutamate transporter VGLUT3 contributes to protection against neonatal hypoxic stress. J. Physiol. 2012, 590, 5183–5198. [Google Scholar] [CrossRef] [PubMed]
- Tatti, R.; Bhaukaurally, K.; Gschwend, O.; Seal, R.P.; Edwards, R.H.; Rodriguez, I.; Carleton, A. A population of glomerular glutamatergic neurons controls sensory information transfer in the mouse olfactory bulb. Nat. Commun. 2014, 5, 3791. [Google Scholar] [CrossRef]
- Rovira-Esteban, L.; Peterfi, Z.; Vikor, A.; Mate, Z.; Szabo, G.; Hajos, N. Morphological and physiological properties of CCK/CB1R-expressing interneurons in the basal amygdala. Brain Struct. Funct. 2017, 222, 3543–3565. [Google Scholar] [CrossRef] [PubMed]
- Ormel, L.; Stensrud, M.J.; Chaudhry, F.A.; Gundersen, V. A distinct set of synaptic-like microvesicles in atroglial cells contain VGLUT3. Glia 2012, 60, 1289–1300. [Google Scholar] [CrossRef]
- Li, D.; Herault, K.; Silm, K.; Evrard, A.; Wojcik, S.; Oheim, M.; Herzog, E.; Ropert, N. Lack of evidence for vesicular glutamate transporter expression in mouse astrocytes. J. Neurosci. 2013, 33, 4434–4455. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Matsumura, K.; Kobayashi, S.; Kaneko, T. Sympathetic premotor neurons mediating thermoregulatory functions. Neurosci. Res. 2005, 51, 1–8. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Hydling, F.; Olsson, E.; Shi, T.; Edwards, R.H.; Fujiyama, F.; Kaneko, T.; Hokfelt, T.; Cullheim, S.; Meister, B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse 2003, 50, 117–129. [Google Scholar] [CrossRef]
- Rosin, D.L.; Chang, D.A.; Guyenet, P.G. Afferent and efferent connections of the rat retrotrapezoid nucleus. J. Comp. Neurol. 2006, 499, 64–89. [Google Scholar] [CrossRef] [PubMed]
- Zerari-Mailly, F.; Braud, A.; Davido, N.; Toure, B.; Azerad, J.; Boucher, Y. Glutamate control of pulpal blood flow in the incisor dental pulp of the rat. Eur. J. Oral Sci. 2012, 120, 402–407. [Google Scholar] [CrossRef]
- Munguba, G.C.; Camp, A.S.; Risco, M.; Tapia, M.L.; Bhattacharya, S.K.; Lee, R.K. Vesicular glutamate transporter 3 in age-dependent optic neuropathy. Mol. Vis. 2011, 17, 413–419. [Google Scholar]
- Stensrud, M.J.; Chaudhry, F.A.; Leergaard, T.B.; Bjaalie, J.G.; Gundersen, V. Vesicular glutamate transporter-3 in the rodent brain: Vesicular colocalization with vesicular gamma-aminobutyric acid transporter. J. Comp. Neurol. 2013, 521, 3042–3056. [Google Scholar] [CrossRef]
- Del Pino, I.; Brotons-Mas, J.R.; Marques-Smith, A.; Marighetto, A.; Frick, A.; Marin, O.; Rico, B. Abnormal wiring of CCK(+) basket cells disrupts spatial information coding. Nat. Neurosci. 2017, 20, 784–792. [Google Scholar] [CrossRef]
- Stensrud, M.J.; Sogn, C.J.; Gundersen, V. Immunogold characteristics of VGLUT3-positive GABAergic nerve terminals suggest corelease of glutamate. J. Comp. Neurol. 2015, 523, 2698–2713. [Google Scholar] [CrossRef] [PubMed]
- Jalabert, M.; Aston-Jones, G.; Herzog, E.; Manzoni, O.; Georges, F. Role of the bed nucleus of the stria terminalis in the control of ventral tegmental area dopamine neurons. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1336–1346. [Google Scholar] [CrossRef]
- Omiya, Y.; Uchigashima, M.; Konno, K.; Yamasaki, M.; Miyazaki, T.; Yoshida, T.; Kusumi, I.; Watanabe, M. VGluT3-expressing CCK-positive basket cells construct invaginating synapses enriched with endocannabinoid signaling proteins in particular cortical and cortex-like amygdaloid regions of mouse brains. J. Neurosci. 2015, 35, 4215–4228. [Google Scholar] [CrossRef]
- Case, D.T.; Burton, S.D.; Gedeon, J.Y.; Williams, S.G.; Urban, N.N.; Seal, R.P. Layer- and cell type-selective co-transmission by a basal forebrain cholinergic projection to the olfactory bulb. Nat. Commun. 2017, 8, 652. [Google Scholar] [CrossRef] [PubMed]
- Nickerson Poulin, A.; Guerci, A.; El Mestikawy, S.; Semba, K. Vesicular glutamate transporter 3 immunoreactivity is present in cholinergic basal forebrain neurons projecting to the basolateral amygdala in rat. J. Comp. Neurol. 2006, 498, 690–711. [Google Scholar] [CrossRef]
- Gras, C.; Amilhon, B.; Lepicard, E.M.; Poirel, O.; Vinatier, J.; Herbin, M.; Dumas, S.; Tzavara, E.T.; Wade, M.R.; Nomikos, G.G.; et al. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nat. Neurosci. 2008, 11, 292–300. [Google Scholar] [CrossRef]
- Higley, M.J.; Gittis, A.H.; Oldenburg, I.A.; Balthasar, N.; Seal, R.P.; Edwards, R.H.; Lowell, B.B.; Kreitzer, A.C.; Sabatini, B.L. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS ONE 2011, 6, e19155. [Google Scholar] [CrossRef]
- Nelson, A.B.; Bussert, T.G.; Kreitzer, A.C.; Seal, R.P. Striatal cholinergic neurotransmission requires VGLUT3. J. Neurosci. 2014, 34, 8772–8777. [Google Scholar] [CrossRef] [PubMed]
