GLP-1a: Going beyond Traditional Use
Abstract
:1. Introduction
2. Clinical Trials That Investigated the Use of GLP-1
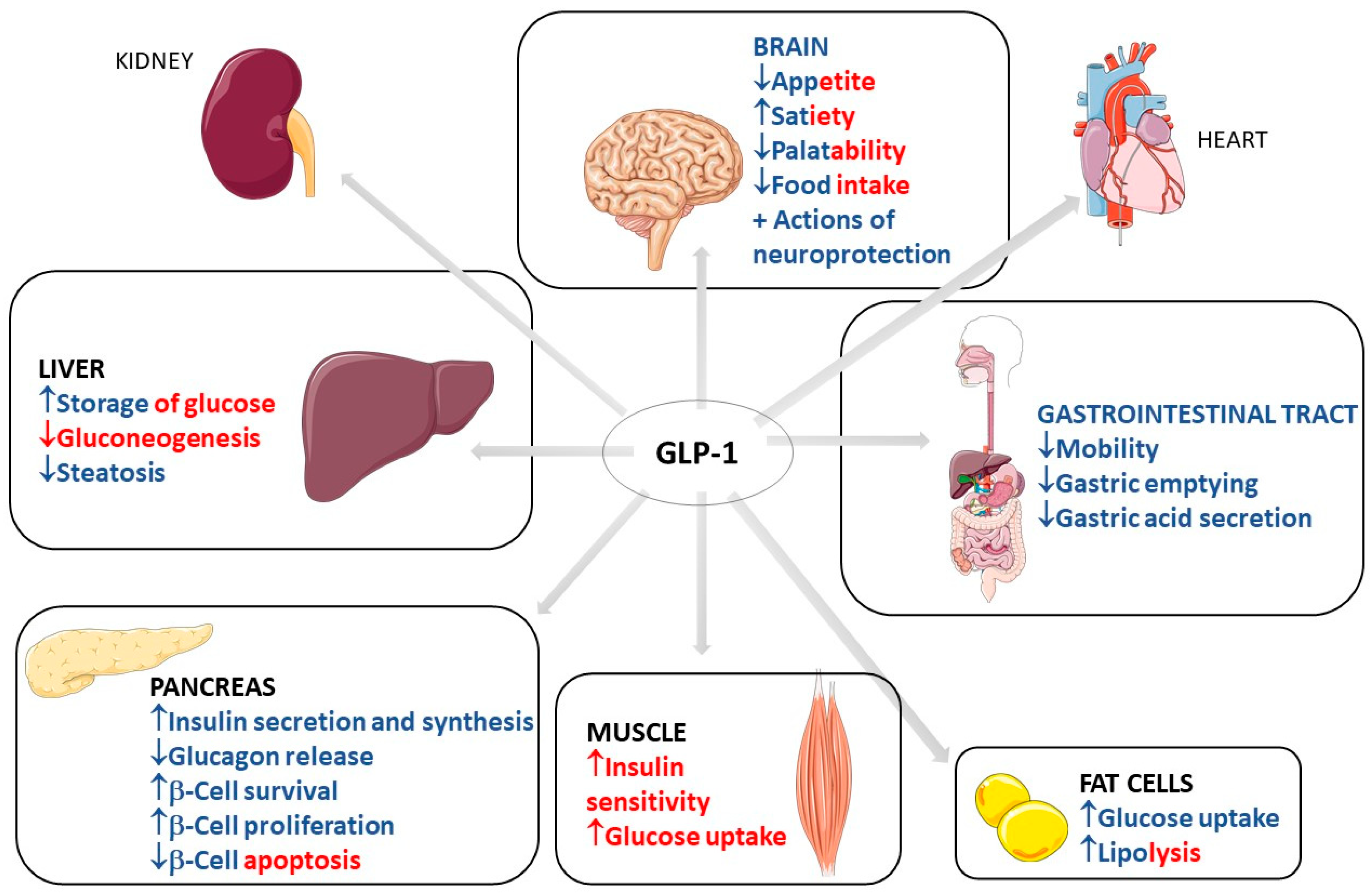
3. Use of GLP1
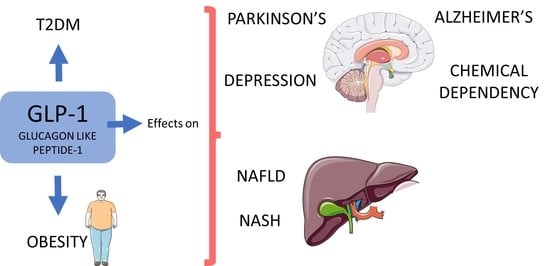
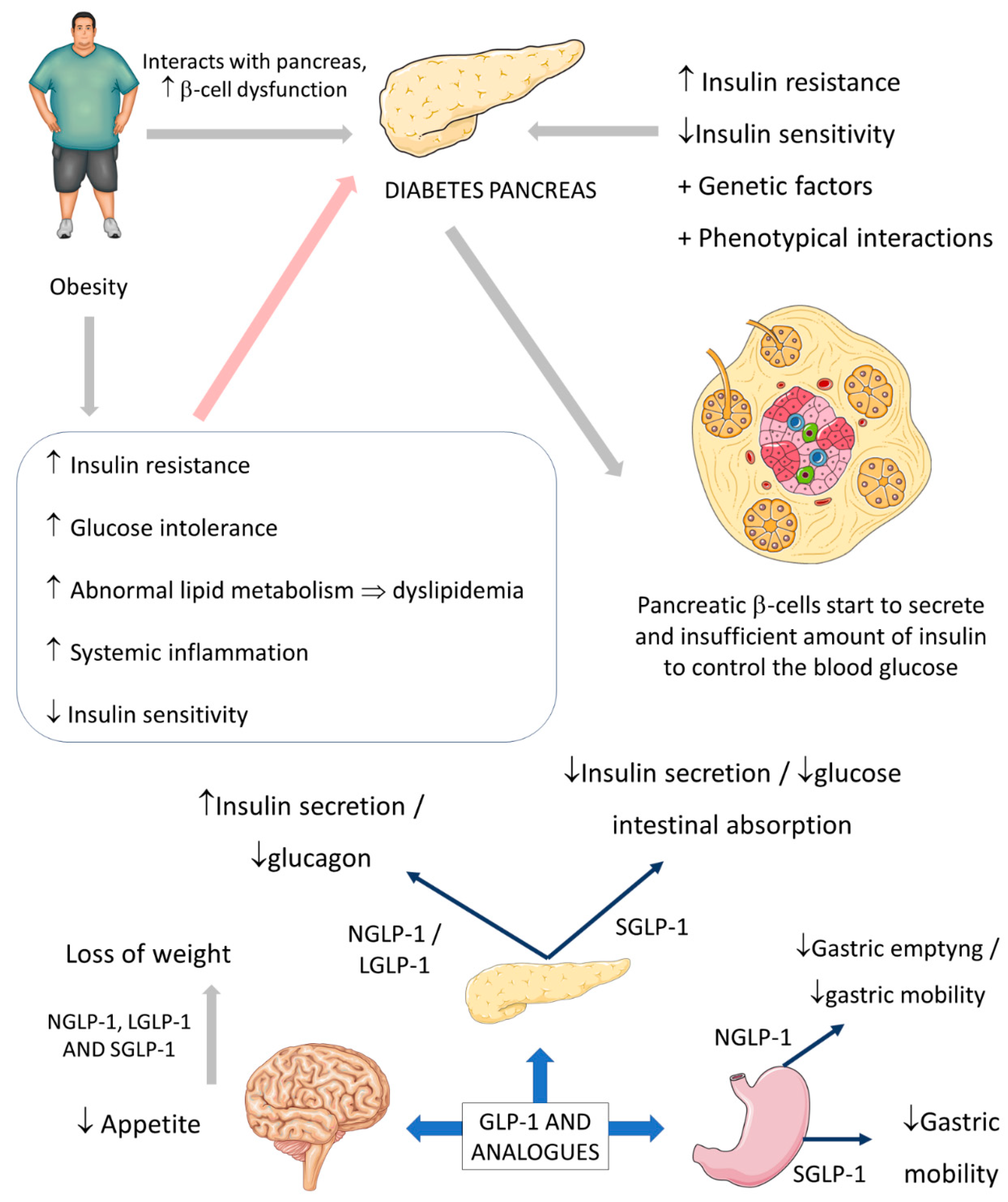
3.1. Common Use of GLP-1: Diabetes and Obesity
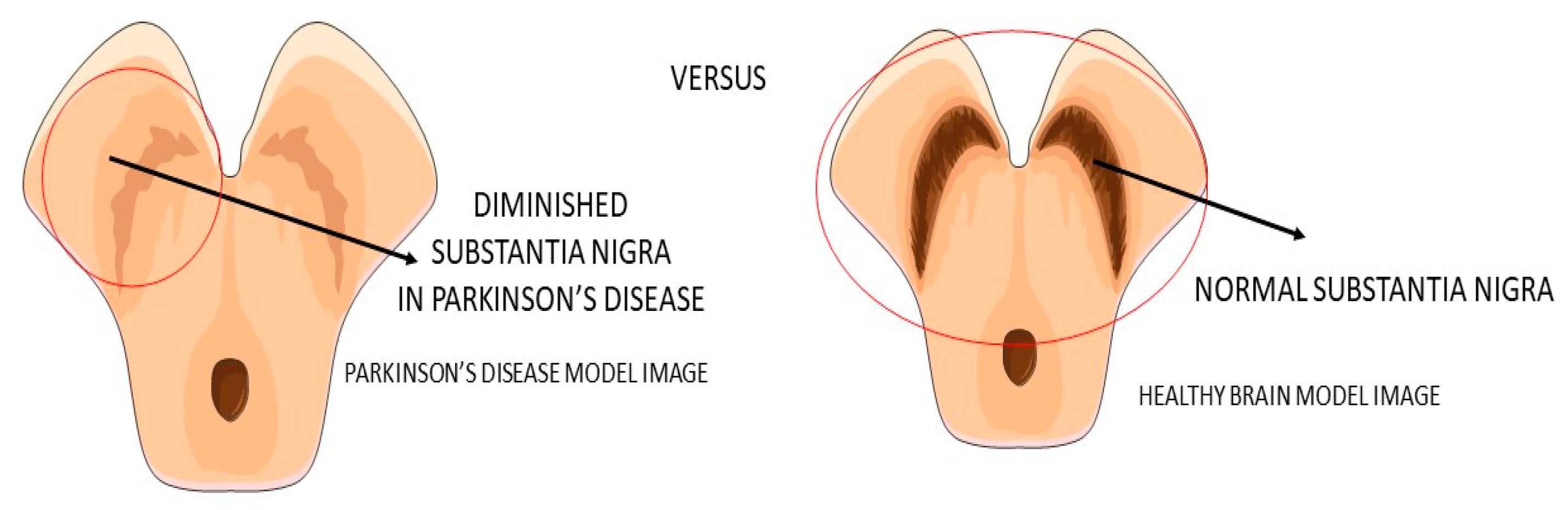
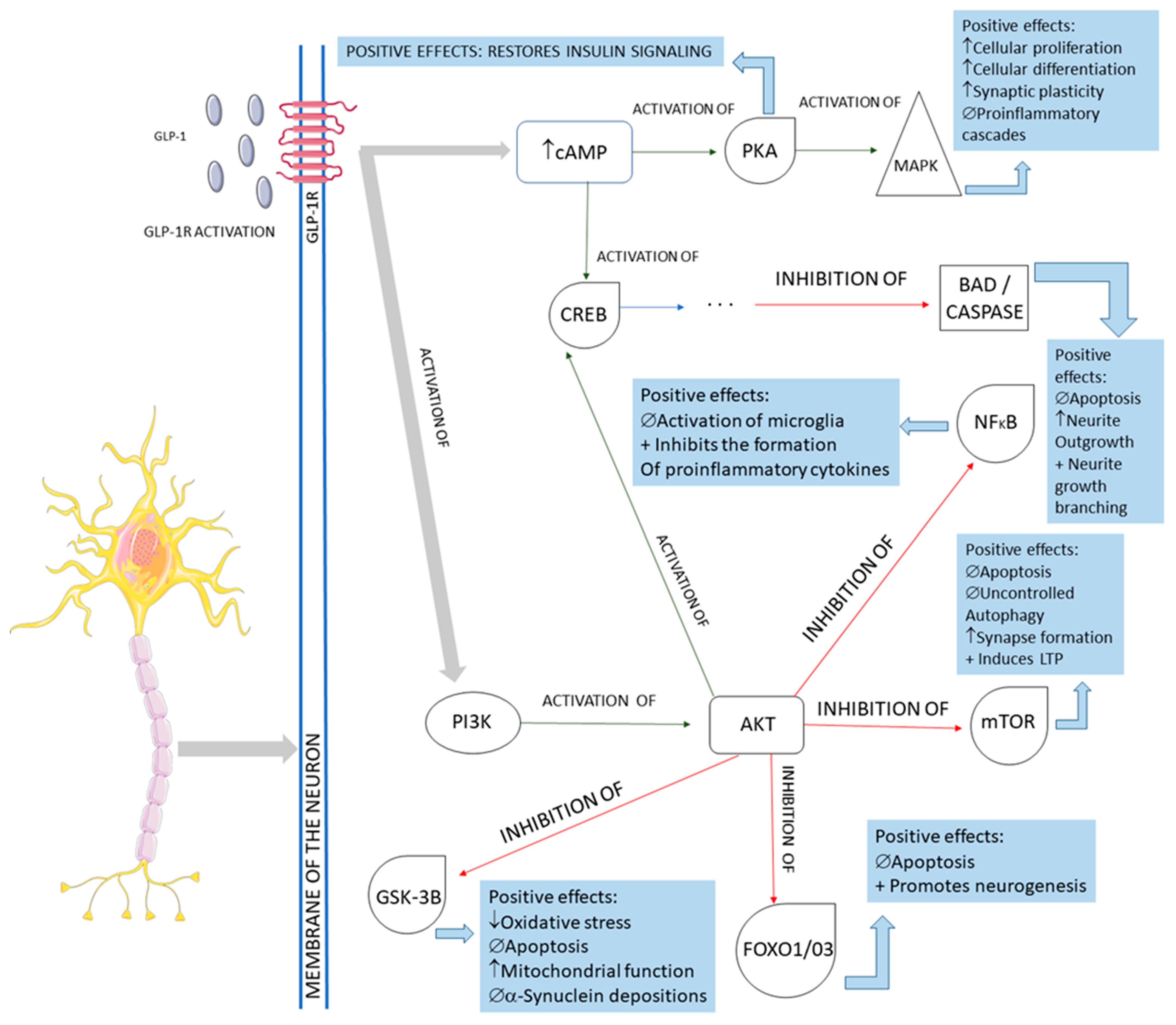
3.2. GLP-1 and Parkinson’s Disease
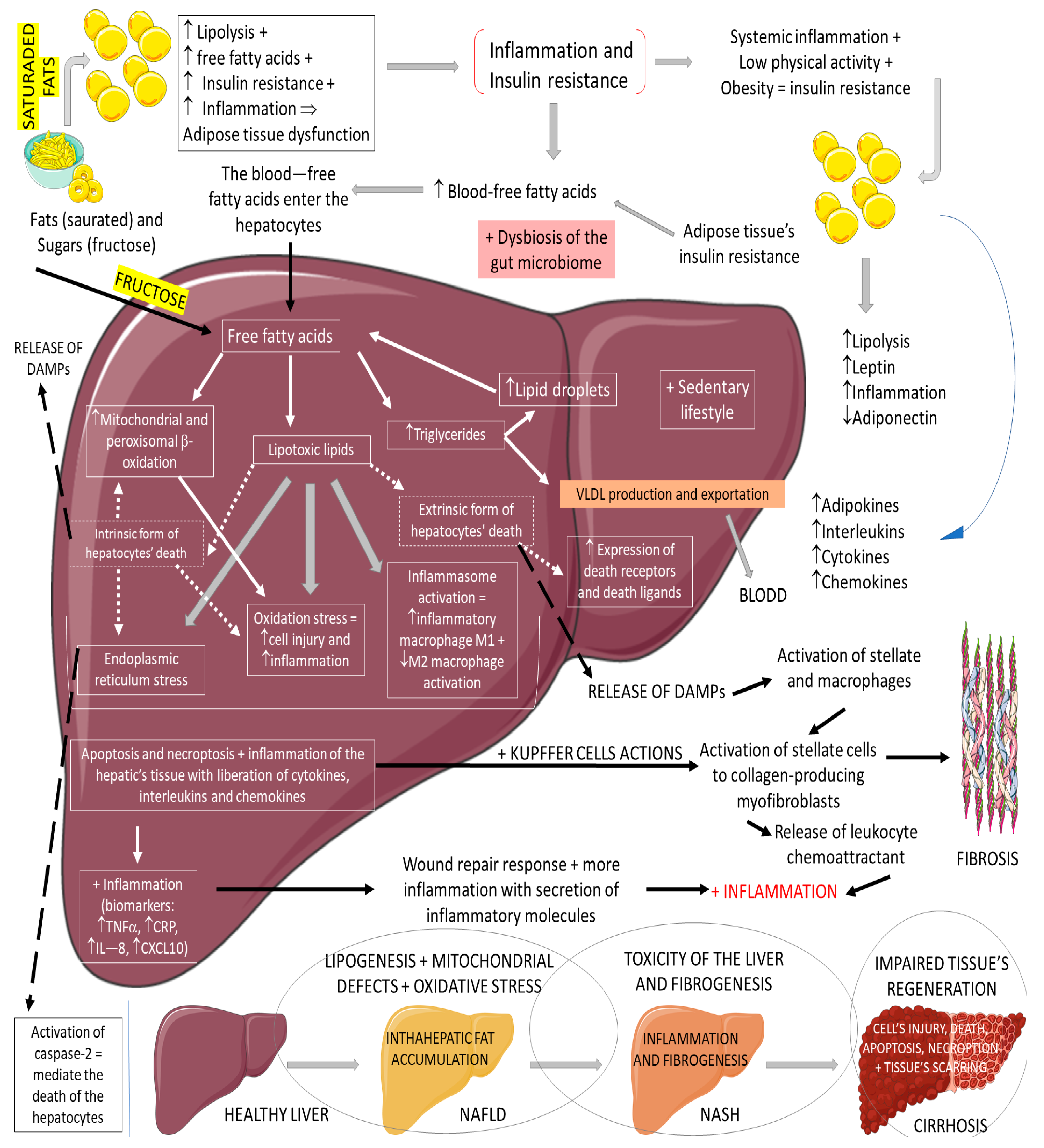
3.3. GLP-1, Non-Alcoholic Fatty Liver Disease, and Non-Alcoholic Steatohepatitis
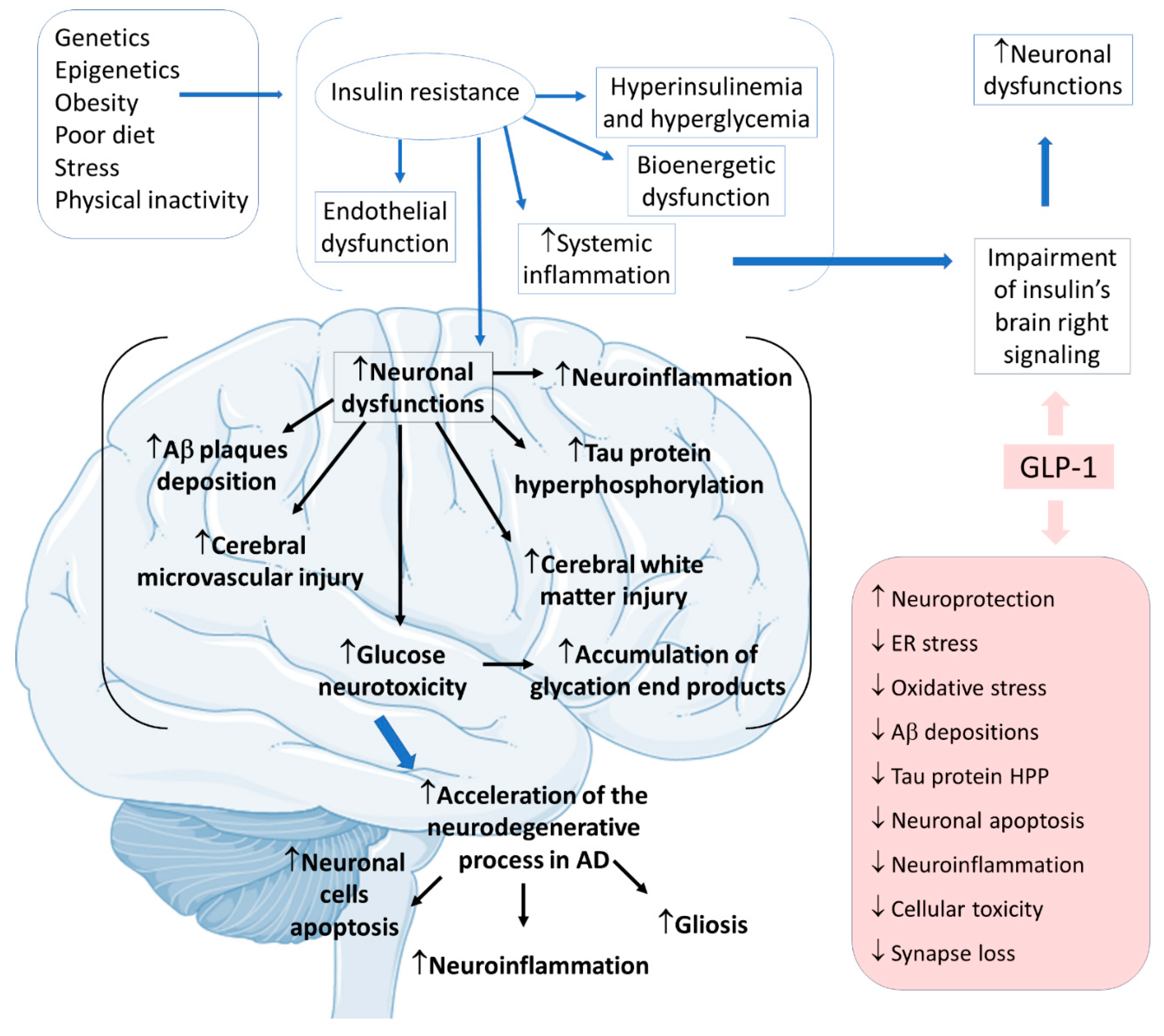
3.4. GLP-1 and Alzheimer’s Disease
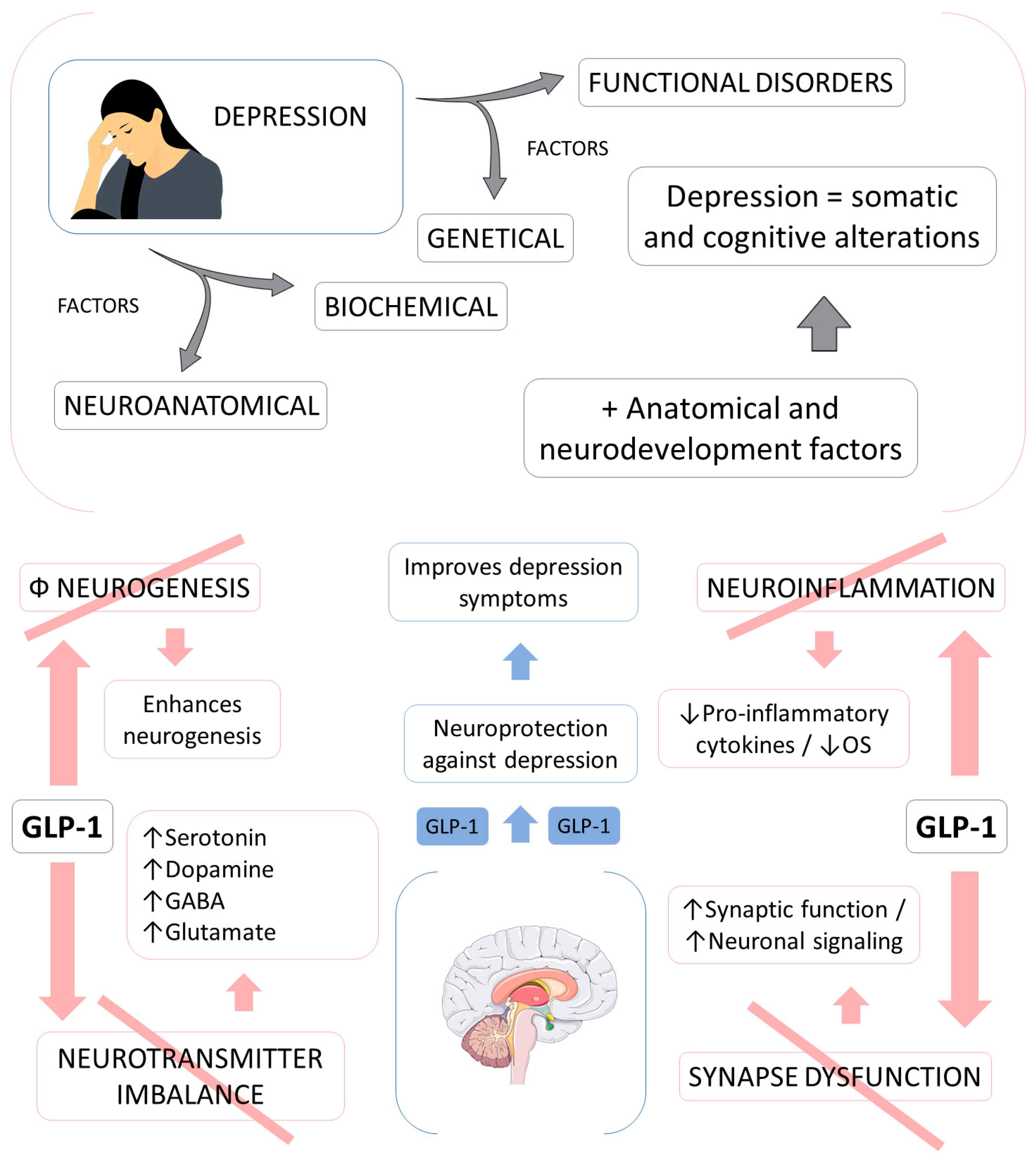
3.5. GLP-1 and Depression
3.6. GLP-1: Other Uses
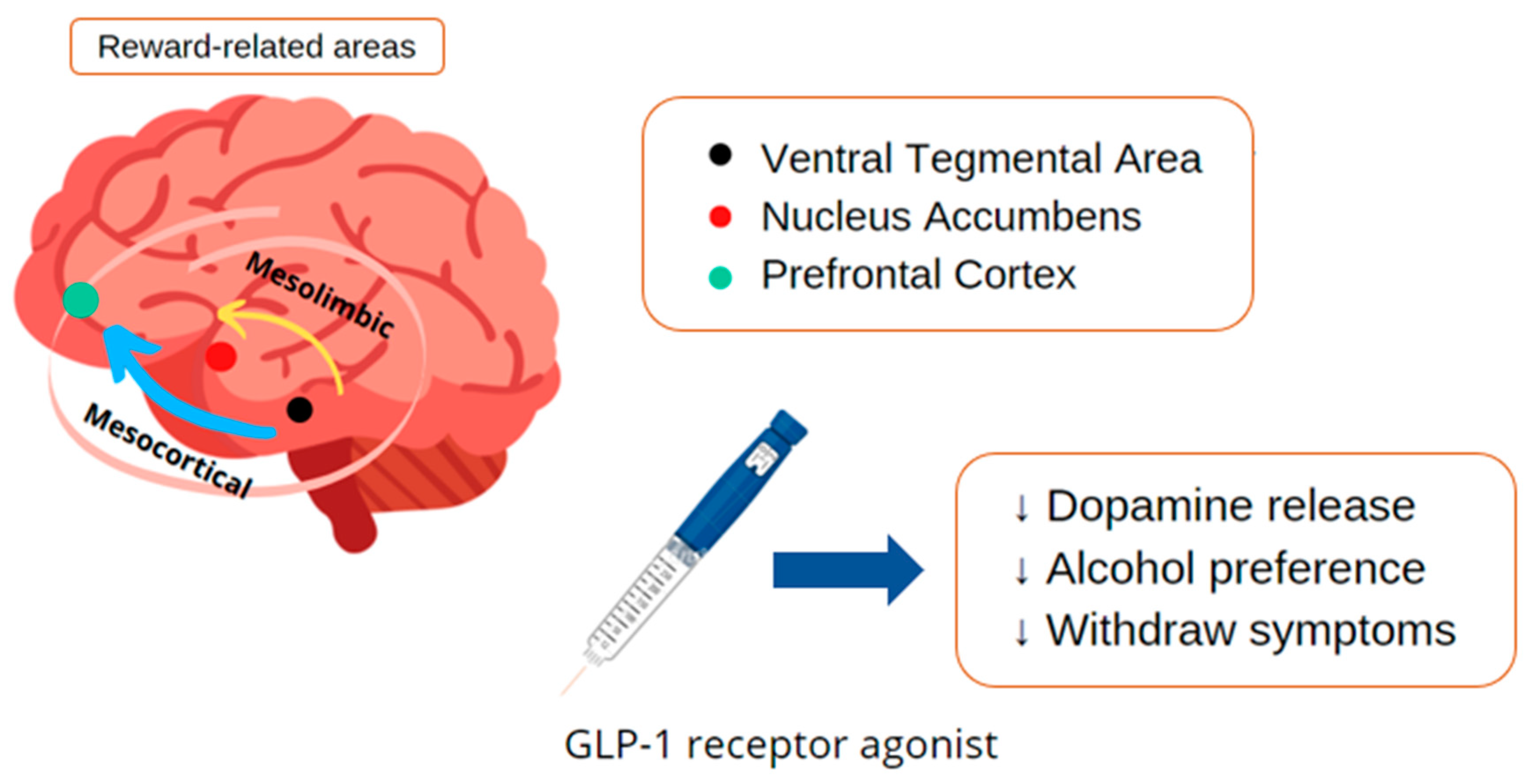
GLP-1 and Chemical Dependency
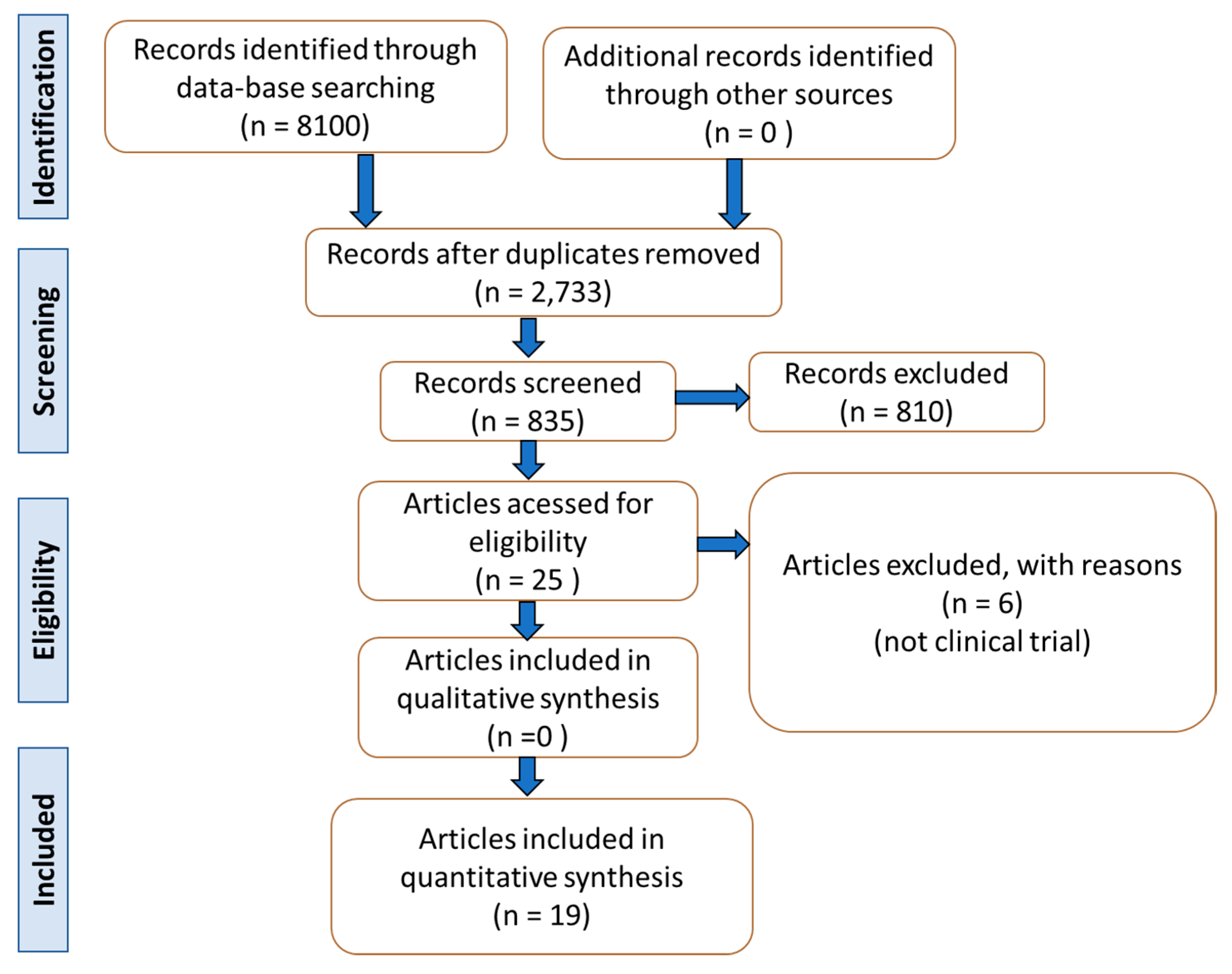
4. Materials and Methods
4.1. Focal Question
4.2. Language
4.3. Databases
4.4. Study Selection
4.5. Data Extraction
4.6. Quality Assessment
5. General Comments
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, N.K.; Hackett, T.A.; Galli, A.; Flynn, C.R. GLP-1: Molecular mechanisms and outcomes of a complex signaling system. Neurochem. Int. 2019, 128, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.W.; Egan, J.M. Incretins in obesity and diabetes. Ann. N. Y. Acad. Sci. 2020, 1461, 104–126. [Google Scholar] [CrossRef] [PubMed]
- Erbil, D.; Eren, C.Y.; Demirel, C.; Küçüker, M.U.; Solaroğlu, I.; Eser, H.Y. GLP-1′s role in neuroprotection: A systematic review. Brain Inj. 2019, 33, 734–819. [Google Scholar] [CrossRef] [PubMed]
- Gribble, F.M.; Reimann, F. Metabolic Messengers: Glucagon-like peptide 1. Nat. Metab. 2021, 3, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, K.H.; Wahlberg, E.A.; Gilbert, M.P. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad. Med. J. 2020, 96, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro-Parenti, L.; Jarry, A.-C.; Cavin, J.-B.; Willemetz, A.; Le Beyec, J.; Sannier, A.; Benadda, S.; Pelletier, A.-L.; Hourseau, M.; Léger, T.; et al. Bariatric surgery induces a new gastric mucosa phenotype with increased functional glucagon-like peptide-1 expressing cells. Nat. Commun. 2021, 12, 110. [Google Scholar] [CrossRef]
- Antonsen, K.K.; Klausen, M.K.; Brunchmann, A.S.; le Dous, N.; Jensen, M.E.; Miskowiak, K.W.; Fisher, P.M.; Thomsen, G.K.; Rindom, H.; Fahmy, T.P.; et al. Does glucagon-like peptide-1 (GLP-1) receptor agonist stimulation reduce alcohol intake in patients with alcohol dependence: Study protocol of a randomised, double-blinded, placebo-controlled clinical trial. BMJ Open 2018, 8, e019562. [Google Scholar] [CrossRef] [Green Version]
- Athauda, D.; Maclagan, K.; Skene, S.S.; Bajwa-Joseph, M.; Letchford, D.; Chowdhury, K.; Hibbert, S.; Budnik, N.; Zampedri, L.; Dickson, J.; et al. Exenatide once weekly versus placebo in Parkinson’s disease: A randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1664–1675. [Google Scholar] [CrossRef]
- Aung, M.M.; Slade, K.; Freeman, L.A.R.; Kos, K.; Whatmore, J.L.; Shore, A.C.; Gooding, K.M. Locally delivered GLP-1 analogues liraglutide and exenatide enhance microvascular perfusion in individuals with and without type 2 diabetes. Diabetologia 2019, 62, 1701–1711. [Google Scholar] [CrossRef] [Green Version]
- Faurschou, A.; Gyldenløve, M.; Rohde, U.; Thyssen, J.P.; Zachariae, C.; Skov, L.; Knop, F.K.; Vilsbøll, T. Lack of effect of the glucagon-like peptide-1 receptor agonist liraglutide on psoriasis in glucose-tolerant patients--a randomized placebo-controlled trial. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Femminella, G.D.; Frangou, E.; Love, S.B.; Busza, G.; Holmes, C.; Ritchie, C.; Lawrence, R.; McFarlane, B.; Tadros, G.; Ridha, B.H.; et al. Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer’s disease: Study protocol for a randomised controlled trial (ELAD study). Trials 2019, 20, 191. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Hartman, M.L.; Sanyal, A.J.; Loomba, R.; Wilson, J.M.; Nikooienejad, A.; Bray, R.; Karanikas, C.A.; Duffin, K.L.; Robins, D.A.; Haupt, A. Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients with Type 2 Diabetes. Diabetes Care 2020, 43, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Pavlidis, G.; Thymis, J.; Birba, D.; Kalogeris, A.; Kousathana, F.; Kountouri, A.; Balampanis, K.; Parissis, J.; Andreadou, I.; et al. Effects of Glucagon-Like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Endothelial Glycocalyx, Arterial Function, and Myocardial Work Index in Patients with Type 2 Diabetes Mellitus after 12-Month Treatment. J. Am. Heart Assoc. 2020, 9, e015716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadouh, H.; Chedid, V.; Halawi, H.; Burton, D.D.; Clark, M.M.; Khemani, D.; Vella, A.; Acosta, A.; Camilleri, M. GLP-1 Analog Modulates Appetite, Taste Preference, Gut Hormones, and Regional Body Fat Stores in Adults with Obesity. J. Clin. Endocrinol. Metab. 2020, 105, 1552–1563. [Google Scholar] [CrossRef]
