Engineering Nanoplatform for Combined Cancer Therapeutics via Complementary Autophagy Inhibition
Abstract
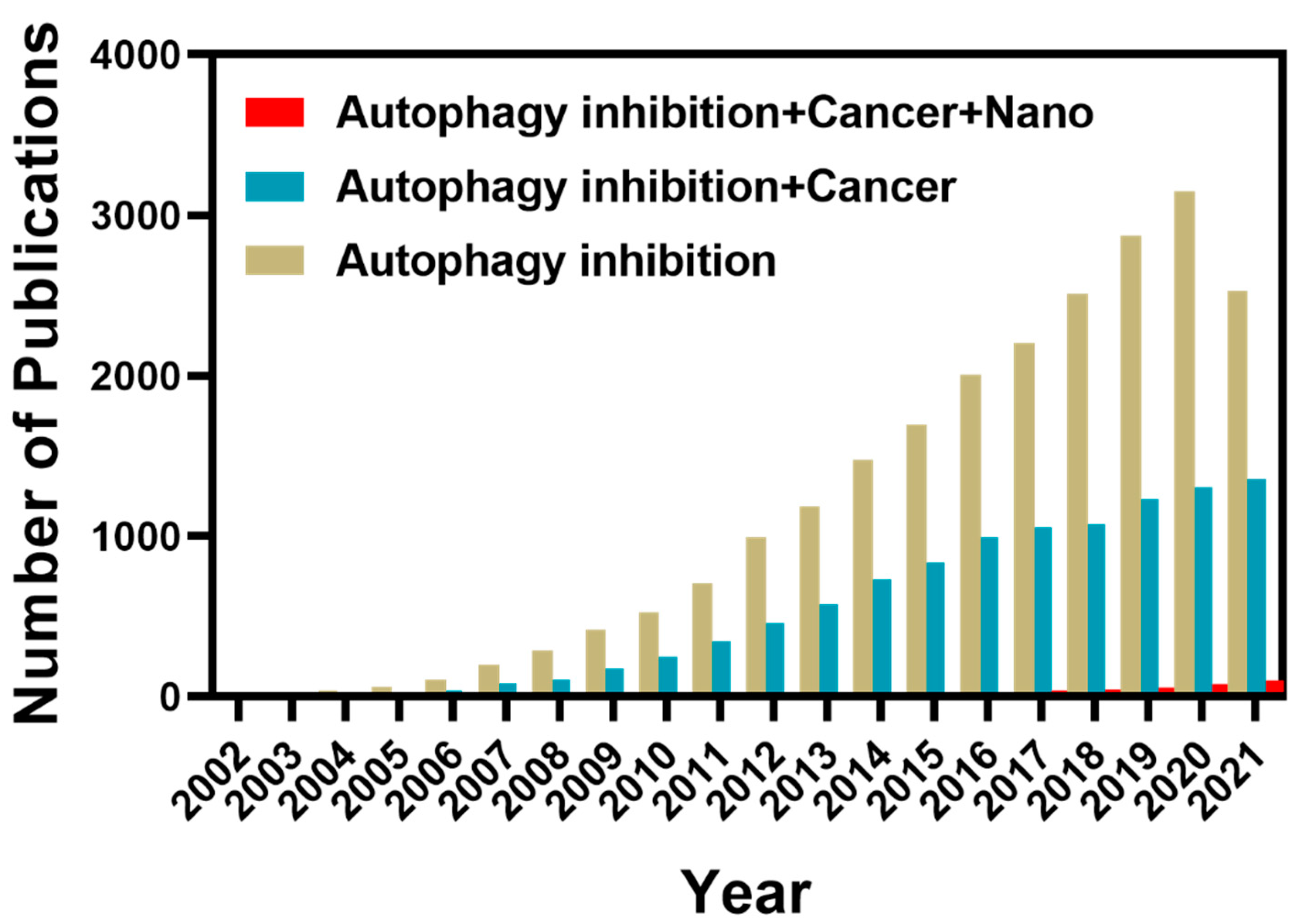
:1. Introduction
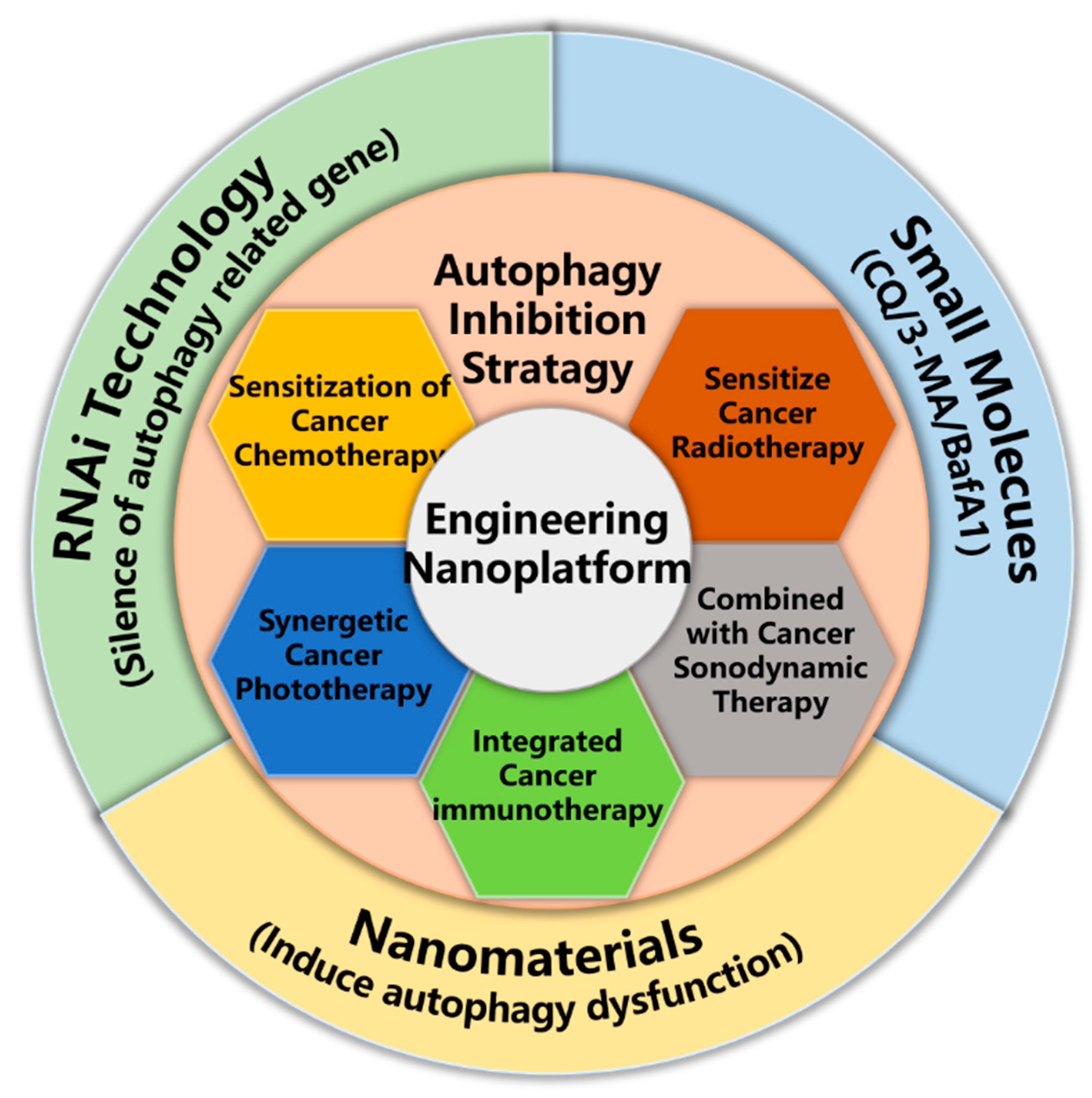
2. Exploitation of Nanoparticle Mediated Autophagy Inhibition Strategy for Cancer Therapy
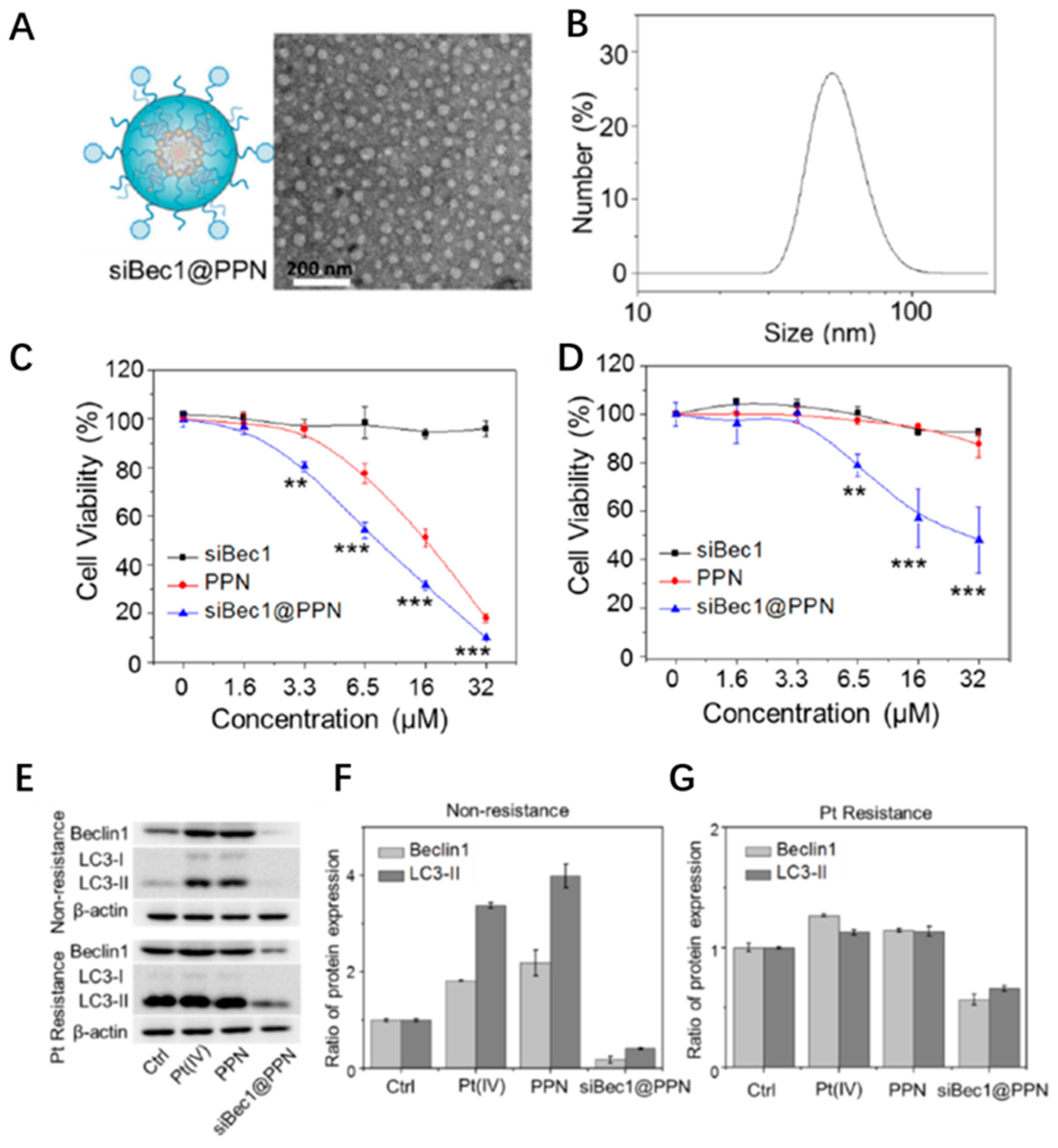
3. Sensitization of Cancer Chemotherapy
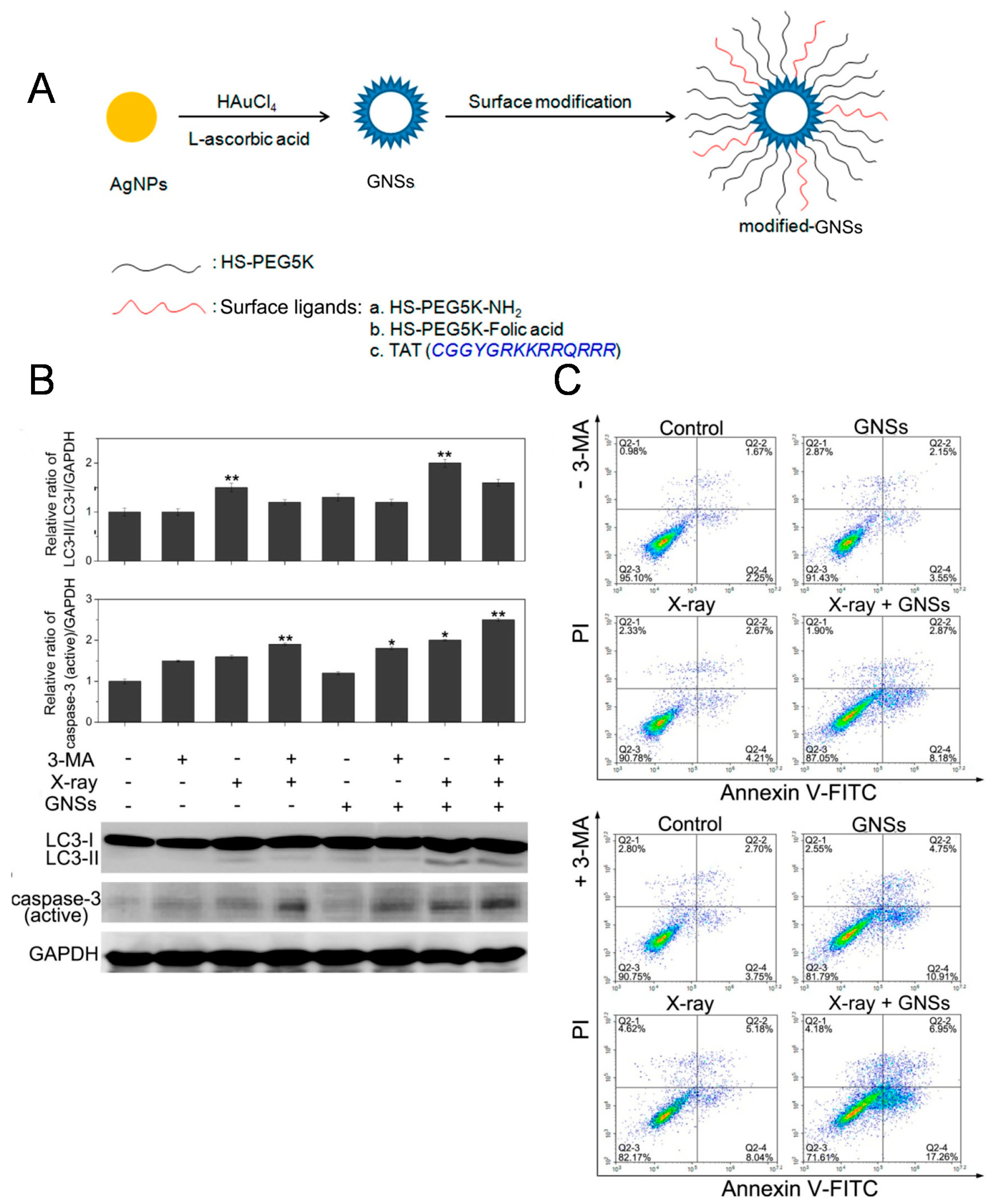
4. Sensitize Cancer Radiotherapy
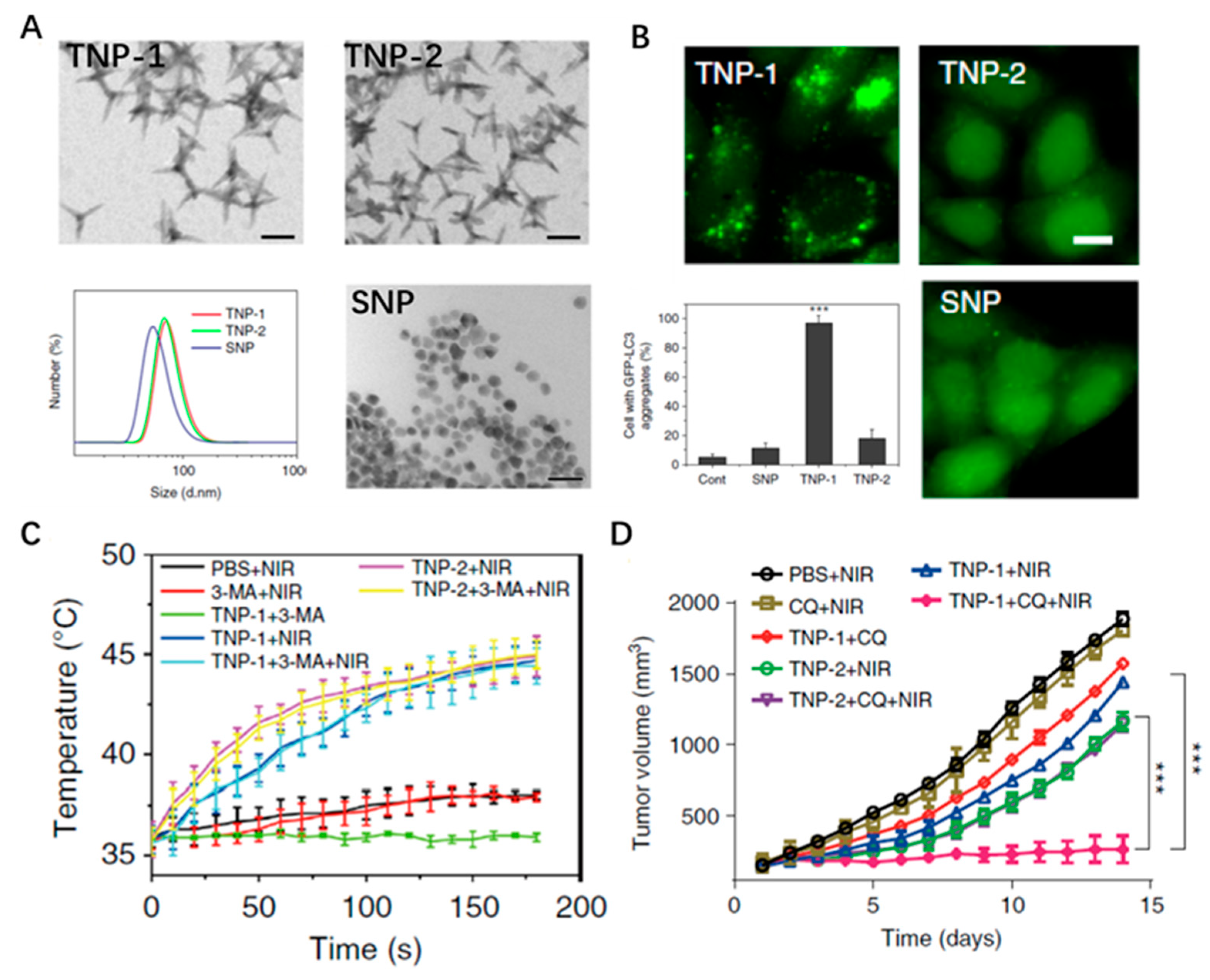
5. Synergetic Cancer Phototherapy
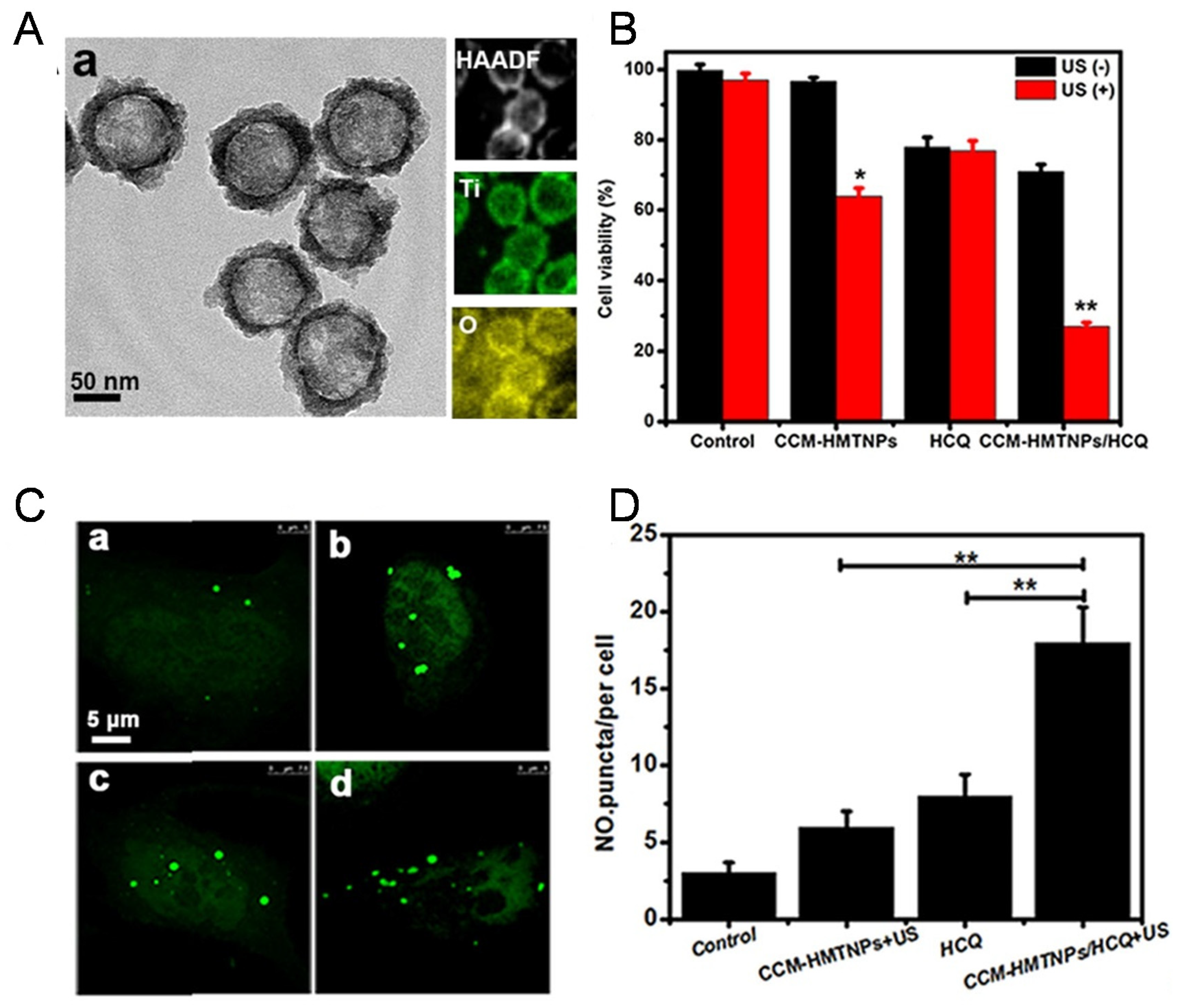
6. Combined with Cancer Sonodynamic Therapy
7. Integrated Cancer Immunotherapy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Peer, D.; Karp, J.M.; Hong, S.; FaroKhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.L.; Kadel, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGF beta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chin. J. Clin. Oncol. 2014, 41, 18–26. [Google Scholar] [CrossRef]
- Li, L.L.; Tang, F.Q.; Liu, H.Y.; Liu, T.L.; Hao, N.J.; Chen, D.; Teng, X.; He, J.Q. In Vivo Delivery of Silica Nanorattle Encapsulated Docetaxel for Liver Cancer Therapy with Low Toxicity and High Efficacy. ACS Nano 2010, 4, 6874–6882. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.J.; Zhao, L.Z.; Zhu, X.J.; Sun, Y.; Feng, W.; Gao, Y.H.; Wang, L.Y.; Li, F.Y. Hollow silica nanoparticles loaded with hydrophobic phthalocyanine for near-infrared photodynamic and photothermal combination therapy. Biomaterials 2013, 34, 7905–7912. [Google Scholar] [CrossRef] [PubMed]
- Bhana, S.; Lin, G.; Wang, L.J.; Starring, H.; Mishra, S.R.; Liu, G.; Huang, X.H. Near-Infrared-Absorbing Gold Nanopopcorns with Iron Oxide Cluster Core for Magnetically Amplified Photothermal and Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2015, 7, 11637–11647. [Google Scholar] [CrossRef] [PubMed]
