Dietary Exposure to Flame Retardant Tris (2-Butoxyethyl) Phosphate Altered Neurobehavior and Neuroinflammatory Responses in a Mouse Model of Allergic Asthma
Abstract
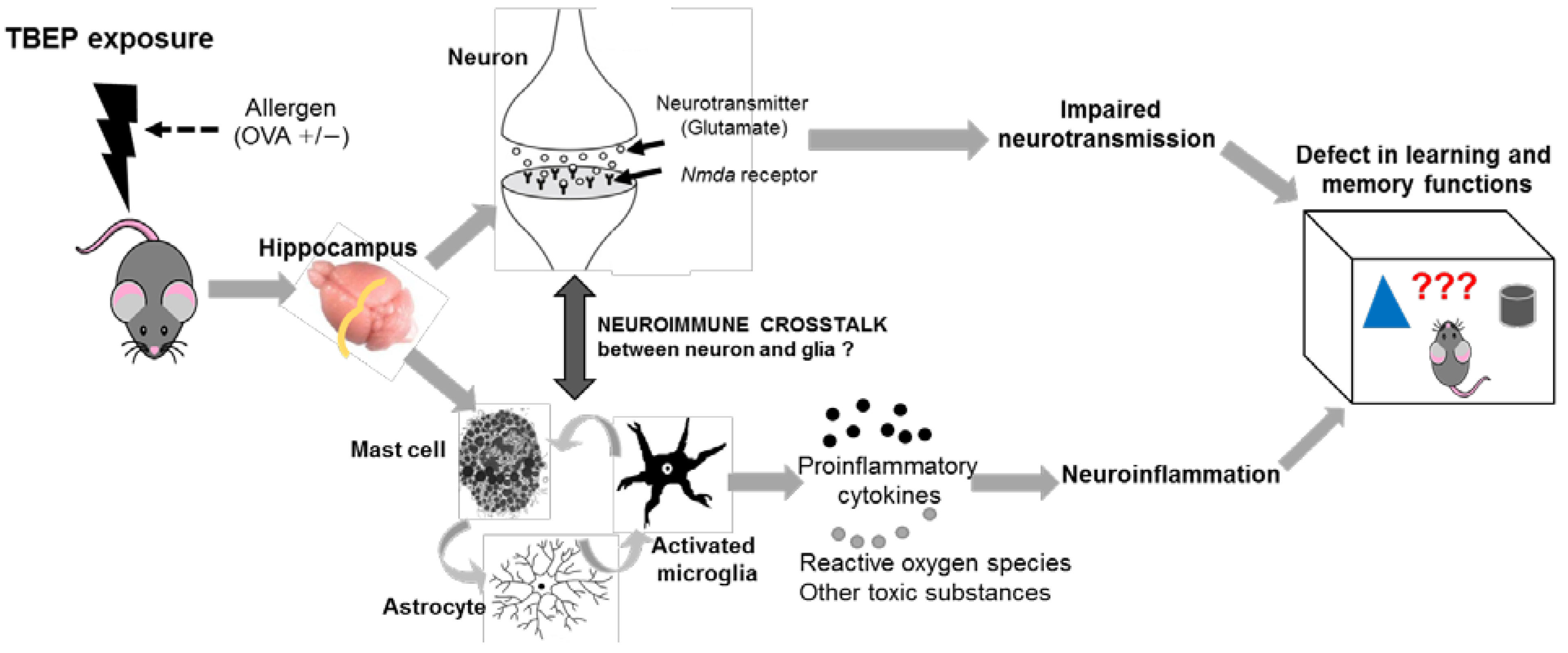
:1. Introduction
2. Results
2.1. Assessment of General Toxicity
2.2. Allergic Asthmatic Mouse Model
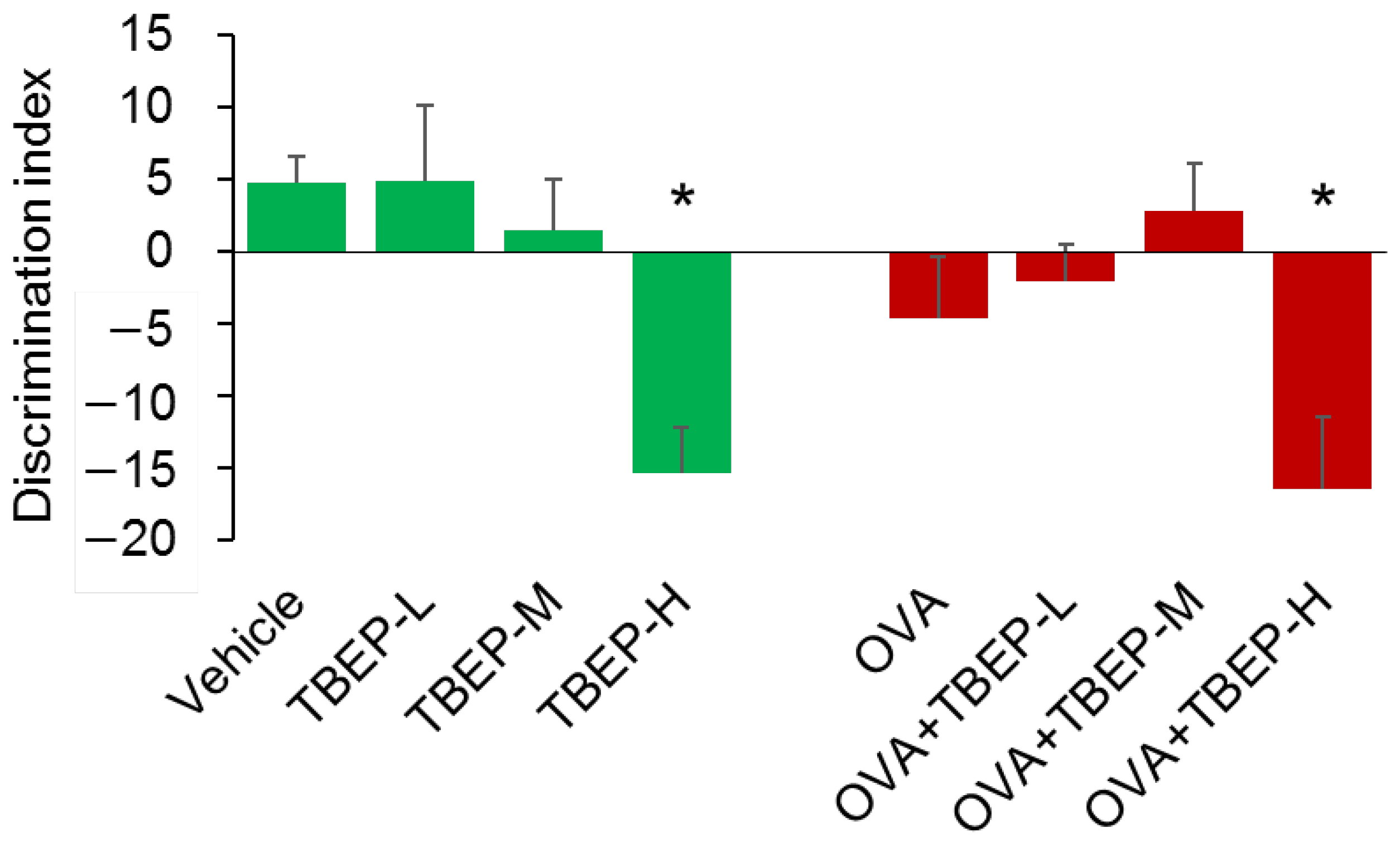
2.3. Effect of TBEP Exposure on Novel Object Recognition Test
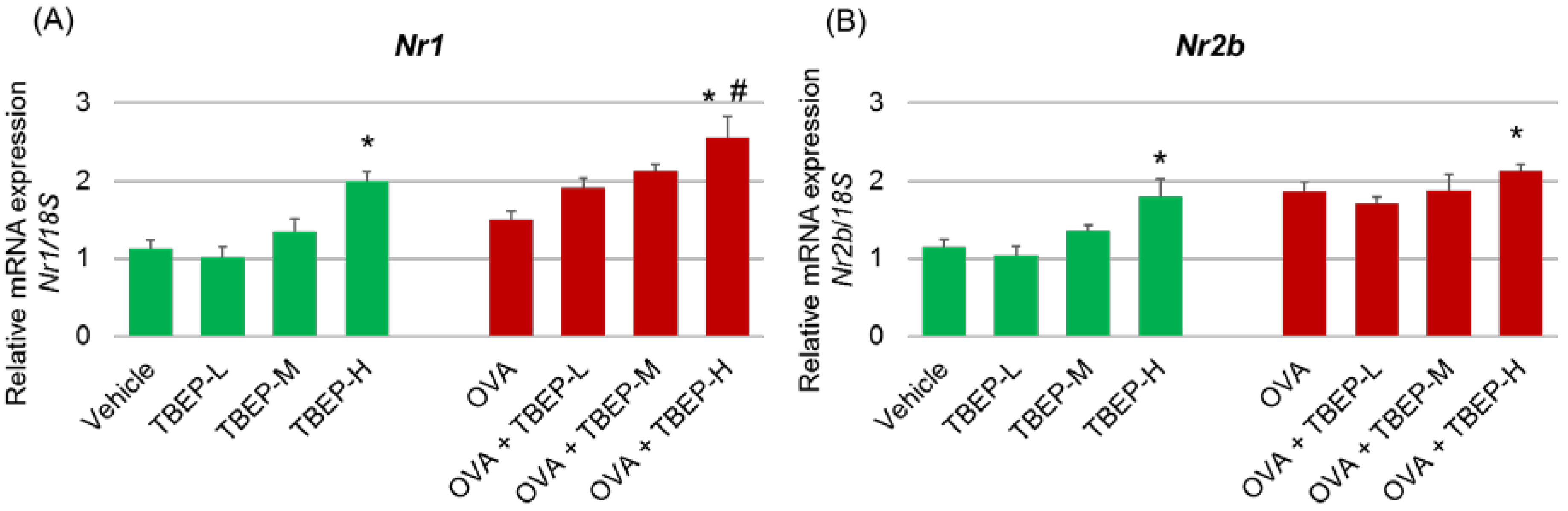
2.4. Effect of TBEP Exposure on Memory Function-Related GeneNmda Receptor Subunits in the Hippocampus
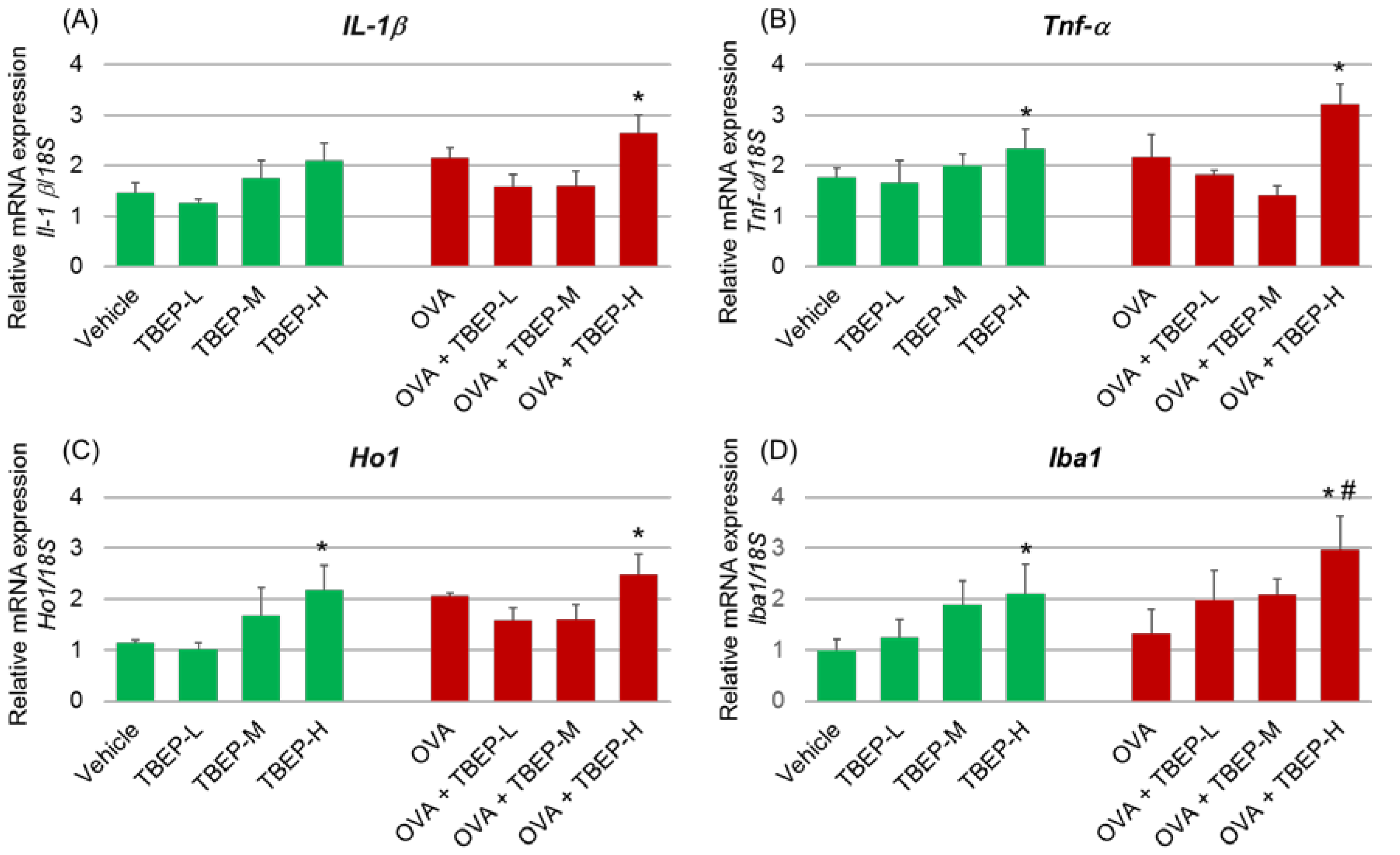
2.5. Effect of TBEP Exposure on Inflammatory Molecular Markers in the Hippocampus
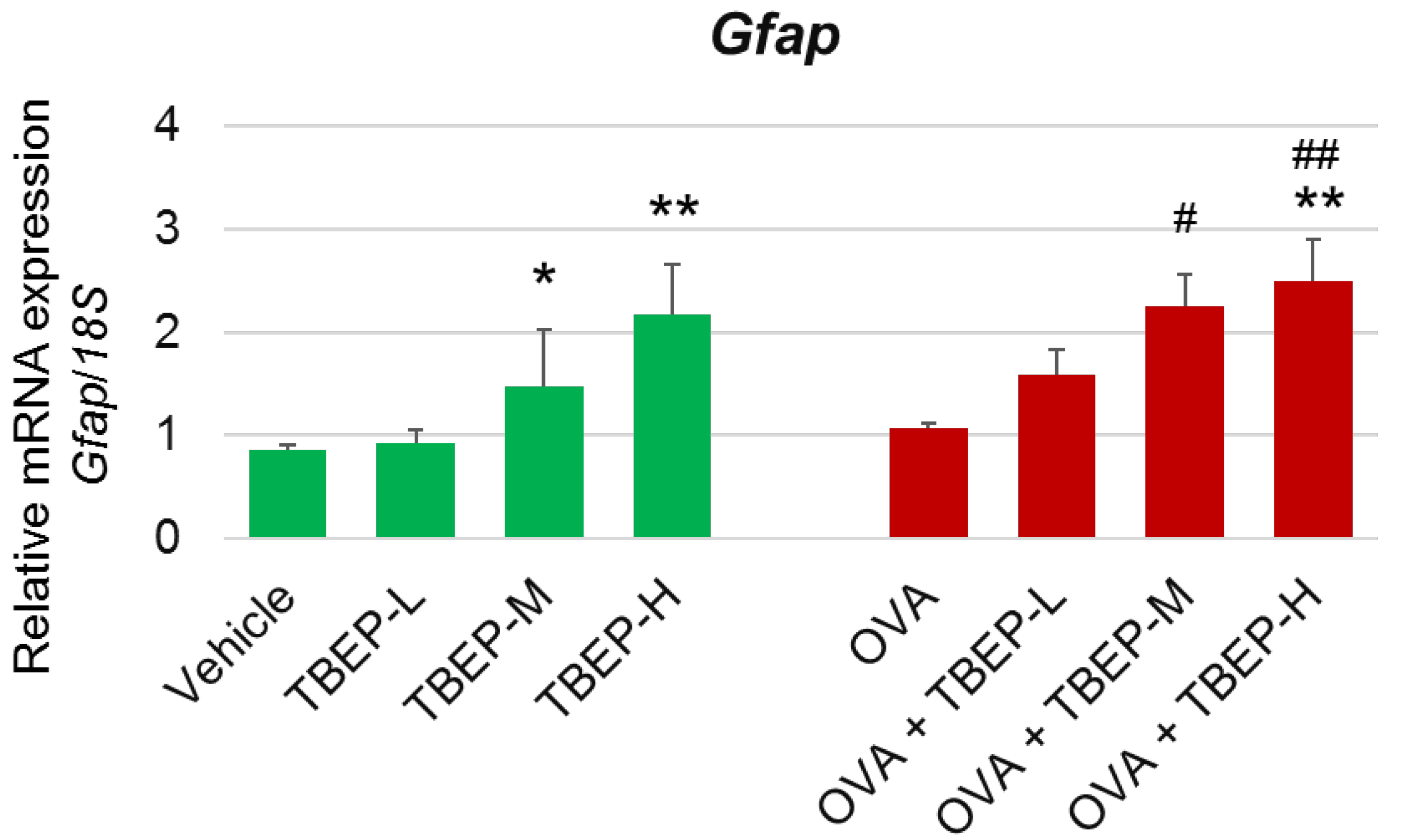
2.6. Effect of TBEP Exposure on Astrocyte Marker Gfap in the Hippocampus
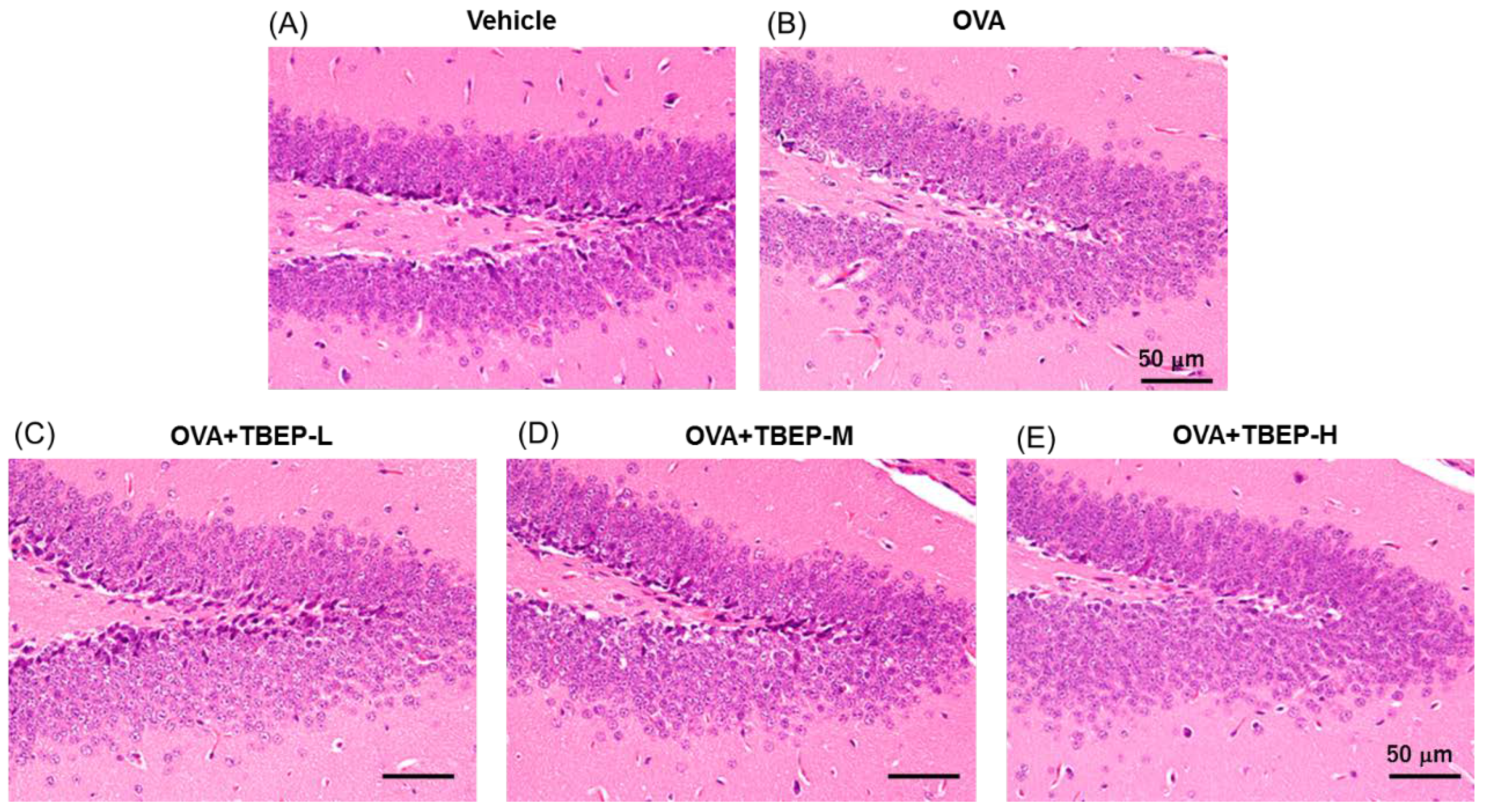
2.7. Effect of TBEP Exposure on Histological Changes in the Hippocampus
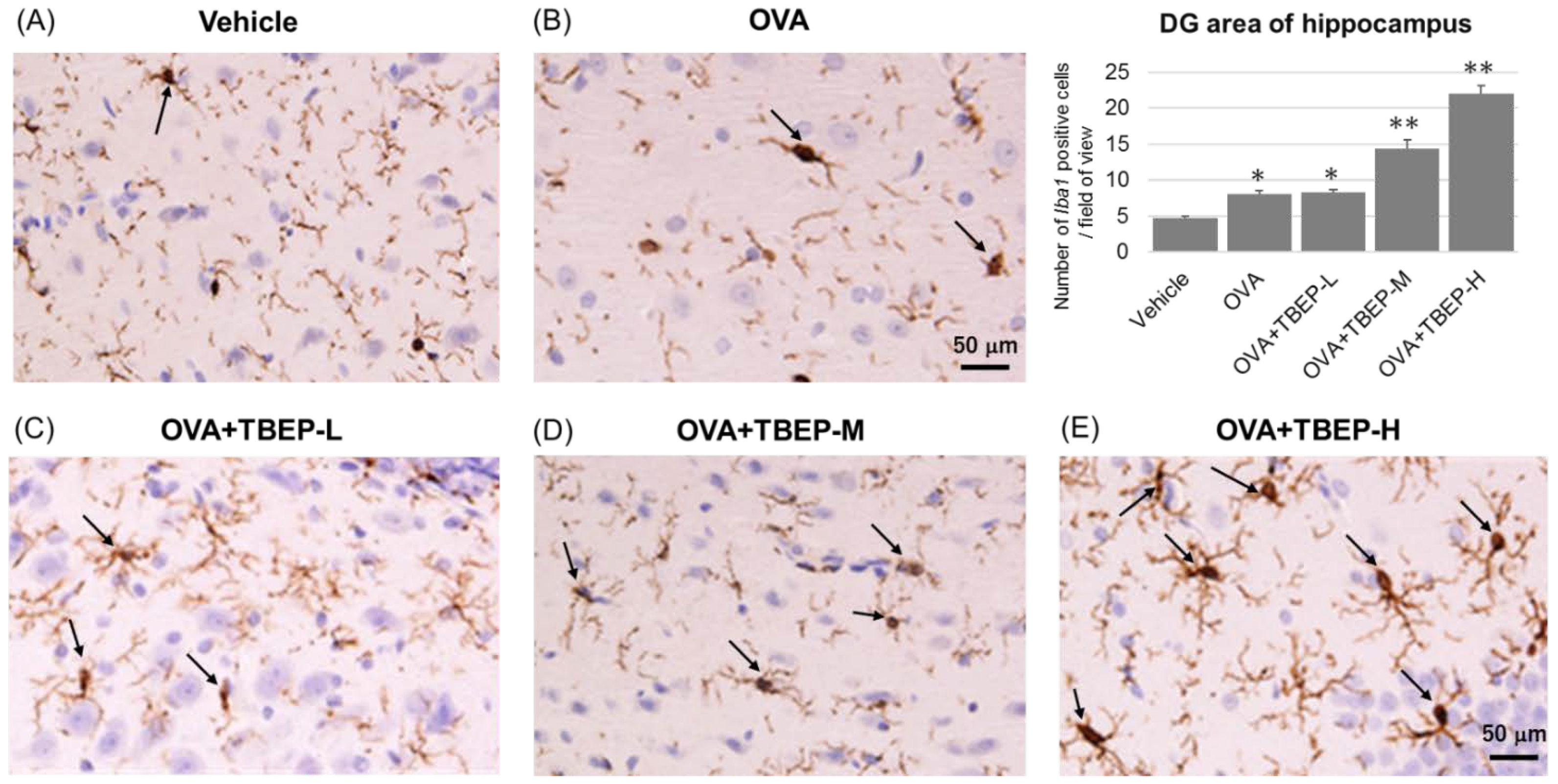
2.8. Effect of TBEP Exposure on Microglia Marker Iba1 Immunoreactivity in the Hippocampus
2.9. Effect of TBEP Exposure on Mast Cell Expression in the Hippocampus
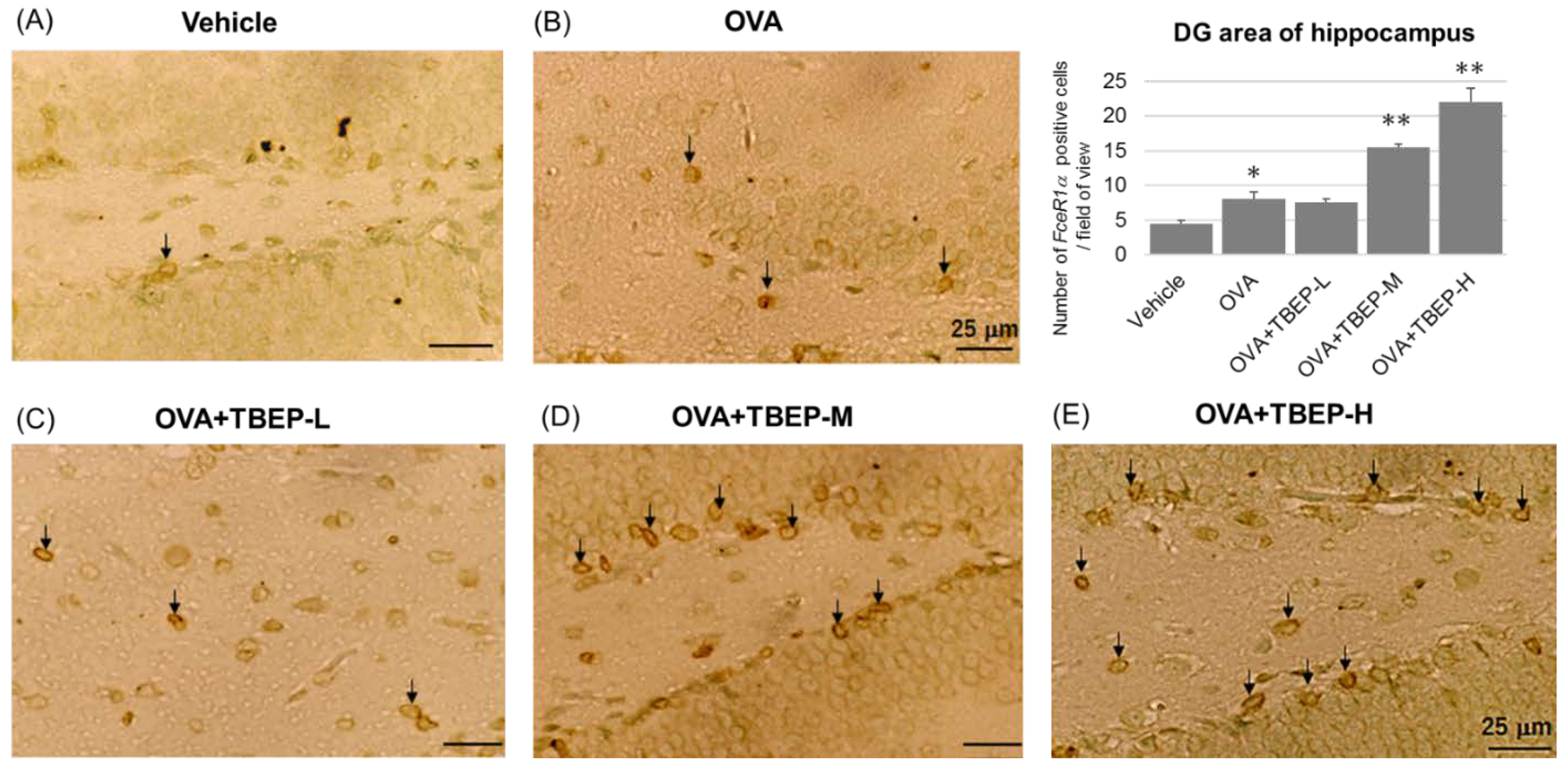
2.10. Effect of TBEP Exposure on Fc Epsilon R1a (FcεR1α) Expression in Mast Cell in the Hippocampus
3. Discussion
4. Materials and Methods
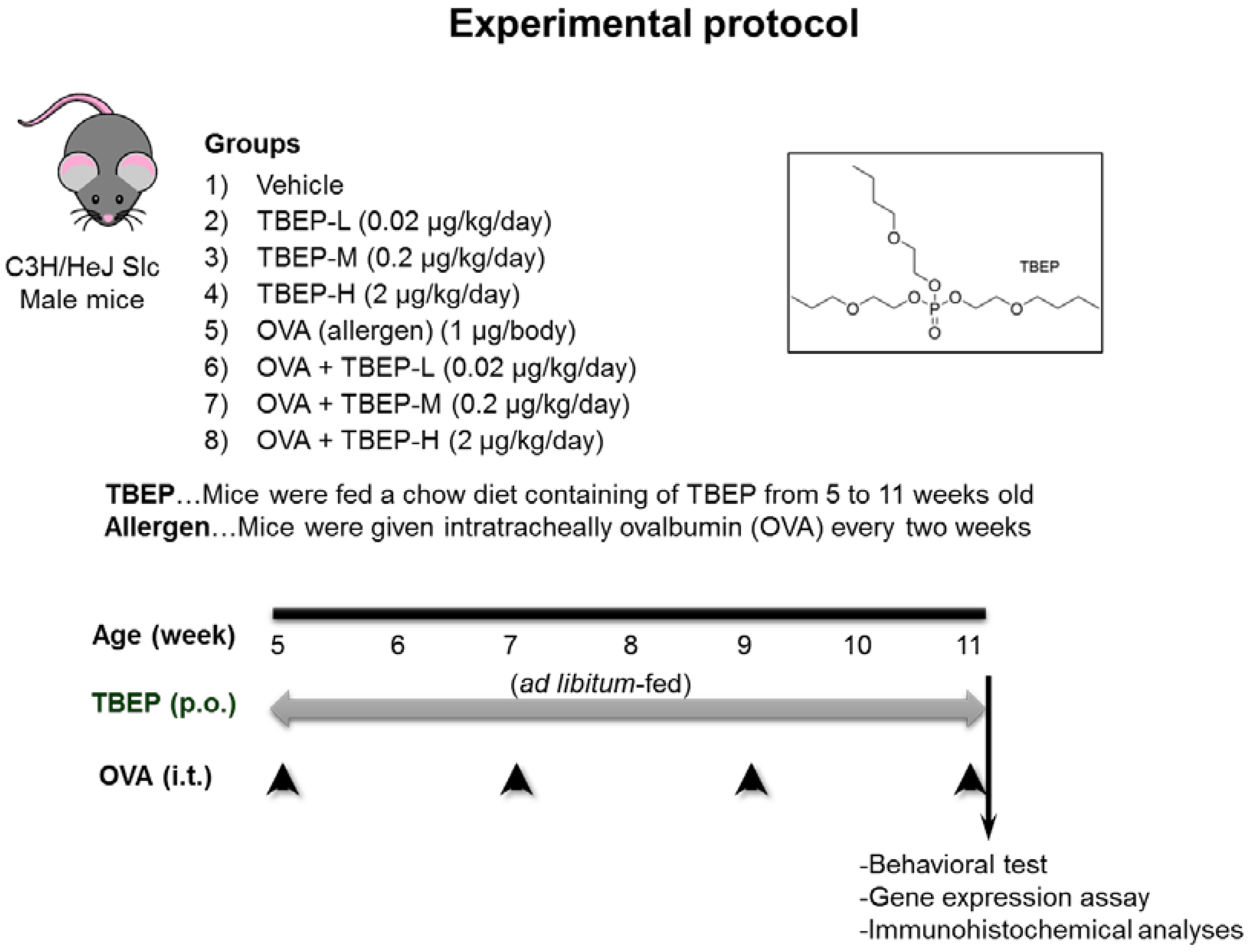
4.1. Animals
4.2. Novel Object Recognition Test
4.3. Quantification of mRNA Expression Levels
4.4. Immunohistochemical Analyses
4.5. Mast Cell Staining
4.6. Immunostaining of Brain Sections with Fc Epsilon R1α (FcεR1α)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TBEP | Tris (2-butoxyethyl) phosphate |