- Shutoh, F.; Ina, A.; Yoshida, S.; Konno, J.; Hisano, S. Two distinct subtypes of serotonergic fibers classified by co-expression with vesicular glutamate transporter 3 in rat forebrain. Neurosci. Lett. 2008, 432, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Voisin, A.N.; Mnie-Filali, O.; Giguere, N.; Fortin, G.M.; Vigneault, E.; El Mestikawy, S.; Descarries, L.; Trudeau, L.E. Axonal segregation and role of the vesicular glutamate transporter VGLUT3 in serotonin neurons. Front. Neuroanat. 2016, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Calizo, L.H.; Akanwa, A.; Ma, X.; Pan, Y.Z.; Lemos, J.C.; Craige, C.; Heemstra, L.A.; Beck, S.G. Raphe serotonin neurons are not homogenous: Electrophysiological, morphological and neurochemical evidence. Neuropharmacology 2011, 61, 524–543. [Google Scholar] [CrossRef]
- Ren, J.; Friedmann, D.; Xiong, J.; Liu, C.D.; Ferguson, B.R.; Weerakkody, T.; DeLoach, K.E.; Ran, C.; Pun, A.; Sun, Y.; et al. Anatomically defined and functionally distinct dorsal raphe serotonin sub-systems. Cell 2018, 175, 472–487 e420. [Google Scholar] [CrossRef]
- Gagnon, D.; Parent, M. Distribution of VGLUT3 in highly collateralized axons from the rat dorsal raphe nucleus as revealed by single-neuron reconstructions. PLoS ONE 2014, 9, e87709. [Google Scholar] [CrossRef]
- Commons, K.G. Locally collateralizing glutamate neurons in the dorsal raphe nucleus responsive to substance P contain vesicular glutamate transporter 3 (VGLUT3). J. Chem. Neuroanat. 2009, 38, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Le Maitre, E.; Fabre, V.; Bernard, J.F.; David Xu, Z.Q.; Hokfelt, T. Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J. Comp. Neurol. 2010, 518, 3464–3494. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Li, Y.; Hu, F.; Lu, Y.; Ma, M.; Feng, Q.; Zhang, J.E.; Wang, D.; Zeng, J.; et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 2014, 81, 1360–1374. [Google Scholar] [CrossRef]
- Zou, W.J.; Song, Y.L.; Wu, M.Y.; Chen, X.T.; You, Q.L.; Yang, Q.; Luo, Z.Y.; Huang, L.; Kong, Y.; Feng, J.; et al. A discrete serotonergic circuit regulates vulnerability to social stress. Nat. Commun. 2020, 11, 4218. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhang, S.; Qi, J.; Wang, H.; Cachope, R.; Mejias-Aponte, C.A.; Gomez, J.A.; Mateo-Semidey, G.E.; Beaudoin, G.M.J.; Paladini, C.A.; et al. Dorsal raphe dual serotonin-glutamate neurons drive reward by establishing excitatory synapses on VTA mesoaccumbens dopamine neurons. Cell Rep. 2019, 26, 1128–1142.e7. [Google Scholar] [CrossRef]
- Sengupta, A.; Holmes, A. A Discrete dorsal raphe to basal amygdala 5-HT circuit calibrates aversive memory. Neuron 2019, 103, 489–505.e7. [Google Scholar] [CrossRef]
- Sengupta, A.; Bocchio, M.; Bannerman, D.M.; Sharp, T.; Capogna, M. Control of Amygdala Circuits by 5-HT Neurons via 5-HT and Glutamate Cotransmission. J. Neurosci. 2017, 37, 1785–1796. [Google Scholar] [CrossRef]
- Martin-Ibanez, R.; Jenstad, M.; Berghuis, P.; Edwards, R.H.; Hioki, H.; Kaneko, T.; Mulder, J.; Canals, J.M.; Ernfors, P.; Chaudhry, F.A.; et al. Vesicular glutamate transporter 3 (VGLUT3) identifies spatially segregated excitatory terminals in the rat substantia nigra. Eur. J. Neurosci. 2006, 23, 1063–1070. [Google Scholar] [CrossRef]
- Sos, K.E.; Mayer, M.I.; Cserep, C.; Takacs, F.S.; Szonyi, A.; Freund, T.F.; Nyiri, G. Cellular architecture and transmitter phenotypes of neurons of the mouse median raphe region. Brain Struct. Funct. 2017, 222, 287–299. [Google Scholar] [CrossRef]
- Belmer, A.; Beecher, K.; Jacques, A.; Patkar, O.L.; Sicherre, F.; Bartlett, S.E. Axonal Non-segregation of the vesicular glutamate transporter VGLUT3 within serotonergic projections in the mouse forebrain. Front. Cell Neurosci. 2019, 13, 193. [Google Scholar] [CrossRef]
- Gaspar, P.; Lillesaar, C. Probing the diversity of serotonin neurons. Philos. Trans. R Soc. Lond B Biol. Sci. 2012, 367, 2382–2394. [Google Scholar] [CrossRef]
- Senft, R.A.; Freret, M.E.; Sturrock, N.; Dymecki, S.M. Neurochemically and hodologically distinct ascending VGLUT3 versus serotonin subsystems comprise the r2-Pet1 median raphe. J. Neurosci. 2021, 41, 2581–2600. [Google Scholar] [CrossRef]
- Amilhon, B.; Lepicard, E.; Renoir, T.; Mongeau, R.; Popa, D.; Poirel, O.; Miot, S.; Gras, C.; Gardier, A.M.; Gallego, J.; et al. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J. Neurosci. 2010, 30, 2198–2210. [Google Scholar] [CrossRef]
- El Mestikawy, S.; Wallen-Mackenzie, A.; Fortin, G.M.; Descarries, L.; Trudeau, L.E. From glutamate co-release to vesicular synergy: Vesicular glutamate transporters. Nat. Rev. Neurosci. 2011, 12, 204–216. [Google Scholar] [CrossRef]
- Fasano, C.; Rocchetti, J.; Pietrajtis, K.; Zander, J.F.; Manseau, F.; Sakae, D.Y.; Marcus-Sells, M.; Ramet, L.; Morel, L.J.; Carrel, D.; et al. Regulation of the hippocampal network by VGLUT3-positive CCK- GABAergic basket cells. Front. Cell Neurosci. 2017, 11, 140. [Google Scholar] [CrossRef]