- Mullins, R.J.; Mustapic, M.; Chia, C.W.; Carlson, O.; Gulyani, S.; Tran, J.; Li, Y.; Mattson, M.P.; Resnick, S.; Egan, J.M.; et al. A Pilot Study of Exenatide Actions in Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.S.; Harrison, S.A. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Pozzi, M.; Mazhar, F.; Peeters, G.; Vantaggiato, C.; Nobile, M.; Clementi, E.; Radice, S.; Carnovale, C. A systematic review of the antidepressant effects of glucagon-like peptide 1 (GLP-1) functional agonists: Further link between metabolism and psychopathology: Special Section on “Translational and Neuroscience Studies in Affective Disorders”. Section Editor, Maria Nobile MD, PhD. This Section of JAD focuses on the relevance of translational and neuroscience studies in providing a better understanding of the neural basis of affective disorders. The main aim is to briefly summaries relevant research findings in clinical neuroscience with particular regards to specific innovative topics in mood and anxiety disorders. J. Affect. Disord. 2019, 257, 774–778. [Google Scholar] [CrossRef]
- Chang, G.; Chen, B.; Zhang, L. Efficacy of GLP-1rA, liraglutide, in plaque psoriasis treatment with type 2 diabetes: A systematic review and meta-analysis of prospective cohort and before-after studies. J. Dermatol. Treat. 2021, 1–10. [Google Scholar] [CrossRef]
- Athauda, D.; Maclagan, K.; Budnik, N.; Zampedri, L.; Hibbert, S.; Skene, S.S.; Chowdhury, K.; Aviles-Olmos, I.; Limousin, P.; Foltynie, T. What Effects Might Exenatide have on Non-Motor Symptoms in Parkinson’s Disease: A Post Hoc Analysis. J. Parkinsons Dis. 2018, 8, 247–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, M.J.; Hull, D.; Guo, K.; Barton, D.; Hazlehurst, J.M.; Gathercole, L.L.; Nasiri, M.; Yu, J.; Gough, S.C.; Newsome, P.N.; et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J. Hepatol. 2016, 64, 399–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchi, R.; Nakano, Y.; Fukuda, T.; Takeuchi, T.; Murakami, M.; Minami, I.; Izumiyama, H.; Hashimoto, K.; Yoshimoto, T.; Ogawa, Y. Reduction of visceral fat by liraglutide is associated with ameliorations of hepatic steatosis, albuminuria, and micro-inflammation in type 2 diabetic patients with insulin treatment: A randomized control trial. Endocr. J. 2017, 64, 269–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Tian, W.; Lin, L.; Xu, X. Liraglutide or insulin glargine treatments improves hepatic fat in obese patients with type 2 diabetes and nonalcoholic fatty liver disease in twenty-six weeks: A randomized placebo-controlled trial. Diabetes Res. Clin. Pract. 2020, 170, 108487. [Google Scholar] [CrossRef]
- Khoo, J.; Hsiang, J.; Taneja, R.; Law, N.M.; Ang, T.L. Comparative effects of liraglutide 3 mg vs structured lifestyle modification on body weight, liver fat and liver function in obese patients with non-alcoholic fatty liver disease: A pilot randomized trial. Diabetes Obes. Metab. 2017, 19, 1814–1817. [Google Scholar] [CrossRef] [PubMed]
- Khoo, J.; Hsiang, J.C.; Taneja, R.; Koo, S.H.; Soon, G.H.; Kam, C.J.; Law, N.M.; Ang, T.L. Randomized trial comparing effects of weight loss by liraglutide with lifestyle modification in non-alcoholic fatty liver disease. Liver Int. 2019, 39, 941–949. [Google Scholar] [CrossRef]
- Petit, J.M.; Cercueil, J.P.; Loffroy, R.; Denimal, D.; Bouillet, B.; Fourmont, C.; Chevallier, O.; Duvillard, L.; Vergès, B. Effect of Liraglutide Therapy on Liver Fat Content in Patients with Inadequately Controlled Type 2 Diabetes: The Lira-NAFLD Study. J. Clin. Endocrinol. Metab. 2017, 102, 407–415. [Google Scholar] [CrossRef]
- Smits, M.M.; Tonneijck, L.; Muskiet, M.H.; Kramer, M.H.; Pouwels, P.J.; Pieters-van den Bos, I.C.; Hoekstra, T.; Diamant, M.; van Raalte, D.H.; Cahen, D.L. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: A randomised placebo-controlled trial. Diabetologia 2016, 59, 2588–2593. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Yao, B.; Kuang, H.; Yang, X.; Huang, Q.; Hong, T.; Li, Y.; Dou, J.; Yang, W.; Qin, G.; et al. Liraglutide, Sitagliptin, and Insulin Glargine Added to Metformin: The Effect on Body Weight and Intrahepatic Lipid in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 2414–2426. [Google Scholar] [CrossRef] [Green Version]
- Gejl, M.; Brock, B.; Egefjord, L.; Vang, K.; Rungby, J.; Gjedde, A. Blood-Brain Glucose Transfer in Alzheimer’s disease: Effect of GLP-1 Analog Treatment. Sci. Rep. 2017, 7, 17490. [Google Scholar] [CrossRef] [Green Version]
- Gejl, M.; Gjedde, A.; Egefjord, L.; Møller, A.; Hansen, S.B.; Vang, K.; Rodell, A.; Brændgaard, H.; Gottrup, H.; Schacht, A.; et al. In Alzheimer’s Disease, 6-Month Treatment with GLP-1 Analog Prevents Decline of Brain Glucose Metabolism: Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Front. Aging Neurosci. 2016, 8, 108. [Google Scholar] [CrossRef]
- Watson, K.T.; Wroolie, T.E.; Tong, G.; Foland-Ross, L.C.; Frangou, S.; Singh, M.; McIntyre, R.S.; Roat-Shumway, S.; Myoraku, A.; Reiss, A.L.; et al. Neural correlates of liraglutide effects in persons at risk for Alzheimer’s disease. Behav. Brain. Res. 2019, 356, 271–278. [Google Scholar] [CrossRef]
- Grant, P.; Lipscomb, D.; Quin, J. Psychological and quality of life changes in patients using GLP-1 analogues. J. Diabetes Complicat. 2011, 25, 244–246. [Google Scholar] [CrossRef]
- Kahal, H.; Kilpatrick, E.; Rigby, A.; Coady, A.; Atkin, S. The effects of treatment with liraglutide on quality of life and depression in young obese women with PCOS and controls. Gynecol. Endocrinol. 2019, 35, 142–145. [Google Scholar] [CrossRef]
