Polyphosphate Kinase 2 (PPK2) Enzymes: Structure, Function, and Roles in Bacterial Physiology and Virulence
Abstract
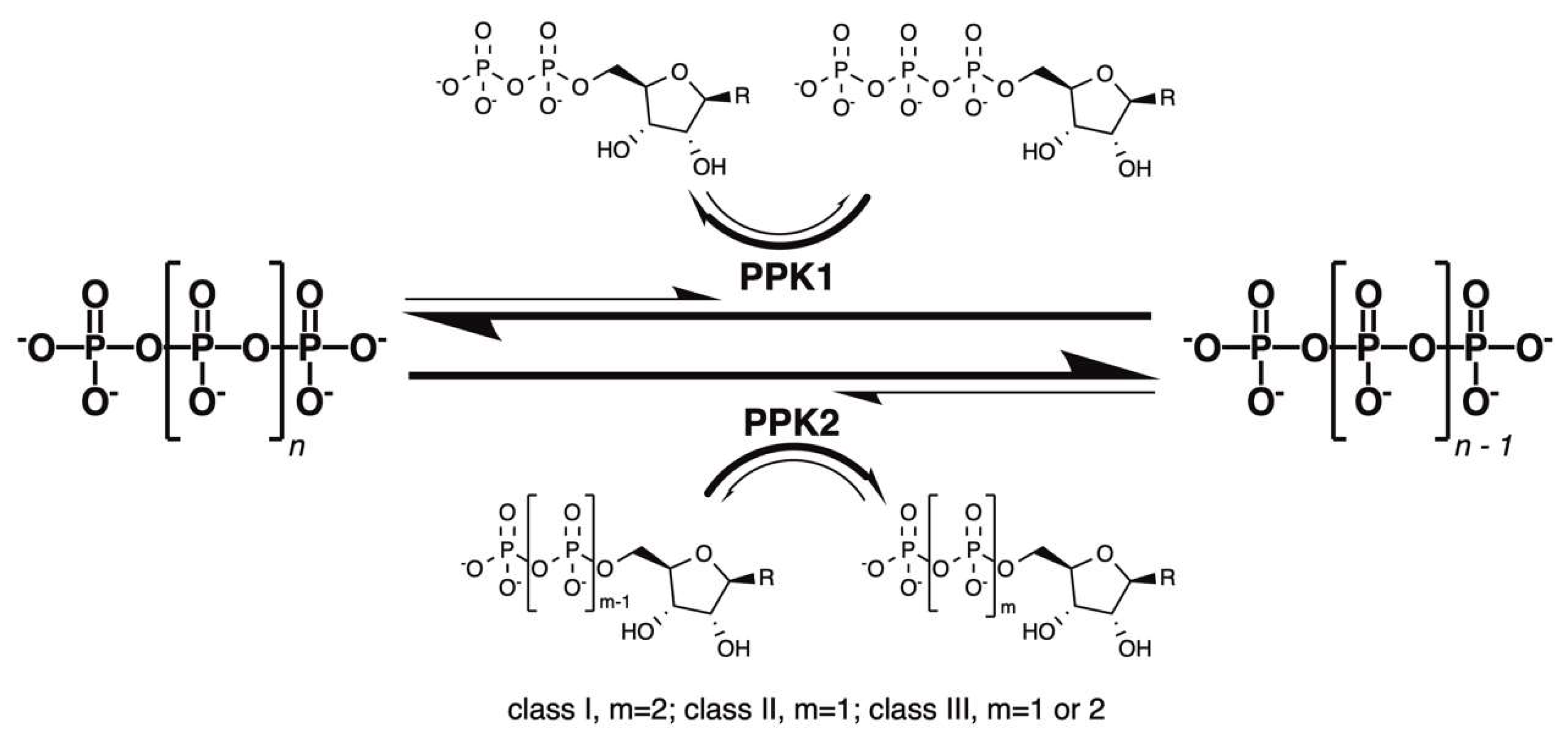
1. Introduction: Inorganic Polyphosphate and Polyphosphate Kinase 1 (PPK1)
2. PPK2: A New Class of PolyP-Metabolizing Enzyme
3. PPK2 Enzymology
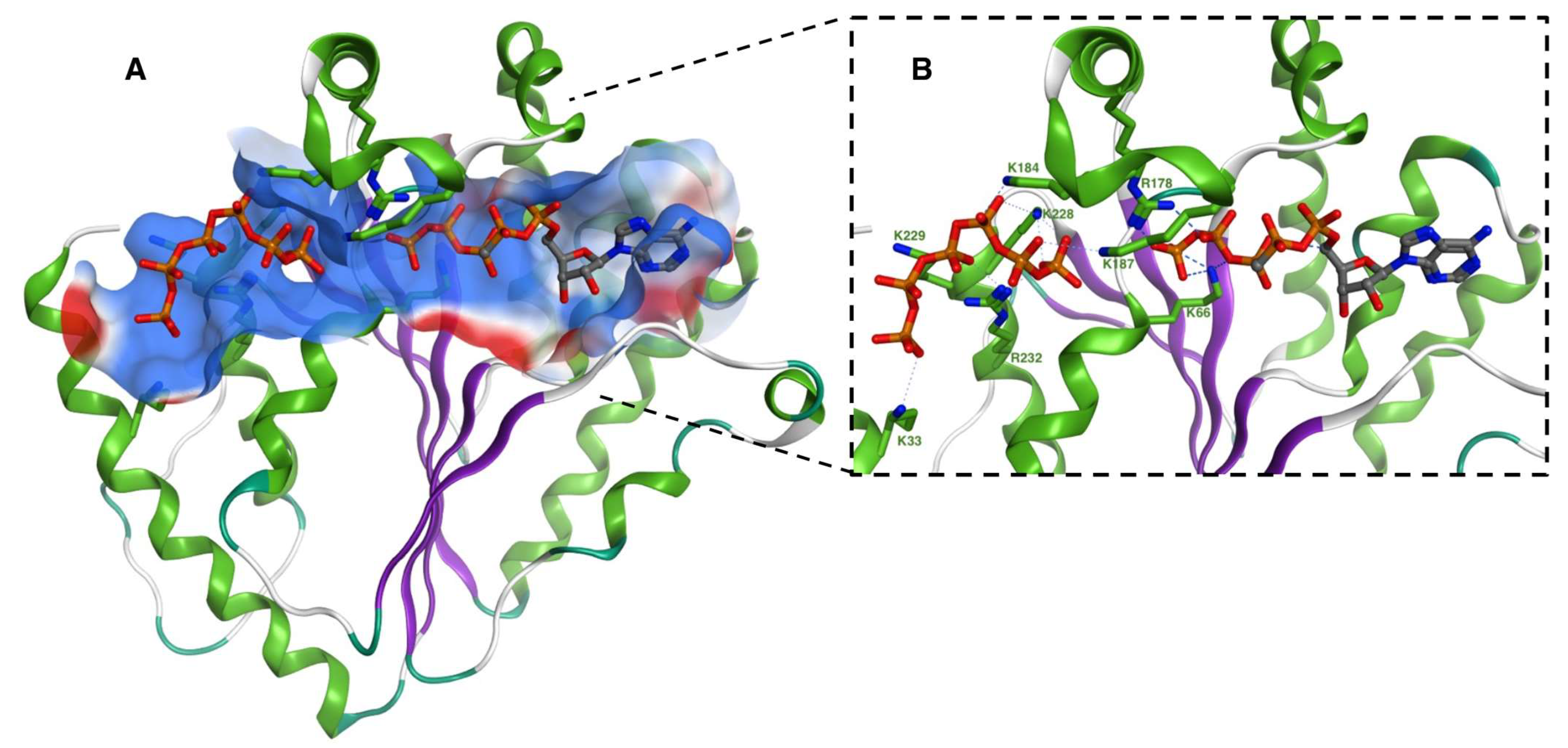
3.1. PPK2 Crystal Structures and Catalytic Mechanism
3.2. Class I PPK2 Enzymology
3.3. Class II PPK2 Enzymology
3.4. Class III PPK2 Enzymology
3.5. Other PPK2 Activities
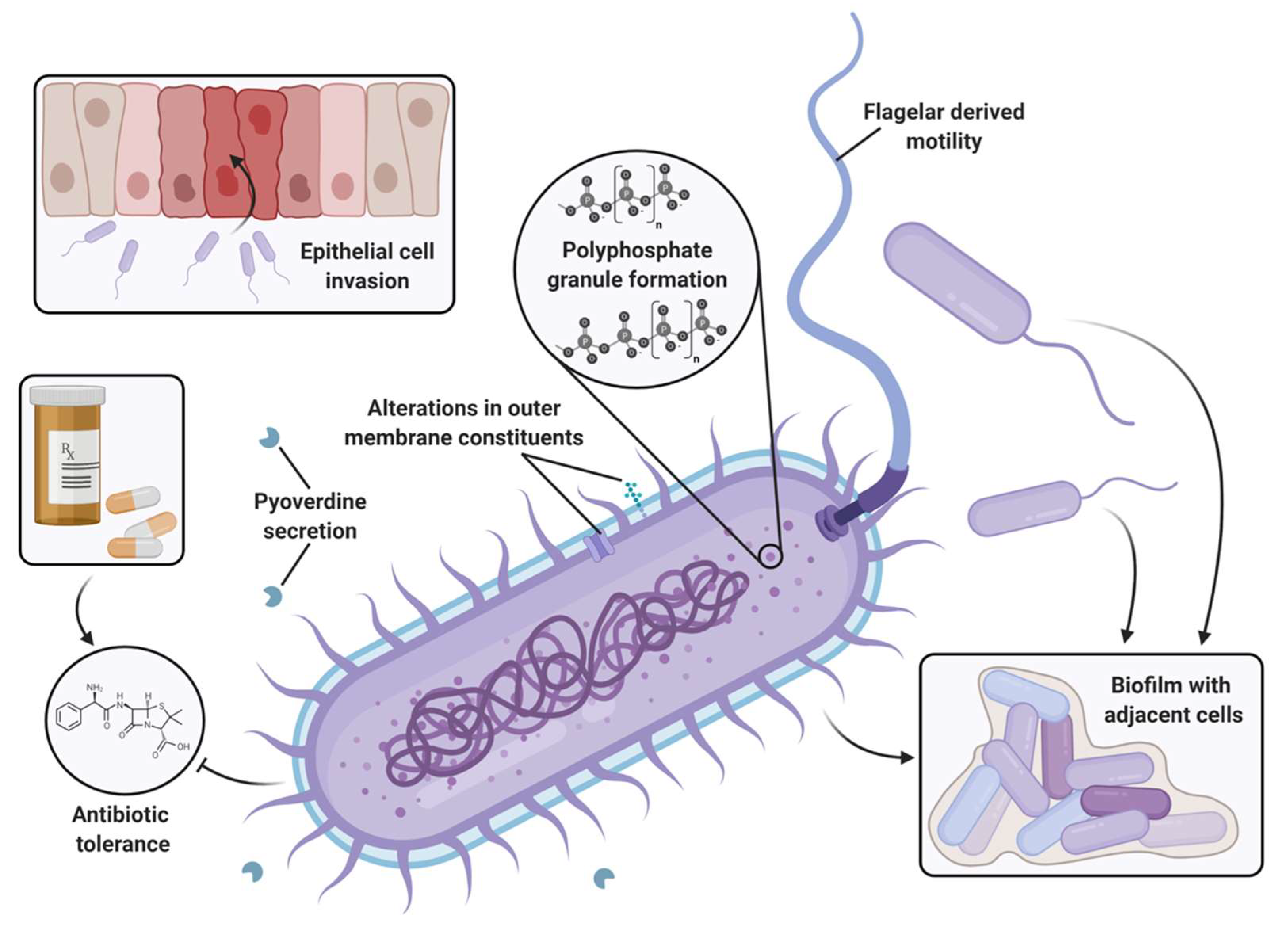
4. Roles of PPK2s in Bacterial Physiology and Virulence
4.1. Bacterial Homeostasis and Stress Response
4.2. Biofilms
4.3. Virulence Factors and Invasion
4.4. Antibiotic Sensitivity
5. Therapeutic Potential: PPK2 Inhibitors
6. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AjPPK2 | Acinetobacter johnsonii PPK2 |
| AMP-PCP | β,γ-methylene adenosine 5ʹ-triphosphate |
| AMP-PCPPP | β,γ-methylene adenosine 5ʹ-pentaphosphate |
| AP4 | adenosine tetraphosphate |
| ChPPK2 | Cytophaga hutchinsonii PPK2 |
| DrPPK2 | Deinococcus radiodurans PPK2 |
| FtPPK2 | Francisella tularensis PPK2 |
| G4P | guanosine 5′-tetraphosphate |
| IC50 | half-maximal inhibition constant |
| Km | Michaelis constant |
| MBP | maltose binding protein |
| MrPPK2 | Meiothermus ruber PPK2 |
| Ndk | nucleoside diphosphate kinase |
| PAP | polyP-AMP phosphotransferase |
| Pi | inorganic phosphate |
| polyP | polyphosphate |
| ppGpp | guanosine tetraphosphate |
| PPK | polyphosphate kinase |
| SmPPK2 | Sinorhizobium meliloti PPK2 |
| Vmax | maximum enzyme velocity |
References
- Xie, L.; Jakob, U. Inorganic polyphosphate, a multifunctional polyanionic protein scaffold. J. Biol. Chem. 2019, 294, 2180–2190. [Google Scholar] [CrossRef]
- Meyer, A. Orientirende Untersuchungen Über Verbreitung Morphologie Und Chemie Des Volutins. Bot Zft 1904, 62, 113–152. [Google Scholar]
- Kornberg, A.; Kornberg, S.; Simms, E.S. Metaphosphate synthesis by an enzyme from Escherichia coli. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 1956, 20, 215–227. [Google Scholar] [CrossRef]
- Ahn, K.; Kornberg, A. Polyphosphate kinase from Escherichia coli. Purification and demonstration of a phosphoenzyme intermediate. J. Biol. Chem. 1990, 265, 11734–11739. [Google Scholar] [CrossRef]
- Akiyama, M.; Crooke, E.; Kornberg, A. The polyphosphate kinase gene of Escherichia coli. Isolation and sequence of the ppk gene and membrane location of the protein. J. Biol. Chem. 1992, 267, 22556–22561. [Google Scholar] [CrossRef]
- Crooke, E.; Akiyama, M.; Rao, N.; Kornberg, A. Genetically altered levels of inorganic polyphosphate in Escherichia coli. J. Biol. Chem. 1994, 269, 6290–6295. [Google Scholar] [CrossRef]
- Kuroda, A.; Murphy, H.; Cashel, M.; Kornberg, A. Guanosine Tetra- and Pentaphosphate Promote Accumulation of Inorganic Polyphosphate in Escherichia coli. J. Biol. Chem. 1997, 272, 21240–21243. [Google Scholar] [CrossRef]
- Rao, N.N.; Kornberg, A. Inorganic polyphosphate supports resistance and survival of stationary-phase Escherichia coli. J. Bacteriol. 1996, 178, 1394–1400. [Google Scholar] [CrossRef]