| OPFR | organophosphate flame retardant |
| FR | flame retardant |
| OVA | ovalbumin |
| BAL | bronchoalveolar |
| Iba-1 | ionized calcium-binding adapter molecule-1 |
| Nmda | N-methyl-D aspartate |
| Ampa | α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid |
| Il-1β | interleukin 1β |
| Tnf-α | tumor necrosis factor α |
| Ho1 | heme oxygenase 1 |
| Cox2 | cyclooxygenase 2 |
| BPA | bisphenol A |
| DEHP | di(2-ethylhexyl) phthalate |
| TCEP | tris(2-chloroethyl)phosphate |
| TBBPA | tetrabromobisphenol A |
| TDCPP | tris (1,3-dichloro-2-propyl) phosphate |
| BDE-47 | tetrabromodiphenyl ether |
| TPHP | triphenyl phosphate |
| Htr1α 5 | hydroxytryptamine receptor 1A |
| Adra1α | alpha-adrenergic receptor |
| FcεR1α | Fc epsilon R1α |
References
- Dishaw, L.; Powers, C.M.; Ryde, I.T.; Roberts, S.C.; Seidler, F.J.; Slotkin, T.A.; Stapleton, H.M. Is the PentaBDE replacement, tris (1,3-dichloro-2-propyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol. Appl. Pharmacol. 2011, 256, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Lam, J.C.; Man, Y.-C.; Lai, N.L.-S.; Kwok, K.Y.; Guo, Y.Y.; Lam, P.K.-S.; Zhou, B. Bioconcentration, metabolism and neurotoxicity of the organophorous flame retardant 1,3-dichloro 2-propyl phosphate (TDCPP) to zebrafish. Aquat. Toxicol. 2015, 158, 108–115. [Google Scholar] [CrossRef]
- Yuan, L.; Li, J.; Zha, J.; Wang, Z. Targeting neurotrophic factors and their receptors, but not cholinesterase or neurotransmitter, in the neurotoxicity of TDCPP in Chinese rare minnow adults (Gobiocypris rarus). Environ. Pollut. 2016, 208, 670–677. [Google Scholar] [CrossRef]
