Diagnostic Accuracy of CT Texture Analysis in Adrenal Masses: A Systematic Review
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Search Strategy
4.2. Review Protocol and Data Extraction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terzolo, M.; Stigliano, A.; Chiodini, I.; Loli, P.; Furlani, L.; Arnaldi, G.; Reimondo, G.; Pia, A.; Toscano, V.; Zini, M.; et al. AME Position Statement on Adrenal Incidentaloma. Eur. J. Endocrinol. 2011, 164, 851–870. [Google Scholar] [CrossRef]
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; de Krijger, R.R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European Society of Endocrinology Clinical Practice Guidelines on the Management of Adrenocortical Carcinoma in Adults, in Collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018, 179, G1–G46. [Google Scholar] [CrossRef]
- Papanicolas, I.; Woskie, L.R.; Jha, A.K. Health Care Spending in the United States and Other High-Income Countries. JAMA 2018, 319, 1024–1039. [Google Scholar] [CrossRef]
- Lenders, J.W.M.; Duh, Q.-Y.; Eisenhofer, G.; Gimenez-Roqueplo, A.-P.; Grebe, S.K.G.; Murad, M.H.; Naruse, M.; Pacak, K.; Young, W.F. Pheochromocytoma and Paraganglioma: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2014, 99, 1915–1942. [Google Scholar] [CrossRef]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef]
- Ebbehoj, A.; Li, D.; Kaur, R.J.; Zhang, C.; Singh, S.; Li, T.; Atkinson, E.; Achenbach, S.; Khosla, S.; Arlt, W.; et al. Epidemiology of Adrenal Tumours in Olmsted County, Minnesota, USA: A Population-Based Cohort Study. Lancet Diabetes Endocrinol. 2020, 8, 894–902. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Zhang, R.; Bai, Y.; Li, C.; Li, B.; Liu, H.; Zhang, T. CT and MRI of Adrenal Gland Pathologies. Quant. Imaging Med. Surg. 2018, 8, 853–875. [Google Scholar] [CrossRef]
- van den Broek, J.; Geenen, R.; Heijnen, L.; Kobus, C.; Schreurs, H. Adrenal Incidentalomas During Diagnostic Work-up of Colorectal Cancer Patients: What Is the Risk of Metastases? Ann. Surg. Oncol. 2018, 25, 1986–1991. [Google Scholar] [CrossRef]
