Immunomodulatory Properties and Osteogenic Activity of Polyetheretherketone Coated with Titanate Nanonetwork Structures
Abstract
:1. Introduction
2. Results
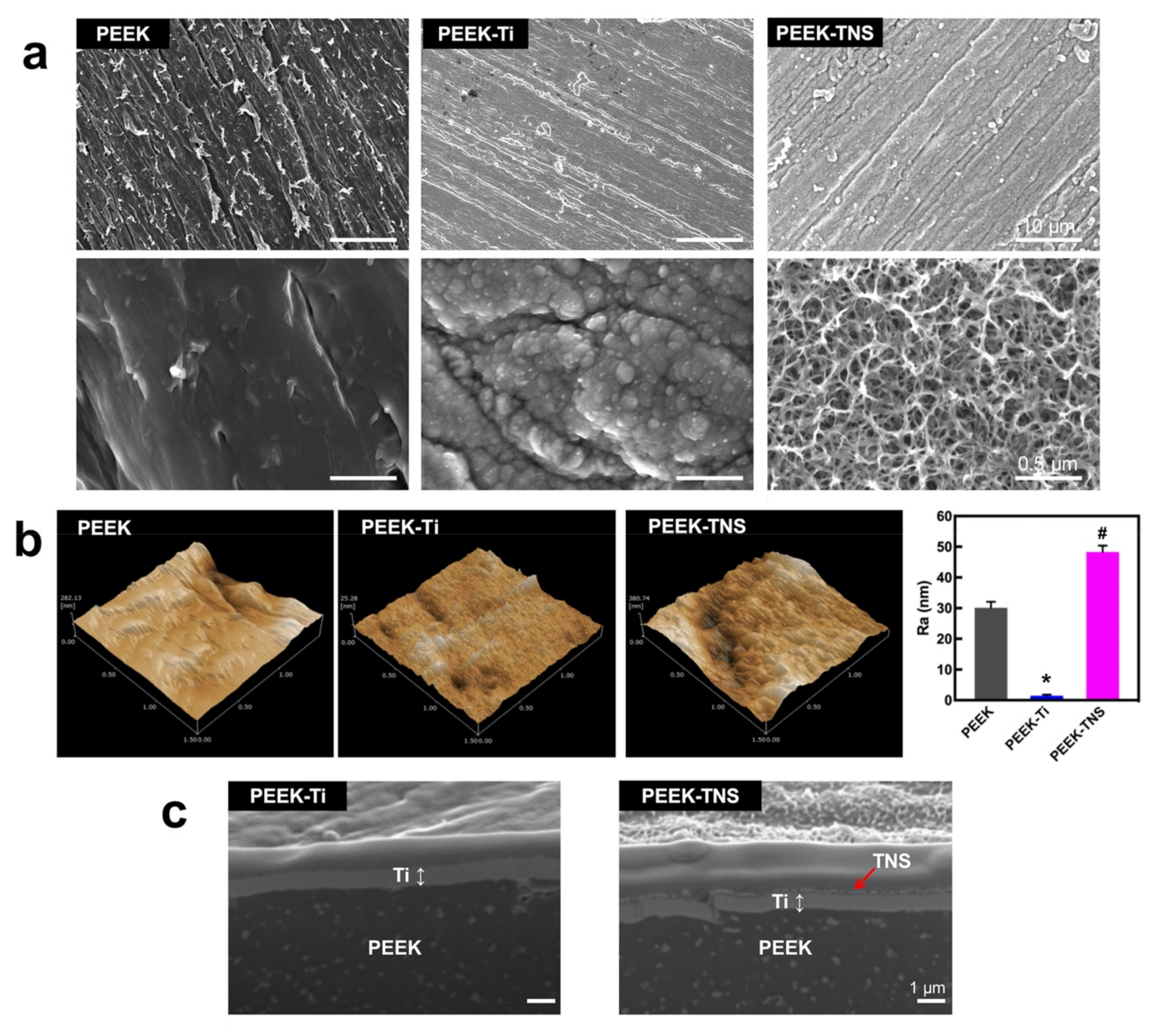
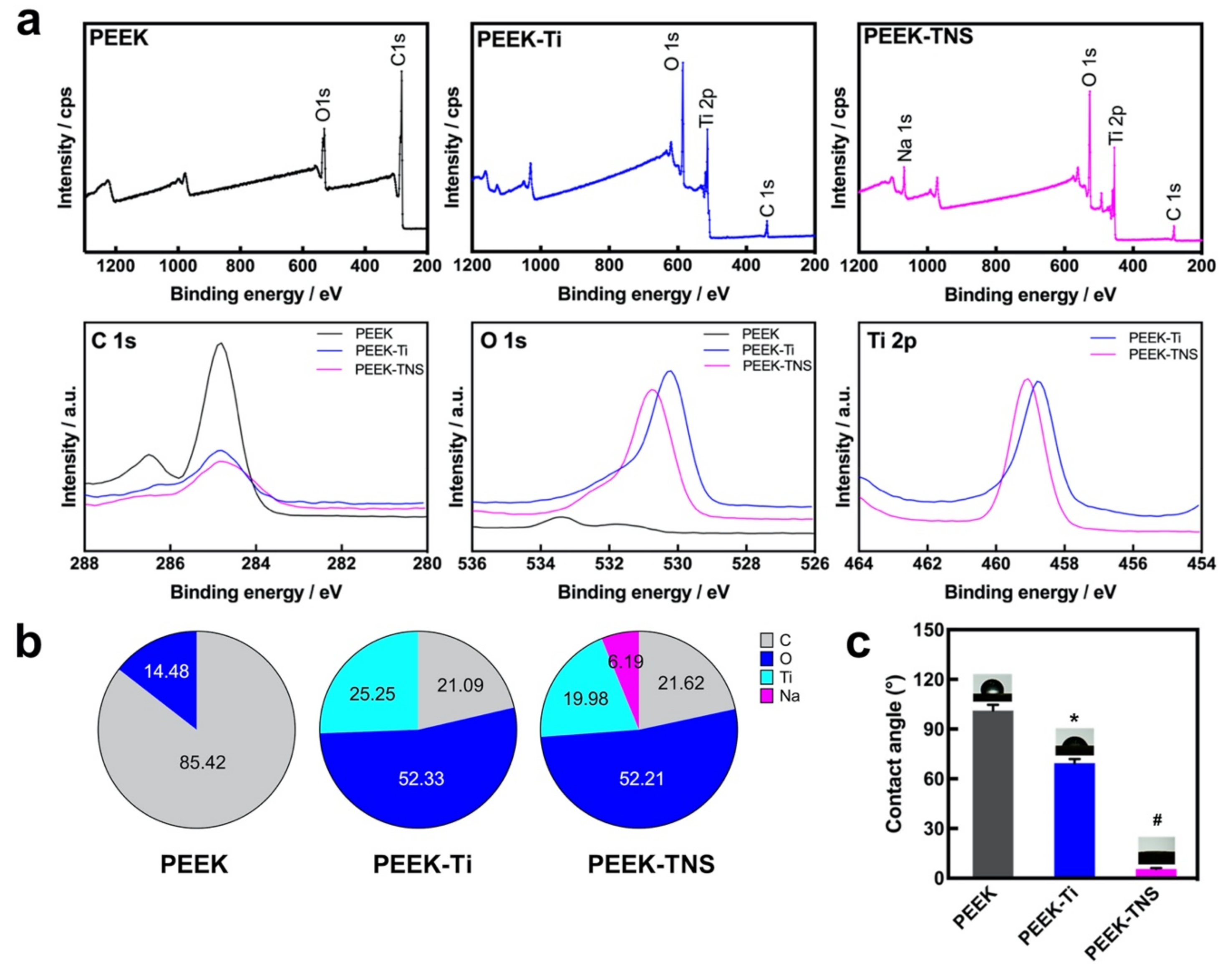
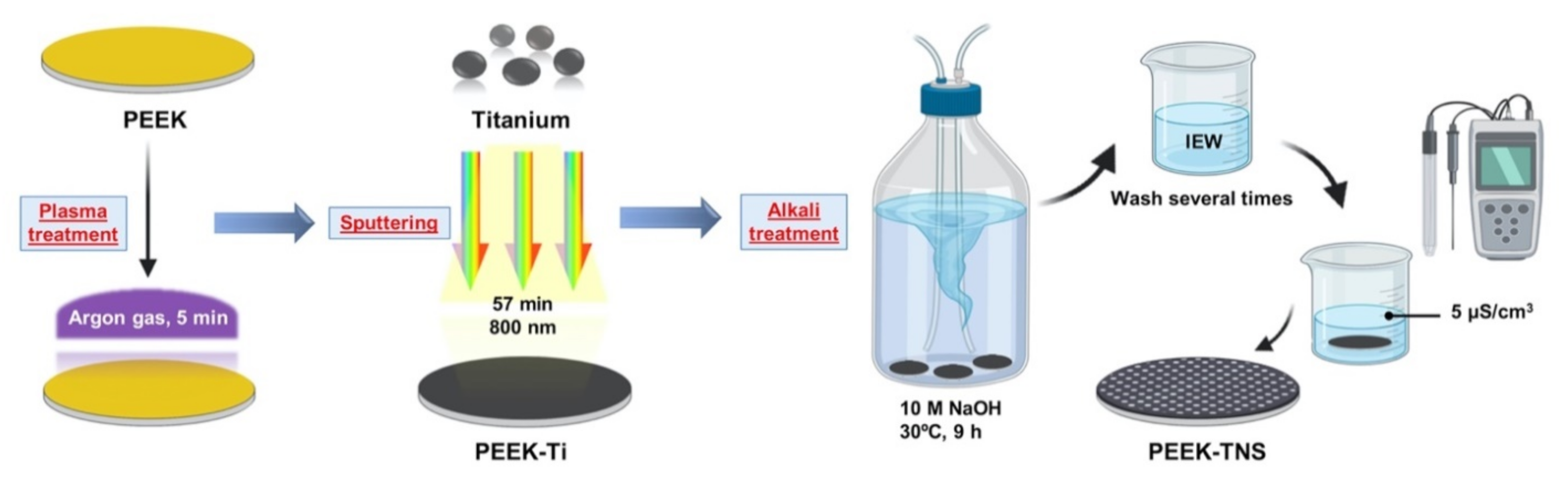
2.1. Surface Characterization
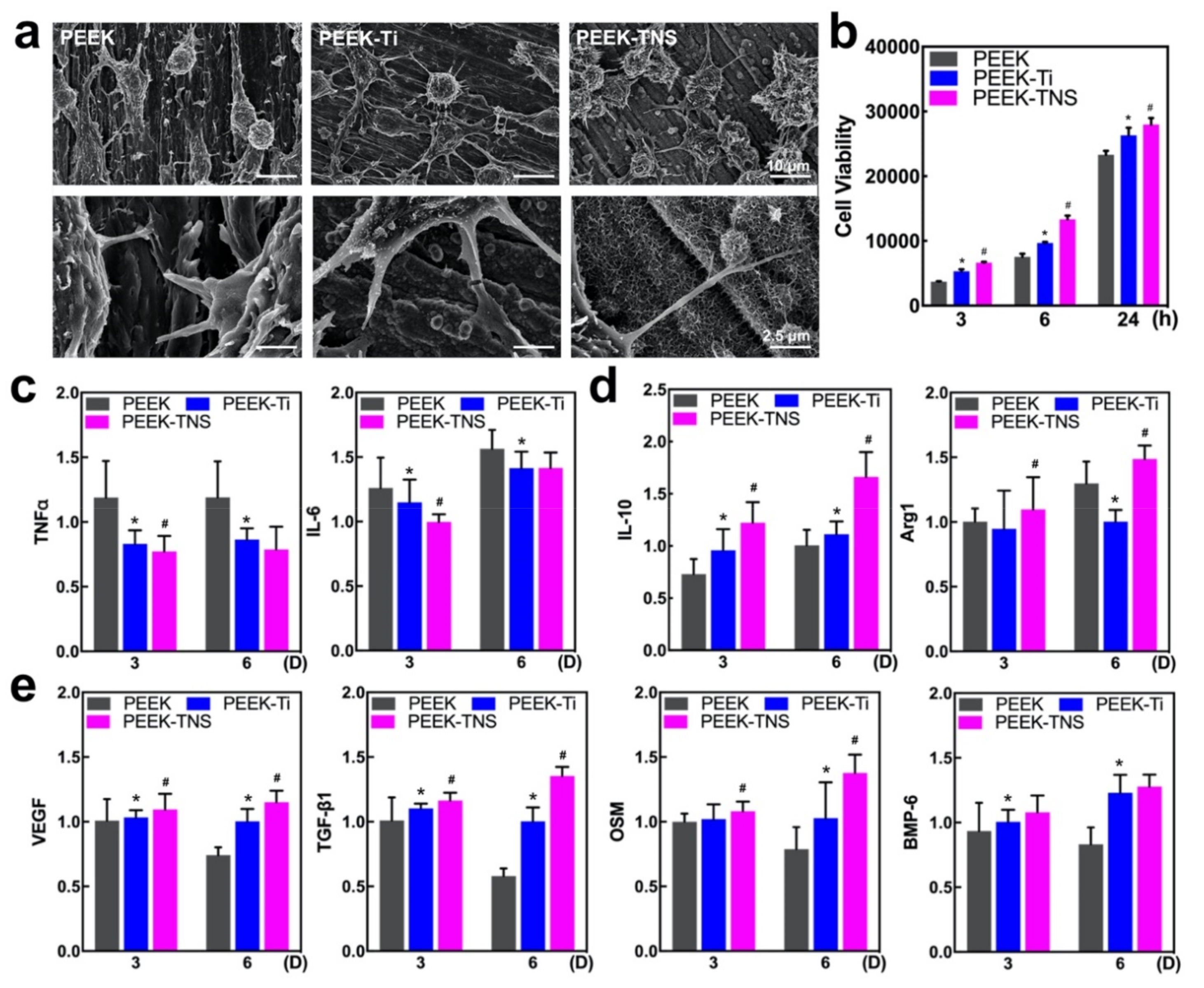
2.2. Inflammatory Response of Macrophages
2.2.1. Cell Morphology and Viability of Macrophages
2.2.2. Polarization of Macrophages
2.2.3. Osteogenic Activities of Macrophages
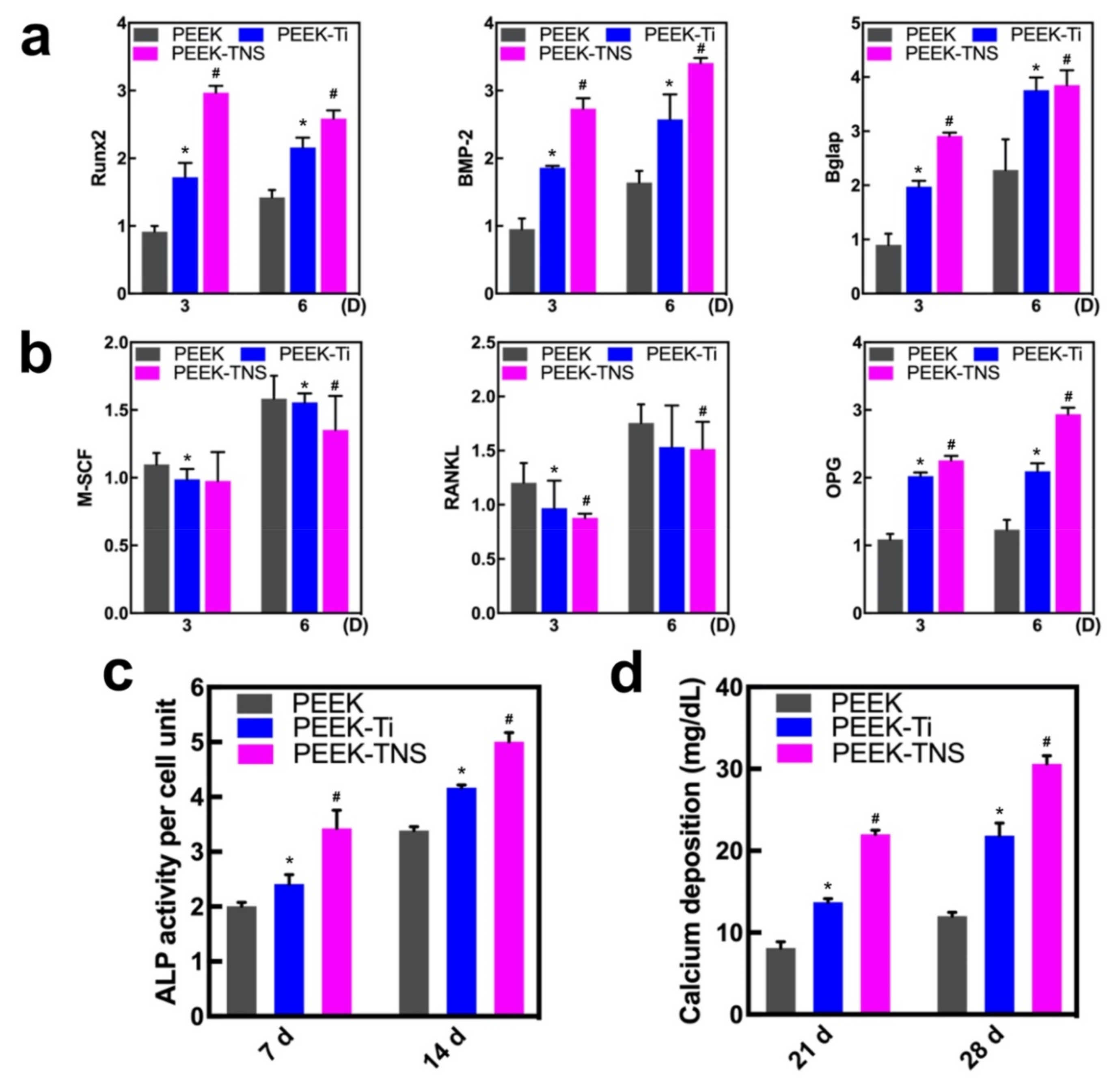
2.3. Osteogenic Differentiation of rBMMSCs Induced by Macrophages
2.3.1. Osteogenesis and Osteoclastogenesis-Related Gene Expression of rBMMSCs
2.3.2. Alkaline Phosphatase (ALP) Activity and ECM Mineralization of rBMMSCs
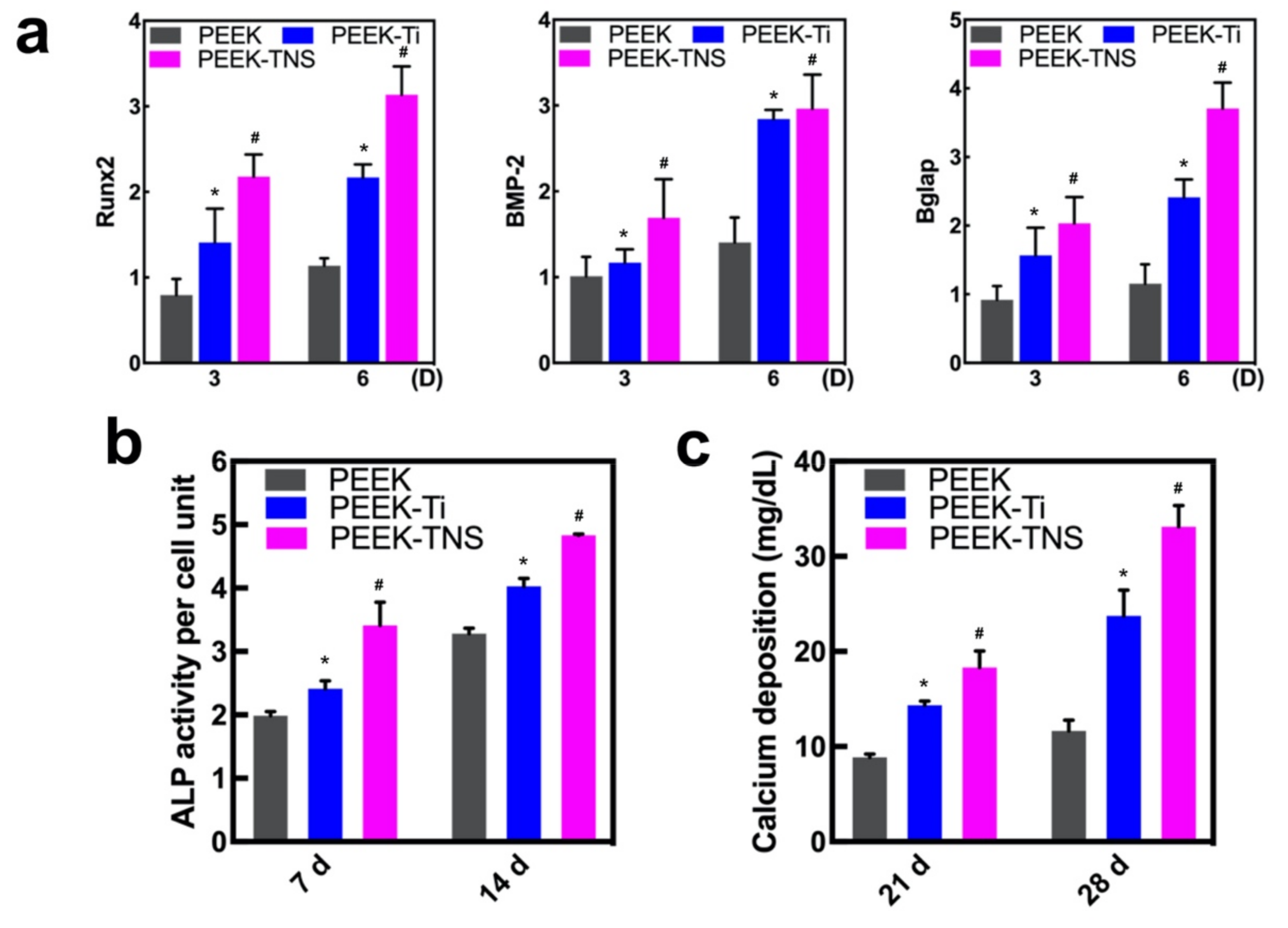
2.4. Osteogenic Differentiation of rBMMSCs on Different Samples
2.4.1. Cell Morphology and Viability of rBMMSCs on Different Samples
2.4.2. Osteogenic Behavior of rBMMSCs on Different Samples
3. Discussion
4. Materials and Methods
4.1. Surface Preparation
4.2. Sample Characterization
4.3. Cell Culture
4.4. Inflammatory Response of Macrophages
4.4.1. Cell Morphology and Viability of Macrophages
4.4.2. Polarization of Macrophages
4.4.3. Osteogenic Gene Expression of Macrophages
4.5. Cell Growth and Osteogenic Behavior of rBMMSCs Induced by Macrophages
4.5.1. Preparation of Macrophage-Conditioned Medium (CM) for rBMMSCs
4.5.2. Osteogenesis and Osteoclastogenesis-Related Gene Expression of rBMMSCs
4.5.3. Alkaline Phosphatase (ALP) Activity of rBMMSCs
4.5.4. ECM Mineralization
4.6. Cell Growth and Osteogenic Behavior of rBMMSCs on Different Samples
4.6.1. Cell Morphology and Viability of rBMMSCs on Different Samples
4.6.2. Osteogenic Behavior of rBMMSCs on Different Samples
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurtz, S.M.; Devine, J.N. PEEK Biomaterials in Trauma, Orthopedic, and Spinal Implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.B.; Lih, E.; Park, K.; Joung, Y.K.; Han, D.K. Biopolymer-Based Functional Composites for Medical Applications. Prog. Polym. Sci. 2017, 68, 77–105. [Google Scholar] [CrossRef]
- Seaman, S.; Kerezoudis, P.; Bydon, M.; Torner, J.C.; Hitchon, P.W. Titanium vs. Polyetheretherketone (PEEK) Interbody Fusion: Meta-Analysis and Review of the Literature. J. Clin. Neurosci. 2017, 44, 23–29. [Google Scholar] [CrossRef]
- Eliades, T.; Pratsinis, H.; Kletsas, D.; Eliades, G.; Makou, M. Characterization and Cytotoxicity of Ions Released From Stainless Steel and Nickel–Titanium Orthodontic Alloys. Am. J. Orthod. Dentofac. Orthop. 2004, 125, 24–29. [Google Scholar] [CrossRef]
- Ortiz, A.J.; Fernández, E.; Vicente, A.; Calvo, J.L.; Ortiz, C. Metallic Ions Released From Stainless Steel, Nickel-Free, and Titanium Orthodontic Alloys: Toxicity and DNA Damage. Am. J. Orthod. Dentofac. Orthop. 2011, 140, e115–e122. [Google Scholar] [CrossRef]