- Moulton, C.D.; Pickup, J.C.; Amiel, S.A.; Winkley, K.; Ismail, K. Investigating incretin-based therapies as a novel treatment for depression in type 2 diabetes: Findings from the South London Diabetes (SOUL-D) Study. Prim. Care Diabetes 2016, 10, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Verma, S.; Vaidya, S.; Kalia, K.; Tiwari, V. Recent updates on GLP-1 agonists: Current advancements & challenges. Biomed. Pharmacother. 2018, 108, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Blair, M. Diabetes Mellitus Review. Urol. Nurs. 2016, 36, 27–36. [Google Scholar] [CrossRef]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.H.; Handelsman, Y.; Insel, R.A.; Mathieu, C.; et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef] [Green Version]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [Green Version]
- McBrayer, D.N.; Tal-Gan, Y. Recent Advances in GLP-1 Receptor Agonists for Use in Diabetes Mellitus. Drug Dev. Res. 2017, 78, 292–299. [Google Scholar] [CrossRef]
- Meier, J.J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 728–742. [Google Scholar] [CrossRef]
- Grill, H.J. A Role for GLP-1 in Treating Hyperphagia and Obesity. Endocrinology 2020, 161. [Google Scholar] [CrossRef]
- D’Alessio, D. Is GLP-1 a hormone: Whether and When? J. Diabetes Investig. 2016, 7 (Suppl. 1), 50–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanoski, S.E.; Hayes, M.R.; Skibicka, K.P. GLP-1 and weight loss: Unraveling the diverse neural circuitry. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R885–R895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Q.; Wang, Y.; Hao, Q.; Vandvik, P.O.; Guyatt, G.; Li, J.; Chen, Z.; Xu, S.; Shen, Y.; Ge, L.; et al. Pharmacotherapy for adults with overweight and obesity: A systematic review and network meta-analysis of randomised controlled trials. Lancet 2021. [Google Scholar] [CrossRef]
- Finelli, C.; Padula, M.C.; Martelli, G.; Tarantino, G. Could the improvement of obesity-related co-morbidities depend on modified gut hormones secretion? World J. Gastroenterol. 2014, 20, 16649–16664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athauda, D.; Foltynie, T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: Mechanisms of action. Drug Discov. Today 2016, 21, 802–818. [Google Scholar] [CrossRef] [Green Version]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Puschmann, A. New Genes Causing Hereditary Parkinson’s Disease or Parkinsonism. Curr. Neurol. Neurosci. Rep. 2017, 17, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.S.; Choi, H.I.; Wang, Y.; Luo, Y.; Hoffer, B.J.; Greig, N.H. A New Treatment Strategy for Parkinson’s Disease through the Gut-Brain Axis: The Glucagon-Like Peptide-1 Receptor Pathway. Cell Transplant. 2017, 26, 1560–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhre, R.E.; Deacon, C.F.; Holst, J.J.; Petersen, N. What Is an L-Cell and How Do We Study the Secretory Mechanisms of the L-Cell? Front. Endocrinol. 2021, 12, 694284. [Google Scholar] [CrossRef] [PubMed]
- Glotfelty, E.J.; Olson, L.; Karlsson, T.E.; Li, Y.; Greig, N.H. Glucagon-like peptide-1 (GLP-1)-based receptor agonists as a treatment for Parkinson’s disease. Expert Opin. Investig. Drugs 2020, 29, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Grieco, M.; Giorgi, A.; Gentile, M.C.; d’Erme, M.; Morano, S.; Maras, B.; Filardi, T. Glucagon-Like Peptide-1: A Focus on Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 1112. [Google Scholar] [CrossRef] [Green Version]
- Hölscher, C. Brain insulin resistance: Role in neurodegenerative disease and potential for targeting. Expert Opin. Investig. Drugs 2020, 29, 333–348. [Google Scholar] [CrossRef]
- Mulvaney, C.A.; Duarte, G.S.; Handley, J.; Evans, D.J.; Menon, S.; Wyse, R.; Emsley, H.C. GLP-1 receptor agonists for Parkinson’s disease. Cochrane Database Syst. Rev. 2020, 7, CD012990. [Google Scholar] [CrossRef]
- Athauda, D.; Foltynie, T. Insulin resistance and Parkinson’s disease: A new target for disease modification? Prog. Neurobiol. 2016, 145–146, 98–120. [Google Scholar] [CrossRef]
- Elbassuoni, E.A.; Ahmed, R.F. Mechanism of the neuroprotective effect of GLP-1 in a rat model of Parkinson’s with pre-existing diabetes. Neurochem. Int. 2019, 131, 104583. [Google Scholar] [CrossRef]
- Feng, P.; Zhang, X.; Li, D.; Ji, C.; Yuan, Z.; Wang, R.; Xue, G.; Li, G.; Hölscher, C. Two novel dual GLP-1/GIP receptor agonists are neuroprotective in the MPTP mouse model of Parkinson’s disease. Neuropharmacology 2018, 133, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, L.; Li, L.; Hölscher, C. Neuroprotective effects of the novel GLP-1 long acting analogue semaglutide in the MPTP Parkinson’s disease mouse model. Neuropeptides 2018, 71, 70–80. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Li, L.; Hölscher, C. Semaglutide is Neuroprotective and Reduces α-Synuclein Levels in the Chronic MPTP Mouse Model of Parkinson’s Disease. J. Parkinsons Dis. 2019, 9, 157–171. [Google Scholar] [CrossRef]
- Foltynie, T.; Aviles-Olmos, I. Exenatide as a potential treatment for patients with Parkinson’s disease: First steps into the clinic. Alzheimers Dement. 2014, 10, S38–S46. [Google Scholar] [CrossRef]