- Atchison, J.; Kamila, S.; Nesbitt, H.; Logan, K.A.; Nicholas, D.M.; Fowley, C.; Davis, J.; Callan, B.; McHale, A.P.; Callan, J.F. Iodinated cyanine dyes: A new class of sensitisers for use in NIR activated photodynamic therapy (PDT). Chem. Commun. 2017, 53, 2009–2012. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Fu, W.; Dong, D.; Zhao, Y. Molecular Mechanisms of Autophagy. Chin. J. Biochem. Mol. Biol. 2017, 33, 448–455. [Google Scholar]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.D.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Klionsky, D.J. Autophagy: From phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 2007, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, Y.; Zhang, C.; Lai, X.; Zhang, Y.; Wu, J.; Hu, C.; Shao, L. Nanomaterial-mediated autophagy: Coexisting hazard and health benefits in biomedicine. Part Fibre Toxicol. 2020, 17, 53. [Google Scholar] [CrossRef]
- Kimura, S.; Fujita, N.; Noda, T.; Yoshimori, T. Chapter 1 Monitoring Autophagy in Mammalian Cultured Cells through the Dynamics of LC3. Methods Enzymol. 2009, 452, 1–12. [Google Scholar] [CrossRef]
- Andón, F.T.; Fadeel, B. Programmed Cell Death: Molecular Mechanisms and Implications for Safety Assessment of Nanomaterials. Acc. Chem. Res. 2013, 46, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, C.L.; Zhao, Z.J.; Zuo, X.X.; Liang, T.S.; Yang, Y.; Ma, S.L.; Yang, D.K. Microwave hyperthermia enhances the sensitivity of lung cancer cells to gemcitabine through reactive oxygen speciesinduced autophagic death. Oncol. Rep. 2019, 41, 3100–3110. [Google Scholar] [CrossRef]
- Mizushima, N. A brief history of autophagy from cell biology to physiology and disease. Nat. Cell Biol. 2018, 20, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Amaravadi, R.K.; Yu, D.N.; Lum, J.J.; Bui, T.; Christophorou, M.A.; Evan, G.I.; Thomas-Tikhonenko, A.; Thompson, C.B. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J. Clin. Investig. 2007, 117, 326–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Livesey, K.M.; Tang, D.; Zeh, H.J.; Lotze, M.T. Autophagy inhibition in combination cancer treatment. Curr. Opin. Investig. Drugs 2009, 10, 1269–1279. [Google Scholar]
- Chen, X.R.; Tong, R.L.; Shi, Z.Q.; Yang, B.; Liu, H.; Ding, S.P.; Wang, X.; Lei, Q.F.; Wu, J.; Fang, W.J. MOF Nanoparticles with Encapsulated Autophagy Inhibitor in Controlled Drug Delivery System for Antitumor. ACS Appl. Mater. Interfaces 2018, 10, 2328–2337. [Google Scholar] [CrossRef]