- Kuroda, A.; Tanaka, S.; Ikeda, T.; Kato, J.; Takiguchi, N.; Ohtake, H. Inorganic polyphosphate kinase is required to stimulate protein degradation and for adaptation to amino acid starvation in Escherichia coli. Proc. Natl. Acad. Sci.USA 1999, 96, 14264–14269. [Google Scholar] [CrossRef]
- Shiba, T.; Tsutsumi, K.; Yano, H.; Ihara, Y.; Kameda, A.; Tanaka, K.; Takahashi, H.; Munekata, M.; Rao, N.N.; Kornberg, A. Inorganic polyphosphate and the induction of rpoS expression. Proc. Natl. Acad. Sci. USA 1997, 94, 11210–11215. [Google Scholar] [CrossRef]
- Rao, N.N.; Gómez-García, M.R.; Kornberg, A. Inorganic Polyphosphate: Essential for Growth and Survival. Annu. Rev. Biochem. 2009, 78, 605–647. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Rao, N.N.; Fraley, C.D.; Kornberg, A. Inorganic polyphosphate is essential for long-term survival and virulence factors in Shigella and Salmonella spp. Proc. Natl. Acad. Sci. USA 2002, 99, 7675–7680. [Google Scholar] [CrossRef] [PubMed]
- Nikel, P.I.; Chavarría, M.; Martínez-García, E.; Taylor, A.C.; de Lorenzo, V. Accumulation of inorganic polyphosphate enables stress endurance and catalytic vigour in Pseudomonas putida KT2440. Microb. Cell Factories 2013, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Fraley, C.D.; Rashid, M.H.; Lee, S.S.K.; Gottschalk, R.; Harrison, J.; Wood, P.J.; Brown, M.R.W.; Kornberg, A. A polyphosphate kinase 1 (ppk1) mutant of Pseudomonas aeruginosa exhibits multiple ultrastructural and functional defects. Proc. Natl. Acad. Sci. USA 2007, 104, 3526–3531. [Google Scholar] [CrossRef]
- Dahl, J.-U.; Gray, M.; Bazopoulou, D.; Beaufay, F.; Lempart, J.; Koenigsknecht, M.J.; Wang, Y.; Baker, J.R.; Hasler, W.L.; Young, V.B.; et al. The anti-inflammatory drug mesalamine targets bacterial polyphosphate accumulation. Nat. Microbiol. 2017, 2, 16267. [Google Scholar] [CrossRef]
- Beaufay, F.; Quarles, E.; Franz, A.; Katamanin, O.; Wholey, W.-Y.; Jakob, U. Polyphosphate Functions In Vivo as an Iron Chelator and Fenton Reaction Inhibitor. mBio 2020, 11, e01017-20. [Google Scholar] [CrossRef]
- Gray, M.J.; Jakob, U. Oxidative stress protection by polyphosphate—New roles for an old player. Curr. Opin. Microbiol. 2015, 24, 1–6. [Google Scholar] [CrossRef]
- Groitl, B.; Dahl, J.-U.; Schroeder, J.W.; Jakob, U. Pseudomonas aeruginosa defense systems against microbicidal oxidants. Mol. Microbiol. 2017, 106, 335–350. [Google Scholar] [CrossRef]
- Gray, M.; Wholey, W.-Y.; Wagner, N.O.; Cremers, C.M.; Mueller-Schickert, A.; Hock, N.T.; Krieger, A.G.; Smith, E.M.; Bender, R.A.; Bardwell, J.C.; et al. Polyphosphate Is a Primordial Chaperone. Mol. Cell 2014, 53, 689–699. [Google Scholar] [CrossRef]
- Brown, M.R.W.; Kornberg, A. Inorganic polyphosphate in the origin and survival of species. Proc. Natl. Acad. Sci. USA 2004, 101, 16085–16087. [Google Scholar] [CrossRef]
- Kumble, K.D.; Kornberg, A. Inorganic Polyphosphate in Mammalian Cells and Tissues. J. Biol. Chem. 1995, 270, 5818–5822. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.A.; Lea, C.R.; Oldfield, E.; Docampo, R.; Gande, R.; Gibson, K.J.C.; Brown, A.K.; Krumbach, K.; Dover, L.G.; Sahm, H.; et al. Human Platelet Dense Granules Contain Polyphosphate and Are Similar to Acidocalcisomes of Bacteria and Unicellular Eukaryotes. J. Biol. Chem. 2004, 279, 44250–44257. [Google Scholar] [CrossRef]
