Abstract
Rice, the main staple food for about half of the world’s population, has had the growth of its production stagnate in the last two decades. One of the ways to further improve rice production is to enhance the associations between rice plants and the microbiome that exists around, on, and inside the plant. This article reviews recent developments in understanding how microorganisms exert positive influences on plant growth, production, and health, focusing particularly on rice. A variety of microbial species and taxa reside in the rhizosphere and the phyllosphere of plants and also have multiple roles as symbiotic endophytes while living within plant tissues and even cells. They alter the morphology of host plants, enhance their growth, health, and yield, and reduce their vulnerability to biotic and abiotic stresses. The findings of both agronomic and molecular analysis show ways in which microorganisms regulate the growth, physiological traits, and molecular signaling within rice plants. However, many significant scientific questions remain to be resolved. Advancements in high-throughput multi-omics technologies can be used to elucidate mechanisms involved in microbial–rice plant associations. Prospectively, the use of microbial inoculants and associated approaches offers some new, cost-effective, and more eco-friendly practices for increasing rice production.
1. Introduction
Rice is one of the most widely consumed grains, and it is a staple food for more than 3.5 billion of the world’s population. The current global paddy production is reported to be around 755 million tonnes, with a global cultivated area of rice around 167 million hectares. The demand for rice will surely continue to increase due to the steady growth of the world’s population []. For some five decades starting in the 1960s, rice yields around the world were improved substantially by the use of high-yielding varieties, pesticides, fertilizers, and improved irrigation systems [,].
However, in the past two decades, increases in rice yield and cultivation area have stagnated due to limited water for irrigation, increases in the costs of cultivation, and degradation of water and soil quality [,,]. In addition, with greater ecological awareness and concerns for the quality of food consumed, consumers have begun to question the use of inorganic nutrients and crop protection [,].
Thus, the development and use of microbial inoculants to stimulate plant growth and health is a propitious option for meeting the expectations of society for more food and a more healthful and sustainable food supply [,]. Plant-beneficial associations with microorganisms are commonplace in nature and have been important for plant survival and evolution over many millennia [,,]. The complex, structured, and interconnected microbial networks that exist in and around plants consist of many different taxa with complementary roles. Keystone species have been identified that are crucial for plant health and ecosystem functioning so that underground ecosystems parallel the ecological complexity and interdependence found above-ground [,,].
A multitude of microorganisms stimulate plant growth and productivity through a variety of mechanisms that include improved nutrient acquisition, altered gene expression, enhanced physiological and biochemical traits, and inhibition of phytopathogens [,,]. The purposeful utilization of microorganisms’ capabilities may become particularly important within sustainable, low-input agricultural cropping systems that rely on biological processes rather than on agrochemicals to maintain soil fertility and protect plant health [,,].
Plant microbiomes are reported to be key factors in maintaining soil quality and rice production, being important influences on the growth of plants from seed to maturity [,]. This highly distinct group of microorganisms can also have profound impacts in stimulating rice plant resistance to disease and abiotic stresses []. Since the 1970s, various studies on rice plant–microbe interactions have shown that harnessing the potential of microorganisms can contribute to sustainable rice production [,].
In the past few years, the advancement in high-throughput multi-omics techniques has allowed researchers to study more comprehensively the physiological, biochemical, and molecular mechanisms that are attributable to the plant microbiome for enhancing and regulating the growth of rice. This article reviews current understandings of the roles that plant microbiomes play in enhancing the of growth, production, and health of this essential crop.
This article also discusses the physiological, biochemical, and molecular mechanisms that underlie the interaction between microbes and rice plants in the context of multi-omics approaches (proteomics, transcriptomics, metagenomics, and metabolomics). Better understanding of microbe–rice plant interactions and of the underlying mechanisms involved will be essential for the utilization of these microorganisms by farmers and agricultural practitioners to meet the ever-increasing demand for sustainable food production.
2. The Diverse and Dynamic Structures of Rice Microbiomes
Rice fields harbor a bewildering diversity of soil microbes and soil fauna—including nitrogen fixers, nitrifiers, methanogens, plant-growth regulators, methane oxidizers, phosphate-dissolving microbes, and sulfur oxidizers along with decomposers and nutrient recyclers [,,]. The composition of species will vary according to chemical, physical, climatic, and other conditions. For example, in comparison with other soil habitats, rice soils have a predominance of actinomycetes and Gram-positive bacteria. The microbial communities found in floodwater have a majority of Gram-negative bacteria plus algae, while in percolating water, only Gram-negative bacteria are common without algae [,,].
Rice–soil microbial communities are composed of huge numbers of bacterial and fungal species that perform a multiplicity of ecological functions []. Various species of archaea, oomycetes, and other microorganisms play salient roles in driving multiple ecosystem functions and ecological processes that maintain soil health and productivity [,,]. However, bacteria and fungi have the leading roles. Bacterial communities are usually dominated by the Proteobacteria, Chloroflexi, Actinobacteria, and Acidobacteria, while the most prominent members in fungal communities are Ascomycota, Basidiomycota, and Glomeromycota [,,].
The microbial communities found in rice field soils are shaped by biotic and abiotic factors such as temperature, precipitation, humidity, pH, agrochemical applications, balance of cations and anions, soil texture, and the rice cultivar planted []. Agricultural management practices such as method of crop establishment (transplanting or direct-seeding) and the duration of drying/flooding stages during crop growth also influence the microbial community structure [,].
For example, Klinnawee et al. [] have documented that under water-saturated soil conditions, the symbiotic relationship between mycorrhizal fungi and lowland (flooded) rice plant roots is diminished because, as aerobic microbes, these organisms similar to other fungi are greatly affected by the amount of water in the soil, which determines the availability of oxygen. ITS2 sequencing analysis has shown that keeping rice paddies inundated inhibits the extent of fungal communities in the soil and reduces the abundance of mycorrhizal fungi in rice roots [].
2.1. The Rhizosphere
Compared to archaea and fungi, bacteria are the predominant group found in the rhizosphere soil that surrounds plant roots; and among the bacterial phyla, Proteobacteria is the major phylum found in most rice rhizospheres []. Studies in China have found that the most abundant bacterial genera associated with the core microbiota of rice rhizospheres under standard crop management there are Anaeromyxobacter, Arenimonas, Arthrobacter, Bacillus, Bellilinea, and 15 other genera [], all of which are known to contribute to the growth and health of rice plants [].
The microbial communities in the root microbiome of rice are influenced by cultivation practices. For example, organically cultivated soils are enriched with certain genera that have potential for promoting plant growth such as Anabaena, Azospirilllum, and Rhodobacter []. Soils on rice farms that are continuously flooded harbor large numbers of methanogenic archaea that generate the greenhouse gas methane. Conversely, in rice-paddy soils that are kept mostly aerobic, methanotrophic bacteria are more dominant. They consume methane for their own needs, keeping this gas from entering the atmosphere [].
Plant roots exude a wide range of compounds into their surrounding rhizosphere such as sugars, polysaccharides, amino acids, aromatic acids, aliphatic acids, and fatty acids that attract microorganisms to form a mutualistic association. The composition and pattern of root exudates definitely will affect the makeup of microbial communities in the rhizosphere [,].
The domestication of rice, both its origins and species, has been influenced by the composition of fungal communities in the rhizosphere. For example, it has been found that five genera of arbuscular mycorrhizal fungi (AMF) (Acaulospora, Claroideoglomus, Pacispora, Redeckera, and Scutellospora) are significantly correlated with other fungi in the rhizosphere of wild rice, whereas only three groups of AMF, a somewhat different set (Claroideoglomus, Gigaspora, and Redeckera), are significantly correlated with the other fungi that exist in the rhizosphere of domesticated rice [].
Arbuscular mycorrhizal fungi are also affected by cultivation practices. When the AMF communities inhabiting rice roots growing under the System of Rice Intensification (SRI) were compared with those found under conventional methods of cultivation, all the AMF in the roots sampled from conventional plots belonged to just one genus, Glomus, while in the roots of rice plants being grown in an SRI environment, there were gene sequences for both Acaulospora and Glomus [].
Numerous studies have reported numerous plant-beneficial microorganisms such as Bacillus, Trichoderma, Aspergillus, Penicillium, Clostridium, and Azotobacter being more abundant in the rhizospheres of rice under SRI crop management [,,,]. This is further evidence that management practices have definite effects on the microbiomes of roots. Figure 1 gives an insight into how agricultural management practices such as SRI affect the soil microbial communities and activities.
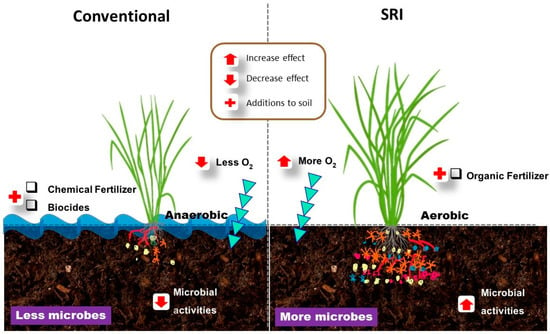
Figure 1.
SRI methods produce more robust plant growth and health (right) than conventional grown plant (left). This is due to the SRI agroecosystems providing a more supportive environment for microbes to grow and to benefit rice plants, while conventional methods limit microbes’ growth and inhibit their effects. If this model is transposed to the larger scale of an SRI rice field, it is understandable why SRI plants achieve higher growth performance and yield as well as more resistance to biotic and abiotic stresses. In SRI rice fields, many and diverse microbes exist and interact with rice plants. Subsequently, this synergetic relationships between SRI methods and microbes affected the rice plant growth, physiological processes, yield, and patterns of gene expression.
2.2. The Phyllosphere
The aerial parts of rice plants, particularly their leaves, provide an open habitat that is colonized by many diverse microorganisms, many of which contribute to plants’ growth and fitness. The phyllosphere is a dynamic habitat with its resident microbes on or around the leaves subjected to varying environmental conditions such as temperature, water availability, moisture, solar radiation, and elevation [].
The phyllospheric microbiome population is also much influenced by cultivation methods and chemical fertilizer applications. Rice plants grown under different methods of cultivation such as SRI and conventional methods exhibited different physiological activity and microbial populations, for example. This can be attributed to their respective soil milieus (aerobic vs. anaerobic) and changes in the quantity and forms of introduced nutrients and in the physiochemical properties of the leaves [,].