- Bryant, K.L.; Stalnecker, C.A.; Zeitouni, D.; Klomp, J.E.; Peng, S.; Tikunov, A.P.; Gunda, V.; Pierobon, M.; Waters, A.M.; George, S.D.; et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat. Med. 2020, 26, 982. [Google Scholar] [CrossRef]
- Zhang, H. Targeting autophagy in lymphomas: A double-edged sword? Int. J. Hematol. 2018, 107, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Vats, S.; Chia, A.Y.Q.; Tan, T.Z.; Deng, S.; Ong, M.S.; Arfuso, F.; Yap, C.T.; Goh, B.C.; Sethi, G.; et al. Dual role of autophagy in hallmarks of cancer. Oncogene 2018, 37, 1142–1158. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, I.; Gkikas, I.; Tavernarakis, N. Hypoxia and Selective Autophagy in Cancer Development and Therapy. Front. Cell. Dev. Biol. 2018, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Ediriwickrema, A.; Saltzman, W.M. Nanotherapy for Cancer: Targeting and Multifunctionality in the Future of Cancer Therapies. ACS Biomater. Sci. Eng 2015, 1, 64–78. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, G.; Zhou, Y.X.; Chen, Y.; Ouyang, L.; Liu, B. Mechanisms of autophagy and relevant small-molecule compounds for targeted cancer therapy. Cell. Mol. Life Sci. 2018, 75, 1803–1826. [Google Scholar] [CrossRef] [PubMed]
- Bhat, P.; Kriel, J.; Priya, B.S.; Basappa; Shivananju, N.S.; Loos, B. Modulating autophagy in cancer therapy: Advancements and challenges for cancer cell death sensitization. Biochem. Pharmacol. 2018, 147, 170–182. [Google Scholar] [CrossRef]
- Li, Y.; Lei, Y.; Yao, N.; Wang, C.; Hu, N.; Ye, W.; Zhang, D.; Chen, Z. Autophagy and multidrug resistance in cancer. Chin. J. Cancer 2017, 36, 342–351. [Google Scholar] [CrossRef]
- Hoare, M.; Young, A.R.J.; Narita, M. Autophagy in cancer: Having your cake and eating it. Semin. Cancer Biol. 2011, 21, 397–404. [Google Scholar] [CrossRef]
- Wu, Y.T.; Tan, H.L.; Shui, G.; Bauvy, C.; Huang, Q.; Wenk, M.R.; Ong, C.N.; Codogno, P.; Shen, H.M. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J. Biol. Chem. 2010, 285, 10850–10861. [Google Scholar] [CrossRef] [Green Version]