- Pavlov, E.; Aschar-Sobbi, R.; Campanella, M.; Turner, R.J.; Gómez-García, M.R.; Abramov, A.Y. Inorganic Polyphosphate and Energy Metabolism in Mammalian Cells. J. Biol. Chem. 2010, 285, 9420–9428. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.H.; Choi, S.H.; Smith, S. Polyphosphate: An ancient molecule that links platelets, coagulation, and inflammation. Blood 2012, 119, 5972–5979. [Google Scholar] [CrossRef] [PubMed]
- Omelon, S.; Georgiou, J.; Henneman, Z.J.; Wise, L.M.; Sukhu, B.; Hunt, T.; Wynnyckyj, C.; Holmyard, D.; Bielecki, R.; Grynpas, M.D. Control of Vertebrate Skeletal Mineralization by Polyphosphates. PLoS ONE 2009, 4, e5634. [Google Scholar] [CrossRef]
- Azevedo, C.; Livermore, T.; Saiardi, A. Protein Polyphosphorylation of Lysine Residues by Inorganic Polyphosphate. Mol. Cell 2015, 58, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Bentley-DeSousa, A.; Holinier, C.; Moteshareie, H.; Tseng, Y.-C.; Kajjo, S.; Nwosu, C.; Amodeo, G.F.; Bondy-Chorney, E.; Sai, Y.; Rudner, A.; et al. A Screen for Candidate Targets of Lysine Polyphosphorylation Uncovers a Conserved Network Implicated in Ribosome Biogenesis. Cell Rep. 2018, 22, 3427–3439. [Google Scholar] [CrossRef] [PubMed]
- Bondy-Chorney, E.; Abramchuk, I.; Nasser, R.; Holinier, C.; Denoncourt, A.; Baijal, K.; McCarthy, L.; Khacho, M.; Lavallée-Adam, M.; Downey, M. A Broad Response to Intracellular Long-Chain Polyphosphate in Human Cells. Cell Rep. 2020, 33, 108318. [Google Scholar] [CrossRef] [PubMed]
- Cremers, C.M.; Knoefler, D.; Gates, S.; Martin, N.; Dahl, J.-U.; Lempart, J.; Xie, L.; Chapman, M.; Galvan, V.; Southworth, D.R.; et al. Polyphosphate: A Conserved Modifier of Amyloidogenic Processes. Mol. Cell 2016, 63, 768–780. [Google Scholar] [CrossRef]
- Roewe, J.; Stavrides, G.; Strueve, M.; Sharma, A.; Marini, F.; Mann, A.; Smith, S.A.; Kaya, Z.; Strobl, B.; Mueller, M.; et al. Bacterial polyphosphates interfere with the innate host defense to infection. Nat. Commun. 2020, 11, 4035. [Google Scholar] [CrossRef]
- Rijal, R.; Cadena, L.A.; Smith, M.R.; Carr, J.F.; Gomer, R.H. Polyphosphate is an extracellular signal that can facilitate bacterial survival in eukaryotic cells. Proc. Natl. Acad. Sci. USA 2020, 117, 31923–31934. [Google Scholar] [CrossRef] [PubMed]
- Bowlin, M.Q.; Gray, M.J. Inorganic polyphosphate in host and microbe biology. Trends Microbiol. 2021, 29, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Rao, N.N.; Kornberg, A. Inorganic Polyphosphate Is Required for Motility of Bacterial Pathogens. J. Bacteriol. 2000, 182, 225–227. [Google Scholar] [CrossRef]
- Ishige, K.; Zhang, H.; Kornberg, A. Polyphosphate kinase (PPK2), a potent, polyphosphate-driven generator of GTP. Proc. Natl. Acad. Sci. USA 2002, 99, 16684–16688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ishige, K.; Kornberg, A. A polyphosphate kinase (PPK2) widely conserved in bacteria. Proc. Natl. Acad. Sci. USA 2002, 99, 16678–16683. [Google Scholar] [CrossRef]