As an open environment that is subject to diverse biotic stimuli, e.g., insect and pathogen invasions, the phyllosphere microbiome is composed of a mixture of commensal, beneficial, and pathogenic species []. Roman-Reyna et al. [] found that the microbial community composition on rice leaves could be delineated in terms of 12 genera that span a range from commensal or pathogenic to beneficial, e.g., Clostridium, Bacillus, Helicobacter, Azotobacter, and Pseudomonas.
Overall, biotic and abiotic stimuli as well as anthropogenic influences, e.g., cultivation methods and fertilizer application, played important roles in shaping both the structural (taxonomical) and functional attributes of phyllosphere microbiomes. These factors make the rice phyllosphere a dynamic and heterogeneous environment.
2.3. The Endosphere
This refers to the plants’ internal domain, which is inhabited by endophytes, both bacteria or fungi, that live most of their life cycle inside plant tissues without causing pathogenic symptoms [,]. As integral parts of plants’ structure and functioning, endophytic microorganisms are known to improve plant health, performance, and adaptation to both biotic and abiotic stresses [,].
Microorganisms in the endosphere can vary over time. Siderophore-producing bacteria belonging to the genera Sphingomonas, Pseudomonas, Burkholderia, and Enterobacter are primarily detected in rice plant tissues during plants’ vegetative stage, when the plants benefit most from siderophores, giving them greater access to iron and other elements in the soil. The endophytic bacterial genus Pantoea, on the other hand, is predominant in roots at the time of tillering and then in the leaves at subsequent stages [].
There can also be locational variations in the inhabitation of plant endospheres. In China, for example, the structure of microbial communities within root endospheres was found to differ significantly between the municipalities of Taoyuan, an ecosite with ultrahigh rice yield, and Jinghongdi. Thirteen phyla, primarily Verrucomicrobia, Proteobacteria, Planctomycetes, and Bacteroidetes, were identified between the two sites. Metagenomic analysis showed that Taoyuan had more taxa, e.g., Thaumarchaeota and Nitrospira, which had nitrogen metabolism functions that correlated with mechanisms for the very high yield [].
In addition to colonizing plant organs, endophytic bacteria are also associated with the seeds (grains) of rice plants. Bacteria genera within the classes Alpha- and Gamma-proteobacteria, Flavobacteria, Bacilli, and Actinobacteria have been observed to reside within rice seeds []. Microbial interactions occurring on, around, and inside seeds are important for subsequent plant fitness, since seed-borne microorganisms are the initial source of inoculum for the plant microbiome and are important for early plant development and early plant vigor [,].
3. Microbial Contributions for Promoting Rice Growth and Yield: From Earlier Views to the Omics Era
The use of microorganisms to enhance rice growth and production is now regarded as an eco-friendly way to carry out intensified agriculture without degrading the environment with agrochemical products or mechanical interventions. Over recent decades, interest in the application of microorganisms in rice production has increased rapidly due to their ability to act as plant-growth regulators [].
Already in the 1970s and 1980s, various studies conducted on the association of N2-fixing bacteria with rice plants indicated that bacterial inoculation could improve plant growth and rice yield [,]. However, as rice plants are not leguminous, the benefits were not from N-fixation in nodules formed on plant roots. The inoculation of rice seedlings with Azospirillum promoted early tillering and the better reproductive performance of rice plants. It was found to significantly increase the grain-filling rate and the grain weight per plant at harvest time [].
In the 1990s, biological N2 fixation was seen as the most effective system for sustaining production in low-input, traditional rice cultivation []. In Egypt, it was found that the association of Rhizobium leguminosarum with rice plants could significantly increase the shoot and root growth of these plants, their grain yield, and their nitrogen-use efficiency []. This was somewhat surprising, because such effects had previously been observed with legumes, not grass-family (Poaceae or Gramineae) plants which include rice, wheat, and most cereal crops.
Further studies on the effects of Rhizobium on rice plants were conducted in the 2000s. The growth and yield responses of rice plants to inoculation with two bacterial strains (R. leguminosarum E11 and Rhizobium sp. IRBG74) showed significantly increased grain and higher straw yields at the maturity stage []. A multinational collaborative study with three field experiments in Egypt reported that the Rhizobium inoculation of rice plants significantly increased their biomass, nutrient uptake (due to more robust root architecture), grain yield, fertilizer efficiency, harvest index, and grain nutritional value. Further experiments of selected rhizobial endophytes on rice showed that they produced cell-bound cellulase and polygalacturonase enzymes that hydrolyze glycosidic bonds in plant cell walls plus certain bacteriocins that can inhibit the growth of undesirable microbes [].
Chi et al. [] examined the infection, dissemination, and colonization patterns of rhizobial bacteria within plant tissues following seedling inoculation with one of five selected species, observing their effects on the same variety of rice grown in the same soil. The different species of rhizobia were tagged with green fluorescent protein (gfp) markers for better ascertaining their influence on agronomic characteristics of rice. The five sets of seedlings respectively inoculated with different bacterial species were raised under the same greenhouse conditions; a sixth set of plants inoculated with dead bacteria was grown alongside them as a control.
This research showed a dynamic process of infection that started with the rhizobia surface colonizing the roots’ rhizoplane. This was followed by the endophytic colonization of rice root tissues and then by the rhizobia endophytically ascending into the stem, leaf sheathes, and leaves of the plants, where their populations reached as high as 9 × 1010 rhizobia per cm3 of infected (inhabited) rice tissue. The rice plants that had been infected with living rhizobia, compared to plants inoculated by dead rhizobia of the same type, produced significantly higher root and shoot biomass with greater rates of photosynthesis, more stomatal conductance, greater transpiration velocity, water-use efficiency, and flag leaf area, plus higher measured levels of phytohormones that stimulate and regulate growth.
A study using strain Azospirillum sp. B510 to evaluate its efficacy with different rice cultivars under field conditions showed that the growth of rice plants, especially tiller number at the early growth stage, varied considerably according to rice genotype, as well as the level of soil nitrogen. So, the plant genotype (cultivar) needs to be considered along with the timing of bacterial inoculation and soil and nutrient management practices when applying Azospirillum-based biofertilizer as a soil amendment [].
Research in Madagascar in 2000 and 2001 on the effects of using SRI vs. conventional crop management on rice plant tillering and yield, considering also the population density of the bacterium Azospirillum inhabiting the plant roots under both cultivation systems, indicated a very large increase in the numbers of endophytic Azospirillum spp. associated with SRI management, with a doubling or tripling of grain yield depending on soil quality. Under SRI crop management, the populations of Azospirillum residing in the roots of SRI rice plants were many times greater than were counted in the roots of rice plants grown conventionally under the same soil and climatic conditions (reported in Uphoff et al. []).
In recent years, microbial consortia have been also developed for improving rice plant productivity, compared with applying single-strain, microbe-based inoculants []. Jha et al. [] have developed a microbial consortium that consisted of Pseudomonas, Azospirillum, and cyanobacteria for improving rice growth. This consortium increased rice plants’ micro- and macronutrient uptake, their growth, and grain yield. Prasanna et al. [] evaluated the capability of three selected microbial strains (Providencia sp. PR3, Brevundimonas sp. PR7, and Ochrobacterium sp. PR10) as well as three strains of cyanobacteria (Anabaena sp. CR1, Calothrix sp. CR2, and Anabaena sp. CR3) and also combinations of them to assess their beneficial effects, if any, on rice growth and yield. The microbial consortia significantly enhanced rice growth and grain yield and improved soil health, making it possible to reduce the application of inorganic nitrogen fertilizer by 40–80 kg ha−1 without sacrificing grain yield.
In recent years, many studies have used multi-omics approaches to elucidate microbial involvement in the promotion of growth and yield of rice plants []. A comparative proteomic study done in China [] indicated that compared to controls, the functional protein profiles in three categories of rice plant tissues (in the roots, leaf sheaths, and leaves) were differentially up-regulated or down-regulated after inoculation with bacterial strain Sinorhizobium meliloti 1021.
Proteins related to photosynthesis capability were up-regulated in the leaf sheath and leaves, while the proteins up-regulated in the roots were mostly linked to plant defense mechanisms. The study also demonstrated an increase in the transport proteins linked to the reaction of chloroplasts to light and dark as well as to the efficient distribution of nutrients within the plant. These enhancements at the cellular level contributed to a higher rate of photosynthesis within the rice plant leaves.
In a subsequent set of trials using transcriptomic analysis [], these researchers evaluated the effects on plants’ gene expression of inoculating rice seedlings with S. meliloti 1021. Their analysis identified more than 2400 differentially-expressed genes (DEGs) in rhizobially-inoculated rice plants vis-à-vis control plants. The genes that were up- or down-regulated in the presence of microbial inoculants were ones known to be involved in processes such as phytohormone production, photosynthetic efficiency, carbohydrate metabolism, cell division, and wall expansion. These effects were induced in rice seedling shoots after the roots had been colonized by S. meliloti 1021, so there was a systemic rather than localized response.
These researchers proposed a model to account for how rice seedling roots interact with the S. meliloti 1021 around and on them. First, S. meliloti 1021 secretes some bioactive signaling chemicals that are recognizable to rice receptor proteins (PRRs, FLS2, and LRR-RLKs). These bioactive signals attach to rice root cells around the area of bacterial colonization and primary infection. After recognizing and transducing these bacterial signals, DEGs that are involved in the production of phytohormones regulating plant growth and development such as auxins, gibberellins, and cytokinins are altered in the rice seedling shoots, and then, the bacteria ascend within the plant into more rice tissues where they live symbiotically, still affecting plant gene expression.
The recognition process between bacterial and plant cells subsequently modulates some plant genes that are involved in the regulation of cell cycles such as CycA, CycB, CycD1, D2, and D3, impacting and accelerating cell division. These cellular changes also enhance several other processes that promote plant growth and development, photosynthesis capacity, C and N metabolisms, phytohormone production, and other agronomic characteristics [].
Tang et al. [] have documented accelerated plant growth and yield enhancement as well as improvement in the quality and mineral nutrition of rice grains after rice plant inoculation with the endophytic fungus Phomopsis liquidambaris. Further transcriptomic analysis showed that genes related to certain transporter genes for N (OsAMT1;4 and OsNRT1;1) and for P (OsPT1 and OsPht1;2) were up-regulated during the seedling stage after inoculation with P. liquidambaris.
The same results were obtained during the heading stage when rice plants were inoculated with P. liquidambaris. This affected the up-regulation of transporter genes in rice roots for P, Zn, and Fe (OsPT1, OsPht1;2, OsZIP3, OsZIP4, OsIRT1, and OsIRT2) and for N, P, Zn, and Fe (OsAMT1;4, OsPht1, OsZIP4, and OsIRT1). Significant alteration was also recorded for genes involved in Zn and Fe transport in rice roots, such as OsZIP3 and OsIRT2, and in the transport of P, Zn, and Fe during the ripening stage (OsPht1;2, OsZIP3, OsZIP4, and OsIRT1).