- Bifari, F.; Manfrini, R.; Dei Cas, M.; Berra, C.; Siano, M.; Zuin, M.; Paroni, R.; Folli, F. Multiple target tissue effects of GLP-1 analogues on non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Pharmacol. Res. 2018, 137, 219–229. [Google Scholar] [CrossRef]
- Brunner, K.T.; Henneberg, C.J.; Wilechansky, R.M.; Long, M.T. Nonalcoholic Fatty Liver Disease and Obesity Treatment. Curr. Obes. Rep. 2019, 8, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Oseini, A.M.; Sanyal, A.J. Therapies in non-alcoholic steatohepatitis (NASH). Liver. Int. 2017, 37 (Suppl. 1), 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid. Med. Cell Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis: A Review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef]
- Sumida, Y.; Yoneda, M.; Tokushige, K.; Kawanaka, M.; Fujii, H.; Yoneda, M.; Imajo, K.; Takahashi, H.; Eguchi, Y.; Ono, M.; et al. Antidiabetic Therapy in the Treatment of Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2020, 21, 1907. [Google Scholar] [CrossRef] [Green Version]
- Seghieri, M.; Christensen, A.S.; Andersen, A.; Solini, A.; Knop, F.K.; Vilsbøll, T. Future Perspectives on GLP-1 Receptor Agonists and GLP-1/glucagon Receptor Co-agonists in the Treatment of NAFLD. Front. Endocrinol. 2018, 9, 649. [Google Scholar] [CrossRef]
- Kazankov, K.; Jørgensen, S.M.D.; Thomsen, K.L.; Møller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Grønbæk, H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef]
- Batista, A.F.; Bodart-Santos, V.; De Felice, F.G.; Ferreira, S.T. Neuroprotective Actions of Glucagon-Like Peptide-1 (GLP-1) Analogues in Alzheimer’s and Parkinson’s Diseases. CNS Drugs 2019, 33, 209–223. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Serý, O.; Povová, J.; Míšek, I.; Pešák, L.; Janout, V. Molecular mechanisms of neuropathological changes in Alzheimer’s disease: A review. Folia Neuropathol. 2013, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Soria Lopez, J.A.; González, H.M.; Léger, G.C. Alzheimer’s disease. Handb. Clin. Neurol. 2019, 167, 231–255. [Google Scholar] [CrossRef]
- Yildirim Simsir, I.; Soyaltin, U.E.; Cetinkalp, S. Glucagon like peptide-1 (GLP-1) likes Alzheimer’s disease. Diabetes Metab. Syndr. 2018, 12, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Murasecco, I.; Mecocci, P. Diabetes drugs in the fight against Alzheimer’s disease. Ageing Res. Rev. 2019, 54, 100936. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.G.; Livingstone, R.; Petrie, J.R. Cardiovascular benefits of GLP-1 agonists in type 2 diabetes: A comparative review. Clin. Sci. (Lond.) 2018, 132, 1699–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellar, D.; Craft, S. Brain insulin resistance in Alzheimer’s disease and related disorders: Mechanisms and therapeutic approaches. Lancet Neurol. 2020, 19, 758–766. [Google Scholar] [CrossRef]
- Kaltenboeck, A.; Harmer, C. The neuroscience of depressive disorders: A brief review of the past and some considerations about the future. Brain Neurosci. Adv. 2018, 2, 2398212818799269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.K.; Kim, O.Y.; Song, J. Alleviation of Depression by Glucagon-Like Peptide 1 Through the Regulation of Neuroinflammation, Neurotransmitters, Neurogenesis, and Synaptic Function. Front. Pharmacol. 2020, 11, 1270. [Google Scholar] [CrossRef]
- Lima-Ojeda, J.M.; Rupprecht, R.; Baghai, T.C. Neurobiology of depression: A neurodevelopmental approach. World J. Biol. Psychiatry 2018, 19, 349–359. [Google Scholar] [CrossRef]
- Stanners, M.N.; Barton, C.A.; Shakib, S.; Winefield, H.R. Depression diagnosis and treatment amongst multimorbid patients: A thematic analysis. BMC Fam. Pract. 2014, 15, 124. [Google Scholar] [CrossRef] [Green Version]
- Weina, H.; Yuhu, N.; Christian, H.; Birong, L.; Feiyu, S.; Le, W. Liraglutide attenuates the depressive- and anxiety-like behaviour in the corticosterone induced depression model via improving hippocampal neural plasticity. Brain Res. 2018, 1694, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Na, K.S.; Myint, A.M.; Leonard, B.E. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 277–284. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K. Synaptic dysfunction in depression: Potential therapeutic targets. Science 2012, 338, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, O.G.; Whyte, M.B. The use of GLP-1 receptor agonists in hospitalised patients: An untapped potential. Diabetes Metab. Res. Rev. 2019, 35, e3191. [Google Scholar] [CrossRef] [Green Version]
- Iepsen, E.W.; Lundgren, J.R.; Hartmann, B.; Pedersen, O.; Hansen, T.; Jørgensen, N.R.; Jensen, J.E.; Holst, J.J.; Madsbad, S.; Torekov, S.S. GLP-1 Receptor Agonist Treatment Increases Bone Formation and Prevents Bone Loss in Weight-Reduced Obese Women. J. Clin. Endocrinol. Metab. 2015, 100, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Hygum, K.; Harsløf, T.; Jørgensen, N.R.; Rungby, J.; Pedersen, S.B.; Langdahl, B.L. Bone resorption is unchanged by liraglutide in type 2 diabetes patients: A randomised controlled trial. Bone 2020, 132, 115197. [Google Scholar] [CrossRef] [PubMed]