- Boland, G.W.; Lee, M.J.; Gazelle, G.S.; Halpern, E.F.; McNicholas, M.M.; Mueller, P.R. Characterization of Adrenal Masses Using Unenhanced CT: An Analysis of the CT Literature. Am. J. Roentgenol. 1998, 171, 201–204. [Google Scholar] [CrossRef]
- Ceccato, F.; Barbot, M.; Scaroni, C.; Boscaro, M. Frequently Asked Questions and Answers (If Any) in Patients with Adrenal Incidentaloma. J. Endocrinol. Investig. 2021, 44, 2749–2763. [Google Scholar] [CrossRef]
- Peña, C.S.; Boland, G.W.L.; Hahn, P.F.; Lee, M.J.; Mueller, P.R. Characterization of Indeterminate (Lipid-Poor) Adrenal Masses: Use of Washout Characteristics at Contrast-Enhanced CT. Radiology 2000, 217, 798–802. [Google Scholar] [CrossRef]
- Bancos, I.; Tamhane, S.; Shah, M.; Delivanis, D.A.; Alahdab, F.; Arlt, W.; Fassnacht, M.; Murad, M.H. DIAGNOSIS OF ENDOCRINE DISEASE: The Diagnostic Performance of Adrenal Biopsy: A Systematic Review and Meta-Analysis. Eur. J. Endocrinol. 2016, 175, R65–R80. [Google Scholar] [CrossRef]
- Lubner, M.G.; Smith, A.D.; Sandrasegaran, K.; Sahani, D.V.; Pickhardt, P.J. CT Texture Analysis: Definitions, Applications, Biologic Correlates, and Challenges. RadioGraphics 2017, 37, 1483–1503. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The Bridge between Medical Imaging and Personalized Medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, G.M.Y.; Xu, M.; Jin, Z.Y.; Sun, H. Distinguishing Metastases from Benign Adrenal Masses: What Can CT Texture Analysis Do? Acta Radiol. 2019, 60, 1553–1561. [Google Scholar] [CrossRef]
- Yu, H.; Parakh, A.; Blake, M.; McDermott, S. Texture Analysis as a Radiomic Marker for Differentiating Benign from Malignant Adrenal Tumors. J. Comput. Assist. Tomogr. 2020, 44, 766–771. [Google Scholar] [CrossRef]