- Freudenthal, R.I.; Henrich, R.T. Chronic Toxicity and Carcinogenic Potential of Tris-(1,3-Dichloro-2-propyl) Phosphate in Sprague-Dawley Rat. Int. J. Toxicol. 2000, 19, 119–125. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Wang, X.; Thai, P.; Baduel, C.; Gallen, C.; Banks, A.; Bainton, P.; English, K.; Mueller, J. Organophosphate and brominated flame retardants in Australian indoor environments: Levels, sources, and preliminary assessment of human exposure. Environ. Pollut. 2018, 235, 670–679. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, X.; Tang, S.; Thai, P.; Li, Z.; Baduel, C.; Mueller, J.F. Concentrations of organophosphate esters and their specific metabolites in food in southeast Queensland, Australia: Is dietary exposure an important pathway of organophosphate esters and their metabolites? Environ. Sci. Technol. 2018, 52, 12765–12773. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.-L.; Li, D.-Q.; Zhuo, M.-N.; Liao, Y.-S.; Xie, Z.-Y.; Guo, T.-L.; Li, J.-J.; Zhang, S.-Y.; Liang, Z.-Q. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environ. Pollut. 2015, 196, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Carrington, C.D.; Lapadula, D.M.; Othman, M.; Farr, C.; Nair, R.S.; Johannsen, F.; Abou-Donia, M.B. Assessment of the Delayed Neurotoxicity of Tributyl Phosphate, Tributoxyethyl Phosphate, and Dibutylphenyl Phosphate. Toxicol. Ind. Health 1990, 6, 415–423. [Google Scholar] [CrossRef]