Fungi belonging to the genus Trichoderma have been frequently reported to be able to colonize rice roots endophytically [,]. For example, a study in Nepal revealed that the inoculation of rice seedlings with Trichoderma significantly increased plant growth and grain yield compared to that of rice plants in adjacent untreated plots [].
A novel isolate growth-promoting fungus, T. asperellum SL2, has been shown to enhance the rice germination, seedling and vegetative growth, plant vigor, grain yield, photosynthetic rate, stomatal conductance, and water-use efficiency of rice plants [,]. Transcriptomic analysis revealed that many genes linked to molecular processes at the cell level in rice plants were significantly up-regulated when inoculated with T. asperellum SL2. These included genes relating to the synthesis of rubisco (RBCS, OsRBCS1, and OsRBCS2); others involved in stress tolerance (CYP38 and CYP20-2); a gene linked to gibberellin regulation (OsGAE1); a gene related to rice tillering (MOC1); and a gene related to the uptake of phosphorus (OsPHR2) [,,].
In addition to being able to modulate biochemical and molecular signals within rice cells, microbes are known to be able to provide a range of services and benefits to the rice plant through a variety of mechanisms, including organic matter mineralization, biological nitrogen fixation, P-solubilization, Fe-chelation, and the secretion of phytohormones such as indole acetic acid, gibberellins, and cytokinins [,]. Documentation regarding the contributions of microorganisms toward the enhancement of rice plant yield and grain production is summarized in Table 1.

Table 1.
Contributions of microorganisms for the enhancement of rice yield.
4. Services of Microbes in Protecting Rice Plants against Plant Diseases
At present, plant diseases are controlled mostly by using chemical biocides and in some cases by cultural practices. However, the widespread use of agrochemical products in agriculture has been a subject of public concern and scrutiny due to potential harmful effects on the environment, undesirable effects on non-target organisms, and the possible carcinogenicity of some chemical elements []. Plus, the intensive use of biocides has led to the development of resistance applications leading to outbreaks of rice diseases (Figure 2). An alternative method for controlling plant diseases is by strengthening plants’ own immunity to diseases through plant–microbial interaction []. Several microorganisms belonging to different genera known to elicit induced systemic resistance (ISR) in plants are reported to be effective tools for the biological control of certain plant pathogens [].


Figure 2.
Excessive use of biocides and chemical fertilizers resulted in the severe outbreaks of various rice diseases in Selangor, Malaysia: (a) Rice plants infected by Pantoea spp. with a yellowish leaf blight disease lesion on the leaves that leads to unfilled, empty, and discolored grains (photo courtesy of Muhammad Nazri Ishak), (b) Close-up view of the infected leaf with a lesion at the edge (adapted from Doni et al. [], the journal does not require permission to use materials), (c) Typical field symptoms of bacterial panicle blight caused by Burkholderia glumae with discoloration and sterility of grain as well as rotting and panicle blanking, (d) A close look at the severely infected panicle. The panicle remains upright rather than bending down with the weight of the grain (photo courtesy of Nurul Shamsinah Mohd Suhaimi). Therefore, the use of beneficial microorganisms for the biological control of plant diseases needs to be encouraged and promoted as a powerful solution to replace toxic chemical biocides.
4.1. Fungal Diseases
Trichoderma spp. are widely used as biocontrol agents for major fungal rice diseases such sheath blight [,], rice blast [], and rice brown spot []. The mechanisms of Trichoderma spp. for protecting rice plants from pathogenic fungi are several, i.e., competition and rhizosphere competence; mycoparasitism and antibiotic production; antibiosis; degradation of toxins produced by pathogens; production of cell wall-degrading enzymes; inactivation of pathogens’ enzymatic pathways; and induction of defense responses in host plants [,]. Transcriptomic analysis has revealed that at least 18 genes related to plant-defense responses are significantly up-regulated after T. asperellum SL2 inoculation in rice plants. Furthermore, T. asperellum SL2 is also able to induce systemic acquired resistance (SAR) in rice plants by reprogramming the molecular signaling of plant cells [].
Pseudomonas spp. have been studied for decades as model microorganisms for biological control, and they have proved to be effective biocontrol agents for resisting various soil-borne plant diseases [,]. It has been known for some time that P. fluorescens inhibits the mycelial growth of the sheath blight fungus R. solani and increases chitinase and peroxidase activity in rice, thereby inducing systemic resistance against R. solani []. A study by Patel et al. [] indicates that Pseudomonas produces a volatile organic compound (pyrazine) that can suppress infection by Magnoporthe oryzae in rice. A talc-based bioformulation of P. fluorescens has also shown biocontrol potential against sheath blight in rice. In field trials, it was found that this microorganism could reduce the incidence of sheath blight in rice by up to 62%, with 12–21% more grain yield [].
Another prominent genus used for biocontrol, Bacillus, can inhibit the spread of major rice diseases such as sheath blight and blast. Antifungal compounds produced by B. subtilis show a strong synergistic inhibitory effect on the hyphal growth of Pyricularia grisea and R. solani, the respective causative agents for blast and sheath blight in rice [].
Another study that employed a strain of endophytic bacterium Bacillus sp. EBPBS4 for managing sheath blight showed it to be antagonistic toward the fungal pathogen R. solani [].
Subsequently, a proteomic study indicated that in rice plants treated with this strain of Bacillus, there was an up-regulation of putative disease-resistance proteins such as RGA1, NBS-LRR, serine threonine protein kinase, chitinase, β 1–3 glucanase, ascorbate peroxidases, hydroxyl methyl CoA ligase, PAL, and iron superoxide dismutase. The up-regulation of these defense proteins in plants treated with Bacillus sp. EBPBS4 was what apparently led to its protective effect against R. solani [].
All of the studies discussed above highlighted that microorganisms could be applied as effective biocontrol agents for more profitable and sustainable rice crop production, especially for controlling fungal diseases while enhancing rice production. Further information on the capability of microbiomes to protect rice plants against different fungal pathogens is summarized in Table 2.

Table 2.
Microbes and their biocontrol regulation activities against fungal diseases in rice.
4.2. Bacterial Diseases
Leaf blight disease caused by Xanthomonas oryzae pv. oryzae and Pantoea spp. is a major disease faced by rice growers in many countries, causing significant economic losses in rice yield world-wide, since the yield loss due to leaf blight can be more than 70% [,]. Over the years, many microbial inoculants have been evaluated as potential biocontrol agents against various bacterial diseases. For example, Burkholderia amyloliquefaciens has been shown to possess biocontrol capacity against X. oryzae pv. oryzae and X. oryzae pv. oryzicola, which are the agents respectively causing leaf blight and bacterial leaf streak in rice. In this case of B. amyloliquefaciens, we know that this bacterium produces two antibiotic compounds (difficidin and bacilysin) that down-regulate the expression of genes affecting Xanthomonas virulence, cell division, and protein and cell wall synthesis [].
The combined application of T. harzianum and P. fluorescens has shown a strong antagonistic effect against X. oryzae pv. oryzae. The inoculation of T. harzianum and P. fluorescens synergistically reduced the incidence of leaf blight in inoculated plants compared to the untreated plant controls. Several parameters of rice plant growth and yield were also increased by the combined application of T. harzianum and P. fluorescens. The protection against X. oryzae pv. oryzae provided by this fungal–bacterial combination is due to an increase in the lignification of cell walls and in the activities of peroxidase, phenylalanine ammonia-lyase, and 4-coumarate-CoA ligase enzymes in rice leaves [,].
Another important bacterial disease in rice is bacterial panicle blight caused by B. glumae. Several Bacillus spp. significantly suppress the development of bacterial panicle blight caused by B. glumae under field conditions []. In recent trials, Bacillus spp. showed a biocontrol capacity in the greenhouse for reducing B. glumae infection by as much as 63.5%, while the populations of B. glumae in the plant were diminished by at least two orders of magnitude [].
The potency of harnessing beneficial microbiomes to control bacterial diseases in rice production has become more apparent every year. Table 3 summarizes the use of microbial inoculants for the biological control of a variety of bacterial pathogens in rice.

Table 3.
Examples of biocontrol effects of beneficial microbes in protecting rice plants from bacterial diseases.
5. Microbial Involvement in Conferring Abiotic Stress Tolerance in Rice
In addition to protecting against various biotic stresses, plant microbiomes also support plants’ resilience against abiotic stresses including drought, salinity, and heavy metals in the soil. Over the years, some microbes, especially fungal endophytes, have come to be widely regarded as important agents that can confer plant stress through plant–microbe symbiosis [].
5.1. Drought Stress
There are numerous reports of fungal symbionts conferring drought-stress resistance on host plants. T. harzianum significantly increases the ability of rice plants to tolerate drought stress with the explanation that this fungus manipulates stomatal conductance, leaf greenness, and net photosynthesis, decreasing proline, malondialdehyde, and H2O2 contents, and it increases superoxide dismutase levels, plant height, total dry matter, and relative chlorophyll content in rice plants [,]. The research indicated that a dose of T. harzianum inoculant (30 g/L) is an effective means for improving drought tolerance in rice [].
Key genes related to metabolic pathways that are affected by inoculation with T. asperellum include phenylpropanoid (PAL), superoxide dismutation (SODs), H2O2 peroxidation (APX, PO), and oxidative defense response (CAT). Under drought conditions, these were over-expressed in rice plants after inoculation with T. asperellum. Enhancement of the expression of drought-adaptation genes such as OSPiP and DHN is also reported as a defense mechanism activated by T. asperellum against drought stress [].
Transcriptomic analysis of drought-challenged rice plants bioprimed by T. harzianum showed substantial alterations in gene expression compared to untreated control plants []. Out of the 2506 DEGs identified by next-generation sequencing analysis, 382 were exclusively expressed in plants inoculated with T. harzianum. Comparative analysis of up-regulated and down-regulated genes under drought conditions showed that 1053 genes were up-regulated and 733 genes were down-regulated in the T. harzianum-inoculated plants. The genes that were exclusively expressed or up-regulated in such plants were mostly genes related to photosynthetic and antioxidative capacities, e.g., plastocyanin, small units of rubisco, PSI subunit Q, PSII subunit PSBY, osmoproteins, proline-rich protein, aquaporins, stress-enhanced proteins, and chaperonins. Hence, biopriming with T. harzianum was seen to aid rice plants in a multifaceted, simultaneous manner that resulted to enhanced drought-stress tolerance.