- Pelkey, K.A.; Calvigioni, D.; Fang, C.; Vargish, G.; Ekins, T.; Auville, K.; Wester, J.C.; Lai, M.; Mackenzie-Gray Scott, C.; Yuan, X.; et al. Paradoxical network excitation by glutamate release from VGluT3(+) GABAergic interneurons. Elife 2020, 9, e51996. [Google Scholar] [CrossRef]
- Zimmermann, J.; Herman, M.A.; Rosenmund, C. Co-release of glutamate and GABA from single vesicles in GABAergic neurons exogenously expressing VGLUT3. Front. Synaptic Neurosci. 2015, 7, 16. [Google Scholar] [CrossRef][Green Version]
- Cropper, E.C.; Jing, J.; Vilim, F.S.; Weiss, K.R. Peptide cotransmitters as dynamic, intrinsic modulators of network activity. Front. Neural Circ. 2018, 12, 78. [Google Scholar] [CrossRef]
- Nusbaum, M.P.; Blitz, D.M.; Marder, E. Functional consequences of neuropeptide and small-molecule co-transmission. Nat. Rev. Neurosci. 2017, 18, 389–403. [Google Scholar] [CrossRef]
- Larsson, M.; Broman, J. Synaptic Organization of VGLUT3 Expressing low-threshold mechanosensitive c fiber terminals in the rodent spinal cord. eNeuro 2019, 6, ENEURO.0007-19.2019. [Google Scholar] [CrossRef]
- Crepel, F.; Galante, M.; Habbas, S.; McLean, H.; Daniel, H. Role of the vesicular transporter VGLUT3 in retrograde release of glutamate by cerebellar Purkinje cells. J. Neurophysiol. 2011, 105, 1023–1032. [Google Scholar] [CrossRef]
- Fuzik, J.; Zeisel, A.; Mate, Z.; Calvigioni, D.; Yanagawa, Y.; Szabo, G.; Linnarsson, S.; Harkany, T. Integration of electrophysiological recordings with single-cell RNA-seq data identifies neuronal subtypes. Nat. Biotechnol. 2016, 34, 175–183. [Google Scholar] [CrossRef]
- Kohus, Z.; Kali, S.; Rovira-Esteban, L.; Schlingloff, D.; Papp, O.; Freund, T.F.; Hajos, N.; Gulyas, A.I. Properties and dynamics of inhibitory synaptic communication within the CA3 microcircuits of pyramidal cells and interneurons expressing parvalbumin or cholecystokinin. J. Physiol. 2016, 594, 3745–3774. [Google Scholar] [CrossRef]
- Grimes, W.N.; Seal, R.P.; Oesch, N.; Edwards, R.H.; Diamond, J.S. Genetic targeting and physiological features of VGLUT3+ amacrine cells. Vis. Neurosci. 2011, 28, 381–392. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, G.P.; Zeng, R.; Guo, J.Y.; Chen, C.F.; Gong, S.S. Temporospatial expression and cellular localization of VGLUT3 in the rat cochlea. Brain Res. 2013, 1537, 100–110. [Google Scholar] [CrossRef]
- Obholzer, N.; Wolfson, S.; Trapani, J.G.; Mo, W.; Nechiporuk, A.; Busch-Nentwich, E.; Seiler, C.; Sidi, S.; Sollner, C.; Duncan, R.N.; et al. Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. J. Neurosci. 2008, 28, 2110–2118. [Google Scholar] [CrossRef]
- Ruel, J.; Emery, S.; Nouvian, R.; Bersot, T.; Amilhon, B.; Van Rybroek, J.M.; Rebillard, G.; Lenoir, M.; Eybalin, M.; Delprat, B.; et al. Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. Am. J. Hum. Genet. 2008, 83, 278–292. [Google Scholar] [CrossRef]
- Noh, J.; Seal, R.P.; Garver, J.A.; Edwards, R.H.; Kandler, K. Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nat. Neurosci. 2010, 13, 232–238. [Google Scholar] [CrossRef]
- Joshi, Y.; Petit, C.P.; Miot, S.; Guillet, M.; Sendin, G.; Bourien, J.; Wang, J.; Pujol, R.; El Mestikawy, S.; Puel, J.L.; et al. VGLUT3-p.A211V variant fuses stereocilia bundles and elongates synaptic ribbons. J. Physiol. 2021, 599, 5397–5416. [Google Scholar] [CrossRef]
- Seal, R.P.; Akil, O.; Yi, E.; Weber, C.M.; Grant, L.; Yoo, J.; Clause, A.; Kandler, K.; Noebels, J.L.; Glowatzki, E.; et al. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron 2008, 57, 263–275. [Google Scholar] [CrossRef]
- Akil, O.; Seal, R.P.; Burke, K.; Wang, C.; Alemi, A.; During, M.; Edwards, R.H.; Lustig, L.R. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 2012, 75, 283–293. [Google Scholar] [CrossRef]
- Kim, K.X.; Payne, S.; Yang-Hood, A.; Li, S.Z.; Davis, B.; Carlquist, J.; Babak, V.-G.; Gantz, J.A.; Kallogjeri, D.; Fitzpatrick, J.A.J.; et al. Vesicular glutamatergic transmission in noise-induced loss and repair of cochlear ribbon synapses. J. Neurosci. 2019, 39, 4434–4447. [Google Scholar] [CrossRef]
- Honsek, S.D.; Seal, R.P.; Sandkuhler, J. Presynaptic inhibition of optogenetically identified VGluT3+ sensory fibres by opioids and baclofen. Pain 2015, 156, 243–251. [Google Scholar] [CrossRef]
- Lou, S.; Duan, B.; Vong, L.; Lowell, B.B.; Ma, Q. Runx1 controls terminal morphology and mechanosensitivity of VGLUT3-expressing C-mechanoreceptors. J. Neurosci. 2013, 33, 870–882. [Google Scholar] [CrossRef]
- Balazsfi, D.; Fodor, A.; Torok, B.; Ferenczi, S.; Kovacs, K.J.; Haller, J.; Zelena, D. Enhanced innate fear and altered stress axis regulation in VGluT3 knockout mice. Stress 2018, 21, 151–161. [Google Scholar] [CrossRef]
- Horvath, H.R.; Fazekas, C.L.; Balazsfi, D.; Jain, S.K.; Haller, J.; Zelena, D. Contribution of vesicular glutamate transporters to stress response and related psychopathologies: Studies in VGluT3 knockout mice. Cell Mol. Neurobiol. 2018, 38, 37–52. [Google Scholar] [CrossRef]