- Sun, L.; Xu, W.; Peng, T.; Chen, H.; Ren, L.; Tan, H.; Xiao, D.; Qian, H.; Fu, Z. Developmental exposure of zebrafish larvae to organophosphate flame retardants causes neurotoxicity. Neurotoxicol. Teratol. 2016, 55, 16–22. [Google Scholar] [CrossRef]
- Sun, L.; Tan, H.; Peng, T.; Wang, S.; Xu, W.; Qian, H.; Jin, Y.; Fu, Z. Developmental neurotoxicity of organophosphate flame retardants in early life stages of Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 2016, 35, 2931–2940. [Google Scholar] [CrossRef]
- Yanagisawa, R.; Koike, E.; Win-Shwe, T.; Kawaguchi, M.; Takano, H. The impact of oral exposure to low-dose tris(2-butoxyethyl) phosphate in allergic asthmatic mice. J. Appl. Toxicol. 2020, 40, 1498–1510. [Google Scholar] [CrossRef]
- Win-Shwe, T.-T.; Yamamoto, S.; Nakajima, D.; Furuyama, A.; Fukushima, A.; Ahmed, S.; Goto, S.; Fujimaki, H. Modulation of neurological related allergic reaction in mice exposed to low-level toluene. Toxicol. Appl. Pharmacol. 2007, 222, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Win-Shwe, T.-T.; Yanagisawa, R.; Koike, E.; Nitta, H.; Takano, H. Expression levels of neuroimmune biomarkers in hypothalamus of allergic mice after phthalate exposure. J. Appl. Toxicol. 2012, 33, 1070–1078. [Google Scholar] [CrossRef]
- Win-Shwe, T.-T.; Yanagisawa, R.; Koike, E.; Takano, H. Memory Function, Neurological, and Immunological Biomarkers in Allergic Asthmatic Mice Intratracheally Exposed to Bisphenol A. Int. J. Environ. Res. Public Health 2019, 16, 3770. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.; Feng, M.; Long, Z.; Ma, J.; Peng, X.; He, G. Allergic Asthma-Induced Cognitive Impairment is Alleviated by Dexamethasone. Front. Pharmacol. 2021, 12, 680815. [Google Scholar] [CrossRef]