- Elmohr, M.M.; Fuentes, D.; Habra, M.A.; Bhosale, P.R.; Qayyum, A.A.; Gates, E.; Morshid, A.I.; Hazle, J.D.; Elsayes, K.M. Machine Learning-Based Texture Analysis for Differentiation of Large Adrenal Cortical Tumours on CT. Clin. Radiol. 2019, 74, 818.e1–818.e7. [Google Scholar] [CrossRef]
- Andersen, M.B.; Bodtger, U.; Andersen, I.R.; Thorup, K.S.; Ganeshan, B.; Rasmussen, F. Metastases or Benign Adrenal Lesions in Patients with Histopathological Verification of Lung Cancer: Can CT Texture Analysis Distinguish? Eur. J. Radiol. 2021, 138, 109664. [Google Scholar] [CrossRef]
- Li, X.; Guindani, M.; Ng, C.S.; Hobbs, B.P. A Bayesian Nonparametric Model for Textural Pattern Heterogeneity. J. R. Stat. Soc. Ser. C Appl. Stat. 2021, 70, 459–480. [Google Scholar] [CrossRef]
- Moawad, A.W.; Ahmed, A.; Fuentes, D.T.; Hazle, J.D.; Habra, M.A.; Elsayes, K.M. Machine Learning-Based Texture Analysis for Differentiation of Radiologically Indeterminate Small Adrenal Tumors on Adrenal Protocol CT Scans. Abdom. Radiol. 2021, 46, 4853–4863. [Google Scholar] [CrossRef]
- Torresan, F.; Crimì, F.; Ceccato, F.; Zavan, F.; Barbot, M.; Lacognata, C.; Motta, R.; Armellin, C.; Scaroni, C.; Quaia, E.; et al. Radiomics: A New Tool to Differentiate Adrenocortical Adenoma from Carcinoma. BJS Open 2021, 5, zraa061. [Google Scholar] [CrossRef]
- Ho, L.M.; Samei, E.; Mazurowski, M.A.; Zheng, Y.; Allen, B.C.; Nelson, R.C.; Marin, D. Can Texture Analysis Be Used to Distinguish Benign from Malignant Adrenal Nodules on Unenhanced CT, Contrast-Enhanced CT, or in-Phase and Opposed-Phase MRI? Am. J. Roentgenol. 2019, 212, 554–561. [Google Scholar] [CrossRef]
- Shoemaker, K.; Hobbs, B.P.; Bharath, K.; Ng, C.S.; Baladandayuthapani, V. Tree-Based Methods for Characterizing Tumor Density Heterogeneity. Pac. Symp. Biocomput. 2018, 23, 216–227. [Google Scholar]
- Voltan, G.; Boscaro, M.; Armanini, D.; Scaroni, C.; Ceccato, F. A Multidisciplinary Approach to the Management of Adrenal Incidentaloma. Expert Rev. Endocrinol. Metab. 2021, 16, 201–212. [Google Scholar] [CrossRef]