- Olivan, A.M.; Perez-Rodriguez, R.; Roncero, C.; Arce, C.; Gonzalez, M.P.; Oset-Gasque, M.J. Plasma membrane and vesicular glutamate transporter expression in chromaffin cells of bovine adrenal medulla. J. Neurosci. Res. 2011, 89, 44–57. [Google Scholar] [CrossRef]
- Balazsfi, D.; Farkas, L.; Csikota, P.; Fodor, A.; Zsebok, S.; Haller, J.; Zelena, D. Sex-dependent role of vesicular glutamate transporter 3 in stress-regulation and related anxiety phenotype during the early postnatal period. Stress 2016, 19, 434–438. [Google Scholar] [CrossRef]
- Prakash, N.; Stark, C.J.; Keisler, M.N.; Luo, L.; Der-Avakian, A.; Dulcis, D. Serotonergic plasticity in the dorsal raphe nucleus characterizes susceptibility and resilience to anhedonia. J. Neurosci. 2020, 40, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Gammelsaeter, R.; Coppola, T.; Marcaggi, P.; Storm-Mathisen, J.; Chaudhry, F.A.; Attwell, D.; Regazzi, R.; Gundersen, V. A role for glutamate transporters in the regulation of insulin secretion. PLoS ONE 2011, 6, e22960. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.L.; Moazzami, A.R.; Longhurst, J.C. Stimulation of cardiac sympathetic afferents activates glutamatergic neurons in the parabrachial nucleus: Relation to neurons containing nNOS. Brain Res. 2005, 1053, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Krzyzanowska, W.; Pomierny, B.; Budziszewska, B.; Filip, M.; Pera, J. N-Acetylcysteine and ceftriaxone as preconditioning strategies in focal brain ischemia: Influence on glutamate transporters expression. Neurotox. Res. 2016, 29, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Alvarez-Sabin, J.; Davalos, A.; Diez-Tejedor, E.; Lizasoain, I.; Martinez-Vila, E.; Vivancos, J.; Zarranz, J.J. Consensus review. Pharmacological neuroprotection in cerebral ischemia: Is it still a therapeutic option? Neurologia 2003, 18, 368–384. [Google Scholar] [PubMed]
- Krzyzanowska, W.; Pomierny, B.; Bystrowska, B.; Pomierny-Chamiolo, L.; Filip, M.; Budziszewska, B.; Pera, J. Ceftriaxone- and N-acetylcysteine-induced brain tolerance to ischemia: Influence on glutamate levels in focal cerebral ischemia. PLoS ONE 2017, 12, e0186243. [Google Scholar] [CrossRef]
- Sanchez-Mendoza, E.; Burguete, M.C.; Castello-Ruiz, M.; Gonzalez, M.P.; Roncero, C.; Salom, J.B.; Arce, C.; Canadas, S.; Torregrosa, G.; Alborch, E.; et al. Transient focal cerebral ischemia significantly alters not only EAATs but also VGLUTs expression in rats: Relevance of changes in reactive astroglia. J. Neurochem. 2010, 113, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Callaerts-Vegh, Z.; Moechars, D.; Van Acker, N.; Daneels, G.; Goris, I.; Leo, S.; Naert, A.; Meert, T.; Balschun, D.; D’Hooge, R. Haploinsufficiency of VGluT1 but not VGluT2 impairs extinction of spatial preference and response suppression. Behav. Brain Res. 2013, 245, 13–21. [Google Scholar] [CrossRef]
- Wallen-Mackenzie, A.; Gezelius, H.; Thoby-Brisson, M.; Nygard, A.; Enjin, A.; Fujiyama, F.; Fortin, G.; Kullander, K. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J. Neurosci. 2006, 26, 12294–12307. [Google Scholar] [CrossRef]
- Wallen-Mackenzie, A.; Wootz, H.; Englund, H. Genetic inactivation of the vesicular glutamate transporter 2 (VGLUT2) in the mouse: What have we learnt about functional glutamatergic neurotransmission? Ups J. Med. Sci. 2010, 115, 11–20. [Google Scholar] [CrossRef]
- Moechars, D.; Weston, M.C.; Leo, S.; Callaerts-Vegh, Z.; Goris, I.; Daneels, G.; Buist, A.; Cik, M.; van der Spek, P.; Kass, S.; et al. Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. J. Neurosci. 2006, 26, 12055–12066. [Google Scholar] [CrossRef]
- Fremeau, R.T., Jr.; Kam, K.; Qureshi, T.; Johnson, J.; Copenhagen, D.R.; Storm-Mathisen, J.; Chaudhry, F.A.; Nicoll, R.A.; Edwards, R.H. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science 2004, 304, 1815–1819. [Google Scholar] [CrossRef]
- Gezelius, H.; Wallen-Mackenzie, A.; Enjin, A.; Lagerstrom, M.; Kullander, K. Role of glutamate in locomotor rhythm generating neuronal circuitry. J. Physiol.-Paris 2006, 100, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Divito, C.B.; Steece-Collier, K.; Case, D.T.; Williams, S.P.; Stancati, J.A.; Zhi, L.; Rubio, M.E.; Sortwell, C.E.; Collier, T.J.; Sulzer, D.; et al. Loss of VGLUT3 Produces circadian-dependent hyperdopaminergia and ameliorates motor dysfunction and l-dopa-mediated dyskinesias in a model of parkinson’s disease. J. Neurosci. 2015, 35, 14983–14999. [Google Scholar] [CrossRef]
- Sakae, D.Y.; Ramet, L.; Henrion, A.; Poirel, O.; Jamain, S.; El Mestikawy, S.; Daumas, S. Differential expression of VGLUT3 in laboratory mouse strains: Impact on drug-induced hyperlocomotion and anxiety-related behaviors. Genes Brain Behav. 2019, 18, e12528. [Google Scholar] [CrossRef] [PubMed]