- Ronan, B.; Flamand, O.; Vescovi, L.; Dureuil, C.; Durand, L.; Fassy, F.; Bachelot, M.F.; Lamberton, A.; Mathieu, M.; Bertrand, T.; et al. A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nat. Chem. Biol. 2014, 10, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.S.; Sun, J.H.; Yu, H.H.; Yu, S.Q. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv. 2017, 24, 18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.J.; Zhang, L.; Gao, J.H.; Wen, L.P. Pro-Death or Pro-Survival: Contrasting Paradigms on Nanomaterial-Induced Autophagy and Exploitations for Cancer Therapy. Acc. Chem. Res. 2019, 52, 3164–3176. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Jin, X.D.; Chen, B.; Li, P.; Li, Q. Autophagy-regulating microRNAs: Potential targets for improving radiotherapy. J. Cancer Res. Clin. Oncol. 2018, 144, 1623–1634. [Google Scholar] [CrossRef]
- Limpert, A.S.; Lambert, L.J.; Bakas, N.A.; Bata, N.; Brun, S.N.; Shaw, R.J.; Cosford, N.D.P. Autophagy in Cancer: Regulation by Small Molecules. Trends Pharmacol. Sci. 2018, 39, 1021–1032. [Google Scholar] [CrossRef]
- Marinkovic, M.; Sprung, M.; Buljubasic, M.; Novak, I. Autophagy Modulation in Cancer: Current Knowledge on Action and Therapy. Oxidative Med. Cell. Longev. 2018, 2018, 8023821. [Google Scholar] [CrossRef] [Green Version]
- Das, C.K.; Mandal, M.; Kogel, D. Pro-survival autophagy and cancer cell resistance to therapy. Cancer Metastasis Rev. 2018, 37, 749–766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Li, X.G.; Wang, D.H. Lysosomes contribute to radioresistance in cancer. Cancer Lett. 2018, 439, 39–46. [Google Scholar] [CrossRef]
- Raju, G.S.R.; Pavitra, E.; Merchant, N.; Lee, H.; Prasad, G.L.V.; Nagaraju, G.P.; Huh, Y.S.; Han, Y.K. Targeting autophagy in gastrointestinal malignancy by using nanomaterials as drug delivery systems. Cancer Lett. 2018, 419, 222–232. [Google Scholar] [CrossRef]