- Fanelli, F.; di Dalmazi, G. Serum Steroid Profiling by Mass Spectrometry in Adrenocortical Tumors: Diagnostic Implications. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 160–165. [Google Scholar] [CrossRef]
- Arlt, W.; Biehl, M.; Taylor, A.E.; Hahner, S.; Libé, R.; Hughes, B.A.; Schneider, P.; Smith, D.J.; Stiekema, H.; Krone, N.; et al. Urine Steroid Metabolomics as a Biomarker Tool for Detecting Malignancy in Adrenal Tumors. J. Clin. Endocrinol. Metab. 2011, 96, 3775–3784. [Google Scholar] [CrossRef]
- Bancos, I.; Taylor, A.E.; Chortis, V.; Sitch, A.J.; Jenkinson, C.; Davidge-Pitts, C.J.; Lang, K.; Tsagarakis, S.; Macech, M.; Riester, A.; et al. Urine Steroid Metabolomics for the Differential Diagnosis of Adrenal Incidentalomas in the EURINE-ACT Study: A Prospective Test Validation Study. Lancet Diabetes Endocrinol. 2020, 8, 773–781. [Google Scholar] [CrossRef]
- Marty, M.; Gaye, D.; Perez, P.; Auder, C.; Nunes, M.L.; Ferriere, A.; Haissaguerre, M.; Tabarin, A. Diagnostic Accuracy of Computed Tomography to Identify Adenomas among Adrenal Incidentalomas in an Endocrinological Population. Eur. J. Endocrinol. 2018, 178, 439–446. [Google Scholar] [CrossRef]
- Schalin-Jäntti, C.; Raade, M.; Hämäläinen, E.; Sane, T. A 5-Year Prospective Follow-Up Study of Lipid-Rich Adrenal Incidentalomas: No Tumor Growth or Development of Hormonal Hypersecretion. Endocrinol. Metab. 2015, 30, 481–487. [Google Scholar] [CrossRef]
- Hong, A.R.; Kim, J.H.; Park, K.S.; Kim, K.Y.; Lee, J.H.; Kong, S.H.; Lee, S.Y.; Shin, C.S.; Kim, S.W.; Kim, S.Y. Optimal Follow-up Strategies for Adrenal Incidentalomas: Reappraisal of the 2016 ESE-ENSAT Guidelines in Real Clinical Practice. Eur. J. Endocrinol. 2017, 177, 475–483. [Google Scholar] [CrossRef]
- Bhandari, A.; Ibrahim, M.; Sharma, C.; Liong, R.; Gustafson, S.; Prior, M. CT-Based Radiomics for Differentiating Renal Tumours: A Systematic Review. Abdom. Radiol. 2021, 46, 2052–2063. [Google Scholar] [CrossRef]