- Huiskes, R.; Weinans, H.; van Rietbergen, B. The Relationship between Stress Shielding and Bone Resorption around Total Hip Stems and the Effects of Flexible Materials. Clin. Orthop. Relat. Res. 1992, 274, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, R.D.; Klosterhoff, B.S.; Torstrick, F.B.; Foley, K.T.; Burkus, J.K.; Lee, C.S.D.; Gall, K.; Guldberg, R.E.; Safranski, D.L. Effect of Porous Orthopaedic Implant Material and Structure on Load Sharing With Simulated Bone Ingrowth: A Finite Element Analysis Comparing Titanium and PEEK. J. Mech. Behav. Biomed. Mater. 2018, 80, 68–76. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of Polyetheretherketone (PEEK) in Oral Implantology and Prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for Medical Applications. J. Mater. Sci. Mater. Med. 2016, 27, 118. [Google Scholar] [CrossRef]
- Noiset, O.; Schneider, Y.J.; Marchand-Brynaert, J. Fibronectin Adsorption Or/and Covalent Grafting on Chemically Modified PEEK Film Surfaces. J. Biomater. Sci. Polym. Ed. 1999, 10, 657–677. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Lu, X.; Yang, L.; Yang, H.; Yuan, W.; Chen, D. Comparison of Titanium and Polyetheretherketone (PEEK) Cages in the Surgical Treatment of Multilevel Cervical Spondylotic Myelopathy: A Prospective, Randomized, Control Study With Over 7-Year Follow-Up. Eur. Spine J. 2013, 22, 1539–1546. [Google Scholar] [CrossRef] [Green Version]
- Dong, T.; Duan, C.; Wang, S.; Gao, X.; Yang, Q.; Yang, W.; Deng, Y. Multifunctional Surface With Enhanced Angiogenesis for Improving Long-Term Osteogenic Fixation of Poly(Ether Ether Ketone) Implants. ACS Appl. Mater. Interfaces 2020, 12, 14971–14982. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Wang, L.; Li, Y.; Pan, J.; Fu, X.; Luo, Z.; Sui, Y.; Zhang, S.; Wang, L.; et al. Triple-Functional Polyetheretherketone Surface With Enhanced Bacteriostasis and Anti-Inflammatory and Osseointegrative Properties for Implant Application. Biomaterials 2019, 212, 98–114. [Google Scholar] [CrossRef]
- Wang, L.; He, S.; Wu, X.; Liang, S.; Mu, Z.; Wei, J.; Deng, F.; Deng, Y.; Wei, S. Polyetheretherketone/Nano-Fluorohydroxyapatite Composite With Antimicrobial Activity and Osseointegration Properties. Biomaterials 2014, 35, 6758–6775. [Google Scholar] [CrossRef]
- Yuan, X.; Ouyang, L.; Luo, Y.; Sun, Z.; Yang, C.; Wang, J.; Liu, X.; Zhang, X. Multifunctional Sulfonated Polyetheretherketone Coating With Beta-defensin-14 for Yielding Durable and Broad-Spectrum Antibacterial Activity and Osseointegration. Acta Biomater. 2019, 86, 323–337. [Google Scholar] [CrossRef]
- Sola, A.; Bellucci, D.; Cannillo, V.; Cattini, A. Bioactive Glass Coatings: A Review. Surf. Eng. 2011, 27, 560–572. [Google Scholar] [CrossRef]