P. fluorescens has also been reported as capable of increasing rice plants’ resistance to drought stress by increasing the activity of protective enzymes, including β-1,3-glucanase, guaiacol peroxidase, peroxidase, phenylalanine ammonia lyase, and superoxide dismutase []. A real-time qPCR analysis showed that six genes known to be related to plants’ stress tolerance (COX1, PKDP, bZIP1, AP2-EREBP, Hsp20, and COC1) were up-regulated in drought-stressed rice plants after inoculation with P. fluorescens [].
B. amyloliquefaciens is also known to have a positive effect for protecting rice plants from drought stress by modulating various biochemical and physiological activities in the plants, such as membrane integrity and the gene expression of drought-related genes []. In addition, AMF were also reported to be effective in enhancing the ability of rice plants to cope with drought stress by decreasing the shoot water potential content of an antioxidant, glutathione []. All these results have showed a remarkable influence from microbial colonization, which appears to be a key element for inducing systemic drought-tolerance.
5.2. Salinity Stress
One approach to solve the constraint of salt stress is by interposing beneficial microbes []. Fungal endophytes have been reported to confer beneficial effects for salt-stressed plants by various direct and indirect mechanisms []. T. harzianum has the ability to increase rice plants’ resistance to salinity, as under saline conditions, plants treated with this symbiotic fungus showed higher relative water content and more stomatal conductance, a greater rate of photosynthesis, and higher pigment and proline concentrations compared to untreated plants [,].
B. pumilus has conferred salt-stress tolerance in rice plants by enhancing their antioxidant enzyme activities, such as catalase by 15.1–32.9% and superoxide dismutase by 8.7–26.6%. B. pumilus also played a role in accelerating the activities of certain soil enzymes such as alkaline phosphatase (by 18.4–53.5%), acid phosphatase (by 28.4–46%), urease (by 14.8–47.8%), and β-glucosidase (by 25.2–56.1%) in inoculated pots as compared with non-inoculated pots, which neutralized the negative effects of salinity [].
A fungal endophyte Piriformospora indica has been found to regulate salt stress in rice plants by up-regulating certain genes related to salt stress, such as OsSTK, OsLEAP, OsAP1, OsMPIX, Os40S27, OsSIP, and OsSOS1 []. In a different set of trials, rice seedlings that had been inoculated with the bacterial endophyte P. alhagi exhibited significantly higher activities of certain antioxidant enzymes (SOD, POD, and CAT) and greater plant biomass and chlorophyll content than did uninoculated seedlings in salt-stress evaluations []. These studies indicate a capacity of certain endophytic microbes to counteract salinity stress in rice plants and thereby to improve rice production on saline soil.
5.3. Heavy Metal Stress
Microorganisms can also play an effective role in acclimatizing plants to grow in metalliferous environments and to improve plants’ tolerance of heavy metals in their soil environment []. Plant-associated microbes can decrease the accumulation of such metals in plant tissues and can help to reduce the bioavailability of metals in the soil through various mechanisms []. A cadmium-tolerant bacterium Cupriavidus taiwanensis was reported to transform a toxic, soluble CdCl2 into a nontoxic, insoluble CdS, and it subsequently decreased the accumulation of cadmium in rice plants by 61% []. Moreover, it has been seen that rice plants treated with Trichoderma spp. had a gain in biomass that appeared correlated with their inducing a lower concentration of the cadmium in these plants that was inhibiting their growth [].
Another study demonstrated that the heavy-metal-resistant bacteria Ochrobactrum spp. and Bacillus spp. increased the germination percentage of rice seeds, relative root elongation of seedlings, and the amylase and protease activities in arsenic/cadmium-treated rice plants. The results showed that the activity of superoxide dismutase and the level of malondialdehyde in rice roots were lowered by bacterial inoculation []. Recent studies conducted separately by Wang et al. [] and Pramanik et al. [] confirmed that Pseudomonas spp. significantly reduces cadmium uptake and accumulation in different parts of rice plants. The capacity of Pseudomonas spp. to mediate the negative effects of cadmium on rice plants’ performance is closely linked to its affecting changes in soil pH and enzyme activities.
Arbuscular mycorrhiza fungi exert some protective effects against the combined toxicity of copper, zinc, lead, and cadmium in contaminated soil, while at the same time increasing the shoot and root biomass of rice plants []. The inoculation of upland rice with AMF also has increased the total phosphorus uptake and decreased arsenic uptake [].
Bacteria belonging to the genus Pantoea have showed a promising capacity to mediate the negative effects of arsenic stress as well as to act as phytostimulants in rice plants. A study conducted by Ghosh et al. []. suggests that the inoculation of rice plants with P. dispersa significantly improves the morpho-biochemical characteristics of the plants such as increased enzymatic activities and reduced arsenic uptake into rice tissues. All of these results indicate that microbes have promising roles to play in bioremediation as well as in improving plants’ tolerance to heavy-metal stress.
6. Conclusions
The continuing increase in global population and the ascendance of environmental threats such as climate change present a strenuous challenge for producing sufficient rice worldwide. To meet global demand, rice yields will need to be increased with diminishing land and water resources per capita.
Current conventional rice farming methods that rely heavily on chemical inputs and varietal improvement have not been increasing the production of rice sufficiently in recent decades, and agrochemical dependence poses serious threats to environmental quality and sustainability. Natural and biological approaches that use beneficial microorganisms in association with other validated agroecological approaches offer untapped opportunities, increasing rice production in eco-friendly ways.
In their association with rice plants, microorganisms can use the plants as habitat or just colonize the rice roots or plant surfaces. Once established, microorganisms release bioactive signals that are recognizable by the rice plant cell receptors, and they subsequently enhance plant growth, confer tolerance to abiotic stress, increase disease resistance, alter patterns of gene expression in beneficial ways, improve physiological and biochemical traits, and enhance the nutrient uptake of rice plants (Figure 3).
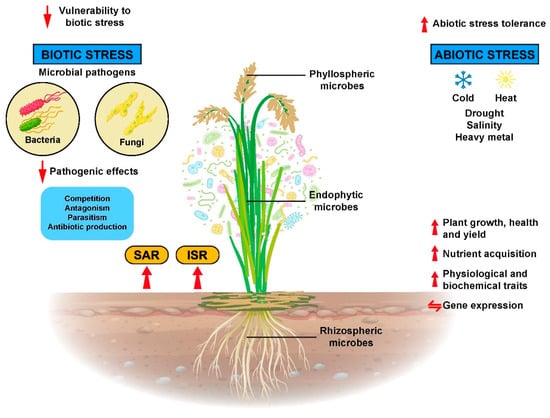
Figure 3.
Microbes that are residing in, on, and inside the rice plants affected rice plant growth, physiological, biochemical, and molecular processes. Thereby, they increase rice plant growth, development, and yield as well as more resistance to biotic and abiotic stresses.
However, still, much investigation remains to be done to better understand rice plant–microbe interactions, especially with regard to: (i) microbiome cross-talk interaction with rice plants; (ii) the isolation and characterization of more compatible strains of microbes that can be utilized in cost-effective ways for commercialization; (iii) understanding the complex composition of the microbiome in the soil, linking microbiome composition with functions; and (iv) understanding of microbial–rice plant associations and their involved mechanisms using omics-based approaches such as proteomics, transcriptomics, metagenomics, and metabolomics.
Author Contributions
Conceptualization: F.D.; writing—original draft preparation: F.D.; writing—review and editing: F.D., N.S.M.S., M.S.M., F.F., B.M.M., J.K., N.U.; supervision and project administration: F.D., B.M.M., J.K.; visualization: F.D., N.S.M.S., N.U., M.S.M., F.F.; funding acquisition: F.D., B.M.M., J.K.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universitas Padjadjaran through Riset Percepatan Lektor Kepala (RPLK), grant number 4895/UN6.3.1/PT.00/2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the financial support provided by Universitas Padjadjaran. The first author also would like to thank Marathur Butarbutar for insightful discussions on agroecology and sustainable food system.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AMF, arbuscular mycorrhizal fungi; APX PO, H2O2 peroxidation; CAT, oxidative defense response; DEGs, differentially expressed genes; gfp, green fluorescent protein; ISR, induced systemic resistance; PAL, phenylpropanoid; POD, peroxidase; qPCR, quantitative polymerase chain reaction; SAR, systemic acquired resistance; SODs, superoxide dismutation; SRI, System of Rice Intensification.
References
- FAO. World Food and Agriculture—Statistical Yearbook 2021; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/3/cb4477en/cb4477en.pdf (accessed on 23 September 2021).