- Whitlock, J.R.; Heynen, A.J.; Shuler, M.G.; Bear, M.F. Learning induces long-term potentiation in the hippocampus. Science 2006, 313, 1093–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Zhao, F.; Fang, Y.; Li, L.; Li, C.; Ta, N. 1H-nuclear magnetic resonance metabolomics revealing the intrinsic relationships between neurochemical alterations and neurobehavioral and neuropathological abnormalities in rats exposed to tris(2-chloroethyl)phosphate. Chemosphere 2018, 200, 649–659. [Google Scholar] [CrossRef]
- Diamandakis, D.; Zieminska, E.; Siwiec, M.; Tokarski, K.; Salinska, E.; Lenart, J.; Hess, G.; Lazarewicz, J.W. Tetrabromobisphenol A-induced depolarization of rat cerebellar granule cells: Ex vivo and in vitro studies. Chemosphere 2019, 223, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, F.; Karakoyun, I.; Sutcu, R.; Savik, E.; Cesur, G.; Orhan, H.; Delibas, N. Chlorpyrifos increases the levels of hippocampal nmda receptor subunits nr2a and nr2b in juvenile and adult rats. Int. J. Neurosci. 2007, 117, 47–62. [Google Scholar] [CrossRef]
- Zhong, X.; Wu, J.; Ke, W.; Yu, Y.; Ji, D.; Kang, J.; Qiu, J.; Wang, C.; Yu, P.; Wei, Y. Neonatal exposure to organophosphorus flame retardant TDCPP elicits neurotoxicity in mouse hippocampus via microglia-mediated inflammation in vivo and in vitro. Arch. Toxicol. 2020, 94, 541–552. [Google Scholar] [CrossRef]