- Smith, A.G.; Macleod, K.F. Autophagy, cancer stem cells and drug resistance. J. Pathol. 2019, 247, 708–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinsey, C.G.; Camolotto, S.A.; Boespflug, A.M.; Guillen, K.P.; Foth, M.; Truong, A.; Schuman, S.S.; Shea, J.E.; Seipp, M.T.; Yap, J.T.; et al. Protective autophagy elicited by RAF -> MEK -> ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat. Med. 2019, 25, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.X.; Wang, Y.; An, H.W.; Qi, B.W.; Wang, J.Q.; Wang, L.; Shi, J.J.; Mei, L.; Wang, H. Peptide-Based Autophagic Gene and Cisplatin Co-delivery Systems Enable Improved Chemotherapy Resistance. Nano Lett. 2019, 19, 2968–2978. [Google Scholar] [CrossRef]
- Gong, C.; Hu, C.L.; Gu, F.F.; Xia, Q.M.; Yao, C.; Zhang, L.J.; Qiang, L.; Gao, S.; Gao, Y. Co-delivery of autophagy inhibitor ATG7 siRNA and docetaxel for breast cancer treatment. J. Control. Release 2017, 266, 272–286. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, W.; Tang, G.; Wang, H.; Wu, M.; Yu, W.; Zhou, Z.; Mou, Y.; Liu, X. Targeted Codelivery of Docetaxel and Atg7 siRNA for Autophagy Inhibition and Pancreatic Cancer Treatment. ACS Applied Bio Materials. 2019, 2, 1168–1176. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liu, Z.J.; Lou, Z.C.; Chen, F.; Chang, S.Q.; Miao, Y.J.; Zhou, Z.; Hu, X.D.; Feng, J.D.; Ding, Q.; et al. Radiosensitivity enhancement of Fe3O4@Ag nanoparticles on human glioblastoma cells. Artif. Cell. Nanomed. Biotechnol. 2018, 46, S975–S984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.J.; Sha, R.; Zhang, L.; Zhang, W.B.; Jin, P.P.; Xu, W.G.; Ding, J.X.; Lin, J.; Qian, J.; Yao, G.Y.; et al. Harnessing copper-palladium alloy tetrapod nanoparticle-induced pro-survival autophagy for optimized photothermal therapy of drug-resistant cancer. Nat. Commun. 2018, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Wan, H.Y.; Chen, J.L.; Zhu, X.Z.; Liu, L.; Wang, J.F.; Zhu, X.M. Titania-Coated Gold Nano-Bipyramids for Blocking Autophagy Flux and Sensitizing Cancer Cells to Proteasome Inhibitor-Induced Death. Adv. Sci. 2018, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.H.; Li, Y.H.; Huang, F.F.; Wang, X.; Lu, J.Q.; Jia, F.; Pan, Z.A.; Cui, X.Y.; Ge, G.L.; Deng, X.W.; et al. Complementary autophagy inhibition and glucose metabolism with rattle -structured polydopamine@mesoporous silica nanoparticles for augmented low -temperature photothermal therapy and in vivo photoacoustic imaging. Theranostics 2020, 10, 7273–7286. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.H.; Cui, Y.K.; Deng, X.W.; Lu, J.Q.; Jia, F.; Pan, Z.A.; Cui, X.Y.; Hu, F.Q.; Hu, W.H.; et al. Hierarchical assembly of dual-responsive biomineralized polydopamine-calcium phosphate nanocomposites for enhancing chemo-photothermal therapy by autophagy inhibition. Biomater. Sci. 2020, 8, 5172–5182. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.S.; Zhang, L.; Zhang, X.Z. An ATP-Regulated Ion Transport Nanosystem for Homeostatic Perturbation Therapy and Sensitizing Photodynamic Therapy by Autophagy Inhibition of Tumors. ACS Cent. Sci. 2019, 5, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.H.; Yang, X.M.; Hao, Y.T.; Wang, N.; Feng, X.B.; Hou, L.; Zhang, Z.Z. Cancer Cell Membrane-Biomimetic Nanoplatform for Enhanced Sonodynamic Therapy on Breast Cancer via Autophagy Regulation Strategy. ACS Appl. Mater. Interfaces 2019, 11, 32729–32738. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, Y.T.; Zhu, J.; Jin, L.B.; Liu, B.; Xia, K.S.; Wang, J.J.; Gao, J.Q.; Liang, C.Z.; Tao, H.M. Autophagy inhibitor enhance ZnPc/BSA nanoparticle induced photodynamic therapy by suppressing PD-L1 expression in osteosarcoma immunotherapy. Biomaterials 2019, 192, 128–139. [Google Scholar] [CrossRef]
- Ruan, S.B.; Xie, R.; Qin, L.; Yu, M.N.; Xiao, W.; Hu, C.; Yu, W.Q.; Qian, Z.Y.; Ouyang, L.; He, Q.; et al. Aggregable Nanoparticles-Enabled Chemotherapy and Autophagy Inhibition Combined with Anti-PD-L1 Antibody for Improved Glioma Treatment. Nano Lett. 2019, 19, 8318–8332. [Google Scholar] [CrossRef]
- Sun, R.; Shen, S.; Zhang, Y.J.; Xu, C.F.; Cao, Z.T.; Wen, L.P.; Wang, J. Nanoparticle-facilitated autophagy inhibition promotes the efficacy of chemotherapeutics against breast cancer stem cells. Biomaterials 2016, 103, 44–55. [Google Scholar] [CrossRef]