- Thawani, R.; McLane, M.; Beig, N.; Ghose, S.; Prasanna, P.; Velcheti, V.; Madabhushi, A. Radiomics and Radiogenomics in Lung Cancer: A Review for the Clinician. Lung Cancer 2018, 115, 34–41. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Piana, M.; Schenone, D.; Lai, R.; Massone, A.M.; Houssami, N. Overview of Radiomics in Breast Cancer Diagnosis and Prognostication. Breast 2020, 49, 74–80. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Oloko, A.; Talreja, H.; Davis, A.; McCormick, B.; Clark, E.; Akbari, A.; Kong, J.; Hiremath, S. Does Iodinated Contrast Affect Residual Renal Function in Dialysis Patients? A Systematic Review and Meta-Analysis. Nephron 2020, 144, 176–184. [Google Scholar] [CrossRef]
- Shams, E.; Mayrovitz, H.N. Contrast-Induced Nephropathy: A Review of Mechanisms and Risks. Cureus 2021, 13, e14842. [Google Scholar] [CrossRef]
- Mantero, F.; Terzolo, M.; Arnaldi, G.; Osella, G.; Masini, A.M.; Alì, A.; Giovagnetti, M.; Opocher, G.; Angeli, A. A Survey on Adrenal Incidentaloma in Italy. J. Clin. Endocrinol. Metab. 2000, 85, 637–644. [Google Scholar] [CrossRef]
- Espinasse, M.; Pitre-Champagnat, S.; Charmettant, B.; Bidault, F.; Volk, A.; Balleyguier, C.; Lassau, N.; Caramella, C. CT Texture Analysis Challenges: Influence of Acquisition and Reconstruction Parameters: A Comprehensive Review. Diagnostics 2020, 10, 258. [Google Scholar] [CrossRef]
- Erasmus, J.J.; Patz, E.F.; McAdams, H.P.; Murray, J.G.; Herndon, J.; Coleman, R.E.; Goodman, P.C. Evaluation of adrenal masses in patients with bronchogenic carcinoma using 18F-fluorodeoxyglucose positron emission tomography. AJR Am. J. Roentgenol. 1997, 168, 25–29. [Google Scholar] [CrossRef]
- Maurea, S.; Mainofli, C.; Bazzicalupo, L.; Panico, M.R.; Imparato, C.; Alfano, B.; Ziviello, M.; Salvatore, M. Imaging of adrenal tumors using FDG PET: Comparison of benign and malignant lesions. AJR Am. J. Roentgenol. 1999, 173, 25–29. [Google Scholar] [CrossRef][Green Version]