- Yuan, S.; Nie, L.; Wang, F.; Huang, J.; Peng, S. Agronomic performance of inbred and hybrid rice cultivars under simplified and reduced-input practices. Field Crops Res. 2017, 210, 129–135. [Google Scholar] [CrossRef]
- Wu, K.; Wang, S.; Song, W.; Zhang, J.; Wang, Y.; Liu, Q.; Yu, J.; Ye, Y.; Li, S.; Chen, J.; et al. Enhanced sustainable green revolution yield via nitrogen-responsive chromatin modulation in rice. Science 2020, 367, eaaz2046. [Google Scholar] [CrossRef]
- Ray, D.K.; Ramankutty, N.; Mueller, N.D.; West, P.C.; Foley, J.A. Recent patterns of crop yield growth and stagnation. Nat. Commun. 2012, 3, 1293. [Google Scholar] [CrossRef] [PubMed]
- Muehe, E.M.; Wang, T.; Kerl, C.F.; Planer-Friedrich, B.; Fendorf, S. Rice production threatened by coupled stresses of climate and soil arsenic. Nat. Commun. 2019, 10, 4985. [Google Scholar] [CrossRef]
- Hussain, S.; Huang, J.; Huang, J.; Ahmad, S.; Nanda, S. Rice production under climate change: Adaptations and mitigating strategies. In Environment, Climate, Plant and Vegetation Growth; Fahad, S., Ed.; Springer: Cham, Switzerland, 2020; pp. 659–686. [Google Scholar]
- Heong, K.L.; Escalada, M.M.; Chien, H.V.; Delos, R.J.H. Are there productivity gains from insecticide applications in rice production? In Rice Planthoppers: Ecology, Management, Socio Economics and Policy; Heong, K.L., Cheng, J.A., Escalada, M.M., Eds.; Zhejiang University Press: Hangzhou, China; Springer Science+Business Media: Dordrecht, The Netherlands, 2015; pp. 181–192. [Google Scholar]
- Li, S.; Tian, Y.; Wu, K.; Ye, Y.; Yu, J.; Zhang, J.; Liu, Q.; Hu, M.; Li, H.; Tong, Y.; et al. Modulating plant growth-metabolism coordination for sustainable agriculture. Nature 2018, 560, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.I.D.S.; Chaibub, A.A.; Sousa, T.P.; Cortes, M.V.; de Souza, A.C.; da Conceição, E.C.; de Filippi, M.C. Formulations of Pseudomonas fluorescens and Burkholderia pyrrocinia control rice blast of upland rice cultivated under no-tillage system. Biol. Control. 2019, 27, 104153. [Google Scholar] [CrossRef]
- Harman, G.; Khadka, R.; Doni, F.; Uphoff, N. Benefits to plant health and productivity from enhancing plant microbial symbionts. Front. Plant Sci. 2021, 11, 610065. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Baird, A.; Cho, S.; Gray, Z.; Groover, E.; Harto, R.; Hsieh, M.; Malmberg, K.; Manglona, R.; Mercer, M.; et al. Programming plants for climate resilience through symbiogenics. In Seed Endophytes; Verma, S., White, J., Jr., Eds.; Springer: Cham, Switzerland, 2019; pp. 127–137. [Google Scholar]
- Nosheen, S.; Ajmal, I.; Song, Y. Microbes as biofertilizers, a potential approach for sustainable crop production. Sustainability 2021, 13, 1868. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.; Hartmann, M. Networking in the plant microbiome. PLoS Biol. 2016, 14, e1002378. [Google Scholar] [CrossRef]
- Fitzpatrick, C.R.; Salas-González, I.; Conway, J.M.; Finkel, O.M.; Gilbert, S.; Russ, D. The plant microbiome: From ecology to reductionism and beyond. Ann. Rev. Microbiol. 2020, 74, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Doni, F.; Suhaimi, N.S.M.; Irawan, B.; Mohamed, Z.; Mispan, M.S. Associations of Pantoea with rice plants: As friends or foes? Agriculture 2021, 11, 1278. [Google Scholar] [CrossRef]
- Harman, G.; Uphoff, N. Symbiotic root-endophytic soil microbes improve crop productivity and provide environmental benefits. Scientifica 2019, 9106395, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.S.; Doni, F.; Mispan, M.S.; Saiman, M.Z.; Yusuf, Y.M.; Oke, M.A.; Suhaimi, N.S.M. Harnessing Trichoderma in agriculture for productivity and sustainability. Agronomy 2021, 11, 2559. [Google Scholar] [CrossRef]
- Raio, A.; Puopolo, G. Pseudomonas chlororaphis metabolites as biocontrol promoters of plant health and improved crop yield. World J. Microbio. Biotechnol. 2021, 37, 1–8. [Google Scholar] [CrossRef]
- Finkel, O.M.; Castrillo, G.; Paredes, S.H.; González, I.S.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant. Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Stoop, W.A.; Sabarmatee, S.; Sivasubramanian, P.; Ravindra, A.; Sen, D.; Shambu, P.C.; Thakur, A.K. Opportunities for ecological intensification: Lessons and insights from the System of Rice/Crop Intensification—Their implications for agricultural research and development approaches. CAB Rev. 2017, 12, 1–19. [Google Scholar] [CrossRef]
- Ma, Y.; Freitas, H.; Vosatka, M. Beneficial microbes alleviate climatic stresses in plants. Front. Plant Sci. 2019, 10, 595. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.H. The rice microbiome: A model platform for crop holobiome. Phytobiomes J. 2020, 4, 5–18. [Google Scholar] [CrossRef]
- Pang, Z.; Zhao, Y.; Xu, P.; Yu, D. Microbial diversity of upland rice roots and their influence on rice growth and drought tolerance. Microorganisms 2020, 8, 1329. [Google Scholar] [CrossRef]
- Ngalimat, M.S.; Mohd Hata, E.; Zulperi, D.; Ismail, S.I.; Ismail, M.R.; Mohd Zainudin, N.A.I. Plant growth-promoting bacteria as an emerging tool to manage bacterial rice pathogens. Microorganisms 2021, 9, 682. [Google Scholar] [CrossRef]
- Prasanna, R.; Sharma, E.; Sharma, P.; Kumar, A.; Kumar, R. Soil fertility and establishment potential of inoculated cyanobacteria in rice crop grown under non-flooded conditions. Paddy Water Environ. 2013, 11, 175–183. [Google Scholar] [CrossRef]
- Diem, G.; Rougier, M.; Hamad-Fares, I.; Balandreau, J.P.; Dommergues, Y.R. Colonization of rice roots by diazotroph bacteria. Ecol. Bull. 1978, 26, 305–311. [Google Scholar]
- Prasanna, R.; Nain, L.; Pandey, A.K.; Saxena, A.K. Microbial diversity and multidimensional interactions in the rice ecosystem. Arch. Agron. Soil Sci. 2012, 58, 723–744. [Google Scholar] [CrossRef]
- Kumar, U.; Shahid, M.; Tripathi, R.; Mohanty, S.; Kumar, A.; Bhattacharyya, P. Variation of functional diversity of soil microbial community in sub-humid tropical rice-rice cropping system under long-term organic and inorganic fertilization. Ecol. Indic. 2017, 73, 536–543. [Google Scholar] [CrossRef]
- Khan, M.I.; Gwon, H.S.; Alam, M.A.; Song, H.J.; Das, S.; Kim, P.J. Short term effects of different green manure amendments on the composition of main microbial groups and microbial activity of a submerged rice cropping system. Appl. Soil Ecol. 2020, 147, 103400. [Google Scholar] [CrossRef]
- Kimura, M.; Asakawa, S. Comparison of community structures of microbiota at main habitats in rice field ecosystems based on phospholipid fatty acid analysis. Biol. Fertil. Soils 2006, 43, 20–29. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, Y.; Song, J.; Rensing, C. Assembly of root-associated microbial community of typical rice cultivars in different soil types. Biol. Fert. Soils 2020, 56, 249–260. [Google Scholar] [CrossRef]
- Liu, L.; Ding, M.; Zhou, L.; Chen, Y.; Li, H.; Zhang, F. Effects of different rice straw on soil microbial community structure. Agron. J. 2021, 113, 794–805. [Google Scholar] [CrossRef]
- Doni, F.; Mispan, M.S.; Suhaimi, N.S.M.; Ishak, N.; Uphoff, N. Roles of microbes in supporting sustainable rice production using the system of rice intensification. Appl. Microbiol. Biotechnol. 2019, 103, 5131–5142. [Google Scholar] [CrossRef]
- Chen, X.P.; Zhu, Y.G.; Xia, Y.; Shen, J.P.; He, J.Z. Ammonia-oxidizing archaea: Important players in paddy rhizosphere soil? Environ. Microbiol. 2008, 10, 1978–1987. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, X.; Wu, L.; Lu, Y. Community composition of ammonia-oxidizing bacteria and archaea in rice field soil as affected by nitrogen fertilization. Syst. Appl. Microbiol. 2009, 32, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Salmaninezhad, F.; Mostowfizadeh-Ghalamfarsa, R. Three new Pythium species from rice paddy fields. Mycologia 2019, 111, 274–290. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Q.; Liu, Y.; Zhang, A.; Pan, G.; Li, L.; Zhang, X.; Song, C.L.; Jin, Z. Variation of bacterial and fungal community structures in the rhizosphere of hybrid and standard rice cultivars and linkage to CO2 flux. FEMS Microbiol. Ecol. 2011, 78, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.J.; Liang, Y.T.; Li, C.M.; Wang, F.; Sui, Y.Y.; Suvannang, N.; Zhou, J.Z.; Sun, B. Crop rotations alter bacterial and fungal diversity in paddy soils across East Asia. Soil Biol. Biochem. 2016, 95, 250–261. [Google Scholar] [CrossRef]
- Yuan, C.L.; Zhang, L.M.; Wang, J.T.; Hu, H.W.; Shen, J.P.; Cao, P.; He, J.Z. Distributions and environmental drivers of archaea and bacteria in paddy soils. J. Soils Sediments 2019, 19, 23–37. [Google Scholar] [CrossRef]
- Thapa, S.; Ranjan, K.; Ramakrishnan, B.; Velmourougane, K.; Prasanna, R. Influence of fertilizers and rice cultivation methods on the abundance and diversity of phyllosphere microbiome. J. Basic Microbiol. 2018, 58, 172–186. [Google Scholar] [CrossRef]
- Klinnawee, L.; Noirungsee, N.; Nopphakat, K.; Runsaeng, P.; Chantarachot, T. Flooding overshadows phosphorus availability in controlling the intensity of arbuscular mycorrhizal colonization in Sangyod Muang Phatthalung lowland indica rice. ScienceAsia 2021, 47, 202–210. [Google Scholar] [CrossRef]
- Somenahally, A.C.; Hollister, E.B.; Loeppert, R.H.; Yan, W.; Gentry, T.J. Microbial communities in rice rhizosphere altered by intermittent and continuous flooding in fields with long-term arsenic application. Soil Biol. Biochem. 2011, 43, 1220–1228. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.T.; Zhang, Z.F.; Li, W.; Chen, W.; Cai, L. Microbiota in the rhizosphere and seed of rice from China, with reference to their transmission and biogeography. Front. Microbiol. 2020, 11, 995. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef] [PubMed]
- Rajkishore, S.K.; Doraisamy, P.; Subramanian, K.S.; Maheswari, M. Methane emissions patterns and their associated microflora with SRI and conventional systems of rice cultivation in Tamil Nadu, India. Taiwan Water Conserv. 2013, 61, 126–134. [Google Scholar]