- Nocek, B.; Kochinyan, S.; Proudfoot, M.; Brown, G.; Evdokimova, E.; Osipiuk, J.; Edwards, A.M.; Savchenko, A.; Joachimiak, A.; Yakunin, A.F. Polyphosphate-dependent synthesis of ATP and ADP by the family-2 polyphosphate kinases in bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 17730–17735. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, W.; Lee, S.S.K.; Xu, W. Crystal structure of a polyphosphate kinase and its implications for polyphosphate synthesis. EMBO Rep. 2005, 6, 681–687. [Google Scholar] [CrossRef]
- Achbergerová, L.; Nahálka, J. PPK1 and PPK2—which polyphosphate kinase is older? Biologia 2014, 69, 263–269. [Google Scholar] [CrossRef]
- Racki, L.R.; Tocheva, E.I.; Dieterle, M.; Sullivan, M.C.; Jensen, G.J.; Newman, D.K. Polyphosphate granule biogenesis is temporally and functionally tied to cell cycle exit during starvation in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2017, 114, E2440–E2449. [Google Scholar] [CrossRef]
- Tumlirsch, T.; Sznajder, A.; Jendrossek, D. Formation of Polyphosphate by Polyphosphate Kinases and Its Relationship to Poly(3-Hydroxybutyrate) Accumulation in Ralstonia eutropha Strain H16. Appl. Environ. Microbiol. 2015, 81, 8277–8293. [Google Scholar] [CrossRef]
- Chuang, Y.-M.; Belchis, D.A.; Karakousis, P.C. The Polyphosphate Kinase Gene ppk2 Is Required for Mycobacterium tuberculosis Inorganic Polyphosphate Regulation and Virulence. mBio 2013, 4, e00039-13. [Google Scholar] [CrossRef]
- Roberge, N.; Neville, N.; Douchant, K.; Noordhof, C.; Boev, N.; Sjaarda, C.; Sheth, P.M.; Jia, Z. Broad-Spectrum Inhibitor of Bacterial Polyphosphate Homeostasis Attenuates Virulence Factors and Helps Reveal Novel Physiology of Klebsiella pneumoniae and Acinetobacter baumannii. Front. Microbiol. 2021, 12, 3229. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Shiba, T. Polyphosphate Synthetic Activity of Polyphosphate:AMP Phosphotransferase in Acinetobacter johnsonii 210A. J. Bacteriol. 2004, 186, 5178–5181. [Google Scholar] [CrossRef] [PubMed]
- Resnick, S.M.; Zehnder, A.J.B. In Vitro ATP Regeneration from Polyphosphate and AMP by Polyphosphate:AMP Phosphotransferase and Adenylate Kinase from Acinetobacter johnsonii 210A. Appl. Environ. Microbiol. 2000, 66, 2045–2051. [Google Scholar] [CrossRef]
- Frank, C.; Teleki, A.; Jendrossek, D. Characterization of Agrobacterium tumefaciens PPKs reveals the formation of oligophosphorylated products up to nucleoside nona-phosphates. Appl. Microbiol. Biotechnol. 2020, 104, 9683–9692. [Google Scholar] [CrossRef] [PubMed]
- Nocek, B.P.; Khusnutdinova, A.N.; Ruszkowski, M.; Flick, R.; Burda, M.; Batyrova, K.; Brown, G.; Mucha, A.; Joachimiak, A.; Berlicki, Ł.; et al. Structural Insights into Substrate Selectivity and Activity of Bacterial Polyphosphate Kinases. ACS Catal. 2018, 8, 10746–10760. [Google Scholar] [CrossRef]
- Shi, X.; Rao, N.N.; Kornberg, A. Inorganic polyphosphate in Bacillus cereus: Motility, biofilm formation, and sporulation. Proc. Natl. Acad. Sci. USA 2004, 101, 17061–17065. [Google Scholar] [CrossRef]