- Baretić, M.; Kušec, V.; Pavlić-Renar, I. Glucagon-Like Peptide-1 Infusion Suppresses Aldosterone Levels in Healthy Normal-Weight Individuals: Double-Blind, Placebo-Controlled Crossover Study. Diabetes Ther. 2018, 9, 2315–2324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jerlhag, E. GLP-1 signaling and alcohol-mediated behaviors; preclinical and clinical evidence. Neuropharmacology 2018, 136, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Vallöf, D.; Maccioni, P.; Colombo, G.; Mandrapa, M.; Jörnulf, J.W.; Egecioglu, E.; Engel, J.A.; Jerlhag, E. The glucagon-like peptide 1 receptor agonist liraglutide attenuates the reinforcing properties of alcohol in rodents. Addict. Biol. 2016, 21, 422–437. [Google Scholar] [CrossRef]
- Jerlhag, E. Alcohol-mediated behaviours and the gut-brain axis; with focus on glucagon-like peptide-1. Brain Res. 2020, 1727, 146562. [Google Scholar] [CrossRef]
- Eren-Yazicioglu, C.Y.; Yigit, A.; Dogruoz, R.E.; Yapici-Eser, H. Can GLP-1 Be a Target for Reward System Related Disorders? A Qualitative Synthesis and Systematic Review Analysis of Studies on Palatable Food, Drugs of Abuse, and Alcohol. Front. Behav. Neurosci. 2020, 14, 614884. [Google Scholar] [CrossRef]
- Hernandez, N.S.; Schmidt, H.D. Central GLP-1 receptors: Novel molecular targets for cocaine use disorder. Physiol. Behav. 2019, 206, 93–105. [Google Scholar] [CrossRef]
- Angarita, G.A.; Matuskey, D.; Pittman, B.; Costeines, J.L.; Potenza, M.N.; Jastreboff, A.M.; Schmidt, H.D.; Malison, R.T. Testing the effects of the GLP-1 receptor agonist exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder. Drug Alcohol. Depend. 2021, 221, 108614. [Google Scholar] [CrossRef]
- Morrison, A.; Polisena, J.; Husereau, D.; Moulton, K.; Clark, M.; Fiander, M.; Mierzwinski-Urban, M.; Clifford, T.; Hutton, B.; Rabb, D. The effect of English-language restriction on systematic review-based meta-analyses: A systematic review of empirical studies. Int. J. Technol. Assess. Health Care 2012, 28, 138–144. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269, w264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]

| Reference | Local | Patients | Intervention | Outcomes | Adverse Effects | Observations |
|---|---|---|---|---|---|---|
| GLP-1 and Parkinson’s Disease | ||||||
| Athauda et al. [9] | England | Randomized, double-blind, placebo-controlled, single-center clinical trial with 62 male and female participants (25–75 y) with idiopathic PD. | Participants were randomized into 2 groups: exenatide (32.2 mg/w) or placebo/48 weeks, followed by a 12 w washout period. | Exenatide represented positive effects on defined off-medication motor scores in PD. | Gastrointestinal symptoms (nausea, constipation, abdominal pain), injection site reaction, lower urinary symptoms, back pain, upper respiratory tract infection, loss of appetite, vomiting, dyskinesia, and anxiety in the exenatide group. Gastrointestinal symptoms and injection site reactions were common in the placebo group. | 62 participants were initially randomized to the study, but 60 participants had their results on the primary analysis. |
| Athauda et al. [21] | England | Post hoc analysis of a randomized, double-blind, placebo-controlled study with 62 male and female patients diagnosed with idiopathic PD. | Participants were randomized into 2 groups: exenatide (2 mg, 1xd weekly for a 48 w period, followed by a washout design of a 12 w period) or placebo. | Participants of the exenatide group had better improvements in mood/depression and mood/apathy. Emotional well-being was improved in the exenatide group. However, these results were not sustained after the 12 w period of exenatide washout. | Gastrointestinal, injection site reaction, lower urinary symptoms, back pain, upper respiratory-tract infection, postural hypotension, loss of appetite, vomiting, dyskinesia, and anxiety in the exenatide group. | 62 participants were initially randomized to the study, but 60 participants had their results on the primary analysis. This information was viewed in the original article of the study. |
| GLP-1, NAFLD, and NASH | ||||||
| Newsome et al. [18] | Multicenter | Randomized, double-blind, placebo-controlled, parallel-group, multicenter clinical trial with 320 male and female subjects (18–75 y, 20–75 y in Japan) affected by histological evidence of NASH, with or without T2DM. All patients had BMI > 25. | Participants were randomized into 4 groups: semaglutide 0.1 mg 1xd/72 w followed by a 7 w follow-up) (n = 80, 55.2 ± 10.9 y, 51 female, BMI average of 36.1 ± 6.4); semaglutide 0.2 mg 1xd/72 w followed by a 7 w follow-up), semaglutide 0.4 mg 1xd/72 w followed by a 7 w follow-up), (n = 82, 54.3 ± 10.2 y, 47 female, BMI average of 35.2 ± 6.6, and placebo (n = 80, 52.4 ± 10.8 y, 44 female, BMI average of 36.1 ± 6.6), and placebo (same administration). | Semaglutide use was significantly related to the percentage of patients’ NASH resolution compared to placebo. The intervention of the study did not show results on the fibrosis stage of the participants. | Nausea, constipation, decreased appetite, abdominal pain, and vomiting in the semaglutide 0.4 mg group. Among other effects, neoplasms were reported in 15% of the semaglutide groups and 8% of the placebo participants. | The clinical trial started with 320 participants, but 302 completed the trial (94% of the initial total of subjects) and 285 participants completed the treatment (89% of the initial total of subjects). 277 subjects (87% of the initial total) had their results compounding the primary and the confirmatory secondary analysis of the outcomes. As a positive point of this study, the authors investigated the effects of different doses of semaglutide against NASH. |