- Costa, L.G.; Pellacani, C.; Dao, K.; Kavanagh, T.J.; Roque, P.J. The brominated flame retardant BDE-47 causes oxidative stress and apoptotic cell death in vitro and in vivo in mice. NeuroToxicology 2015, 48, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- da Silva, E.Z.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Boyce, J.A. Mast cells and eicosanoid mediators: A system of reciprocal paracrine and autocrine regulation. Immunol. Rev. 2007, 217, 168–185. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, H.; Wang, Y.; Zhang, X.; Zhang, X.; Qian, Y.; Ding, H.; Zhang, S. Stabilization of Brain Mast Cells Alleviates LPS-Induced Neuroinflammation by Inhibiting Microglia Activation. Front. Cell. Neurosci. 2019, 13, 191. [Google Scholar] [CrossRef]
- Koike, E.; Yanagisawa, R.; Win-Shwe, T.-T.; Takano, H. Exposure to low-dose bisphenol A during the juvenile period of development disrupts the immune system and aggravates allergic airway inflammation in mice. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418774897. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, R.; Koike, E.; Win-Shwe, T.-T.; Takano, H. Oral exposure to low dose bisphenol A aggravates allergic airway inflammation in mice. Toxicol. Rep. 2019, 6, 1253–1262. [Google Scholar] [CrossRef]
- Win-Shwe, T.-T.; Yanagisawa, R.; Koike, E.; Takano, H. Dietary exposure to bisphenol A affects memory function and neuroimmune biomarkers in allergic asthmatic mice. J. Appl. Toxicol. 2021, 41, 1527–1536. [Google Scholar] [CrossRef]
- Zhong, X.; Yu, Y.; Wang, C.; Zhu, Q.; Wu, J.; Ke, W.; Ji, D.; Niu, C.; Yang, X.; Wei, Y. Hippocampal proteomic analysis reveals the disturbance of synaptogenesis and neurotransmission induced by developmental exposure to organophosphate flame retardant triphenyl phosphate. J Hazard Mater. 2021, 404 Pt B, 124111. [Google Scholar] [CrossRef]