- Yun, M.; Kim, W.; Alnafisi, N.; Lacorte, L.; Jang, S.; Alavi, A. 18F-FDG PET in characterizing adrenal lesions detected on CT or MRI. J. Nucl. Med. 2001, 42, 1795–1799. [Google Scholar]
- Tenenbaum, F.; Groussin, L.; Foehrenbach, H.; Tissier, F.; Gouya, H.; Bertherat, J.; Dousset, B.; Legmann, P.; Richard, B.; Bertagna, X. 18F-fluorodeoxyglucose positron emission tomography as a diagnostic tool for malignancy of adrenocortical tumours? Preliminary results in 13 consecutive patients. Eur. J. Endocrinol. 2004, 150, 789–792. [Google Scholar] [CrossRef][Green Version]
- Groussin, L.; Bonardel, G.; Silvéra, S.; Tissier, F.; Coste, J.; Abiven, G.; Libé, R.; Bienvenu, M.; Alberini, J.L.; Salenave, S.; et al. 18F-Fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: A prospective study in 77 operated patients. J. Clin. Endocrinol. Metab. 2009, 94, 1713–1722. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Whiting, P.F. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
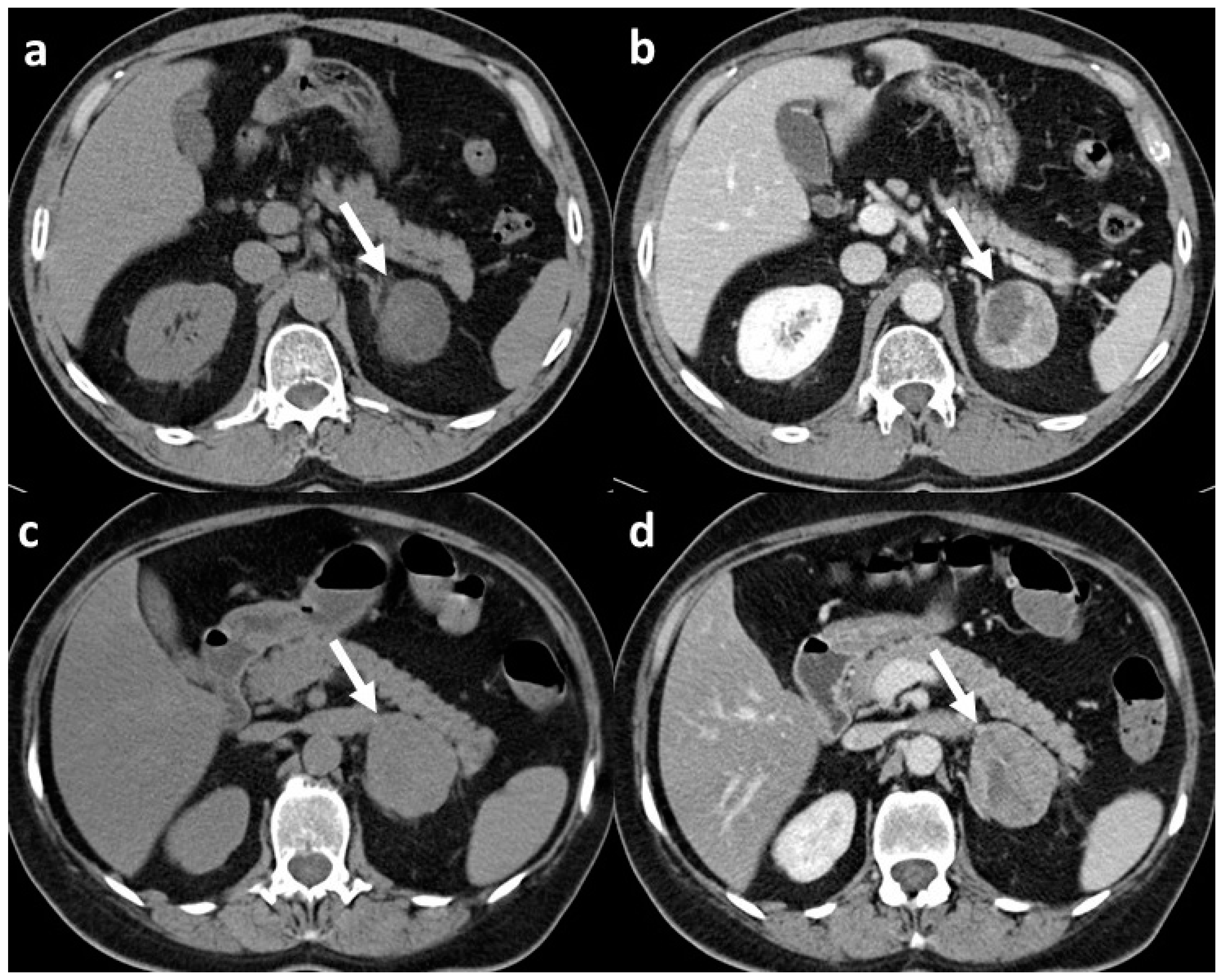
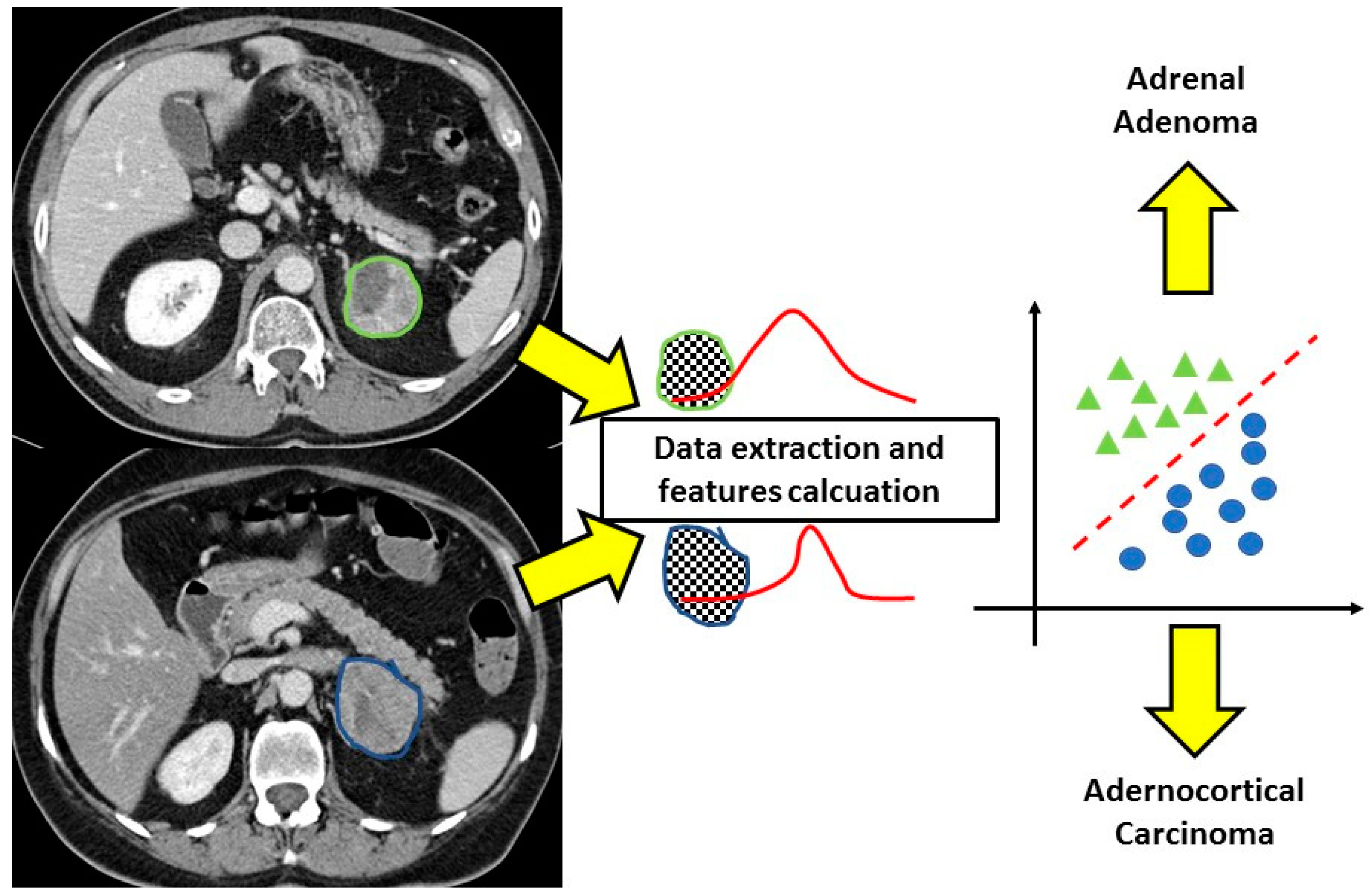
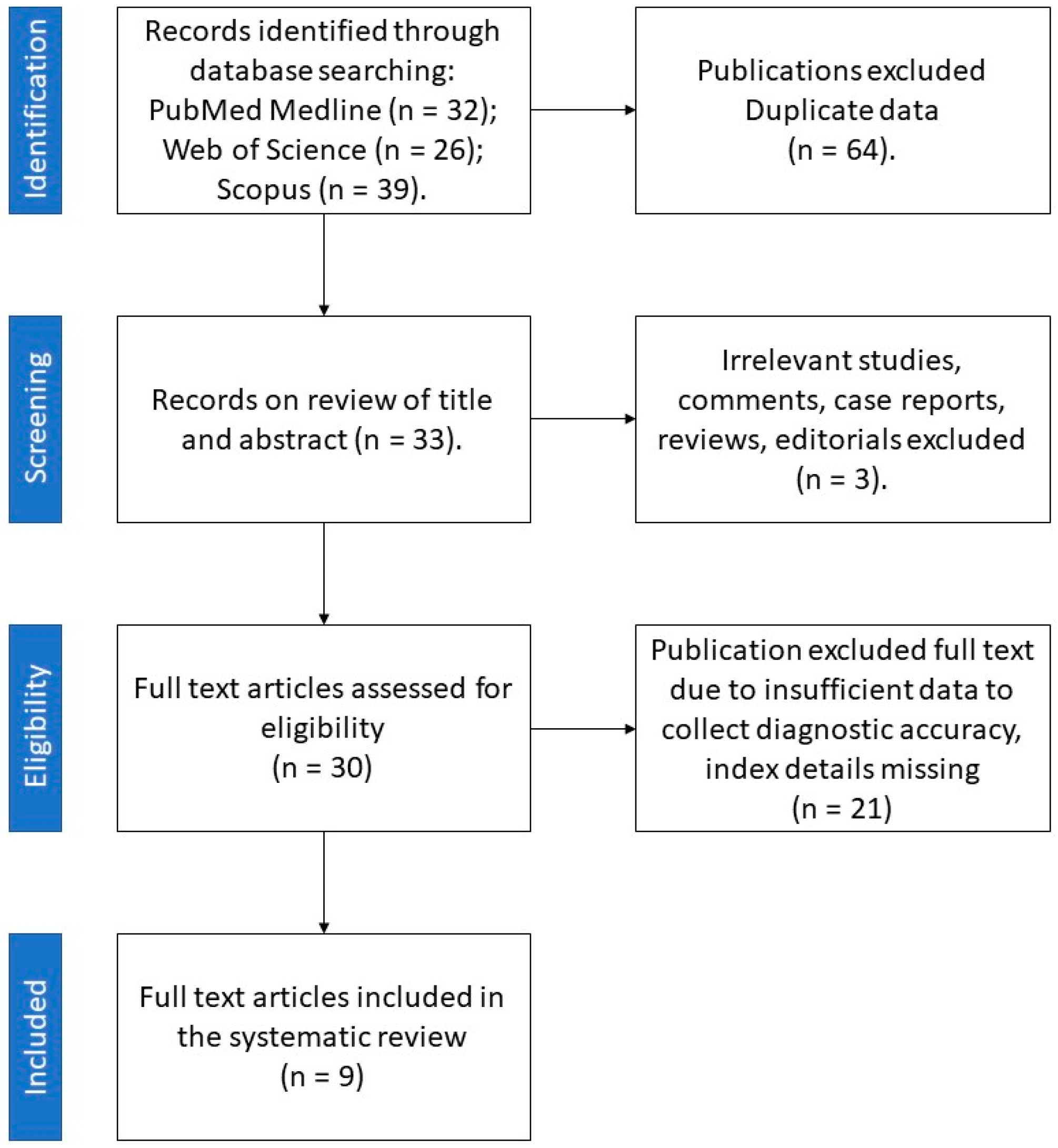
| First Author | Year of Publication | Study Type | Number of Patients | Type of Adrenal Lesions | Unenhanced CT/Contrast-Enhanced CT | Number of Radiologists Involved | Observers Blinded to Clinical/Histopathological Data | Reference Standard | Texture Analysis Software | Spatial Scaling Factors | Number of Textural Features Selected (Unenhanced CT/Contrast-Enhanced CT) | Pooled AUC of the Study (Benign vs. Malignant Lesion) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shi B | 2018 | Observational retrospective | 225 | Lipid poor adenomas, metastases, pheochromocytoma | +/+ | 2 | Yes | Histology | TexRAD | 0, 2, 3, 4, 5, 6 | 5/4 | 0.81 |
| Yu HS | 2020 | Observational retrospective | 125 | Benign (not specified), metastases | −/+ | 1 | Yes | Histology | TexRAD | 0, 2, 3, 4, 5, 6 | 0/36 | 0.89 |
| Elmohr MM | 2019 | Observational retrospective | 54 | Adenomas, adrenocortical carcinomas | +/+ | 2 | Yes | Histology | PyRadiomics | NA | 3/2 | 0.86 |