- Xin, X.F.; Du, X.Q.; Xiao, Q.Q.; Azevedo, H.S.; He, W.; Yin, L.F. Drug Nanorod-Mediated Intracellular Delivery of microRNA-101 for Self-sensitization via Autophagy Inhibition. Nano-Micro Lett. 2019, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.W.; Gao, G.; Jia, H.R.; Zhang, X.D.; Zhao, J.; Ma, N.N.; Liu, J.B.; Liu, P.D.; Wu, F.G. Copper Oxide Nanoparticles Induce Enhanced Radiosensitizing Effect via Destructive Autophagy. ACS Biomater. Sci. Eng. 2019, 5, 1569–1579. [Google Scholar] [CrossRef]
- Ma, N.N.; Liu, P.D.; He, N.Y.; Gu, N.; Wu, F.G.; Chen, Z. Action of Gold Nanospikes-Based Nanoradiosensitizers: Cellular Internalization, Radiotherapy, and Autophagy. ACS Appl. Mater. Interfaces 2017, 9, 31526–31542. [Google Scholar] [CrossRef]
- Gong, F.; Cheng, L.; Yang, N.L.; Betzer, O.; Feng, L.Z.; Zhou, Q.; Li, Y.G.; Chen, R.H.; Popovtzer, R.; Liu, Z. Ultrasmall Oxygen-Deficient Bimetallic Oxide MnWOX Nanoparticles for Depletion of Endogenous GSH and Enhanced Sonodynamic Cancer Therapy. Adv. Mater. 2019, 31, 9. [Google Scholar] [CrossRef]
- Yue, W.W.; Chen, L.; Yu, L.D.; Zhou, B.G.; Yin, H.H.; Ren, W.W.; Liu, C.; Guo, L.H.; Zhang, Y.F.; Sun, L.P.; et al. Checkpoint blockade and nanosonosensitizer- augmented noninvasive sonodynamic therapy combination reduces tumour growth and metastases in mice. Nat. Commun. 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinleye, A.; Rasool, Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J. Hematol. Oncol. 2019, 12, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.Q.; Liu, P.P.; Wang, K.F.; Glorieux, C.; Hu, Y.M.; Wen, S.J.; Jiang, W.Q.; Huang, P. Chemotherapy induces tumor immune evasion by upregulation of programmed cell death ligand 1 expression in bone marrow stromal cells. Mol. Oncol. 2017, 11, 358–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Autophagy Inhibition Mediated Nanoplatform | Combined Therapy | Strategy for Autophagy Inhibition | Reference |
|---|---|---|---|
| Self-assemble nano-prodrug platform siBec1@PPN | Chemotherapy | Beclin1 siRNA | [44] |
| A crosslinked redox-sensitive polypeptide micellar system | Chemotherapy | ATG7 siRNA | [45] |