- Gangaiah, D.; Liu, Z.; Arcos, J.; Kassem, I.; Sanad, Y.; Torrelles, J.B.; Rajashekara, G. Polyphosphate Kinase 2: A Novel Determinant of Stress Responses and Pathogenesis in Campylobacter jejuni. PLoS ONE 2010, 5, e12142. [Google Scholar] [CrossRef]
- Lindner, S.N.; Vidaurre, D.; Willbold, S.; Schoberth, S.M.; Wendisch, V.F. NCgl2620 Encodes a Class II Polyphosphate Kinase in Corynebacterium glutamicum. Appl. Environ. Microbiol. 2007, 73, 5026–5033. [Google Scholar] [CrossRef]
- Motomura, K.; Hirota, R.; Okada, M.; Ikeda, T.; Ishida, T.; Kuroda, A. A New Subfamily of Polyphosphate Kinase 2 (Class III PPK2) Catalyzes both Nucleoside Monophosphate Phosphorylation and Nucleoside Diphosphate Phosphorylation. Appl. Environ. Microbiol. 2014, 80, 2602–2608. [Google Scholar] [CrossRef]
- Ogawa, M.; Uyeda, A.; Harada, K.; Sato, Y.; Kato, Y.; Watanabe, H.; Honda, K.; Matsuura, T. Class III Polyphosphate Kinase 2 Enzymes Catalyze the Pyrophosphorylation of Adenosine-5′-Monophosphate. ChemBioChem 2019, 20, 2961–2967. [Google Scholar] [CrossRef]
- Batten, L.E.; Parnell, A.E.; Wells, N.J.; Murch, A.L.; Oyston, P.C.F.; Roach, P.L. Biochemical and structural characterization of polyphosphate kinase 2 from the intracellular pathogen Francisella tularensis. Biosci. Rep. 2016, 36, e00294. [Google Scholar] [CrossRef]
- Parnell, A.; Mordhorst, S.; Kemper, F.; Giurrandino, M.; Prince, J.P.; Schwarzer, N.J.; Hofer, A.; Wohlwend, D.; Jessen, H.; Gerhardt, S.; et al. Substrate recognition and mechanism revealed by ligand-bound polyphosphate kinase 2 structures. Proc. Natl. Acad. Sci. USA 2018, 115, 3350–3355. [Google Scholar] [CrossRef]
- Sureka, K.; Sanyal, S.; Basu, J.; Kundu, M. Polyphosphate kinase 2: A modulator of nucleoside diphosphate kinase activity in mycobacteria. Mol. Microbiol. 2009, 74, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Tiwari, P.; Arora, G.; Agarwal, S.; Kidwai, S.; Singh, R. Establishing Virulence Associated Polyphosphate Kinase 2 as a drug target for Mycobacterium tuberculosis. Sci. Rep. 2016, 6, 26900. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kimura, Y.; Kamatani, S. Catalytic activity profile of polyP:AMP phosphotransferase from Myxococcus xanthus. J. Biosci. Bioeng. 2021, 131, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Neville, N.; Roberge, N.; Ji, X.; Stephen, P.; Lu, J.L.; Jia, Z. A Dual-Specificity Inhibitor Targets Polyphosphate Kinase 1 and 2 Enzymes To Attenuate Virulence of Pseudomonas aeruginosa. mBio 2021, 12, e0059221. [Google Scholar] [CrossRef]
- Rosigkeit, H.; Kneißle, L.; Obruča, S.; Jendrossek, D. The Multiple Roles of Polyphosphate in Ralstonia eutropha and Other Bacteria. Microb. Physiol. 2021, 31, 163–177. [Google Scholar] [CrossRef]
- Nahálka, J.; Pätoprstý, V. Enzymatic synthesis of sialylation substrates powered by a novel polyphosphate kinase (PPK3). Org. Biomol. Chem. 2009, 7, 1778–1780. [Google Scholar] [CrossRef]
- Leipe, D.D.; Koonin, E.V.; Aravind, L. Evolution and Classification of P-loop Kinases and Related Proteins. J. Mol. Biol. 2003, 333, 781–815. [Google Scholar] [CrossRef]