| Ho LM | 2019 | Observational retrospective | 20 | Lipid poor adenomas, metastases, adrenocortical carcinomas | +/+ | 1 | NA | Histology or radiological features (rapid size increase) | Lesion Tool | NA | 9/18 | 0.85 |
| Shoemaker K | 2018 | Observational retrospective | 356 | Adrenocortical carcinomas, adenomas, benign (not specified), haemorrhages, adrenal hyperplasia, lymphoma, malignant (not specified), metastases, myelolipomas, neurogenic tumours, pheochromocytomas | NA | NA | NA | NA | C++ program | NA | 37 | 0.78 0.93 (functioning vs. non-functioning) 1 (calcified vs. non-calcified) |
| Andersen MB | 2021 | Observational retrospective | 160 | Benign (not specified), metastases of lung tumours (adenocarcinoma, squamous cell carcinoma, neuroendocrine tumours, unclassified NSCLC or SCLC) | −/+ | 2 | Yes | Histology | TexRAD | NA | 0/25 | 0.67 |
| Li X | 2021 | Observational retrospective | 204 | Benign (not specified), malignant (not specified) | +/+ | 1 | NA | Histology | HRGSDP method | NA | 5/5 | NA |
| Moawad AW | 2021 | Observational retrospective | 40 | Benign (not specified), metastases, adrenocortical carcinomas | +/+ | 2 | NA | Histology | PyRadiomics | NA | 1/3 | 0.85 |
| Torresan F | 2021 | Observational retrospective | 30 | Adenomas, benign incidentalomas, adrenocortical carcinomas | +/+ | 2 | Yes | Histology or follow-up | PMOD | NA | 16/24 | NA |
| Risk of Bias | Applicability Concern | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author | Year | Setting | Type of Study | Design | Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard |
| Shoemaker | 2018 | University hospital | Observational | Retrospective | Unclear | Unclear | Low | Unclear | High | Low | Low |
| Elmohr | 2019 | University hospital | Observational | Retrospective | Low | Low | Low | Low | Low | Low | Low |
| Ho | 2019 | University hospital | Observational | Retrospective | Low | Low | High | Low | Low | Low | High |