- Sakae, D.Y.; Marti, F.; Lecca, S.; Vorspan, F.; Martin-Garcia, E.; Morel, L.J.; Henrion, A.; Gutierrez-Cuesta, J.; Besnard, A.; Heck, N.; et al. The absence of VGLUT3 predisposes to cocaine abuse by increasing dopamine and glutamate signaling in the nucleus accumbens. Mol. Psychiatry 2015, 20, 1448–1459. [Google Scholar] [CrossRef]
- Gangarossa, G.; Guzman, M.; Prado, V.F.; Prado, M.A.; Daumas, S.; El Mestikawy, S.; Valjent, E. Role of the atypical vesicular glutamate transporter VGLUT3 in l-DOPA-induced dyskinesia. Neurobiol. Dis. 2016, 87, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Mansouri-Guilani, N.; Bernard, V.; Vigneault, E.; Vialou, V.; Daumas, S.; El Mestikawy, S.; Gangarossa, G. VGLUT3 gates psychomotor effects induced by amphetamine. J. Neurochem. 2019, 148, 779–795. [Google Scholar] [CrossRef]
- Fontaine, H.M.; Silva, P.R.; Neiswanger, C.; Tran, R.; Abraham, A.D.; Land, B.B.; Neumaier, J.F.; Chavkin, C. Stress decreases serotonin tone in the nucleus accumbens in male mice to promote aversion and potentiate cocaine preference via decreased stimulation of 5-HT1B receptors. Neuropsychopharmacology 2021. [Google Scholar] [CrossRef] [PubMed]
- Ramet, L.; Zimmermann, J.; Bersot, T.; Poirel, O.; De Gois, S.; Silm, K.; Sakae, D.Y.; Mansouri-Guilani, N.; Bourque, M.J.; Trudeau, L.E.; et al. Characterization of a human point mutation of VGLUT3 (p.A211V) in the rodent brain suggests a nonuniform distribution of the transporter in synaptic vesicles. J. Neurosci. 2017, 37, 4181–4199. [Google Scholar] [CrossRef]
- Fazekas, C.L.; Balazsfi, D.; Horvath, H.R.; Balogh, Z.; Aliczki, M.; Puhova, A.; Balagova, L.; Chmelova, M.; Jezova, D.; Haller, J.; et al. Consequences of VGluT3 deficiency on learning and memory in mice. Physiol. Behav. 2019, 212, 112688. [Google Scholar] [CrossRef]
- Cheng, X.R.; Yang, Y.; Zhou, W.X.; Zhang, Y.X. Expression of VGLUTs contributes to degeneration and acquisition of learning and memory. Neurobiol. Learn. Mem. 2011, 95, 361–375. [Google Scholar] [CrossRef]
- Cossart, R.; Khazipov, R. How development sculpts hippocampal circuits and function. Physiol. Rev. 2022, 102, 343–378. [Google Scholar] [CrossRef] [PubMed]
- Wirth, S.; Soumier, A.; Eliava, M.; Derdikman, D.; Wagner, S.; Grinevich, V.; Sirigu, A. Territorial blueprint in the hippocampal system. Trends Cogn. Sci. 2021, 25, 831–842. [Google Scholar] [CrossRef]
- Sachser, R.M.; Haubrich, J.; Lunardi, P.S.; de Oliveira Alvares, L. Forgetting of what was once learned: Exploring the role of postsynaptic ionotropic glutamate receptors on memory formation, maintenance, and decay. Neuropharmacology 2017, 112, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Manahan-Vaughan, D. Role of metabotropic glutamate receptors in persistent forms of hippocampal plasticity and learning. Neuropharmacology 2013, 66, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Femenia, T.; Gomez-Galan, M.; Lindskog, M.; Magara, S. Dysfunctional hippocampal activity affects emotion and cognition in mood disorders. Brain Res. 2012, 1476, 58–70. [Google Scholar] [CrossRef]
- Frey, B.N.; Andreazza, A.C.; Nery, F.G.; Martins, M.R.; Quevedo, J.; Soares, J.C.; Kapczinski, F. The role of hippocampus in the pathophysiology of bipolar disorder. Behav. Pharmacol. 2007, 18, 419–430. [Google Scholar] [CrossRef]
- Szonyi, A.; Mayer, M.I.; Cserep, C.; Takacs, V.T.; Watanabe, M.; Freund, T.F.; Nyiri, G. The ascending median raphe projections are mainly glutamatergic in the mouse forebrain. Brain Struct. Funct. 2016, 221, 735–751. [Google Scholar] [CrossRef]
- Klausberger, T.; Somogyi, P. Neuronal diversity and temporal dynamics: The unity of hippocampal circuit operations. Science 2008, 321, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.D.; Hochgerner, H.; Skene, N.G.; Magno, L.; Katona, L.; Bengtsson Gonzales, C.; Somogyi, P.; Kessaris, N.; Linnarsson, S.; Hjerling-Leffler, J. Classes and continua of hippocampal CA1 inhibitory neurons revealed by single-cell transcriptomics. PLoS Biol. 2018, 16, e2006387. [Google Scholar] [CrossRef]
- Semyanov, A.; Kullmann, D.M. Modulation of GABAergic signaling among interneurons by metabotropic glutamate receptors. Neuron 2000, 25, 663–672. [Google Scholar] [CrossRef]
- Aznar, S.; Qian, Z.X.; Knudsen, G.M. Non-serotonergic dorsal and median raphe projection onto parvalbumin- and calbindin-containing neurons in hippocampus and septum. Neuroscience 2004, 124, 573–581. [Google Scholar] [CrossRef]
- McQuade, R.; Sharp, T. Functional mapping of dorsal and median raphe 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. J. Neurochem. 1997, 69, 791–796. [Google Scholar] [CrossRef]
- Kusljic, S.; van den Buuse, M. Functional dissociation between serotonergic pathways in dorsal and ventral hippocampus in psychotomimetic drug-induced locomotor hyperactivity and prepulse inhibition in rats. Eur. J. Neurosci. 2004, 20, 3424–3432. [Google Scholar] [CrossRef] [PubMed]