| Armstrong et al. [23] | England | Randomized, double-blind, placebo-controlled clinical trial with 14 participants (18–70 y, BMI ≥ 25 kg/m2) diagnosed with NASH. | Participants were randomized into 2 groups: liraglutide (n = 7, 1.8 mg liraglutide injections 1xd/12 weeks) and placebo (n = 7). The dose started at 0.6 mg daily until reaching 1.8 mg/d a few days after the clinical trial. | Liraglutide was associated with reductions of metabolic dysfunctions, insulin resistance, and lipotoxicity in key organs related to the pathogenesis of NASH. | NR | This study presented a small number of participants. |
| Armstrong et al. [22] | England | Randomized, double-blind, placebo-controlled phase 2 clinical trial with 52 participants (18–70 y) affects by NASH and with BMI ≥ 25 kg/m2. | Participants were randomized into liraglutide (1.8 mg, n = 26) and placebo (n = 26) groups 1xd/48 weeks. | The use of liraglutide led to histological resolution of NASH. | Gastrointestinal disorders: diarrhea, constipation, and loss of appetite. | There were 3 participants in the liraglutide group who abandoned the treatment. |
| Bouchi et al. [24] | Japan | Single-center, randomized, open-label, comparative study with 19 patients with T2DM older than 20 years. | Participants were randomized into 2 groups: liraglutide + insulin (n = 9, liraglutide, increasing doses until 0.9 mg/d, 63% male, 57 ± 16 y) and insulin alone (n = 10, 33 male, 60 ± 22 y)/36 w; some participants had their doses adjusted between the 24th and the 36th w. | The use of liraglutide could reduce visceral adiposity and attenuate fat deposition in the liver. | No severe adverse events were observed in either group. | There were 2 patients who did not conclude the study, 1 in the liraglutide group and the other in the placebo group. This study presented a small number of participants. |
| Khoo et al. [26] | Singapore | Prospective study with 24 participants (21–65 y) diagnosed with NAFLD and NASH had the criteria of obesity. | Diet and exercise group (n = 12): each individual was told to reduce caloric intake and to exercise moderately over 26 weeks. Liraglutide group (n = 12): not given individual diet or exercise and advised to continue the pre-study routine/26 weeks, starting at 0.6 mg until 3 mg. | Weight, total fat mass, and insulin resistance decreased significantly in both groups. Reductions in serum ALT and AST, LFF, and liver stiffness were also significant and similar. CRP decreased only in liraglutide group. | Liraglutide group: nausea, transient abdominal discomfort, and bloating with every dose increment. | This study presented a small number of participants. |
| Smits et al. [29] | Netherlands | Single-center, randomized, double-blind, placebo-controlled, double-dummy, three-armed, parallel group clinical trial with 52 overweight subjects (35–75 y) with diagnosis of T2DM. | The participants were randomized into 3 groups: 1xd liraglutide (n = 17, 1.8 mg, 60.8 ± 1.8 y, 12 men), 1xd sitagliptin (n = 18, 100 mg, 61.5 ± 1.7 y, 14 men) and 1xd placebo (n = 17, 65.8 ± 1.4 y, 13 men)/12 w. | Treatment with liraglutide and sitagliptin did not reduce hepatic steatosis or fibrosis in the T2DM patients. | No serious adverse effects occurred. | 51 participants completed the study (one participant of the sitagliptin group did not conclude the trial). |
| Petit et al. [28] | France | Prospective single-center parallel-group study with 68 patients (56.9 ± 11.3 y, 31 female) with uncontrolled T2DM. 80 participants initiated the trial. | Liraglutide 1.2 mg/day for 6 m (dose that started at 0.6 mg/d and after 1 week reached 1.2 mg/d, 37 men, 56.9 ± 11.3 y). The study intervention took 6 m to be completed. | Liraglutide reduced liver fat content. The effects may be derived from the loss of weight. | 6 participants in the liraglutide group did not complete the study because of gastrointestinal adverse effects. | 80 participants initiated the study, 68 participants completed the trial. |
| Khoo et al. [27] | Singapore | Prospective randomized study with 30 obese participants with NAFLD (40.7 ± 9.1 y), BMI 33.2 ± 3.6 kg/m2 (90% male). | Participants were randomized to a supervised program of diet + exercise to induce ≥5% of weight loss (n = 15) or to liraglutide 3 mg daily (n = 15, the dose started at 0.6 mg and reached 3 mg at the end of 4 w)/26 w. | Liraglutide could decrease body weight, hepatic steatosis, and hepatocellular apoptosis in obese participants with NAFLD. There were decreases in serum alanine aminotransferases and reductions in caspase cleaved cytokeratin-18. | Nausea, abdominal discomfort and bloating, diarrhea, flatulence, constipation, dizziness, and muscle aches in the liraglutide group. | 3 participants did not conclude the study (1 of the liraglutide group). |