- Qin, W.; Li, Y.; Ma, J.; Liang, Q.; Cui, X.; Jia, H.; Tang, B. Osseointegration and Biosafety of Graphene Oxide Wrapped Porous CF/PEEK Composites as Implantable Materials: The Role of Surface Structure and Chemistry. Dent. Mater. 2020, 36, 1289–1302. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Pan, Z.; Ma, M.; Xu, Z.; Mei, S.; Jiang, Z.; Yin, F. Nanostructured Coating of Non-Crystalline Tantalum Pentoxide on Polyetheretherketone Enhances RBMS Cells/HGE Cells Adhesion. Int. J. Nanomed. 2021, 16, 725–740. [Google Scholar] [CrossRef]
- Ding, R.; Chen, T.; Xu, Q.; Wei, R.; Feng, B.; Weng, J.; Duan, K.; Wang, J.; Zhang, K.; Zhang, X. Mixed Modification of the Surface Microstructure and Chemical State of Polyetheretherketone to Improve Its Antimicrobial Activity, Hydrophilicity, Cell Adhesion, and Bone Integration. ACS Biomater. Sci. Eng. 2020, 6, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wang, J.; Li, C.; Ma, K.; Wei, J.; Yang, P.; Guo, D.; Wang, K.; Wang, W. Effects of Different Sulfonation Times and Post-Treatment Methods on the Characterization and Cytocompatibility of Sulfonated PEEK. J. Biomater. Appl. 2020, 35, 342–352. [Google Scholar] [CrossRef]
- Riveiro, A.; Soto, R.; Comesaña, R.; Boutinguiza, M.D.; del Val, J.; Quintero, F.; Lusquiños, F.; Pou, J. Laser Surface Modification of PEEK. Appl. Surf. Sci. 2012, 258, 9437–9442. [Google Scholar] [CrossRef]
- Wang, W.; Luo, C.J.; Huang, J.; Edirisinghe, M. PEEK Surface Modification by Fast Ambient-Temperature Sulfonation for Bone Implant Applications. J. R. Soc. Interface 2019, 16, 20180955. [Google Scholar] [CrossRef] [Green Version]
- Hassan, E.A.M.; Elagib, T.H.H.; Memon, H.; Yu, M.; Zhu, S. Surface Modification of Carbon Fibers by Grafting Peek-nh2 for Improving Interfacial Adhesion with Polyetheretherketone. Materials 2019, 12, 778. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Xiong, C.; Wang, Z.; Li, X.; Zhang, L. A Combination of CO2 Laser and Plasma Surface Modification of Poly(Etheretherketone) to Enhance Osteoblast Response. Appl. Surf. Sci. 2015, 344, 79–88. [Google Scholar] [CrossRef]
- Ourahmoune, R.M.S.; Salvia, M.; Mathia, T.G.; Mesrati, N. Surface Morphology and Wettability of Sandblasted PEEK and Its Composites. Scanning 2014, 36, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Parvaiz, M.R.; Mohanty, S.; Nayak, S.K.; Mahanwar, P.A. Effect of surface modification of fly ash on the mechanical, thermal, electrical and morphological properties of polyetheretherketone composites. Mater. Sci. Eng. A 2011, 528, 4277–4286. [Google Scholar] [CrossRef]
- Torstrick, F.B.; Lin, A.S.P.; Potter, D.; Safranski, D.L.; Sulchek, T.A.; Gall, K.; Guldberg, R.E. Porous PEEK Improves the Bone-Implant Interface Compared to Plasma-Sprayed Titanium Coating on PEEK. Biomaterials 2018, 185, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Torstrick, F.B.; Klosterhoff, B.S.; Westerlund, L.E.; Foley, K.T.; Gochuico, J.; Lee, C.S.D.; Gall, K.; Safranski, D.L. Impaction Durability of Porous Polyether-Ether-Ketone (PEEK) and Titanium-Coated PEEK Interbody Fusion Devices. Spine J. 2018, 18, 857–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, S.D.; Rust-Dawicki, A.M. Preliminary Evaluation of Titanium-Coated PEEK Dental Implants. J. Oral Implantol. 1995, 21, 176–181. [Google Scholar]