- Molliver, M.E. Serotonergic neuronal systems: What their anatomic organization tells us about function. J. Clin. Psychopharmacol. 1987, 7, 3S–23S. [Google Scholar] [CrossRef] [PubMed]
- Commons, K.G. Ascending serotonin neuron diversity under two umbrellas. Brain Struct. Funct. 2016, 221, 3347–3360. [Google Scholar] [CrossRef]
- McKenna, J.T.; Vertes, R.P. Collateral projections from the median raphe nucleus to the medial septum and hippocampus. Brain Res. Bull. 2001, 54, 619–630. [Google Scholar] [CrossRef]
- Vertes, R.P.; Fortin, W.J.; Crane, A.M. Projections of the median raphe nucleus in the rat. J. Comp. Neurol. 1999, 407, 555–582. [Google Scholar] [CrossRef]
- Jackson, J.; Bland, B.H.; Antle, M.C. Nonserotonergic projection neurons in the midbrain raphe nuclei contain the vesicular glutamate transporter VGLUT3. Synapse 2009, 63, 31–41. [Google Scholar] [CrossRef]
- Freund, T.F.; Gulyas, A.I.; Acsady, L.; Gorcs, T.; Toth, K. Serotonergic control of the hippocampus via local inhibitory interneurons. Proc. Natl. Acad. Sci. USA 1990, 87, 8501–8505. [Google Scholar] [CrossRef]
- Halasy, K.; Miettinen, R.; Szabat, E.; Freund, T.F. GABAergic Interneurons are the major postsynaptic targets of median raphe afferents in the rat dentate gyrus. Eur. J. Neurosci. 1992, 4, 144–153. [Google Scholar] [CrossRef]
- Hornung, J.P.; Celio, M.R. The selective innervation by serotoninergic axons of calbindin-containing interneurons in the neocortex and hippocampus of the marmoset. J. Comp. Neurol. 1992, 320, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Varga, V.; Losonczy, A.; Zemelman, B.V.; Borhegyi, Z.; Nyiri, G.; Domonkos, A.; Hangya, B.; Holderith, N.; Magee, J.C.; Freund, T.F. Fast synaptic subcortical control of hippocampal circuits. Science 2009, 326, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Domonkos, A.; Nikitidou Ledri, L.; Laszlovszky, T.; Cserep, C.; Borhegyi, Z.; Papp, E.; Nyiri, G.; Freund, T.F.; Varga, V. Divergent in vivo activity of non-serotonergic and serotonergic VGluT3-neurones in the median raphe region. J. Physiol. 2016, 594, 3775–3790. [Google Scholar] [CrossRef]
- Leranth, C.; Vertes, R.P. Median raphe serotonergic innervation of medial septum/diagonal band of broca (MSDB) parvalbumin-containing neurons: Possible involvement of the MSDB in the desynchronization of the hippocampal EEG. J. Comp. Neurol. 1999, 410, 586–598. [Google Scholar] [CrossRef]
- Alreja, M. Excitatory actions of serotonin on GABAergic neurons of the medial septum and diagonal band of Broca. Synapse 1996, 22, 15–27. [Google Scholar] [CrossRef]
- Liu, W.; Alreja, M. Atypical antipsychotics block the excitatory effects of serotonin in septohippocampal neurons in the rat. Neuroscience 1997, 79, 369–382. [Google Scholar] [CrossRef]
- Assaf, S.Y.; Miller, J.J. The role of a raphe serotonin system in the control of septal unit activity and hippocampal desynchronization. Neuroscience 1978, 3, 539–550. [Google Scholar] [CrossRef]
- Kinney, G.G.; Kocsis, B.; Vertes, R.P. Injections of excitatory amino acid antagonists into the median raphe nucleus produce hippocampal theta rhythm in the urethane-anesthetized rat. Brain Res. 1994, 654, 96–104. [Google Scholar] [CrossRef]
- Okaty, B.W.; Freret, M.E.; Rood, B.D.; Brust, R.D.; Hennessy, M.L.; deBairos, D.; Kim, J.C.; Cook, M.N.; Dymecki, S.M. Multi-Scale molecular deconstruction of the serotonin neuron system. Neuron 2015, 88, 774–791. [Google Scholar] [CrossRef]
- Tavares, L.C.S.; Tort, A.B.L. Hippocampal-prefrontal interactions during spatial decision-making. Hippocampus 2022, 32, 38–54. [Google Scholar] [CrossRef]
- Ego-Stengel, V.; Wilson, M.A. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 2010, 20, 1–10. [Google Scholar] [CrossRef]
- Girardeau, G.; Benchenane, K.; Wiener, S.I.; Buzsaki, G.; Zugaro, M.B. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 2009, 12, 1222–1223. [Google Scholar] [CrossRef]
- Mizuseki, K.; Miyawaki, H. Hippocampal information processing across sleep/wake cycles. Neurosci. Res. 2017, 118, 30–47. [Google Scholar] [CrossRef]
- Khodagholy, D.; Gelinas, J.N.; Buzsaki, G. Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science 2017, 358, 369–372. [Google Scholar] [CrossRef]
- Szucs, A.; Ratkai, A.; Schlett, K.; Huerta, R. Frequency-dependent regulation of intrinsic excitability by voltage-activated membrane conductances, computational modeling and dynamic clamp. Eur. J. Neurosci. 2017, 46, 2429–2444. [Google Scholar] [CrossRef] [PubMed]