- Reier, P.J. Gliosis following CNS injury: The anatomy of astrocytic scars and their influences on axonal elongation. Astrocytes 1986, 3, 263–324. [Google Scholar]
- Tani, M.; Glabinski, A.R.; Tuohy, V.K.; Stoler, M.H.; Estes, M.L.; Ransohoff, R.M. In situ hybridization analysis of glial fibrillary acidic protein mRNA reveals evidence of biphasic astrocyte activation during acute experimental autoimmune encephalomyelitis. Am. J. Pathol. 1996, 148, 889–896. [Google Scholar]
- Eng, L.F.; Ghirnikar, R.S. GFAP and Astrogliosis. Brain Pathol. 1994, 4, 229–237. [Google Scholar] [CrossRef] [PubMed]
- BAG (Swiss Federal Health Ministry). Phosphoabasierte Flammschutzmittel in der Innenraumluft–Schlussbericht; Phosphorus-Based Flame Retardants in Indoor Air—Final Report; BAG: Zurich, Switzerland, 24 June 2002. [Google Scholar]
- Win-Shwe, T.-T.; Fujitani, Y.; Kyi-Tha-Thu, C.; Furuyama, A.; Michikawa, T.; Tsukahara, S.; Nitta, H.; Hirano, S. Effects of Diesel Engine Exhaust Origin Secondary Organic Aerosols on Novel Object Recognition Ability and Maternal Behavior in BALB/C Mice. Int. J. Environ. Res. Public Health 2014, 11, 11286–11307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dix, S.L.; Aggleton, J. Extending the spontaneous preference test of recognition: Evidence of object-location and object-context recognition. Behav. Brain Res. 1999, 99, 191–200. [Google Scholar] [CrossRef]