- Venturi, V.; Keel, C. Signaling in the rhizosphere. Trends Plant Sci. 2016, 21, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.F.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.B. Root exudate metabolites drive plant-soil feedbacks on growth and defence by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Sun, Y.; Tian, L.; Ji, L.; Luo, S. The structure of rhizosphere fungal communities of wild and domesticated rice: Changes in diversity and co-occurrence patterns. Front. Microbiol. 2021, 12, 45. [Google Scholar] [CrossRef]
- Watanarojanaporn, N.; Boonkerd, N.; Tittabutr, P.; Longtonglang, A.; Young, J.P.W.; Teaumroong, N. Effect of rice cultivation systems on indigenous arbuscular mycorrhizal fungal community structure. Microbes Environ. 2013, 28, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, R.; Jaiswal, P.; Nayak, S.; Sood, A.; Kaushik, B.D. Cyanobacterial diversity in the rhizosphere of rice and its ecological significance. Indian J. Microbiol. 2009, 49, 89–97. [Google Scholar] [CrossRef]
- Anas, I.; Rupela, O.P.; Thiyagarajan, T.M.; Uphoff, N. A review of studies on SRI effects on beneficial organisms in rice soil rhizospheres. Paddy Water Environ. 2011, 9, 53–64. [Google Scholar] [CrossRef]
- Mwajita, M.R.; Murage, H.; Tani, A.; Kahangi, E.M. Evaluation of rhizosphere, rhizoplane and phyllosphere bacteria and fungi isolated from rice in Kenya for plant growth promoters. Springerplus 2013, 2, 606. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Kumar, R.; Humayun, P.; Srinivas, V.; Kumari, B. Assessment of different methods of rice (Oryza sativa. L) cultivation affecting growth parameters, soil chemical, biological, and microbiological properties, water saving, and grain yield in rice–rice system. Paddy Water Environ. 2014, 12, 79–87. [Google Scholar] [CrossRef]
- Leveau, J.H. A brief from the leaf: Latest research to inform our understanding of the phyllosphere microbiome. Curr. Opin. Microbiol. 2019, 49, 41–49. [Google Scholar] [CrossRef]
- Venkatachalam, S.; Ranjan, K.; Prasanna, R.; Ramakrishnan, B.; Thapa, S.; Kanchan, A. Diversity and functional traits of culturable microbiome members, including cyanobacteria in the rice phyllosphere. Plant Biol. 2016, 18, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brettell, L.E.; Singh, B. Linking the phyllosphere microbiome to plant health. Trends Plant Sci. 2020, 25, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Roman-Reyna, V.; Pinili, D.; Borja, F.N.; Quibod, I.L.; Groen, S.C.; Alexandrov, N. Characterization of the leaf microbiome from whole-genome sequencing data of the 3000 rice genomes project. Rice 2020, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Mano, H.; Morisaki, H. Endophytic bacteria in the rice plant. Microbes Environ. 2008, 23, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S.; Babalola, O.O. Roles of plant endosphere microbes in agriculture—A review. J. Plant Growth Regul. 2021. [Google Scholar] [CrossRef]
- Chebotar, V.K.; Malfanova, N.V.; Shcherbakov, A.V.; Ahtemova, G.A.; Borisov, A.Y.; Lugtenberg, B.; Tikhonovich, I.A. Endophytic bacteria in microbial preparations that improve plant development (review). Appl. Biochem. Microbiol. 2015, 51, 271–277. [Google Scholar] [CrossRef]
- Loaces, I.; Ferrando, L.; Scavino, A.F. Dynamics, diversity and function of endophytic siderophore-producing bacteria in rice. Microb. Ecol. 2010, 61, 606–618. [Google Scholar] [CrossRef]
- Zhong, Y.; Hu, J.; Xia, Q.; Zhang, S.; Li, X. Soil microbial mechanisms promoting ultrahigh rice yield. Soil Biol. Biochem. 2020, 143, 107741. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef]
- Torres-Cortés, G.; Bonneau, S.; Bouchez, O.; Genthon, C.; Briand, M.; Jacques, M.A.; Barret, M. Functional microbial features driving community assembly during seed germination and emergence. Front. Plant Sci. 2018, 9, 902. [Google Scholar] [CrossRef]
- Rodríguez, C.E.; Antonielli, L.; Mitter, B.; Trognitz, F.; Sessitsch, A. Heritability and functional importance of the Setaria viridis bacterial seed microbiome. Phytobiomes J. 2020, 4, 40–52. [Google Scholar] [CrossRef]
- Watanabe, I.; Barraquio, W.L.; de Guzman, M.; Cabrera, D.A. Nitrogen fixation (acetylene reduction) activity and population of aerobic heterotrophic nitrogen-fixing bacteria associated with wetland rice. Appl. Environ. Microbiol. 1979, 37, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.R.; Jena, P.K.; Adhya, T.K. Inoculation of rice with nitrogen-fixing bacteria—problems and perspectives. Biol. Fertil. Soils 1987, 4, 21–26. [Google Scholar]
- Watanabe, I.; Lin, C. Response of wetland rice to inoculation with Azospirillum lipoferum and Pseudomonas sp. Soil Sci. Plant Nutr. 1984, 30, 117–124. [Google Scholar] [CrossRef]
- Roger, P.A. Biological N2-fixation and its management in wetland rice cultivation. Fertil. Res. 1995, 42, 261–276. [Google Scholar] [CrossRef]
- Yanni, Y.G.; Rizk, R.Y.; Corich, V.; Squartini, A.; Ninke, K.; Philip-Hollingsworth, S. Natural endophytic association between Rhizobium leguminosarum bv. trifolii and rice roots and assessment of its potential to promote rice growth. Plant Soil 1997, 194, 99–114. [Google Scholar] [CrossRef]
- Biswas, J.C.; Ladha, J.K.; Dazzo, F.B.; Yanni, Y.G.; Rolfe, B.G. Rhizobial inoculation influences seedling vigor and yield of rice. J. Agron. 2000, 92, 880–886. [Google Scholar] [CrossRef]
- Yanni, Y.; Rizk, R.Y.; Abd El-Fattah, F.K. The beneficial plant growth-promoting association of Rhizobium leguminosarum bv. trifolii with rice roots. Funct. Plant Biol. 2001, 28, 845–870. [Google Scholar] [CrossRef]
- Chi, F.; Shen, S.H.; Cheng, H.; Jing, Y.X.; Yanni, Y.G.; Dazzo, F.B. Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. Appl. Environ. Microbiol. 2005, 71, 7271–7278. [Google Scholar] [CrossRef]
- Sasaki, K.; Ikeda, S.; Eda, S.; Mitsui, H.; Hanzawa, E.; Kisara, C. Impact of plant genotype and nitrogen level on rice growth response to inoculation with Azospirillum sp. strain B510 under paddy field conditions. Soil Sci. Plant Nutr. 2010, 56, 636–644. [Google Scholar] [CrossRef]
- Uphoff, N.; Chi, F.; Dazzo, F.B.; Rodriguez, R.J. Soil fertility as a contingent rather than inherent characteristic: Considering the contributions of crop–symbiotic soil biota. In Principles of Sustainable Soil Management in Agroecosystems; Lal, R., Stewart, B., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2013; pp. 141–166. [Google Scholar]
- Mondal, S.; Halder, S.K.; Yadav, A.N.; Mondal, K.C. Microbial consortium with multifunctional plant growth-promoting attributes: Future perspective in agriculture. In Advances in Plant Microbiome and Sustainable Agriculture: Microorganisms for Sustainability; Yadav, A., Rastegari, A., Yadav, N., Kour, D., Eds.; Springer: Singapore, 2020; pp. 219–258. [Google Scholar]
- Jha, M.; Chourasia, S.; Sinha, S. Microbial consortium for sustainable rice production. Agroecol. Sustain. Food Syst. 2013, 37, 340–362. [Google Scholar] [CrossRef]
- Prasanna, R.; Joshi, M.; Rana, A.; Shivay, Y.S.; Nain, L. Influence of co-inoculation of bacteria-cyanobacteria on crop yield and C–N sequestration in soil under rice crop. World J. Microbiol. Biotechnol. 2012, 28, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Gupta, R.; Kwon, S.J.; Wang, Y.; Agrawal, G.K.; Rakwal, R. Transcriptomic analysis of Oryza sativa leaves reveals key changes in response to Magnaporthe oryzae MSP1. Plant Pathol. J. 2018, 34, 257. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.; Yang, P.; Han, F.; Jing, Y.; Shen, S. Proteomic analysis of rice seedlings infected by Sinorhizobium meliloti 1021. Proteomics 2010, 10, 1861–1874. [Google Scholar] [CrossRef]
- Wu, Q.; Peng, X.; Yang, M.; Zhang, W.; Dazzo, F.B.; Uphoff, N.; Jing, Y.; Shen, S. Rhizobia promote the growth of rice shoots by targeting cell signalling, division and expansion. Plant Molec. Biol. 2018, 97, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.J.; Lu, F.; Yang, Y.; Sun, K.; Zhu, Q.; Xu, F.J.; Zang, W.; Dai, C.C. Benefits of endophytic fungus Phomopsis liquidambaris inoculation for improving mineral nutrition, quality, and yield of rice grains under low nitrogen and phosphorus condition. J. Plant Growth Regul. 2021, 1–15. [Google Scholar] [CrossRef]
- Harman, G.E.; Doni, F.; Khadka, R.B.; Uphoff, N. Endophytic strains of Trichoderma increase plants’ photosynthetic capability. J. Appl. Microbiol. 2021, 130, 529–546. [Google Scholar] [CrossRef]
- Anhar, A.; Putri, D.H.; Advinda, L.; Atika, V.; Amimi, S.; Aldo, W.; Ruchi, W. Molecular characterization of Trichoderma strains from West Sumatera, Indonesia and their beneficial effects on rice seedling growth. J. Crop Sci. Biotechnol. 2021, 24, 441–448. [Google Scholar] [CrossRef]
- Khadka, R.B.; Uphoff, N. Effects of Trichoderma seedling treatment with System of Rice Intensification management and with conventional management of transplanted rice. PeerJ 2019, 7, e5877. [Google Scholar] [CrossRef]
- Doni, F.; Che Radziah, C.M.Z.; Anizan, I.; Norela, S.; Fathurrahman, F.; Uphoff, N.; Wan Mohtar, W.Y. Relationships observed between Trichoderma inoculation and characteristics of rice grown under System of Rice Intensification (SRI) vs. conventional methods of cultivation. Symbiosis 2017, 72, 45–59. [Google Scholar] [CrossRef]
- Doni, F.; Fathurrahman, F.; Mispan, M.S.; Suhaimi, N.S.M.; Yusoff, W.M.W.; Uphoff, N. Transcriptomic profiling of rice seedlings inoculated with the symbiotic fungus Trichoderma asperellum SL2. J. Plant Growth Regul. 2019, 38, 1507–1515. [Google Scholar] [CrossRef]
- Doni, F.; Isahak, A.; Yusoff, W.M.W.; Uphoff, N. Physiological Effects and Transcriptomic Profiling of Rice Plant-Microbe Interactions in System of Rice Intensification (SRI) Management. In Proceedings of the 5th International Rice Congress, Singapore, 16 October 2018; Available online: https://www.slideshare.net/SRI.CORNELL/1809-physiological-effects-and-transcriptomic-profiling-of-rice-plant-microbe-interatctions-in-system-of-rice-intensification-sri-management (accessed on 23 September 2021).