- Amar, D.; Berger, I.; Amara, N.; Tafa, G.; Meijler, M.M.; Aharoni, A. The Transition of Human Estrogen Sulfotransferase from Generalist to Specialist Using Directed Enzyme Evolution. J. Mol. Biol. 2012, 416, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, E.; Haouz, A.; Ursby, T.; Munier-Lehmann, H.; Delarue, M.; Bourgeois, D. Mycobacterium tuberculosis Thymidylate Kinase: Structural Studies of Intermediates along the Reaction Pathway. J. Mol. Biol. 2003, 327, 1077–1092. [Google Scholar] [CrossRef]
- Tavanti, M.; Hosford, J.; Lloyd, R.C.; Brown, M.J.B. Recent Developments and Challenges for the Industrial Implementation of Polyphosphate Kinases. ChemCatChem 2021, 13, 3565–3580. [Google Scholar] [CrossRef]
- Shum, K.T.; Lui, E.L.H.; Wong, S.C.K.; Yeung, P.; Sam, L.; Wang, Y.; Watt, R.M.; Tanner, J.A. Aptamer-Mediated Inhibition of Mycobacterium tuberculosis Polyphosphate Kinase 2. Biochemistry 2011, 50, 3261–3271. [Google Scholar] [CrossRef] [PubMed]
- Rexer, T.F.; Schildbach, A.; Klapproth, J.; Schierhorn, A.; Mahour, R.; Pietzsch, M.; Rapp, E.; Reichl, U. One pot synthesis of GDP-mannose by a multi-enzyme cascade for enzymatic assembly of lipid-linked oligosaccharides. Biotechnol. Bioeng. 2017, 115, 192–205. [Google Scholar] [CrossRef]
- Hildenbrand, J.C.; Teleki, A.; Jendrossek, D. A universal polyphosphate kinase: PPK2c of Ralstonia eutropha accepts purine and pyrimidine nucleotides including uridine diphosphate. Appl. Microbiol. Biotechnol. 2020, 104, 6659–6667. [Google Scholar] [CrossRef]
- Mahour, R.; Klapproth, J.; Rexer, T.F.; Schildbach, A.; Klamt, S.; Pietzsch, M.; Rapp, E.; Reichl, U. Establishment of a five-enzyme cell-free cascade for the synthesis of uridine diphosphate N-acetylglucosamine. J. Biotechnol. 2018, 283, 120–129. [Google Scholar] [CrossRef]
- Gottschalk, J.; Blaschke, L.; Aßmann, M.; Kuballa, J.; Elling, L. Integration of a Nucleoside Triphosphate Regeneration System in the One-pot Synthesis of UDP-sugars and Hyaluronic Acid. ChemCatChem 2021, 13, 3074–3083. [Google Scholar] [CrossRef]
- Bonting, C.F.; Kortstee, G.J.; Zehnder, A.J. Properties of polyphosphate: AMP phosphotransferase of Acinetobacter strain 210A. J. Bacteriol. 1991, 173, 6484–6488. [Google Scholar] [CrossRef]
- Zhang, H.; Rao, N.N.; Shiba, T.; Kornberg, A. Inorganic polyphosphate in the social life of Myxococcus xanthus: Motility, development, and predation. Proc. Natl. Acad. Sci. USA 2005, 102, 13416–13420. [Google Scholar] [CrossRef]
- Mordhorst, S.; Singh, J.; Mohr, M.K.F.; Hinkelmann, R.; Keppler, M.; Jessen, H.J.; Andexer, J.N. Several Polyphosphate Kinase 2 Enzymes Catalyse the Production of Adenosine 5′-Polyphosphates. ChemBioChem 2018, 20, 1019–1022. [Google Scholar] [CrossRef]
- Dürr-Mayer, T.; Qiu, D.; Eisenbeis, V.B.; Steck, N.; Häner, M.; Hofer, A.; Mayer, A.; Siegel, J.S.; Baldridge, K.K.; Jessen, H.J. The chemistry of branched condensed phosphates. Nat. Commun. 2021, 12, 5368. [Google Scholar] [CrossRef]
- Mandala, V.S.; Loh, D.M.; Shepard, S.M.; Geeson, M.B.; Sergeyev, I.V.; Nocera, D.G.; Cummins, C.C.; Hong, M. Bacterial Phosphate Granules Contain Cyclic Polyphosphates: Evidence from 31P Solid-State NMR. J. Am. Chem. Soc. 2020, 142, 18407–18421. [Google Scholar] [CrossRef]