| Shi | 2019 | University hospital | Observational | Retrospective | Low | Low | Low | Low | Low | Low | Low |
| Yu | 2020 | University hospital | Observational | Retrospective | Low | Low | Low | Low | Low | Low | Low |
| Andersen | 2021 | University hospital | Observational | Retrospective | Low | Low | Low | Low | Low | Low | Low |
| Li | 2021 | University hospital | Observational | Retrospective | Low | Unclear | Low | Low | Low | Unclear | Low |
| Moawad | 2021 | University hospital | Observational | Retrospective | Low | Low | Low | Low | Low | Low | Low |
| Torresan | 2021 | University hospital | Observational | Retrospective | Low | Low | Low | Low | Low | Low | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crimì, F.; Quaia, E.; Cabrelle, G.; Zanon, C.; Pepe, A.; Regazzo, D.; Tizianel, I.; Scaroni, C.; Ceccato, F. Diagnostic Accuracy of CT Texture Analysis in Adrenal Masses: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 637. https://doi.org/10.3390/ijms23020637
Crimì F, Quaia E, Cabrelle G, Zanon C, Pepe A, Regazzo D, Tizianel I, Scaroni C, Ceccato F. Diagnostic Accuracy of CT Texture Analysis in Adrenal Masses: A Systematic Review. International Journal of Molecular Sciences. 2022; 23(2):637. https://doi.org/10.3390/ijms23020637
Chicago/Turabian StyleCrimì, Filippo, Emilio Quaia, Giulio Cabrelle, Chiara Zanon, Alessia Pepe, Daniela Regazzo, Irene Tizianel, Carla Scaroni, and Filippo Ceccato. 2022. "Diagnostic Accuracy of CT Texture Analysis in Adrenal Masses: A Systematic Review" International Journal of Molecular Sciences 23, no. 2: 637. https://doi.org/10.3390/ijms23020637
APA StyleCrimì, F., Quaia, E., Cabrelle, G., Zanon, C., Pepe, A., Regazzo, D., Tizianel, I., Scaroni, C., & Ceccato, F. (2022). Diagnostic Accuracy of CT Texture Analysis in Adrenal Masses: A Systematic Review. International Journal of Molecular Sciences, 23(2), 637. https://doi.org/10.3390/ijms23020637