- Han, C.M.; Lee, E.J.; Kim, H.E.; Koh, Y.H.; Kim, K.N.; Ha, Y.; Kuh, S.U. The Electron Beam Deposition of Titanium on Polyetheretherketone (PEEK) and the Resulting Enhanced Biological Properties. Biomaterials 2010, 31, 3465–3470. [Google Scholar] [CrossRef]
- Vogel, D.; Dempwolf, H.; Baumann, A.; Bader, R. Characterization of Thick Titanium Plasma Spray Coatings on PEEK Materials Used for Medical Implants and the Influence on the Mechanical Properties. J. Mech. Behav. Biomed. Mater. 2018, 77, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Ushirozako, H.; Shigeto, E.; Ohba, T.; Oba, H.; Mukaiyama, K.; Shimizu, S.; Yamato, Y.; Ide, K.; Shibata, Y.; et al. The Titanium-Coated PEEK Cage Maintains Better Bone Fusion With the Endplate Than the PEEK Cage 6 Months After PLIF Surgery: A Multicenter, Prospective, Randomized Study. Spine 2020, 45, E892–E902. [Google Scholar] [CrossRef]
- Sargin, F.; Erdogan, G.; Kanbur, K.; Turk, A. Investigation of In Vitro Behavior of Plasma Sprayed Ti, TiO2 and HA Coatings on PEEK. Surf. Coat. Technol. 2021, 411. [Google Scholar] [CrossRef]
- Kotsias, A.; Mularski, S.; Kühn, B.; Hanna, M.; Suess, O. Does Partial Coating With Titanium Improve the Radiographic Fusion Rate of Empty PEEK Cages in Cervical Spine Surgery? A Comparative Analysis of Clinical Data. Patient Saf. Surg. 2017, 11, 13. [Google Scholar] [CrossRef]
- Walsh, W.R.; Bertollo, N.; Christou, C.; Schaffner, D.; Mobbs, R.J. Plasma-Sprayed Titanium Coating to Polyetheretherketone Improves the Bone-Implant Interface. Spine J. 2015, 15, 1041–1049. [Google Scholar] [CrossRef]
- Taherian, M.H.; Rezazadeh, M.; Taji, A. Optimum Surface Roughness for Titanium-Coated PEEK Produced by Electron Beam PVD for Orthopedic Applications. Mater. Technol. 2021, 1–14. [Google Scholar] [CrossRef]
- Su, Y.; Komasa, S.; Sekino, T.; Nishizaki, H.; Okazaki, J. Nanostructured Ti6Al4V Alloy Fabricated Using Modified Alkali-Heat Treatment: Characterization and Cell Adhesion. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yang, Y.; Chen, L.; Yin, D.; Zhang, H.; Tashiro, Y.; Inui, S.; Kusumoto, T.; Nishizaki, H.; Sekino, T.; et al. Optimized Surface Characteristics and Enhanced In Vivo Osseointegration of Alkali-Treated Titanium With Nanonetwork Structures. Int. J. Mol. Sci. 2019, 20, 1127. [Google Scholar] [CrossRef] [Green Version]
- Tashiro, Y.; Komasa, S.; Miyake, A.; Nishizaki, H.; Okazaki, J. Analysis of titania Nanosheet Adsorption Behavior Using a Quartz Crystal Microbalance Sensor. Adv. Mater. Sci. Eng. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kusumoto, T.; Yin, D.; Zhang, H.; Chen, L.; Nishizaki, H.; Komasa, Y.; Okazaki, J.; Komasa, S. Evaluation of the Osteointegration of a Novel Alkali-Treated Implant System In Vivo. J. Hard Tissue Biol. 2017, 26, 355–360. [Google Scholar] [CrossRef]