- Chuang, Y.-M.; Dutta, N.K.; Hung, C.-F.; Wu, T.-C.; Rubin, H.; Karakousis, P.C. Stringent Response Factors PPX1 and PPK2 Play an Important Role in Mycobacterium tuberculosis Metabolism, Biofilm Formation, and Sensitivity to Isoniazid In Vivo. Antimicrob. Agents Chemother. 2016, 60, 6460–6470. [Google Scholar] [CrossRef]
- Hildenbrand, J.C.; Reinhardt, S.; Jendrossek, D. Formation of an Organic–Inorganic Biopolymer: Polyhydroxybutyrate–Polyphosphate. Biomacromolecules 2019, 20, 3253–3260. [Google Scholar] [CrossRef]
- Cross, A.S. What is a virulence factor? Crit. Care 2008, 12, 197. [Google Scholar] [CrossRef]
- Kang, D.; Revtovich, A.V.; Chen, Q.; Shah, K.N.; Cannon, C.L.; Kirienko, N.V. Pyoverdine-Dependent Virulence of Pseudomonas aeruginosa Isolates From Cystic Fibrosis Patients. Front. Microbiol. 2019, 10, 2048. [Google Scholar] [CrossRef]
- Pina-Mimbela, R.; Madrid, J.A.; Kumar, A.; Torrelles, J.B.; Rajashekara, G. Polyphosphate Kinases Modulate Campylobacter Jejuni Outer Membrane Constituents and Alter Its Capacity to Invade and Survive in Intestinal Epithelial Cells In Vitro. Emerg. Microbes Infect. 2015, 4, e77. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.I.; Michell, S.L.; Oyston, P.C.F. An intracellularly inducible gene involved in virulence and polyphosphate production in Francisella. J. Med. Microbiol. 2008, 57, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Severín, J.; Varas, M.; Bravo-Toncio, C.; Guiliani, N.; Chávez, F.P. Multiple antibiotic susceptibility of polyphosphate kinase mutants (ppk1 and ppk2) from Pseudomonas aeruginosa PAO1 as revealed by global phenotypic analysis. Biol. Res. 2015, 48, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Burda-Grabowska, M.; Macegoniuk, K.; Flick, R.; Nocek, B.P.; Joachimiak, A.; Yakunin, A.F.; Mucha, A.; Berlicki, Ł. Bisphosphonic acids and related compounds as inhibitors of nucleotide- and polyphosphate-processing enzymes: A PPK1 and PPK2 case study. Chem. Biol. Drug Des. 2019, 93, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Baijal, K.; Downey, M. Targeting Polyphosphate Kinases in the Fight against Pseudomonas aeruginosa. mBio 2021, 12, 0147721. [Google Scholar] [CrossRef] [PubMed]
| Species | Number of PPK2s | PPK2 Class | PDB ID | Reference |
|---|---|---|---|---|
| Acinetobacter baumannii | 1 | II | [42] | |
| Acinetobacter johnsonii | 1 | II | [43,44] | |
| Agrobacterium tumefaciens | 1 | I | [36,45] | |
| Arthrobacter aurescens | 1 | III | 3RHF | [46] |
| Bacillus cereus | 1 | II | [47] | |
| Campylobacter jejuni | 1 | I | [48] | |
| Corynebacterium glutamicum | 2 | I | [49] | |
| Cytophaga hutchinsonii | 1 | III | 6ANG, 6ANH, 6ANQ, 6AUO, 6AN9, 6B18 | [46] |
| Deinococcus geothermalis | 1 | III | [50] | |
| Deinococcus radiodurans | 1 | III | 6AQE, 7NMJ, 7BMM | [46,50] |
| Delftia tsuruhatensis | 1 | III | [51] | |
| Francisella tularensis | 1 | I | 4YEG, 5LLB, 5LL0, 5LLF | [52,53] |
| Klebsiella pneumoniae | 1 | I | [42] | |
| Meiothermus ruber | 1 | III | 5LC9 | [50,53] |