- Pati, B.R. Effect of spraying nitrogen fixing phyllospheric bacterial isolates on rice plants. Zentralbl. Mikrobiol. 1992, 147, 441–446. [Google Scholar] [CrossRef]
- Biswas, J.C.; Ladha, J.K.; Dazzo, F.B. Rhizobia inoculation improves nutrient uptake and growth of lowland rice. Soil Sci. Soc. Am. J. 2000, 64, 1644–1650. [Google Scholar] [CrossRef]
- Alam, M.S.; Cui, Z.J.; Yamagishi, T.; Ishii, R. Grain yield and related physiological characteristics of rice plants (Oryza sativa L.) inoculated with free-living rhizobacteria. Plant Prod. Sci. 2001, 4, 126–130. [Google Scholar] [CrossRef]
- Cuevas, V.C. Soil inoculation with Trichoderma pseudokoningii Rifai enhances yield of rice. Philip. J. Sci. 2006, 135, 31–37. [Google Scholar]
- Dhar, D.W.; Prasanna, R.; Singh, B.V. Comparative performance of three carrier-based blue green algal biofertilizers for sustainable rice cultivation. J. Sust. Agric. 2007, 30, 41–50. [Google Scholar] [CrossRef]
- Razie, F.; Anas, I. Effect of Azotobacter and Azospirillum on growth and yield of rice grown on tidal swamp rice fields in south Kalimantan. J. Tanah Lingk. 2008, 10, 41–45. [Google Scholar] [CrossRef]
- Isawa, T.; Yasuda, M.; Awazaki, H.; Minamisawa, K.; Shinozaki, S.; Nakashita, H. Azospirillum sp. strain B510 enhances rice growth and yield. Microbes Environ. 2010, 25, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Seenivasan, N. Efficacy of Pseudomonas fluorescens and Paecilomyces lilacinus against Meloidogyne graminicola infesting rice under system of rice intensification. Arch. Phytopathol. Plant. Prot. 2011, 44, 1467–1482. [Google Scholar] [CrossRef]
- Lavakush, L.; Yadav, J.; Verma, J.P.; Jaiswal, D.K.; Kumar, A. Evaluation of PGPR and different concentration of phosphorus level on plant growth, yield and nutrient content of rice (Oryza sativa). Ecol. Eng. 2014, 62, 123–128. [Google Scholar] [CrossRef]
- Duy, M.; Hoi, N.; Ve, N.; Thuc, L.; Trang, N. Influence of cellulomonas Flavigena, Azospirillum sp. and Psudomonas sp. on rice growth and yield grown in submerged soil amended in rice straw. Recent Trends PGPR Res. Sust. Crop. Product. 2016, 8, 238–242. [Google Scholar]
- Doni, F.; Zain, C.R.; Isahak, A.; Fathurrahman, F.; Anhar, A.; Mohamad, W.N.; Yusoff, W.M.; Uphoff, N. A simple, efficient, and farmer-friendly Trichoderma-based biofertilizer evaluated with the SRI rice management system. Org. Agric. 2018, 8, 207–223. [Google Scholar] [CrossRef]
- Nascente, A.S.; Lanna, A.C.; de Sousa, T.P.; Chaibub, A.A.; de Souza, A.C.; de Filippi, M.C. N fertilizer dose-dependent efficiency of Serratia spp. for improving growth and yield of upland rice (Oryza sativa L.). Int. J. Plant Prod. 2019, 13, 217–226. [Google Scholar] [CrossRef]
- Ríos-Ruiz, W.F.; Torres-Chávez, E.E.; Torres-Delgado, J.; Rojas-García, J.C.; Bedmar, E.J.; Valdez-Nuñez, R.A. Inoculation of bacterial consortium increases rice yield (Oryza sativa L.) reducing applications of nitrogen fertilizer in San Martin region, Peru. Rhizosphere 2020, 14, 100200. [Google Scholar] [CrossRef]
- Fitriatin, B.N.; Sofyan, E.T.; Turmuktini, T. Increasing soil P and yield of upland rice through application phosphate solubilizing microbes. Haya Saudi J. Life. Sci. 2021, 6, 163–167. [Google Scholar]
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Labuschagne, N.; Dubery, I.A. The chemistry of plant–microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Front. Plant Sci. 2018, 9, 112. [Google Scholar] [CrossRef]
- Rahman, S.F.S.A.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef]
- Mathivanan, N.; Prabavathy, V.R.; Vijayanandraj, V.R. Application of talc formulations of Pseudomonas fluorescens Migula and Trichoderma viride Pers. Ex S.F. gray decrease the sheath blight disease and enhance the plant growth and yield in rice. J. Phytopathol. 2005, 153, 697–701. [Google Scholar] [CrossRef]
- França, S.K.S.; Cardoso, A.F.; Lustosa, D.C.; Ramos, M.L.S.; Filippi, M.C.; Silva, G.B. Biocontrol of sheath blight by Trichoderma asperellum in tropical lowland rice. Agron. Sustain. Dev. 2015, 35, 317–324. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, A.K.; Singh, H.B.; Dhakal, B.K. Biological control of rice blast disease with Trichoderma harzianum in direct-seeded rice under medium low land rainfed conditions. Environ. Ecol. 2012, 30, 834–837. [Google Scholar]
- Abdel-Fattah, G.M.; Shabana, Y.M.; Ismail, A.E.; Rashad, Y.M. Trichoderma harzianum: A biocontrol agent against Bipolaris oryzae. Mycopathology 2007, 164, 81–89. [Google Scholar] [CrossRef]
- Harman, G.E. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef]
- Stockwell, V.O.; Stack, J.P. Using Pseudomonas spp. for integrated biological control. Phytopathology 2007, 97, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Oni, F.E.; Olorunleke, O.F.; Höfte, M. Phenazines and cyclic lipopeptides produced by Pseudomonas sp. CMR12a are involved in the biological control of Pythium myriotylum on cocoyam (Xanthosoma sagittifolium). Biol. Control. 2019, 129, 109–114. [Google Scholar] [CrossRef]
- Nandakumar, R.; Babu, S.; Viswanathan, R.; Raguchander, T.; Samiyappan, R. Induction of systemic resistance in rice against sheath blight disease by Pseudomonas fluorescens. Soil Biol. Biochem. 2001, 33, 603–612. [Google Scholar] [CrossRef]
- Patel, A.; Kumar, A.; Sheoran, N.; Kumar, M.; Sahu, K.P. Antifungal and defense elicitor activities of pyrazines identified in endophytic Pseudomonas putida BP25 against fungal blast incited by Magnaporthe oryzae in rice. J. Plant Dis. Prot. 2021, 128, 261–272. [Google Scholar] [CrossRef]
- Radjacommare, R.; Nandakumar, R.; Kandan, A.; Suresh, S.; Bharathi, M.; Raguchander, T.; Samiyappan, R. Pseudomonas fluorescens based bioformulation for the management of sheath blight and leaffolder in rice. Crop Prot. 2002, 21, 671–677. [Google Scholar] [CrossRef]
- Leelasuphakul, W.; Sivanunsakul, P.; Phongpaichit, S. Purification, characterization and synergistic activity of β-1,3-glucanase and antibiotic extract from an antagonistic Bacillus subtilis NSRS 89-24 against rice blast and sheath blight. Enzyme Microb. Technol. 2006, 38, 990–997. [Google Scholar] [CrossRef]
- Durgadevi, D.; Harish, S.; Manikandan, R.; Prabhukarthikeyan, S.R.; Alice, D.; Raguchander, T. Proteomic profiling of defense/resistant genes induced during the tripartite interaction of Oryza sativa, Rhizoctonia solani AG1-1A, and Bacillus subtilis against rice sheath blight. Physiol. Mol. Plant Pathol. 2021, 115, 101669. [Google Scholar] [CrossRef]
- Vidhyasekaran, P.; Rabindran, R.; Muthamilan, R.; Nayar, M.; Rajappan, K.; Subramanian, K.; Vasumathi, K. Development of powder formulation of Pseudomonas fluorescens for control of rice blast. Plant Pathol. 1997, 46, 291–297. [Google Scholar] [CrossRef]
- Someya, N.; Nakajima, M.; Watanabe, K.; Hibi, T.; Akutsu, K. Potential of Serratia marcescens strain B2 for biological control of rice sheath blight. Biocontrol Sci. Technol. 2005, 15, 105–109. [Google Scholar] [CrossRef]
- Yang, D.; Wang, B.; Wang, J.; Chen, Y.; Zhou, M. Activity and efficacy of Bacillus subtilis strain NJ-18 against rice sheath blight and Sclerotinia stem rot of rape. Biol. Control. 2009, 51, 61–65. [Google Scholar] [CrossRef]
- Campos-Soriano, L.; Garcia-Martinez, J.; San, S.B. The arbuscular mycorrhizal symbiosis promotes the systemic induction of regulatory defence-related genes in rice leaves and confers resistance to pathogen infection. Mol. Plant Pathol. 2012, 13, 579–592. [Google Scholar] [CrossRef]
- Hossain, M.T.; Khan, A.; Chung, E.J.; Rashid, M.H.; Chung, Y.R. Biological control of rice bakanae by an endophytic Bacillus oryzicola YC7007. Plant Pathol. J. 2016, 32, 228–241. [Google Scholar] [CrossRef]
- Shao, Z.; Li, Z.; Fu, Y.; Wen, Y.; Wei, S. Induction of defense responses against Magnaporthe oryzae in rice seedling by a new potential biocontrol agent Streptomyces JD211. J. Basic Microbiol. 2018, 58, 686–697. [Google Scholar] [CrossRef]
- Chaibub, A.A.; Carvalho, J.C.B.; Silva, C.S.; Collevatti, R.G. Defence responses in rice plants in prior and simultaneous applications of Cladosporium sp. during leaf blast suppression. Environ. Sci. Pollut. Res. 2016, 23, 21554–21564. [Google Scholar] [CrossRef] [PubMed]
- Chaibub, A.A.; de Sousa, T.P.; de Araújo, L.G.; de Filippi, M.C. Cladosporium cladosporioides C24G modulates gene expression and enzymatic activity during leaf blast suppression in rice plants. J. Plant Growth Regul. 2020, 39, 1140–1152. [Google Scholar] [CrossRef]
- Chaibub, A.A.; de Sousa, T.P.; de Oliveira, M.I.; Arriel-Elias, M.T.; de Araújo, L.G.; de Filippi, M.C. Efficacy of Cladosporium cladosporioides C24G as a multifunctional agent in upland rice in agroecological systems. Int. J. Plant Prod. 2020, 14, 463–474. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, H.; Ren, Z.; Liu, E. Control of Magnaporthe oryzae and rice growth promotion by Bacillus subtilis JN005. J. Plant Growth Regul. 2021, 1–9. [Google Scholar] [CrossRef]
- Abbas, A.; Fu, Y.; Qu, Z.; Zhao, H.; Sun, Y. Isolation and evaluation of the biocontrol potential of Talaromyces spp. against rice sheath blight guided by soil microbiome. Environ. Microbiol. 2021, 23, 5946–5961. [Google Scholar] [CrossRef]