- Lorenzo, J.; Horowitz, M.; Choi, Y. Osteoimmunology: Interactions of the Bone and Immune System. Endocr. Rev. 2008, 29, 403–440. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Hu, C.; Feng, Y.; Li, D.; Ai, T.; Huang, Y.; Xiaodan, C.; Lijia, H.; Jiali, T. Osteoimmunomodulatory Effects of Biomaterial Modification Strategies on Macrophage Polarization and Bone Regeneration. Regen. Biomater. 2020, 7, 233–245. [Google Scholar] [CrossRef]
- Bai, J.; Wang, H.; Chen, H.; Ge, G.; Wang, M.; Gao, A.; Tong, L.; Xu, Y.; Yang, H.; Pan, G.; et al. Biomimetic Osteogenic Peptide With Mussel Adhesion and Osteoimmunomodulatory Functions to Ameliorate Interfacial Osseointegration Under Chronic Inflammation. Biomaterials 2020, 255, 120197. [Google Scholar] [CrossRef]
- Avery, S.J.; Ayre, W.N.; Sloan, A.J.; Waddington, R.J. Interrogating the Osteogenic Potential of Implant Surfaces In Vitro: A Review of Current Assays. Tissue Eng. Part B Rev. 2020, 26, 217–229. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving Concepts in Bone-Immune Interactions in Health and Disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef]
- Okamoto, K.; Nakashima, T.; Shinohara, M.; Negishi-Koga, T.; Komatsu, N.; Terashima, A.; Sawa, S.; Nitta, T.; Takayanagi, H. Osteoimmunology: The Conceptual Framework Unifying the Immune and Skeletal Systems. Physiol. Rev. 2017, 97, 1295–1349. [Google Scholar] [CrossRef]
- Takayanagi, H. Osteoimmunology: Shared Mechanisms and Crosstalk between the Immune and Bone Systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef]
- Chen, Z.; Klein, T.; Murray, R.Z.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. Osteoimmunomodulation for the Development of Advanced Bone Biomaterials. Mater. Today 2016, 19, 304–321. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage Polarization: An Opportunity for Improved Outcomes in Biomaterials and Regenerative Medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage Activation and Polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Böse, T.; Unger, R.E.; Jansen, J.A.; Kirkpatrick, C.J.; van den Beucken, J.J.J.P. Macrophage Type Modulates Osteogenic Differentiation of Adipose Tissue MSCs. Cell Tissue Res. 2017, 369, 273–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.; Zhang, Q.; Li, J.; Zhang, L.; Jokerst, J.V. A gold/silver hybrid nanoparticle for treatment and photoacoustic imaging of bacterial infection. ACS Nano 2018, 12, 5562–5615. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, F.; Chen, H.; Tan, H.; Yang, L.; Zhang, L.; Xie, J.; Sun, J.; Hiang, X.; Huang, Y. Postnatal exposure to DINP was associated with greater alterations of lipidomic markers for hepatic steatosis than DEHP in postweaning mice. Sci. Total Environ. 2021, 758, 143631. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Guo, G.; Wang, Q.; Tang, J.; Zhao, Y.; Qin, H.; Wahafu, T.; Shen, H.; Liu, X.; et al. Silver-nanoparticles-modified biomaterial surface resistant to staphylococcus: New insight into the antimicrobial action of silver. Sci. Rep. 2016, 6, 32699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A Critical Review of Multifunctional Titanium Surfaces: New Frontiers for Improving Osseointegration and Host Response, Avoiding Bacteria Contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Saruwatari, L.; Nakamura, H.K.; Yang, J.M.; Ogawa, T. Enhanced Intrinsic Biomechanical Properties of Osteoblastic Mineralized Tissue on Roughened Titanium Surface. J. Biomed. Mater. Res. A 2005, 72, 296–305. [Google Scholar] [CrossRef]
- Ogawa, T.; Nishimura, I. Genes Differentially Expressed in Titanium Implant Healing. J. Dent. Res. 2006, 85, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Butz, F.; Aita, H.; Wang, C.J.; Ogawa, T. Harder and Stiffer Bone Osseointegrated to Roughened Titanium. J. Dent. Res. 2006, 85, 560–565. [Google Scholar] [CrossRef]
- Klein, M.O.; Bijelic, A.; Ziebart, T.; Koch, F.; Kämmerer, P.W.; Wieland, M.; Al-Nawas, B. Submicron scale-structured hydrophilic titanium surfaces promote early osteogenic gene response for cell adhesion and cell differentiation. Clin. Implant. Dent. Relat. Res. 2013, 15, 166–175. [Google Scholar] [CrossRef]
- Yang, G.; Feng, X.; Wang, W.; Ou Yang, Q.; Liu, L. Effective interlaminar reinforcing and delamination monitoring of carbon fibrous composites using a novel nano-carbon woven grid. Compos. Sci. Technol. 2021, 213, 108959. [Google Scholar] [CrossRef]
- Yan, S.; Yan, J.; Liu, D.; Li, X.; Kang, Q.; You, W.; Zhang, J.; Wang, L.; Tian, Z.; Lu, W.; et al. A nano-predator of pathological MDMX construct by clearable supramolecular gold(I)-thiol-peptide complexes achieves safe and potent anti-tumor activity. Theranostics 2021, 11, 6833–6846. [Google Scholar] [CrossRef]
- Eriksson, C.; Nygren, H. Polymorphonuclear Leukocytes in Coagulating Whole Blood Recognize Hydrophilic and Hydrophobic Titanium Surfaces by Different Adhesion Receptors and Show Different Patterns of Receptor Expression. J. Lab. Clin. Med. 2001, 137, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, D.; Li, Y.; Yang, F.; Hou, L.; Yu, Y.; Sun, C.; Duan, G.; Meng, C.; Yan, H.; et al. Paraquat Promotes Acute Lung Injury in Rats by Regulating Alveolar Macrophage Polarization Through Glycolysis. Ecotoxicol. Environ. Saf. 2021, 223, 112571. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.L.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Bueno, D.F.; Amano, M.T. Macrophage: A Potential Target on Cartilage Regeneration. Front. Immunol. 2020, 11, 111. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, Y.; Wang, Y.; Wang, Y.; Yang, Y.; Zhao, J.; Li, M.; Tian, X.; Zeng, X. Association between Acute Phase Reactants, Interleukin-6, Tumor Necrosis Factor-α, and Disease Activity in Takayasu’s Arteritis Patients. Arthritis Res. Ther. 2020, 22, 285. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Cui, F.; Cao, C.; Wang, Q.; Zou, Q. Single-cell RNA analysis reveals the potential risk of organ-specific cell types vulnerable to SARS-CoV-2 infections. Comput. Biol. Med. 2021, 140, 105092. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor Necrosis Factor-α Signaling in Macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Hanada, T.; Yoshimura, A. Regulation of Cytokine Signaling and Inflammation. Cytokine Growth Factor Rev. 2002, 13, 413–421. [Google Scholar] [CrossRef]
- Vukicevic, S.; Grgurevic, L. BMP-6 and Mesenchymal Stem Cell Differentiation. Cytokine Growth Factor Rev. 2009, 20, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Nicolaidou, V.; Wong, M.M.; Redpath, A.N.; Ersek, A.; Baban, D.F.; Williams, L.M.; Cope, A.P.; Horwood, N.J. Monocytes Induce STAT3 Activation in Human Mesenchymal Stem Cells to Promote Osteoblast Formation. PLoS ONE 2012, 7, e39871. [Google Scholar] [CrossRef] [Green Version]
- Omar, O.; Lennerås, M.; Svensson, S.; Suska, F.; Emanuelsson, L.; Hall, J.; Nannmark, U.; Thomsen, P. Integrin and Chemokine Receptor Gene Expression in Implant-Adherent Cells During Early Osseointegration. J. Mater. Sci. Mater. Med. 2010, 21, 969–980. [Google Scholar] [CrossRef]
- Simunek, A.; Kopecka, D.; Brazda, T.; Strnad, I.; Capek, L.; Slezak, R. Development of Implant Stability During Early Healing of Immediately Loaded Implants. Int. J. Oral Maxillofac. Implants 2012, 27, 619–627. [Google Scholar]
- Borciani, G.; Montalbano, G.; Baldini, N.; Cerqueni, G.; Vitale-Brovarone, C.; Ciapetti, G. Co-Culture Systems of Osteoblasts and Osteoclasts: Simulating In Vitro Bone Remodeling in Regenerative Approaches. Acta Biomater. 2020, 108, 22–45. [Google Scholar] [CrossRef]
- Bächle, M.; Kohal, R.J. A Systematic Review of the Influence of Different Titanium Surfaces on Proliferation, Differentiation and Protein Synthesis of Osteoblast-Like MG63 Cells. Clin. Oral Implants Res. 2004, 15, 683–692. [Google Scholar] [CrossRef]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High Surface Energy Enhances Cell Response to Titanium Substrate Microstructure. J. Biomed. Mater. Res. A 2005, 74, 49–58. [Google Scholar] [CrossRef]
- Boyan, B.D.; Bonewald, L.F.; Paschalis, E.P.; Lohmann, C.H.; Rosser, J.; Cochran, D.L.; Dean, D.D.; Schwartz, Z.; Boskey, A.L. Osteoblast-Mediated Mineral Deposition in Culture Is Dependent on Surface Microtopography. Calcif. Tissue Int. 2002, 71, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.S.; Lian, J.B. Molecular Mechanisms Mediating Proliferation/Differentiation Interrelationships During Progressive Development of the Osteoblast Phenotype. Endocr. Rev. 1993, 14, 424–442. [Google Scholar] [CrossRef]