| Guo et al. [25] | China | Single-center, prospective, randomized, placebo-controlled stud with 96 T2DM and NASH patients (30–60 y), BMI greater than 25 kg/m2, and treated with metformin as monotherapy. | Participants were randomized into 3 groups: insulin glargine group (n = 32, 18 male, 52.0 ± 8.7 y), liraglutide (n = 32, 16 male, 53.1 ± 6.3 y) and placebo (n = 32, 20 male, 52.6 ± 3.9). The study intervention took 26 weeks to be completed. | Compared with the placebo group, treatment with liraglutide plus an adequate dose of metformin for 26 weeks is more effective in the reductions of intrahepatic content of lipids, subcutaneous adipose tissue, and visceral adipose tissue. | Nausea, vomiting, and diarrhea were gastrointestinal effects reported. Hypoglycemia was one adverse effect reported in the liraglutide group. | 91 patients completed the trial: 30 in the insulin group, 31 in the liraglutide group and 30 in the placebo group. |
| Yan et al. [30] | China | Randomized, double-blind, placebo-controlled trial with 75 participants (30–75 y) with T2DM and affected with NAFLD. The participants had been treated with metformin monotherapy. | Participants were randomized into 3 groups: liraglutide group (n = 24, 7 female, 1.8 mg 1xd), sitagliptin (n = 27, 6 female, 100 mg 1xd), and insulin glargine (n = 24, 10 female)/26 w. | Metformin and liraglutide reduced body weight, intrahepatic lipids, and visceral adipose tissue. There was an improvement in glycemic control with the use of liraglutide. | Nausea, vomiting, and headache were reported to the use of liraglutide. | 65 participants completed the trial: 18 in the liraglutide group, 26 in the sitagliptin group, and 21 in the insulin glargine group. |
| GLP-1 and Alzheimer’s Disease | ||||||
| Mullins et al. [17] | United States | Randomized, double-blind, placebo-controlled phase 2 clinical trial with 27 male and female subjects (older than 60 years) with absence of DM diagnosed with high probability for AD. | Participants were randomized into 2 groups: placebo (n = 10, 74.0 ± 6.4 y, 4 male) and exenatide (n = 11, 71.7 ± 6.9 y, 7 male, initial dose of 5 mcg/2xd, which was augmented after 1 week of the start of the study for 10 mcg/2xd)/18 w. | Exenatide demonstrated no differences or trends compared to placebo in the parameters of comparison used by the study. The treatment with the GLP-1 receptor agonist did not produce differences in comparison with the placebo intervention in clinical and cognitive measurements, in magnetic resonance imaging cortical thickness, or in cortical volume, nor were there differences in plasma biomarkers and in plasma neuronal extracellular vesicles, except with Aβ42 present in the extracellular vesicles. | Nausea, diarrhea, abdominal pain, transient asymptomatic elevation of pancreatic enzymes, loss of appetite, loss of weight, hypoglycemia. | 27 participants were randomized, but only 18 participants completed all the study. |
| Gejl et al. [31] | Denmark | Randomized, double-blind, placebo-controlled trial with 38 participants diagnosed with AD. | Participants were randomized into 2 groups: liraglutide (n = 18) and placebo (n = 20, 1,8 mg/d)/6 w. The dose of liraglutide was increased from 0.6 mg to 1.8 mg daily in the initial weeks. | Liraglutide treatment improved glucose transport by the blood–brain barrier. | NR | 17 participants in the placebo group and 14 participants in the liraglutide group completed the study. |
| Gejl et al. [32] | Denmark | Randomized, double-blind, placebo-controlled clinical trial with 38 male and female participants with AD. | Participants were randomized into 2 groups: placebo (n = 20, 66.6 y, 5 female) and liraglutide 1.8 mg d (n = 18). The dose was increased from 0.6 mg to 1.8 mg in the initial weeks of the study/26 w. | No differences between the placebo and liraglutide groups with respect to amyloid depositions or cognition, but the treatment with liraglutide prevented the decline of glucose metabolism. | Liraglutide: gastrointestinal effects such as nausea, loss of weight, a decrease of systolic blood pressure, and reduction of adipose tissue. | 38 participants initiated the study, 34 participants completed the trial. |
| Watson et al. [33] | United States | Randomized, double-blind, placebo-controlled clinical trial with 43 male and females subjects (45–75 y) affected by subjective cognitive complaints, but with a mini-mental exam greater than 27. | Participants were randomized into 2 groups: placebo (n = 21) and liraglutide (n = 22). The dose of liraglutide was increased from 0.6 mg to 1.8 mg d/12 w. | There were no cognitive differences between the groups after the 12 weeks. | NR | The first analysis of the study counted 41 participants, although the second counted 32 participants. |
| GLP-1 and Depression | ||||||