- Win-Shwe, T.-T.; Nway, N.C.; Imai, M.; Lwin, T.-T.; Mar, O.; Watanabe, H. Social behavior, neuroimmune markers and glutamic acid decarboxylase levels in a rat model of valproic acid-induced autism. J. Toxicol. Sci. 2018, 43, 631–643. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Win-Shwe, T.-T.; Yanagisawa, R.; Lwin, T.-T.; Kawakami, F.; Koike, E.; Takano, H. Dietary Exposure to Flame Retardant Tris (2-Butoxyethyl) Phosphate Altered Neurobehavior and Neuroinflammatory Responses in a Mouse Model of Allergic Asthma. Int. J. Mol. Sci. 2022, 23, 655. https://doi.org/10.3390/ijms23020655
Win-Shwe T-T, Yanagisawa R, Lwin T-T, Kawakami F, Koike E, Takano H. Dietary Exposure to Flame Retardant Tris (2-Butoxyethyl) Phosphate Altered Neurobehavior and Neuroinflammatory Responses in a Mouse Model of Allergic Asthma. International Journal of Molecular Sciences. 2022; 23(2):655. https://doi.org/10.3390/ijms23020655
Chicago/Turabian StyleWin-Shwe, Tin-Tin, Rie Yanagisawa, Thet-Thet Lwin, Fumitaka Kawakami, Eiko Koike, and Hirohisa Takano. 2022. "Dietary Exposure to Flame Retardant Tris (2-Butoxyethyl) Phosphate Altered Neurobehavior and Neuroinflammatory Responses in a Mouse Model of Allergic Asthma" International Journal of Molecular Sciences 23, no. 2: 655. https://doi.org/10.3390/ijms23020655
APA StyleWin-Shwe, T.-T., Yanagisawa, R., Lwin, T.-T., Kawakami, F., Koike, E., & Takano, H. (2022). Dietary Exposure to Flame Retardant Tris (2-Butoxyethyl) Phosphate Altered Neurobehavior and Neuroinflammatory Responses in a Mouse Model of Allergic Asthma. International Journal of Molecular Sciences, 23(2), 655. https://doi.org/10.3390/ijms23020655