- Owen, T.A.; Aronow, M.; Shalhoub, V.; Barone, L.M.; Wilming, L.; Tassinari, M.S.; Kennedy, M.B.; Pockwinse, S.; Lian, J.B.; Stein, G.S. Progressive Development of the Rat Osteoblast Phenotype In Vitro: Reciprocal Relationships in Expression of Genes Associated With Osteoblast Proliferation and Differentiation During Formation of the Bone Extracellular Matrix. J. Cell. Physiol. 1990, 143, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Alborzi, A.; Mac, K.; Glackin, C.A.; Murray, S.S.; Zernik, J.H. Endochondral and Intramembranous Fetal Bone Development: Osteoblastic Cell Proliferation, and Expression of Alkaline Phosphatase, m-Twist, and Histone H4. J. Craniofac. Genet. Dev. Biol. 1996, 16, 94–106. [Google Scholar] [PubMed]
- Siddhanti, S.R.; Quarles, L.D. Molecular to Pharmacologic Control of Osteoblast Proliferation and Differentiation. J. Cell. Biochem. 1994, 55, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takayanagi, H. Osteoclasts, Rheumatoid Arthritis, and Osteoimmunology. Curr. Opin. Rheumatol. 2006, 18, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Takegahara, N.; Kim, H.; Choi, Y. Updating Osteoimmunology: Regulation of Bone Cells by Innate and Adaptive Immunity. Nat. Rev. Rheumatol. 2018, 14, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, M. Rankl and Osteoimmunology in Periodontitis. J. Bone Miner. Metab. 2021, 39, 82–90. [Google Scholar] [CrossRef]
- Zhang, H.; Komasa, S.; Mashimo, C.; Sekino, T.; Okazaki, J. Effect of Ultraviolet Treatment on Bacterial Attachment and Osteogenic Activity to Alkali-Treated Titanium With Nanonetwork Structures. Int. J. Nanomed. 2017, 12, 4633–4646. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, H.; Komasa, S.; Kusumoto, T.; Kuwamoto, S.; Okunishi, T.; Kobayashi, Y.; Hashimoto, Y.; Sekino, T.; Okazaki, J. Immunomodulatory Properties and Osteogenic Activity of Polyetheretherketone Coated with Titanate Nanonetwork Structures. Int. J. Mol. Sci. 2022, 23, 612. https://doi.org/10.3390/ijms23020612
Yang Y, Zhang H, Komasa S, Kusumoto T, Kuwamoto S, Okunishi T, Kobayashi Y, Hashimoto Y, Sekino T, Okazaki J. Immunomodulatory Properties and Osteogenic Activity of Polyetheretherketone Coated with Titanate Nanonetwork Structures. International Journal of Molecular Sciences. 2022; 23(2):612. https://doi.org/10.3390/ijms23020612
Chicago/Turabian StyleYang, Yuanyuan, Honghao Zhang, Satoshi Komasa, Tetsuji Kusumoto, Shinsuke Kuwamoto, Tohru Okunishi, Yasuyuki Kobayashi, Yoshiya Hashimoto, Tohru Sekino, and Joji Okazaki. 2022. "Immunomodulatory Properties and Osteogenic Activity of Polyetheretherketone Coated with Titanate Nanonetwork Structures" International Journal of Molecular Sciences 23, no. 2: 612. https://doi.org/10.3390/ijms23020612
APA StyleYang, Y., Zhang, H., Komasa, S., Kusumoto, T., Kuwamoto, S., Okunishi, T., Kobayashi, Y., Hashimoto, Y., Sekino, T., & Okazaki, J. (2022). Immunomodulatory Properties and Osteogenic Activity of Polyetheretherketone Coated with Titanate Nanonetwork Structures. International Journal of Molecular Sciences, 23(2), 612. https://doi.org/10.3390/ijms23020612