- Chukwu, S.C.; Rafii, M.Y.; Ramlee, S.; Ismail, S.I. Bacterial leaf blight resistance in rice: A review of conventional breeding to molecular approach. Mol. Biol. Rep. 2019, 46, 1519–1532. [Google Scholar] [CrossRef]
- Wu, L.M.; Wu, H.J.; Chen, L.; Yu, X.F.; Borriss, R.; Gao, X.W. Difficidin and bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens. Sci. Rep. 2015, 5, 12975. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, G.P.; Sinha, A.P. Evaluation of Trichoderma spp. and fluorescent pseudomonads for the management of bacterial leaf blight of rice. Indian Phytopath. 2012, 65, 89–91. [Google Scholar]
- Jambhulkar, P.P.; Sharma, P.; Manokaran, R.; Lakshman, D.K.; Rokadia, P.; Jambhulkar, N. Assessing synergism of combined applications of Trichoderma harzianum and Pseudomonas fluorescens to control blast and bacterial leaf blight of rice. Eur. J. Plant Pathol. 2018, 152, 747–757. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Karki, H.S.; Groth, D.E.; Jungkhun, N.; Ham, J.H. Biological control activities of rice-associated Bacillus sp. strains against sheath blight and bacterial panicle blight of rice. PLoS ONE 2016, 11, e0146764. [Google Scholar]
- Pedraza-Herrera, L.A.; Bautista, J.P.; Cruz-Ramírez, C.A.; Uribe-Vélez, D. IBUN2755 Bacillus strain controls seedling root and bacterial panicle blight caused by Burkholderia glumae. Biol. Control. 2021, 153, 104494. [Google Scholar] [CrossRef]
- Chung, E.J.; Hossain, M.T.; Khan, A.; Kim, K.H.; Jeon, C.O. Bacillus oryzicola sp. nov., an endophytic bacterium isolated from the roots of rice with antimicrobial, plant growth promoting, and systemic resistance inducing activities in rice. Plant Pathol. J. 2015, 31, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Elshakh, A.S.; Anjum, S.I.; Qiu, W.; Almoneafy, A.A.; Li, W.; Yang, Z.; Cui, Z.Q.; Li, B.; Sun, G.C.; Xie, G.L. Controling and defence-related mechanisms of Bacillus strains against bacterial leaf blight of rice. J. Phytopathol. 2016, 164, 534–546. [Google Scholar] [CrossRef]
- Yasmin, S.; Hafeez, F.Y.; Mirza, M.S.; Rasul, M.; Arshad, H.M.; Zubair, M.; Iqbal, M. Biocontrol of bacterial leaf blight of rice and profiling of secondary metabolites produced by rhizospheric Pseudomonas aeruginosa BRp3. Front. Microbiol. 2017, 8, 1895. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Moreno, Z.R.; Vinchira-Villarraga, D.M.; Vergara-Morales, D.I.; Castellanos, L. Plant-growth promotion and biocontrol properties of three Streptomyces spp. isolates to control bacterial rice pathogens. Front. Microbiol. 2019, 10, 290. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Chatterjee, A.; Das, S. Synergistic consortium of beneficial microorganisms in rice rhizosphere promotes host defense to blight-causing Xanthomonas oryzae pv. oryzae. Planta 2020, 252, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Saikia, K.; Bora, L.C. Exploring actinomycetes and endophytes of rice ecosystem for induction of disease resistance against bacterial blight of rice. Eur. J. Plant. Pathol. 2021, 159, 67–79. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.; Awasthi, R.P.; Rawat, L.; Kumar, J. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol. Biochem. 2012, 54, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Ansari, M.W.; Tula, S.; Yadav, S.; Sahoo, R.K.; Shukla, N. Dose-dependent response of Trichoderma harzianum in improving drought tolerance in rice genotypes. Planta 2016, 243, 1251–1264. [Google Scholar] [CrossRef]
- Singh, D.P.; Singh, V.; Gupta, V.K.; Shukla, R.; Prabha, R.; Sarma, B.K.; Patel, J.S. Microbial inoculation in rice regulates antioxidative reactions and defense-related genes to mitigate drought stress. Sci. Rep. 2020, 10, 4818. [Google Scholar] [CrossRef]
- Bashyal, B.M.; Parmar, P.; Zaidi, N.W.; Aggarwal, R. Molecular programming of drought-challenged Trichoderma harzianum-bioprimed rice (Oryza sativa L.). Front. Microbiol. 2021, 12, 655165. [Google Scholar] [CrossRef]
- Prathuangwong, S.; Chuaboon, W.; Chatnaparat, T.; Kladsuwan, L.; Choorin, M.; Kasem, S. Induction of disease and drought resistance in rice by Pseudomonas fluorescens SP007s. CMU J. Nat. Sci. Special Issue Agric. Nat. Res. 2012, 11, 45–55. [Google Scholar]
- Saakre, M.; Baburao, T.M.; Salim, A.P.; Ffancies, R.M.; Achuthan, V.P.; Thomas, G.; Sivarajan, S.R. Identification and characterization of genes responsible for drought tolerance in rice mediated by Pseudomonas Fluorescens. Rice Sci. 2017, 24, 291–298. [Google Scholar] [CrossRef]
- Tiwari, S.; Prasad, V.; Chauhan, P.S.; Lata, C. Bacillus amyloliquefaciens confers tolerance to various abiotic stresses and modulates plant response to phytohormones through osmoprotection and gene expression regulation in rice. Front. Plant Sci. 2017, 8, 1510. [Google Scholar] [CrossRef]
- Ruiz-Sanchez, M.; Armada, E.; Munoz, Y.; Garcia de Salamone, I.E.; Aroca, R.; Ruiz-Lozano, J.M.; Azcon, R. Azospirillum and arbuscular mycorrhizal colonization enhance rice growth and physiological traits under well-watered and drought conditions. J. Plant Physiol. 2011, 168, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.J.; Woodward, C.J.; Redman, R.S. Fungal influence on plant tolerance to stress. In Biocomplexity of Plant–Fungal Interactions; Southworth, D., Ed.; Wiley-Blackwell: Oxford, UK, 2012; pp. 155–163. [Google Scholar]
- Redman, R.S.; Rodriguez, R.J. The symbiogenic tango: Achieving climate-resilient crops via mutualistic plant-fungal relationships. In Functional Importance of the Plant Microbiome; Doty, S.L., Ed.; Springer: Cham, Switzerland, 2017; pp. 71–87. [Google Scholar]
- Rawat, L.; Singh, Y.; Shukla, N.; Kumar, J. Seed biopriming with salinity tolerant isolates of Trichoderma harzianum alleviates salt stress in rice: Growth, physiological and biochemical characteristics. J. Plant Pathol. 2012, 94, 353–365. [Google Scholar]
- Yasmeen, R.; Siddiqui, Z. Physiological responses of crop plants against Trichoderma harzianum in saline environment. Acta Bot. Croat. 2017, 76, 154–162. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef] [PubMed]
- Jogawat, A.; Vadassery, J.; Verma, N.; Oelmüller, R. PiHOG1, a stress regulator MAP kinase from the root endophyte fungus Piriformospora indica, confers salinity stress tolerance in rice plants. Sci. Rep. 2016, 6, 36765. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lei, P.; Wang, Q.; Ma, J.; Zhan, Y.; Jiang, K.; Xu, Z.; Xu, H. The endophyte Pantoea alhagi NX-11 alleviates salt stress damage to rice seedlings by secreting exopolysaccharides. Front. Microbiol. 2020, 10, 3112. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Singh, R.; Arora, N.K. Alleviation of heavy metal stress in plants and remediation of soil by rhizosphere microorganisms. Front. Microbiol. 2017, 8, 1706. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C. Heavy metal stress, signaling, and tolerance due to plant-associated microbes: An overview. J. Front. Plant. Sci. 2018, 9, 452. [Google Scholar] [CrossRef]
- Siripornadulsil, S.; Siripornadulsil, W. Cadmium-tolerant bacteria reduce the uptake of cadmium in rice: Potential for microbial bioremediation. Ecotoxicol. Environ. Saf. 2013, 94, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Nongmaithem, N.; Roy, A.; Bhattacharya, P.M. Potential of Trichoderma spp. on growth promotion and mitigating cadmium uptake in rice plant under the metal stress ecosystem. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 992–1010. [Google Scholar] [CrossRef][Green Version]
- Pandey, S.; Ghosh, P.K.; Ghosh, S. Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J. Microbiol. 2013, 51, 11–17. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, X.; He, X.; Lü, Q.; Qian, X.; Xiao, Q.; Lin, R. Effects of Pseudomonas TCd-1 on rice (Oryza sativa L.) cadmium uptake, rhizosphere soils enzyme activities and cadmium bioavailability under cadmium contamination. Ecotoxicol. Environ. Saf. 2021, 218, 112249. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.; Mandal, S.; Banerjee, S.; Ghosh, A.; Maiti, T.K.; Mandal, N.C. Unraveling the heavy metal resistance and biocontrol potential of Pseudomonas sp. K32 strain facilitating rice seedling growth under Cd stress. Chemosphere 2021, 274, 129819. [Google Scholar] [CrossRef]
- Zhang, X.H.; Zhu, Y.G.; Chen, B.D.; Lin, A.J.; Smith, S.E.; Smith, F.A. Arbuscular mycorrhizal fungi contribute to resistance of upland rice to combined metal contamination of soil. J. Plant Nutr. 2005, 28, 2065–2077. [Google Scholar] [CrossRef]
- Chan, W.F.; Li, H.; Wu, F.Y. Arsenic uptake in upland rice inoculated with a combination or single arbuscular mycorrhizal fungi. J. Hazard Mater. 2013, 262, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Pramanik, K.; Bhattacharya, S.; Mondal, S.; Ghosh, S.K.; Ghosh, P.K.; Maiti, T.K. Abatement of arsenic-induced phytotoxic effects in rice seedlings by an arsenic-resistant Pantoea dispersa strain. Environ. Sci. Pollut. Res. 2021, 28, 21633–21649. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).