| Kahal et al. [35] | United Kingdom | Interventional case–control study with 36 obese women with or without polycystic ovary syndrome (PCOS). | Participants had POS diagnosis (n = 19, 33.9 ± 6.7 y, 102.1 ± 17.1 kg) or not (control, n = 17, 33.5 ± 7.1 y, 100.4 ± 15.1 kg). All participants received liraglutide: 0.6 mg 1xd/1 w, followed by 1.2 mg once daily for 1 w, followed by 1.8 mg 1xd/6 m. | The treatment with liraglutide improved the quality of life in the obese participants affected by PCOS, but no differences were found for risk of depression or need for treatment. | Nausea and vomiting. | 25 participants completed the trial: 13 in the PCOS group and 12 in the control group. |
| Moulton et al. [36] | United Kingdom | Prospective analysis of baseline and of 1-year period of data from 862 male and female participants newly diagnosed with T2DM (within 6 months prior to the study’s recruitment, 55.2 ± 10.5 y). | There were 2 groups: incretin (n = 25, 50.2 ± 8.8 y, 14 male, 15 sitagliptin, 6 saxagliptin, 3 exenatide, and 1 vidagliptin) and control (n = 837, oral hypoglycemic agents or insulin, 476 male, 55.4 ± 10.5 y)/12 m. | The use of an incretin-based therapy for newly diagnosed patients with T2DM was associated with improvements in depressive symptoms. | NR | 1735 participants were eligible to be part of the study and 1444 participants completed the follow-up after 1 year. The 1444 participants who completed the 1-year follow-up were more likely to have prescribed anti-depressant medications. |
| Grant et al. [34] | United Kingdom | Matched group design study with 138 male and female participants diagnosed with T2DM and did not reach adequate glycemic levels with glucose-lowering oral therapy. | Participants were divided into 2 groups: insulin (n = 67, 59.12 ± 8.6 y, 33 male) and exenatide (n = 71, 57.81 ± 9.5 y, 41 male)/18 w, but the data were collected over the 6 m of study duration. | Participants treated with exenatide had significant reductions in the depression scale used in the study compared to the insulin group. | NR | No dropouts were registered during the study. |
| Study | Question Focus | Appropriate Randomization | Allocation Blinding | Double-Blind | Losses (<20%) | Prognostics or Demographic Characteristics | Outcomes | Intention to Treat Analysis | Sample Calculation | Adequate Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Athauda et al. [9] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Athauda et al. [21] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Newsome et al. [18] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Nr | Yes |
| Armstrong et al. [23] | Yes | No | Yes | Yes | Nr | Yes | Yes | Yes | No | Yes |
| Armstrong et al. [22] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Bouchi et al. [24] | Yes | No | No | No | Yes | Yes | Yes | No | No | Yes |
| Khoo et al. [26] | Yes | Yes | No | No | NR | Yes | Yes | Yes | Nr | Yes |
| Smits et al. [29] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Petit et al. [28] | This study is not randomized. Therefore, COCHRANE guidelines do not apply here. | |||||||||
| Khoo et al. [27] | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Nr | Yes |
| Guo et al. [25] | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes | Yes |
| Yan et al. [30] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Mullins et al. [17] | Yes | No | Yes | Yes | No | Yes | Yes | No | No | Yes |
| Gejl et al. [31] | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| Gejl et al. [32] | Yes | No | Yes | Yes | Yes | Yes | Yes | No | NR | Yes |
| Watson et al. [33] | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes |
| Kahal et al. [35] | This study is not randomized. Therefore, COCHRANE guidelines do not apply here. | |||||||||
| Moulton et al. [36] | This study is not randomized. Therefore, COCHRANE guidelines do not apply here. | |||||||||
| Grant et al. [34] | This study is not randomized. Therefore, COCHRANE guidelines do not apply here. | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurindo, L.F.; Barbalho, S.M.; Guiguer, E.L.; da Silva Soares de Souza, M.; de Souza, G.A.; Fidalgo, T.M.; Araújo, A.C.; de Souza Gonzaga, H.F.; de Bortoli Teixeira, D.; de Oliveira Silva Ullmann, T.; et al. GLP-1a: Going beyond Traditional Use. Int. J. Mol. Sci. 2022, 23, 739. https://doi.org/10.3390/ijms23020739
Laurindo LF, Barbalho SM, Guiguer EL, da Silva Soares de Souza M, de Souza GA, Fidalgo TM, Araújo AC, de Souza Gonzaga HF, de Bortoli Teixeira D, de Oliveira Silva Ullmann T, et al. GLP-1a: Going beyond Traditional Use. International Journal of Molecular Sciences. 2022; 23(2):739. https://doi.org/10.3390/ijms23020739
Chicago/Turabian StyleLaurindo, Lucas Fornari, Sandra Maria Barbalho, Elen Landgraf Guiguer, Maricelma da Silva Soares de Souza, Gabriela Achete de Souza, Thiago Marques Fidalgo, Adriano Cressoni Araújo, Heron F. de Souza Gonzaga, Daniel de Bortoli Teixeira, Thais de Oliveira Silva Ullmann, and et al. 2022. "GLP-1a: Going beyond Traditional Use" International Journal of Molecular Sciences 23, no. 2: 739. https://doi.org/10.3390/ijms23020739
APA StyleLaurindo, L. F., Barbalho, S. M., Guiguer, E. L., da Silva Soares de Souza, M., de Souza, G. A., Fidalgo, T. M., Araújo, A. C., de Souza Gonzaga, H. F., de Bortoli Teixeira, D., de Oliveira Silva Ullmann, T., Sloan, K. P., & Sloan, L. A. (2022). GLP-1a: Going beyond Traditional Use. International Journal of Molecular Sciences, 23(2), 739. https://doi.org/10.3390/ijms23020739