| Meiothermus silvanus | 1 | III | [50] | |
| Mycobacterium smegmatis | 1 | I | [54] | |
| Mycobacterium tuberculosis | 1 | I | [41,55] | |
| Myxococcus xanthus | 1 | II | [56] | |
| Pseudomonas aeruginosa | 3 | I (PPK2A/PA0141) | [34,57] | |
| I (PPK2B/PA2428) | [39,57] | |||
| II (PPK2C/PA3455) | 3CZP | [36] | ||
| Ralstonia eutropha | 5 | Not yet classified | [40,58] | |
| Rhodopseudomonas palustris | 1 | I | [36] | |
| Ruegeria pomeroyi | 1 | I | [59] | |
| Sinorhizobium meliloti | 3 | I | 3CZQ, 6DZG | [36] |
| Thermosynechococcus elongatus | 1 | III | [50] |
| Inhibitor | Structure | Reaction Tested | Inhibition Potency | Reference |
|---|---|---|---|---|
| NSC 35676 |  | ATP synthesis from ADP | >80% inhibition at 100 µM of M. tuberculosis PPK2–MBP fusion | [55] |
| NSC 30205 |  | ATP synthesis from ADP | >80% inhibition at 100 µM of M. tuberculosis PPK2–MBP fusion | |
| NSC 345647 | 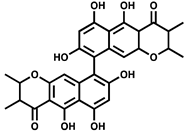 | ATP synthesis from ADP | >80% inhibition at 100 µM of M. tuberculosis PPK2–MBP fusion | |
| NSC 9037 | 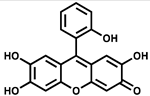 | ATP synthesis from ADP | >80% inhibition at 100 µM of M. tuberculosis PPK2–MBP fusion | |
| 11f |  | ATP synthesis from ADP | IC50 = 60.2 µM for C. hutchinsonii PPK2 | [81] |
| 11g | 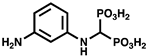 | ATP synthesis from ADP | IC50 = 70.5 µM for C. hutchinsonii PPK2 | |
| 11i |  | ATP synthesis from ADP | IC50 = 58.0 µM for C. hutchinsonii PPK2 | |
| 14b | 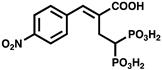 | ATP synthesis from ADP | IC50 = 85.4 µM for C. hutchinsonii PPK2 | |
| Gallein | 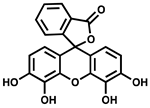 | ATP synthesis from ADP | IC50 = 16 µM for P. aeruginosa PPK2A | [57] |
| PolyP synthesis from ATP | IC50 = 20 µM for P. aeruginosa PPK2B | |||
| ADP synthesis from AMP | IC50 = 165 µM for P. aeruginosa PPK2C | |||
| ADP synthesis from AMP | IC50 = 40.7 µM for A. baumannii PPK2 | [42] | ||
| ATP synthesis from ADP | IC50 = 50.4 µM for K. pneumoniae MBP-PPK2 | |||
| Aptamer G9 | N/A | ATP synthesis from ADP | IC50 = 39.3 nM for M. tuberculosis PPK2 | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neville, N.; Roberge, N.; Jia, Z. Polyphosphate Kinase 2 (PPK2) Enzymes: Structure, Function, and Roles in Bacterial Physiology and Virulence. Int. J. Mol. Sci. 2022, 23, 670. https://doi.org/10.3390/ijms23020670
Neville N, Roberge N, Jia Z. Polyphosphate Kinase 2 (PPK2) Enzymes: Structure, Function, and Roles in Bacterial Physiology and Virulence. International Journal of Molecular Sciences. 2022; 23(2):670. https://doi.org/10.3390/ijms23020670
Chicago/Turabian StyleNeville, Nolan, Nathan Roberge, and Zongchao Jia. 2022. "Polyphosphate Kinase 2 (PPK2) Enzymes: Structure, Function, and Roles in Bacterial Physiology and Virulence" International Journal of Molecular Sciences 23, no. 2: 670. https://doi.org/10.3390/ijms23020670
APA StyleNeville, N., Roberge, N., & Jia, Z. (2022). Polyphosphate Kinase 2 (PPK2) Enzymes: Structure, Function, and Roles in Bacterial Physiology and Virulence. International Journal of Molecular Sciences, 23(2), 670. https://doi.org/10.3390/ijms23020670