- Amilhon, B.; Huh, C.Y.; Manseau, F.; Ducharme, G.; Nichol, H.; Adamantidis, A.; Williams, S. parvalbumin interneurons of hippocampus tune population activity at theta frequency. Neuron 2015, 86, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Soltani Zangbar, H.; Ghadiri, T.; Seyedi Vafaee, M.; Ebrahimi Kalan, A.; Fallahi, S.; Ghorbani, M.; Shahabi, P. Theta oscillations through hippocampal/prefrontal pathway: Importance in cognitive performances. Brain Connect. 2020, 10, 157–169. [Google Scholar] [CrossRef]
- Wang, D.V.; Yau, H.J.; Broker, C.J.; Tsou, J.H.; Bonci, A.; Ikemoto, S. Mesopontine median raphe regulates hippocampal ripple oscillation and memory consolidation. Nat. Neurosci. 2015, 18, 728–735. [Google Scholar] [CrossRef]
- Willner, P. Reliability of the chronic mild stress model of depression: A user survey. Neurobiol. Stress 2017, 6, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Willner, P.; Benton, D.; Brown, E.; Cheeta, S.; Davies, G.; Morgan, J.; Morgan, M. “Depression” increases “craving” for sweet rewards in animal and human models of depression and craving. Psychopharmacology 1998, 136, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Beecher, K.; Wang, J.; Jacques, A.; Chaaya, N.; Chehrehasa, F.; Belmer, A.; Bartlett, S.E. Sucrose consumption alters serotonin/glutamate co-localisation within the prefrontal cortex and hippocampus of mice. Front. Mol. Neurosci. 2021, 14, 678267. [Google Scholar] [CrossRef] [PubMed]
- Tukey, D.S.; Lee, M.; Xu, D.; Eberle, S.E.; Goffer, Y.; Manders, T.R.; Ziff, E.B.; Wang, J. Differential effects of natural rewards and pain on vesicular glutamate transporter expression in the nucleus accumbens. Mol. Brain 2013, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, Y.S.; Liu, L.; Tang, L.; Yang, H.; Meng, P.; Zhao, H.Q.; Wang, Y.H. Abnormal Glu/mGluR2/3/PI3K pathway in the hippocampal neurovascular unit leads to diabetes-related depression. Neural. Regen. Res. 2021, 16, 727–733. [Google Scholar] [CrossRef]
- Abela, A.R.; Browne, C.J.; Sargin, D.; Prevot, T.D.; Ji, X.D.; Li, Z.; Lambe, E.K.; Fletcher, P.J. Median raphe serotonin neurons promote anxiety-like behavior via inputs to the dorsal hippocampus. Neuropharmacology 2020, 168, 107985. [Google Scholar] [CrossRef]
- Van Liefferinge, J.; Jensen, C.J.; Albertini, G.; Bentea, E.; Demuyser, T.; Merckx, E.; Aronica, E.; Smolders, I.; Massie, A. Altered vesicular glutamate transporter expression in human temporal lobe epilepsy with hippocampal sclerosis. Neurosci. Lett. 2015, 590, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Llorente, I.L.; Perez-Rodriguez, D.; Burgin, T.C.; Gonzalo-Orden, J.M.; Martinez-Villayandre, B.; Fernandez-Lopez, A. Age and meloxicam modify the response of the glutamate vesicular transporters (VGLUTs) after transient global cerebral ischemia in the rat brain. Brain Res. Bull. 2013, 94, 90–97. [Google Scholar] [CrossRef]
- McLellan, M.A.; Rosenthal, N.A.; Pinto, A.R. Cre-loxP-Mediated recombination: General principles and experimental considerations. Curr. Protoc. Mouse Biol. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Sternberg, N.; Hamilton, D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J. Mol. Biol. 1981, 150, 467–486. [Google Scholar] [CrossRef]
- Zelena, D.; Demeter, K.; Haller, J.; Balazsfi, D. Considerations for the use of virally delivered genetic tools for in-vivo circuit analysis and behavior in mutant mice: A practical guide to optogenetics. Behav. Pharmacol. 2017, 28, 598–609. [Google Scholar] [CrossRef]
- Vooijs, M.; Jonkers, J.; Berns, A. A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep. 2001, 2, 292–297. [Google Scholar] [CrossRef]
- Wunderlich, F.T.; Wildner, H.; Rajewsky, K.; Edenhofer, F. New variants of inducible Cre recombinase: A novel mutant of Cre-PR fusion protein exhibits enhanced sensitivity and an expanded range of inducibility. Nucleic Acids Res. 2001, 29, E47. [Google Scholar] [CrossRef]
- Karray, S.; Kress, C.; Cuvellier, S.; Hue-Beauvais, C.; Damotte, D.; Babinet, C.; Levi-Strauss, M. Complete loss of Fas ligand gene causes massive lymphoproliferation and early death, indicating a residual activity of gld allele. J. Immunol. 2004, 172, 2118–2125. [Google Scholar] [CrossRef]
- Raab, S.; Beck, H.; Gaumann, A.; Yuce, A.; Gerber, H.P.; Plate, K.; Hammes, H.P.; Ferrara, N.; Breier, G. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb. Haemost. 2004, 91, 595–605. [Google Scholar] [CrossRef]
- Schmidt, E.E.; Taylor, D.S.; Prigge, J.R.; Barnett, S.; Capecchi, M.R. Illegitimate Cre-dependent chromosome rearrangements in transgenic mouse spermatids. Proc. Natl. Acad. Sci. USA 2000, 97, 13702–13707. [Google Scholar] [CrossRef] [PubMed]
- Papathanou, M.; Dumas, S.; Pettersson, H.; Olson, L.; Wallen-Mackenzie, A. Off-target effects in transgenic mice: Characterization of dopamine transporter (DAT)-Cre transgenic mouse lines exposes multiple non-dopaminergic neuronal clusters available for selective targeting within limbic neurocircuitry. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Schmidt-Supprian, M.; Rajewsky, K. Vagaries of conditional gene targeting. Nat. Immunol. 2007, 8, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Heffner, C.S.; Herbert Pratt, C.; Babiuk, R.P.; Sharma, Y.; Rockwood, S.F.; Donahue, L.R.; Eppig, J.T.; Murray, S.A. Supporting conditional mouse mutagenesis with a comprehensive cre characterization resource. Nat. Commun. 2012, 3, 1218. [Google Scholar] [CrossRef] [PubMed]