| A multifunctional micelle vehicle | Chemotherapy | ATG7 siRNA | [46] |
| A carrier-free PTX nanocrystals (PNs) | Chemotherapy | MiR-101 | [45] |
| Radiosensitizer based on gold nanoparticles (GNSs) | Radiotherapy | 3-MA | [47] |
| Copper (Cu)-palladium (Pd) alloy tetrapod nanoparticles | Photothermal therapy | 3-MA/CQ | [48] |
| Titania-coated gold nano-bipyramid (NBP/TiO2) nanostructures | Photothermal therapy | Nanomaterials | [49] |
| Rattle-structured polydopamine@mesoporous silica nanoparticles (PDA@hm@CQ@GOx) | Photothermal-Starvation therapy | HCQ | [50] |
| Biomineralized nanocomposites (PCNPs/DC) | Photothermal-Chemotherapy | HCQ | [51] |
| An ATP-regulated ion transport nano-system (SQU@PCN) | Photodynamic-Homeostatic perturbation therapy | Nanomaterials | [52] |
| CCM biomimetic nanoplatform based on HMTNPs | Sonodynamic therapy | HCQ | [53] |
| Bovine serum albumin-Zinc phthalocyanine (ZnPc/BSA) nanoparticles | Immuno-Photodynamic therapy | 3-MA | [54] |
| Functional gold nanoparticles (D&H-A-A&C) | Immuno-Chemotherapy | HCQ | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, Y.; Lu, J.; Deng, X.; Wu, Y. Engineering Nanoplatform for Combined Cancer Therapeutics via Complementary Autophagy Inhibition. Int. J. Mol. Sci. 2022, 23, 657. https://doi.org/10.3390/ijms23020657
Wang X, Li Y, Lu J, Deng X, Wu Y. Engineering Nanoplatform for Combined Cancer Therapeutics via Complementary Autophagy Inhibition. International Journal of Molecular Sciences. 2022; 23(2):657. https://doi.org/10.3390/ijms23020657
Chicago/Turabian StyleWang, Xuan, Yunhao Li, Jianqing Lu, Xiongwei Deng, and Yan Wu. 2022. "Engineering Nanoplatform for Combined Cancer Therapeutics via Complementary Autophagy Inhibition" International Journal of Molecular Sciences 23, no. 2: 657. https://doi.org/10.3390/ijms23020657
APA StyleWang, X., Li, Y., Lu, J., Deng, X., & Wu, Y. (2022). Engineering Nanoplatform for Combined Cancer Therapeutics via Complementary Autophagy Inhibition. International Journal of Molecular Sciences, 23(2), 657. https://doi.org/10.3390/ijms23020657