- Guru, A.; Post, R.J.; Ho, Y.Y.; Warden, M.R. Making sense of optogenetics. Int. J. Neuropsychopharmacol. 2015, 18, pyv079. [Google Scholar] [CrossRef]
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 2003, 100, 13940–13945. [Google Scholar] [CrossRef]
- Jiang, J.; Cui, H.; Rahmouni, K. Optogenetics and pharmacogenetics: Principles and applications. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R633–R645. [Google Scholar] [CrossRef]
- Chen, R.; Gore, F.; Nguyen, Q.A.; Ramakrishnan, C.; Patel, S.; Kim, S.H.; Raffiee, M.; Kim, Y.S.; Hsueh, B.; Krook-Magnusson, E.; et al. Deep brain optogenetics without intracranial surgery. Nat. Biotechnol. 2021, 39, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.M.; Rogan, S.C.; Abbas, A.I.; Armbruster, B.N.; Pei, Y.; Allen, J.A.; Nonneman, R.J.; Hartmann, J.; Moy, S.S.; Nicolelis, M.A.; et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 2009, 63, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, B.N.; Li, X.; Pausch, M.H.; Herlitze, S.; Roth, B.L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA 2007, 104, 5163–5168. [Google Scholar] [CrossRef]
- Campbell, E.J.; Marchant, N.J. The use of chemogenetics in behavioural neuroscience: Receptor variants, targeting approaches and caveats. Br. J. Pharmacol. 2018, 175, 994–1003. [Google Scholar] [CrossRef]
- Liguz-Lecznar, M.; Skangiel-Kramska, J. Vesicular glutamate transporters (VGLUTs): The three musketeers of glutamatergic system. Acta Neurobiol. Exp. (Wars) 2007, 67, 207–218. [Google Scholar]
- Kehrl, J.; Althaus, J.C.; Showalter, H.D.; Rudzinski, D.M.; Sutton, M.A.; Ueda, T. Vesicular glutamate transporter inhibitors: Structurally modified brilliant yellow analogs. Neurochem. Res. 2017, 42, 1823–1832. [Google Scholar] [CrossRef]
- Thompson, C.M.; Chao, C.K. VGLUT substrates and inhibitors: A computational viewpoint. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183175. [Google Scholar] [CrossRef] [PubMed]
- Gegelashvili, G.; Bjerrum, O.J. Glutamate transport system as a key constituent of glutamosome: Molecular pathology and pharmacological modulation in chronic pain. Neuropharmacology 2019, 161, 107623. [Google Scholar] [CrossRef]
| GABAergic Interneurons in the Cortex | GABAergic Interneurons in the Hippocampus | VGLUT3+ Interneurons in the Amygdala | VGLUT3+ Interneurons in the Hippocampus | |
|---|---|---|---|---|
| Resting membrane potential | −57.48–−49.40 mV | NA | NA | −59.00–−56.90 mV |
| Input resistance | 219.77–419.61 MΩ | 107.89 MΩ | 168.10 MΩ | 149.70–158.50 MΩ |
| Action potential threshold | −32.67–−27.82 mV | −42.81 mV | −38.80 mV | −41.90–−39.86 mV |
| Action potential amplitude | 71.30–86.11 mV | 74.27 mV | 71.60 mV | 55.70–57.40 mV |
| Firing frequency | 19.34–52.48 Hz (2×) | 15.00 Hz (steady trace) | 31.50 Hz (2×) | 31.30–34.90 Hz (2×) |
| Amplitude of after-hyperpolarisation | 8.60–17.63 mV | 12.68 mV (new method) | 14.70 mV | −11.80–−10.30 mV |
| Co-transmitters | CCK | CCK | CCK, GABA | CCK, GABA |
| Reference | [71], all subtypes displayed | [72] | [27] | [65], both subtypes displayed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazekas, C.L.; Szabó, A.; Török, B.; Bánrévi, K.; Correia, P.; Chaves, T.; Daumas, S.; Zelena, D. A New Player in the Hippocampus: A Review on VGLUT3+ Neurons and Their Role in the Regulation of Hippocampal Activity and Behaviour. Int. J. Mol. Sci. 2022, 23, 790. https://doi.org/10.3390/ijms23020790
Fazekas CL, Szabó A, Török B, Bánrévi K, Correia P, Chaves T, Daumas S, Zelena D. A New Player in the Hippocampus: A Review on VGLUT3+ Neurons and Their Role in the Regulation of Hippocampal Activity and Behaviour. International Journal of Molecular Sciences. 2022; 23(2):790. https://doi.org/10.3390/ijms23020790
Chicago/Turabian StyleFazekas, Csilla Lea, Adrienn Szabó, Bibiána Török, Krisztina Bánrévi, Pedro Correia, Tiago Chaves, Stéphanie Daumas, and Dóra Zelena. 2022. "A New Player in the Hippocampus: A Review on VGLUT3+ Neurons and Their Role in the Regulation of Hippocampal Activity and Behaviour" International Journal of Molecular Sciences 23, no. 2: 790. https://doi.org/10.3390/ijms23020790
APA StyleFazekas, C. L., Szabó, A., Török, B., Bánrévi, K., Correia, P., Chaves, T., Daumas, S., & Zelena, D. (2022). A New Player in the Hippocampus: A Review on VGLUT3+ Neurons and Their Role in the Regulation of Hippocampal Activity and Behaviour. International Journal of Molecular Sciences, 23(2), 790. https://doi.org/10.3390/ijms23020790







